Abstract
Developmental progress of germ cells through meiotic phases is closely tied to ongoing meiotic recombination. In mammals, recombination preferentially occurs in genomic regions known as hotspots; the protein that activates these hotspots is PRDM9, containing a genetically variable zinc-finger domain and a PR-SET domain with histone H3K4 trimethyltransferase activity. PRDM9 is required for fertility in mice, but little is known about its localization and developmental dynamics. Application of spermatogenic stage-specific markers demonstrates that PRDM9 accumulates in male germ-cell nuclei at pre-leptonema to early leptonema, but is no longer detectable in nuclei by late zygonema. By the pachytene stage, PRDM9-dependent histone H3K4 trimethyl marks on hotspots also disappear. PRDM9 localizes to nuclei concurrently with the deposition of meiotic cohesin complexes, but is not required for incorporation of cohesin complex proteins into chromosomal axial elements, or accumulation of normal numbers of RAD51 foci on meiotic chromatin by late zygonema. Germ cells lacking PRDM9 exhibit inefficient homology recognition and synapsis, with aberrant repair of meiotic DNA double-strand breaks and transcriptional abnormalities characteristic of meiotic silencing of unsynapsed chromatin. Together, these results on the developmental time course for nuclear localization of PRDM9 establish its direct window of function, and demonstrate the independence of chromosome axial element formation from the concurrent PRDM9-mediated activation of recombination hotspots.
Keywords: meiosis, recombination, PRDM9, cohesin, synapsis, spermatogenesis
Introduction
The genetic hallmark of gametogenesis is meiosis, during which a protracted meiosis I prophase sets up meiotic and developmental competence of the gamete. Meiotic prophase I includes complex chromatin remodeling events that enable pairing, synapsis and recombination between homologous chromosomes. In mammalian males, meiosis is initiated by post-mitotic germ cells, the pre-leptotene spermatocytes, with a protracted period of DNA replication producing two sister chromatids linked by cohesin complexes, some of which are meiosis-specific (Bolcun-Filas and Schimenti 2012), contributing to the axial elements (AEs) of each homolog. The period beginning with the pre-leptotene S phase and culminating in homologous synapsis at the beginning of the pachytene stage is marked by dramatic chromatin remodeling, involving recombination site activation, condensation of chromatin, and assembly of a unique array of chromosomal proteins. After selection and activation of recombination sites, meiotic recombination-initiating double-strand DNA breaks (DSBs) are created by SPO11 (Bolcun-Filas and Schimenti 2012; Handel and Schimenti 2010). During leptonema, before chromosome pairing and synapsis, RAD51, DMC1 and phosphorylated histone H2AFX (P-H2AFX, also known as γH2AX) accumulate in chromatin surrounding the DSBs. Chromosome ends attach to the nuclear envelope and cluster into a “meiotic bouquet,” the function of which is unknown. Nuclear envelope association and/or the bouquet may facilitate chromosome movement and/or positioning (Scherthan 2001) leading to homologous chromosome pairing and initiation of synapsis during the zygotene stage. Both homologous chromosome synapsis and progress of recombination-based DSB repair occur in the context of a unique meiotic chromosomal scaffold, the synaptonemal complex (SC), consisting of the lateral elements (LEs), derived from the AEs, and a central element (CE) that “zips” the homologs into the intimate juxtaposition termed synapsis. The subsequent pachytene stage, defined cytologically by full synapsis, is when DNA exchange (crossover recombination) between homologous non-sister chromatids is manifest in focal structures termed recombination nodules, which contain the mismatch repair proteins MLH1 and MLH3. Recombination events are resolved toward the end of meiosis I prophase, when chromosomes condense further, with chiasmata are clearly visible at the diplotene stage, followed by the two meiotic divisions (Handel and Schimenti 2010).
The protein responsible for selection and activation of specific mammalian recombination sites is PRDM9 (Baudat, et al. 2010; Baudat, et al. 2013; Parvanov, et al. 2010), a multi-domain protein (Fig. 1a) characterized by C-terminal DNA-binding zinc finger (ZNF) domain, a PR-SET domain and both KRAB and SSXRD domains. PRDM9 has been shown by genetic and physical assays to bind to recombination hotspots and the different allelic variants of PRDM9 account for variability in hotspot selection in mice (Baker, et al. 2014; Baudat, et al. 2010; Billings, et al. 2013; Borde and de Massy 2013; de Massy 2013; Paigen and Petkov 2010; Parvanov, et al. 2010). As predicted by its SET domain, PRDM9 has histone H3 lysine 4 (H3K4) trimethyltransferase activity (Hayashi, et al. 2005) and is responsible for H3K4me3 marks at hotspots of mouse spermatocytes and subsequent nucleosome rearrangement (Baker, et al. 2014; Baudat, et al. 2013; Brick, et al. 2012; de Massy 2013).
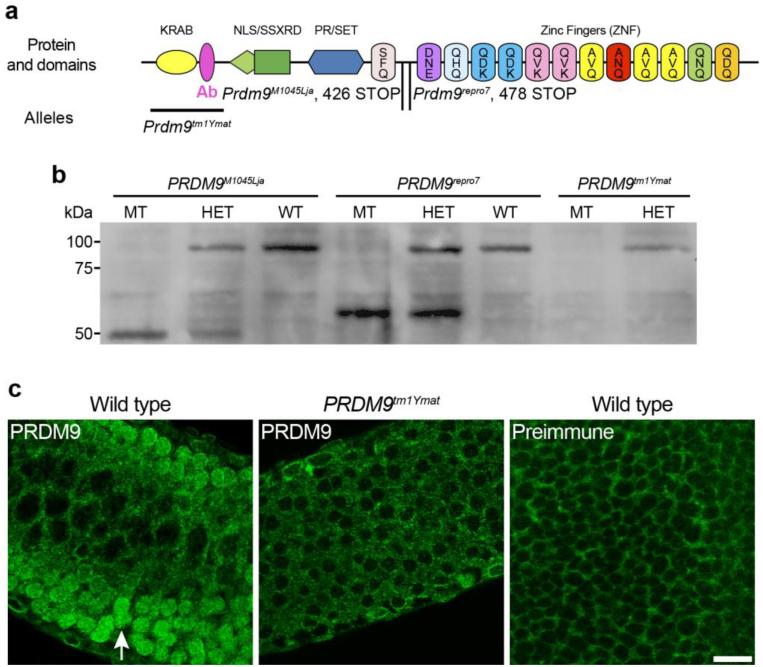
Fig. 1.
PRDM9 protein domains and characterization of the antibody. a: Protein domain structure of PRDM9, showing the position of the antigen (pink, Ab) used for antibody production. Illustrated below the protein diagram are the extent of the deletion in the Prdm9tm1Ymat allele, and the position of the STOP codon in the Prdm9M1045Lja and Prdm9repro7 alleles. b: Western-blot analysis of testis extracts from the three distinct Prdm9 mutants, using the custom-produced PRDM9 antibody. PRDM9 protein (~97 kDa) is detected in whole testis extracts of wild type and heterozygous mice, but is absent in extracts from homozygous mutants bearing any of the three alleles. Bands indicating a truncated protein (~45-55 kDa) are detected in homozygous and heterozygous mice bearing either the Prdm9M1045Lja or the Prdm9repro7 allele, but not in extracts of Prdm9tm1Ymat mutants, where the antigenic peptide is deleted. c: PRDM9 is detected in nuclei of germ cells in seminiferous tubules from wild type mice (arrow in left panel) at 8 dpp, but not in Prdm9tm1Ymat germ cells (middle panel) or in testes labeled with a preimmune control antiserum (right panel). Scale bar = 20 μm
At the same time that recombination site activation occurs, there is dramatic remodeling of chromatin, homology recognition and assembly of the SC. Together, these events lead to homologous synapsis, which facilitates reciprocal recombination and accurate segregation of chromosomes. Post-replicative DNA strands (chromatids) are bundled by the cohesin protein complex. Cohesin is a ring-shaped protein complex comprised of two structural-maintenance-of-chromosomes ATPase proteins, SMC1A (also known as SMC1α) and SMC3, with non-SMC subunits, the kleisin RAD21 and a HEAT-repeat domain STAG protein (Revenkova and Jessberger 2006). In addition to these components expressed ubiquitously in somatic cells, there are meiosis-specific cohesin subunits assembled during early meiotic prophase I concurrently with recombination hotspot activation. The meiosis-specific SMC1A paralog is SMC1B (also known as SMC1β) (Revenkova, et al. 2001; Revenkova and Jessberger 2006); the meiosis-specific kleisins are REC8 (Eijpe, et al. 2003) and RAD21L (Gutierrez-Caballero, et al. 2011; Herran, et al. 2011; Ishiguro, et al. 2011); and STAG3 is the meiosis-specific STAG1/2 (also known as SA1/SA2) paralog (Pezzi, et al. 2000; Prieto, et al. 2001). Few details are available on the precise timing of deposition of these proteins in meiotic chromatin, but they exhibit early meiotic prophase I co-localization with proteins (e.g., SYCP3) that form the meiotic chromosome AEs, which are precursors of the LEs of the mature SC. Indeed, absence of both meiosis-specific kleisins can lead to failure to form the AEs and spermatogenic arrest at a leptotene-like stage, earlier than the arrest of other known meiotic mutations (Llano, et al. 2012).
PRDM9 clearly plays a key initiating role in the progression of meiotic events, but the cellular dynamics and mechanisms(s) by which it does so are not known. Moreover, the consequences of its absence for the concurrent events of meiotic chromatin remodeling are poorly understood. The phenotype of Prdm9-deficient mice is informative. Both males and females are infertile. In spermatocytes, meiotic arrest occurs at an abnormal “late zygotene/pachytene-like” stage, with aberrant synapsis and apparent impairment of DNA DSB repair (Hayashi, et al. 2005). In Prdm9 mutant testes, there is cytological evidence of DSB formation with nuclear accumulation of P-H2AFX and DMC1 (Hayashi, et al. 2005). Interestingly, molecular evidence suggests that meiotic DSBs in Prdm9-mutant germ cells are not at hotspot sequences, but directed to other sites, such as promoters (Brick, et al. 2012). Given the multi-domain nature of PRDM9, it is not clear which aspects of the null developmental phenotype derive directly from failed initiation of recombination or indirectly, perhaps from aberrant chromatin modifications. Establishing the developmental and subcellular localization of PRDM9 in nuclei, a primary objective of this study, sets limits on the time frame and mechanisms by which it functions. We also determined the consequences of PRDM9 deficiency for meiotic chromatin axis formation and homologous synapsis. We find that PRDM9 is present in prophase I nuclei transiently, during the leptotene and zygotene stages when the meiotic chromosomal axis is elaborated. While nuclear PRDM9 is not required for concurrent chromosome AE assembly, it is required for subsequent synapsis and meiotic progress.
Results
Transient nuclear localization of PRDM9 during early meiotic prophase I
Both Prdm9 transcript and PRDM9 protein are expressed during meiotic prophase I. Transcription of Prdm9 is developmentally regulated during the juvenile onset of spermatogenesis and meiosis, appearing at 7 dpp and 9 dpp, coinciding with the first appearance of preleptotene spermatocytes, and then decreasing when pachytene spermatocytes become the most abundant germ cell type in the testicular cell population (Supplemental Fig. 1a), suggesting transcription by early prophase I spermatocytes. We produced an antibody against an N-terminal domain peptide of PRDM9 (Materials and Methods, Fig. 1a). The specificity of this antibody was tested by western-blot analysis (Fig. 1b) of testis extracts from three distinct Prdm9 mutants (see below for description of the mutant alleles). This analysis revealed that the antibody recognizes a protein of the anticipated molecular weight for PRDM9 (~97 kDa). The full-length protein is diminished in lysates from heterozygous mice and absent in lysates from Prdm9-deficient testis, although in mutants with an allele truncating the protein before the ZNF domain, a lower molecular weight band is detected (Fig. 1b). Western blot analysis of developmental expression of PRDM9 protein (Supplemental Fig. 1b) revealed a developmental pattern of protein expression similar to the Prdm9 transcript expression.
To determine if PRDM9 demonstrates inherent nuclear localization and if it can be recognized in situ by our antibody, we transfected HEK293 cells with a FLAG-tagged Prdm9 construct encompassing the entire open reading frame and then immunolabeled the cells with either anti-PRDM9 or anti-FLAG antibodies (Supplemental Fig. 1c). We found that signals for both anti-PRDM9 and anti-FLAG co-localized with DAPI-stained DNA, suggesting inherent nuclear localization of PRDM9, even in heterologous cells. Indirect immunofluorescence labeling of seminiferous tubules with the antibody demonstrated both cytoplasmic and nuclear signals (Fig. 1c, left panel); however, the nuclear PRDM9 signal was absent in spermatocytes in Prdm9-deficient mice (Fig. 1c, middle panel). Additionally, pre-immune serum also gave non-specific background cytoplasmic signal (Fig. 1c, right panel). Therefore we consider that while the cytoplasmic signal is non-specific, the specific nuclear signal is biologically informative.
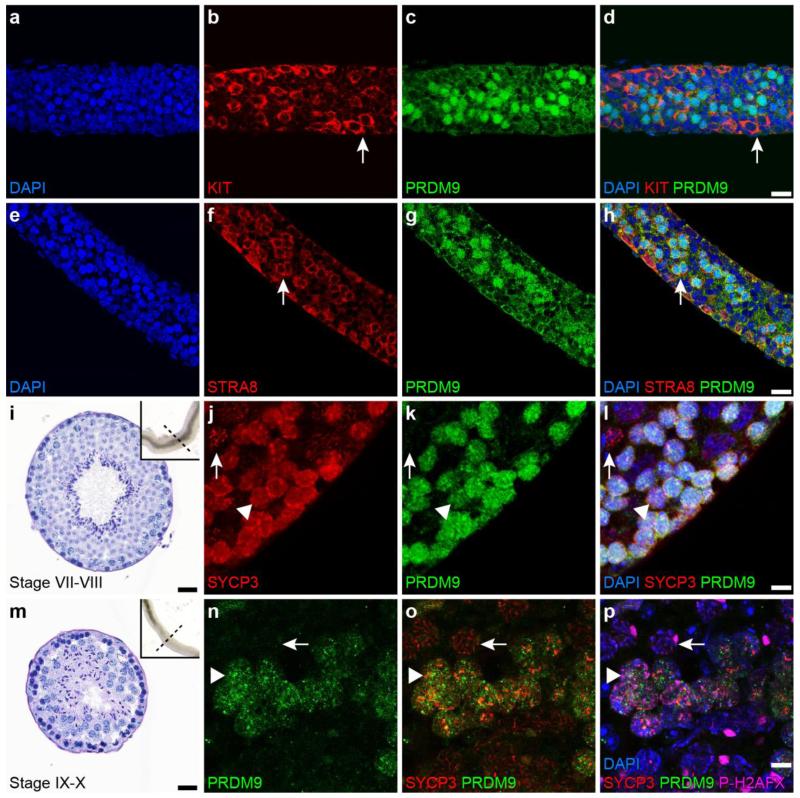
To determine the meiotic time span when PRDM9 is detectable in germ-cell nuclei, we examined different kinds of histological and cytological preparations of testes and germ cells, immunolabeled with antibodies detecting stage-specific proteins. These preparations included micro-dissected and whole-mounted tubules (Fig. 2, Supplemental Fig. 2c-f), whole mounts of germ cells (Supplemental Fig. 2a-b), and standard histological sections (Fig. 3). Despite considerable effort, we were unable to detect PRDM9 in any of a variety of procedures for spreading spermatocyte chromatin (Cobb, et al. 1999; Peters, et al. 1997), possibly indicating that these ionic conditions and disruptive procedures result in dissociation of PRDM9 from its DNA targets. To determine if PRDM9 is present in spermatogonia, we examined whole-mounted seminiferous tubules retrieved from mice at 8 dpp, which contain undifferentiated spermatogonia, differentiating spermatogonia, and preleptotene spermatocytes. To discriminate among these cell stages, preparations were immunolabled with anti-KIT, which labels differentiating spermatogonia but not preleptotene spermatocytes, and anti-STRA8, which labels both differentiating spermatogonia and preleptotene spermatocytes (Yoshinaga, et al. 1991; Zhou, et al. 2008). Double labeling with anti-PRDM9 and either anti-KIT or anti-STRA8 revealed that PRDM9-positive cells are KIT-negative, and STRA8-positive, although not all STRA8-positive cells are PRDM9-positive (Fig. 2a-h), indicating that PRDM9 is present in nuclei of pre-leptotene spermatocytes. We also used EdU (a thymidine analog) labeling at 8 dpp to track S-phase and post-S-phase preleptotene spermatocytes (Boateng, et al. 2013). The juvenile males were injected with EdU for a 6-hr pulse, followed by removal of testes for immunolabeling. Under these conditions, we found that some but not all EdU labeled cells were positive for nuclear PRDM9 (Supplemental Fig. 2c-f). Taken together, these observations suggest that PRDM9 initially localizes to S-phase and post-S-phase spermatocytes (pre-leptotene and leptotene).
Fig. 2.
PRDM9 in the nuclei of germ cells during early meiotic prophase I. a-h: Immunolabeling of whole-mounted seminiferous tubules retrieved from mice at 8 dpp; antibodies used recognize KIT (red in b and d), PRDM9 (green in c, d, g and h), and STRA8 (red in f and h). Arrows in b and d denote spermatogonia, and in f and h, the arrows indicate preleptotene spermatocytes. i-l: Seminiferous tubules retrieved from adult mice at stage VII-VIII (i) were immunolabeled with anti-SYCP3 (red in j and l) and anti-PRDM9 (green in k and l). Both SYCP3 (red) and PRDM9 (green) are localized in preleptotene spermatocytes (arrow head in j-l), but PRDM9 (green) is not present in pachytene spermatocytes (arrow in j-l). m-p: Squashed germ cell preparations from adult seminiferous tubules at stage IX-X (m) were immunolabeled with anti-PRDM9 (green in n-p), anti-SYCP3 (red in o and p), and P-H2AFX (pink in p). PRDM9 (green) and SYCP3 (red) are localized in zygotene spermatocytes (arrow head in n-p), but PRDM9 (green) is not present in pachytene spermatocytes (arrow in n-p). Nuclei were counterstained with DAPI in blue (a, d, e, h, l and p). Scale bars = 20 μm
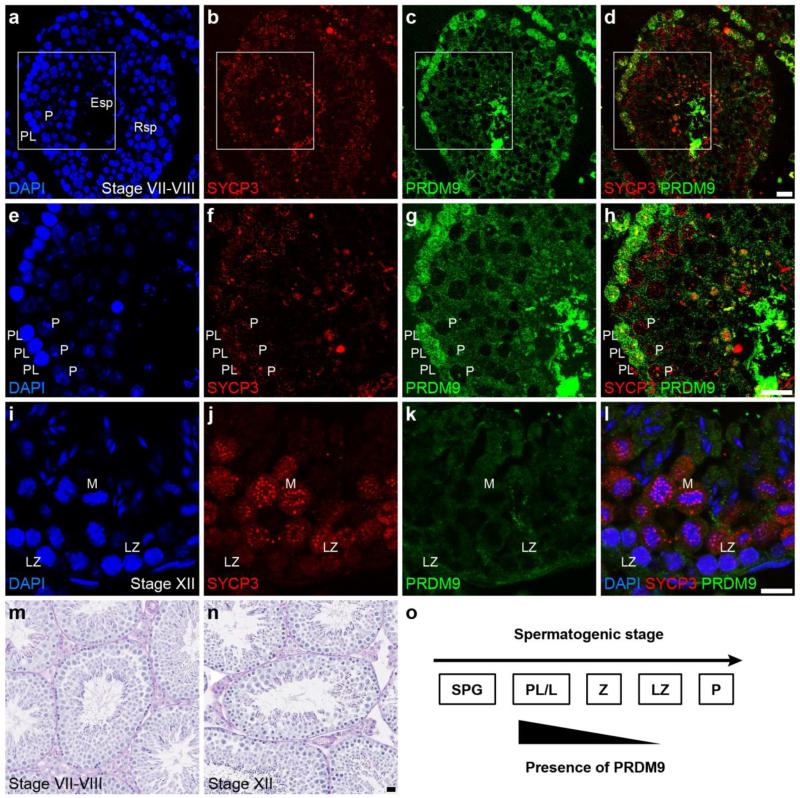
Fig. 3.
Stage-specific expression of PRDM9 in seminiferous tubules of adult mice. a-h and m: Adult testis cross sections at stages VII-VIII (m) were immunolabeled with anti-PRDM9 (green in c, d, g and h) and anti-SYCP3 (red in b, d, f and h). Localization of PRDM9 (green) was observed in preleptotene (PL) but not pachytene (P) spermatocytes as identified by both the seminiferous tubule stage and the labeling pattern of SYCP3 (red). i-l and n: Adult testis cross sections at stages XII (n) were immunolabeled with anti-PRDM9 (green in k-l) and anti-SYCP3 (red in j and l). Localization of PRDM9 (green) was not detected in late zygotene (LZ) and metaphase (M) spermatocytes as identified by the seminiferous tubule stage and the labeling pattern of SYCP3 (red). Nuclei were counterstained with DAPI in blue (a, e, i and l). Scale bar = 20 μm. o: Summary of nuclear localization pattern of PRDM9 in spermatogonia and early meiotic prophase I spermatocytes. SPG = spermatogonia, PL/L = pre-leptotene to leptotene spermatocytes, Z = zygotene spermatocytes, LZ = late zygotene spermatocytes, P = pachytene spermatocytes
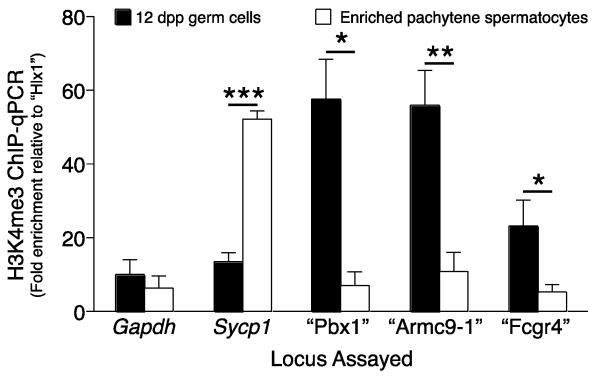
To determine when PRDM9 ceases to be detectable in spermatocyte nuclei, a combination of anti-PRDM9 and antibody recognizing SYCP3, a protein component of the AE of chromosomes and lateral element (LE) of the SC, was used to label specific stages of the adult seminiferous epithelium (Figs. 2 and 3) as well as whole-mounted isolated germ cells (Supplemental Fig. 2a-b). In microdissected stage VII-VIII tubule whole mounts (Parvinen, et al. 1993) (Fig. 2i-l) and histological cross sections of the same stages from adult mice (Fig. 3a-h, m), nuclear PRDM9 signal was found in SYCP3-positive pre-leptotene spermatocytes located adjacent to the seminiferous tubule basement membrane, but not in spermatocytes with SYCP3 morphology typical of pachytene spermatocytes (Fig. 2i-l, Fig. 3f-h). In stage IX-X tubule whole mounts, immunolabeling revealed nuclear PRDM9 signal in mid-zygotene spermatocytes, but not in pachytene cells (Fig. 2m-p). In histological cross-sections at stage XII, there was no detectable PRDM9 signal in either the late zygotene spermatocytes or the metaphase spermatocytes (Fig. 3 i-l, n). Together these observations indicate that PRDM9 localizes to pre-leptotene germ cell nuclei and is no longer detectable by late zygotene (Fig. 3o). An interesting corollary to this is whether the known functional consequence of PRDM9, tri-methylation on histone H3K4, persists into the pachytene stage when nuclear PRDM9 is not longer detectable. To assess this, we conducted chromatin-immunoprecipitation (ChIP) for PRDM9-dependent histone H3K4me3 marks on known hotspots in both germ cells enriched from testes of 12 dpp males, which are predominantly leptotene to zygotene, and in mid- to late-pachytene spermatocytes enriched from adult testes by sedimentation at unit gravity. We examined both recombination hotspot and transcribed gene sequences. The sequences assessed included 1) “Hlx1,” a recombination cold spot in the region of the Hlx gene, not exhibiting H3K4 trimethylation in B6 males (Baker, et al. 2014), used for normalization; 2) recombination hotspots known to exhibit PRDM9-dependent H3K4 trimethylation in B6 males (Baker, et al. 2014), briefly designated by nearby gene names: “Pbx1,” “Armc9-1” and “Fcgr4;” and 3) promoter sequences of Gapdh, a housekeeping gene, and Sycp1, a gene transcribed in pachytene spermatocytes (unpublished transcriptome data), both of which exhibit histone methylation not dependent on PRDM9. As shown in Fig. 4, enrichment of “Pbx1” “Armc9-1” and “Fcgr4” in the H3K4me3 ChIP product was high in early spermatocytes from 12 dpp mouse testes, but significantly diminished in pachytene spermatocytes. In contrast, non-PRDM9-dependent histone H3K4 methyl marks on actively transcribed Sycp1 was enriched in pachytene spermatocytes. Thus we conclude that PRDM9 methyl marks are not enriched in nuclei of pachytene spermatocytes, consistent with the fact that presence of PRDM9 is not detected.
Fig. 4.
Meiotic prophase H3K4me3 marks on recombination hotspots. ChIP-qPCR analysis was performed to assess H3K4me3 levels in C57BL/6J testicular germ cells on Gapdh and Sycp1 promoter regions, on three known recombination hotspots (“Pbx1”, “Armc9-1” and “Fcgr4”), and on a recombination cold spot (“Hlx1”). For ease of graphical referral, the recombination spots are designated by the symbol for an associated or nearby gene (in quotes and not italicized, because the recombination spot is not the actual gene sequence); the actual recombination spots are defined by precise genomic position (Table 2). Early meiosis I prophase spermatocytes were enriched from 12 dpp testes of three biological samples, and subjected to ChIP-qPCR. Pachytene spermatocytes were enriched (85% purity) by unit gravity sedimentation from adult testes of three biological samples, and subjected to ChIP-qPCR. The ChIP-qPCR signal for each sequence is normalized to that for “Hlx1”, a cold spot in C57BL/6J background, used as the negative control, and shown on the y-axis as fold enrichment compared to “Hlx1”; the x-axis designates the loci assayed. *, p < 0.05; **, p < 0.01; ***, p < 0. 0.00005
Together, these cytological and molecular observations demonstrate that PRDM9 is in nuclei of preleptotene, leptotene, and early to mid-zygotene spermatocytes (a period of roughly 48 hours), but is not detected by late zygonema or during the pachytene stage (which has a duration of approximately one week in male mouse germ cells) (Fig. 3o).
Early meiosis prophase I arrest, chromosome axis formation and synapsis in Prdm9-mutant spermatocytes
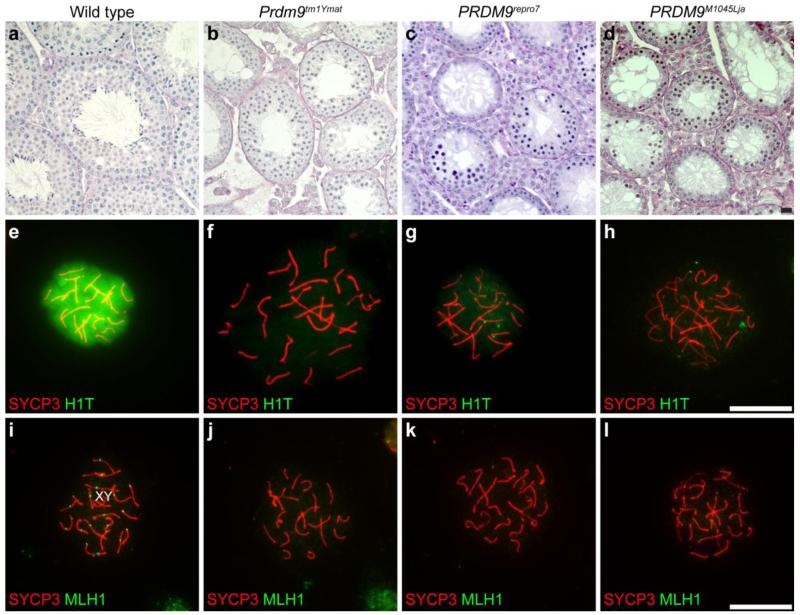
To confirm and extend previous analysis (Hayashi, et al. 2005) of the consequences of PRDM9 deficiency, we analyzed phenotypes of three distinct Prdm9 mutant alleles. Overall, each of the three alleles causes infertility phenotypes with meiotic prophase I arrest of spermatocytes. One allele is the previously published Prdm9 targeted mutation, Prdm9tm1Ymat (Hayashi, et al. 2005), with a deletion of exons 2-5 and no protein production (Fig. 1a-b). A second allele is an ENU-induced mutation produced by the Reproductive Genomics Program at The Jackson Laboratory (Handel, et al. 2006) designated Prdm9repro7. This is a nonsense mutation, a G to A transition changing Q478 to a premature stop codon (Fig. 1a-b). The third allele is another ENU-induced mutation (Weiss, et al. 2012). Contrary to the original publication, we found that this allele, Prdm9M1045Lja, is an A1276T mutation, with a stop codon at K426 (Fig. 1a-b). Although no full-length PRDM9 protein is detected in testes of homozygous Prdm9tm1Ymat mice, the Prdm9repro7 and Prdm9M1045Lja mutant germ cells produce a truncated protein detected by our antibody that is presumably the N-terminal domains without the ZNF domain. The infertility phenotypes of these two alleles reveal the essential nature of the DNA binding domain for PRDM9 meiotic function. The testis histological phenotypes of the two ENU-induced alleles (Prdm9repro7, Prdm9M1045Lja) recapitulate those of the original allele (Prdm9tm1Ymat). We found that spermatogonia and spermatocytes were present, but no post-meiotic spermatids were observed in testes of mice homozygous for any of the three Prdm9 alleles (Fig. 5a-d). In the present study, all observations were confirmed for each of the three distinct mutations.
Fig. 5.
Spermatogenic arrest at early meiotic prophase I in Prdm9-deficient mice. Genotypes are designated for each vertical column. a-d: Adult testis cross-sections, stained with PAS, from wild type (a) and Prdm9-deficient (b-d) mice. In contrast to normal wild type histology, no post meiotic germ cells are present in Prdm9-deficient testes, with all three alleles presenting a similar arrest. e-h: Chromatin spreads were prepared from wild type (e) and Prdm9-deficient (f-h) mice and immunolabeled with antibodies to histone H1T (green in e-h), a marker of the mid-pachytene stage, and with antibody to SYCP3 (red in e-h) for visualization of the SC. Histone H1T (green) signal was absent or at only a weak background level in spermatocytes of Prdm9-deficient mice compared to wild type. i-l: Chromatin spreads were prepared from wild type (i) and Prdm9-mutant mice (j-l) and immunolabeled with anti-MLH1 (green in i-l) to mark crossover recombination sites and anti-SYCP3 (red in i-l) for visualization of the SC. No MLH1foci were present in Prdm9-deficient spermatocytes. Scale bars = 20 μm
Stage-specific markers were used to pinpoint the developmental time of spermatogenesis arrest with respect to the time window for nuclear localization of PRDM9. Chromatin spreads of spermatocytes isolated from 17 dpp mutants and littermate controls were immunolabeled with anti-histone HIST1H1T (herein referred to its more common abbreviated designation, H1T), a marker of mid-late pachynema (Cobb, et al. 1999) and anti-SYCP3. At 17 dpp, many wild type spermatocytes were strongly positive for H1T, whereas there was only weak or background labeling of Prdm9-mutant spermatocytes (Fig. 5e-h), indicating arrest before mid-pachynema. This conclusion was supported by immunolabeling to detect MLH1, a marker for the recombination nodules that form in mid-pachynema (Cohen, et al. 2006); no MLH1 foci were observed in the Prdm9-mutant spermatocytes (Fig. 5i-l). These analyses demonstrate that absence of PRDM9 does not cause arrest spermatogenesis at the leptotene and zygotene stages when PRDM9 is localized in nuclei; instead the effects of PRDM9 absence are not apparent until later in meiosis I prophase, after SC formation.
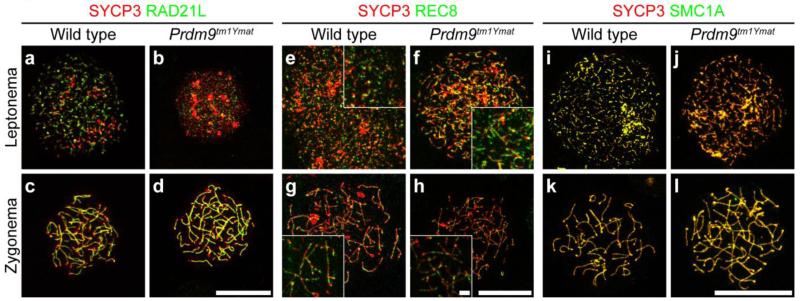
PRDM9 is localized to spermatocyte nuclei at roughly the same time as the appearance of meiosis-specific cohesin proteins, which are required for formation of the chromosomal AEs (Llano, et al. 2012). We tested whether localization of cohesin-complex proteins and chromatid axis formation are compromised due to the loss of PRDM9. We immunolabeled spermatocyte surface-spread chromatin prepared from the leptotene and zygotene stages for SYCP3 (a staging marker) in combination with different subunits of the cohesin complexes, including two meiosis-specific cohesin subunits, RAD21L and REC8, and SMC1A, a structural maintenance of chromosomes (SMC) protein that is a mitotic cohesin subunit and also present on AEs in spermatocytes. As shown in Fig. 6, the foci-like nuclear localization of the three cohesin subunits were detected in leptotene spermatocytes retrieved from Prdm9-deficient mice. The labeling pattern of the three cohesin subunits was indistinguishable from that in wild type leptotene spermatocytes in terms of colocalization of many foci with SYCP3. At the zygotene stage, the colocalization of SYCP3 and the three cohesin subunits became more obvious in both wild type and Prdm9-deficient spermatocytes, with no consistent abnormality observed in Prdm9-deficient spermatocytes. These observations indicate that cohesin loading on meiotic chromatin in early prophase I spermatocytes is apparently not affected by the absence of PRDM9, suggesting that cohesin loading occurs before or independently of nuclear localization of PRDM9.
Fig. 6.
Localization of cohesin complex proteins in Prdm9-deficient spermatocytes. Chromatin spreads from spermatocytes (meiotic substage indicated on left) isolated from wild type (a, c, e, g, i and k) and Prdm9-deficient (b, d, f, h, j and l) testes were immunolabeled with anti-SYCP3 antibody (red) to mark the SC, combined with anti-RAD21L (green in a-d), anti-REC8 (green in e-h), or anti-SMC1A (green in i-l), to assess cohesin-complex proteins. The assembly and morphological localization of these cohesin-related proteins is apparently normal in homozygous Prdm9tm1Ymat-mutant spermatocytes. Scale bars = 20 μm for main panels and scale bar = 2 μm for insets
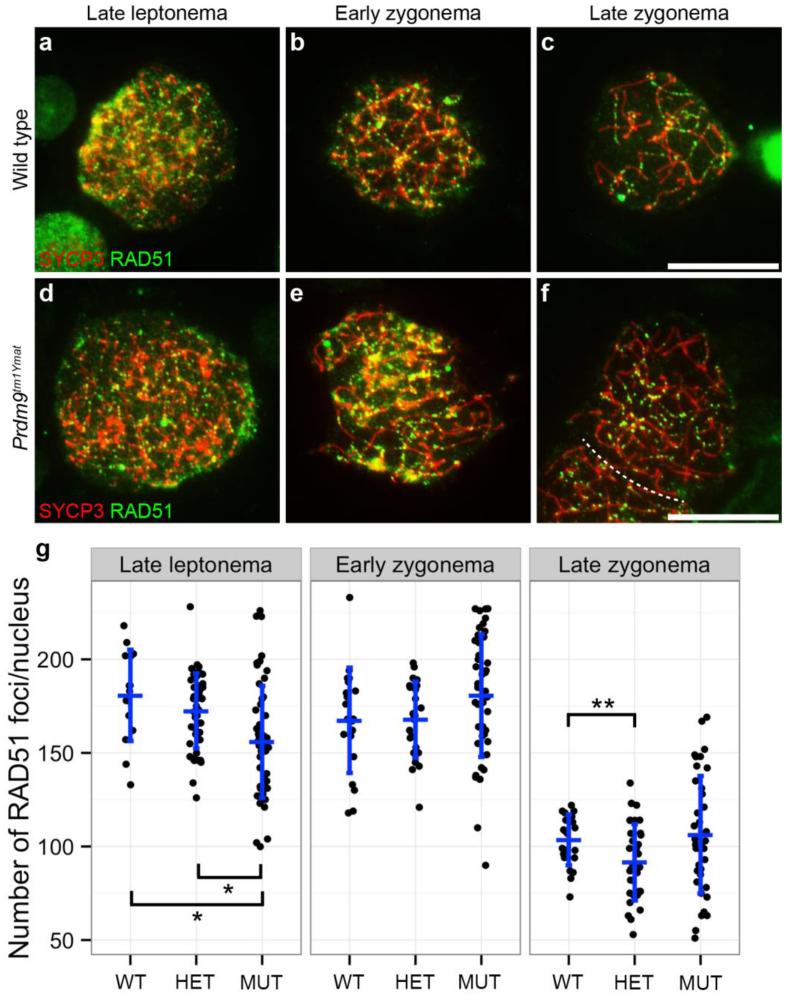
It is thought that SPO11 is recruited and creates meiosis-specific DSBs after binding of PRDM9 to hotspots. Single-stranded DNA produced by resection of DSB ends becomes coated with RAD51, a single-strand DNA binding protein that promotes the strand invasion step of recombination. Because it is known that the number of RAD51 foci is dependent on Spo11 gene dose (Kauppi, et al. 2013), we asked if the number of RAD51 foci is dependent on Prdm9 gene dose. RAD51 foci on spermatocyte chromatin were counted at three meiotic prophase I substages (late leptotene, early zygotene, and late zygotene, differentiated by SYCP3 labeling and extent of synapsis) for WT, heterozygous and homozygous mutant Prdm9tm1Ymat mice, as seen in Fig. 7a. Scoring data across these early meiotic stages are summarized in Fig. 7b. At early leptonema, RAD51 foci were not distinct and too numerous (>500 per nucleus) to count across all three genotypes. For this presentation, cell counts from all mice of the same genotype were pooled (data were also analyzed on a per-mouse basis, with the same conclusions, data not shown). Across all three genotypes, the number RAD51 foci decreased with progress of meiosis I prophase as expected and observed by others (Ashley, et al. 1995). There were some small, but statistically significant (p<0.03), differences in some genotype comparisons. However, there were no large differences in RAD51 counts/cell among the three genotypes, indicating that a similar number of DSBs per cell are being processed in each case. This suggests that the number of RAD51 foci is not dependent on Prdm9-gene dose. However, there was greater variability in RAD51 counts/cell among mutant spermatocytes (Fig. 7b), suggesting possibly aberrant processing. Interestingly in this context, the disappearance of RAD51 foci from autosomal chromatin, especially those that exhibit pairing failures, was delayed or inhibited in pachytene-like mutant spermatocytes (see below, Fig. 9a-b).
Fig. 7.
Localization and number of RAD51 foci in Prdm9tm1Ymat-mutant spermatocytes. Chromatin spreads were prepared from wild type spermatocytes (a-c) and homozygous Prdm9tm1Ymat-mutant spermatocytes (d-f) and immunolabeled with anti-RAD51 (green) and anti-SYCP3 (red) to mark the SC for staging purposes. These panels (a-f) reveal that RAD51 localizes to both wild type and Prdm9-mutant chromatin in early meiosis I prophase (meiotic substages indicated across the top and the genotype on the left). Scale bars = 20 μm. g. In late leptonema (LL), early zygonema (EZ) and late zygonema (LZ), the numbers of RAD51 foci associated with SYCP3 were assessed in Prdm9+/+ wild type spermatocytes (WT, where N = 4 animals and the total number of cells scored was 15 for LL, 46 for EZ and 54 for LZ), Prdm9tm1Ymat/+ spermatocytes (HET, where N = 3 animals and the total number of cells scored was 20 for LL, 23 for EZ and 46 for LZ), and in Prdm9tm1Ymat/Prdm9tm1Ymat spermatocytes (MUT, where N = 4 animals and the total number of cells scored was 22 for LL, 36 for EZ and 38 for LZ). The vertical bars represent the SD and the horizontal bars designate the mean number of RAD51 foci. The statistical significance was assessed among genotypes by a Mann-Whitney test. *, p < 0.005; **, p < 0.05
Fig. 9.
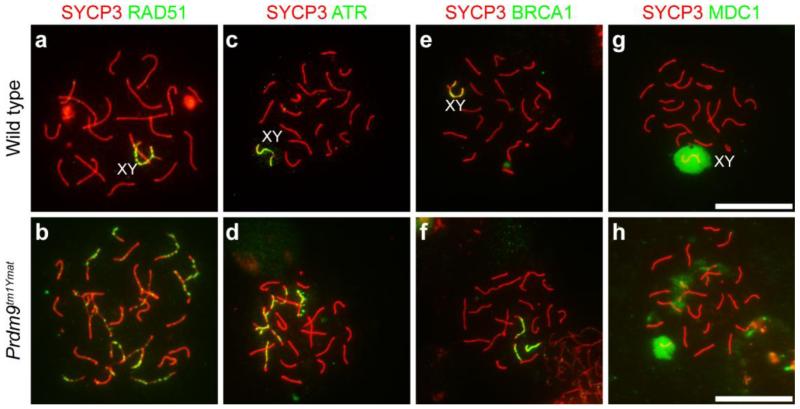
Aberrant DSB repair in Prdm9-deficient spermatocytes. Genotypes are designated for each horizontal row. For these images, chromatin spreads were prepared from juvenile (17 dpp) testes from wild type (a, c, e and g) and Prdm9-mutants (b, d, f and h) were immunolabeled with anti-SYCP3 (red) for staging and antibodies to different DSB-response proteins (green): RAD51 in a-b, ATR in c-d, BRCA1 in e-f, and MDC1 in g-h. Ectopic presence of RAD51, ATR, BRCA1, and MDC1 in autosomal chromatin of Prdm9-deficient spermatocytes suggests the failure of DSB repair. Scale bars = 20 μm
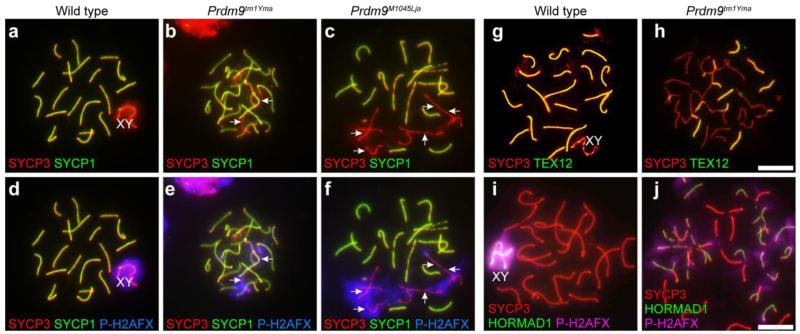
In spite of apparently normal localization of RAD51 and AE proteins in Prdm9-mutant spermatocytes, pairing, assembly of a tripartite SC, and synapsis were not morphologically or quantitatively normal in mutants. Surface-spread chromatin from spermatocytes was immunolabeled with anti-SYCP3 (AE protein) and anti-SYCP1 or TEX12 (CE proteins). In contrast to wild type spermatocytes, many unsynapsed chromosomes and chromosomal regions, detected by absence of SYCP1 (Fig. 8a-f) and TEX12 signal (Fig. 8g-h), were observed in Prdm9-mutant spermatocytes. These unsynapsed regions co-labeled with HORMAD1 (Fig. 8i-j) and P-H2AFX (Fig. 8d-f, i-j), both markers of unsynapsed chromatin. In mutants, normal homologous synapsis was observed in only about 20% of the germ cells at 17 dpp, and by 21 dpp, less than 10% of all mutant spermatocytes exhibited completely normal paring. The abnormal pairing configurations observed included partial synapsis, heterologous synapsis, and tangled LEs, but there was no evidence for sister-chromatid synapsis as seen in some meiosis-specific cohesin mutants, e.g., in Rec8 mutants (Xu, et al. 2005). Interestingly, the sex chromosomes of mutant germ cells exhibited pairing at the pseudoautosomal region, although, as was the case for autosomal chromosomes in mutant spermatocytes, there was some X-Y dissociation. Thus, although chromosomal LEs are formed in Prdm9 mutant spermatocytes, nuclei with fully normal synapsis were rare.
Fig. 8.
Aberrant synapsis in Prdm9-deficient spermatocytes. For these images chromatin spreads were prepared from juvenile testes and immunolabeled for components of the SC and DNA damage markers. Genotypes are designated for each vertical column. a-f: Spread chromatin preparations from wild type testes (a and d) and Prdm9-deficient testes (b-c and e-f) were immunolabeled with anti-SYCP1 (green), anti-SYCP3 (red), and anti-P-H2AFX (blue), revealing colocalization in mutant germ cells of asynapsis detected by absence of SYCP1 signal (arrows b-c) with P-H2AFX (arrows in e-f). a and d are the same cell, as are b and e, and c and f, respectively. g-j: Spread chromatin preparations from wild type testes (g and i) and Prdm9-deficient testes (h and j) were immunolabeled with anti-SYCP3 (red), anti-TEX12 (green in g and h), a component of the SC CE, or anti-P-H2AFX (pink in i and j) and anti-HORMAD1 (green in i and j), which marks regions of asynapsis. Patchy TEX12 label and persistence of HORMAD1 label on mutant chromosomes indicate numerous regions of asynapsis. Scale bars = 20 μm
Compromised repair of DNA double-strand breaks and aberrant transcription in PRDM9-deficient germ cells
Arrest of spermatogenesis by the pachytene stage suggested that DNA DSB repair might be affected by loss of PRDM9. We used P-H2AFX as a marker of DSBs to follow repair in Prdm9-mutant spermatocytes. In both wild type and mutant spermatocytes, positive signals for P-H2AFX were observed throughout the chromatin at the leptotene stage and zygotene stages, confirming previous evidence for DSB formation in Prdm9-mutant spermatocytes (Brick, et al. 2012; Hayashi, et al. 2005). In subsequent stages of meiosis in wild type spermatocytes, disappearance of P-H2AFX signal was observed on the autosomal chromatin, with P-H2AFX becoming restricted to the XY body by the pachytene stage (Fig. 8d and j). This pattern of P-H2AFX localization constitutes evidence for DSB repair on autosomal chromatin in wild type spermatocytes. In contrast, in Prdm9-mutant spermatocytes irregular and persistent P-H2AFX signal was observed, suggesting incomplete DSB repair (Fig. 8e-f, j). Because the persistence of markers of DNA DSBs in Prdm9-mutant spermatocytes suggests abnormal repair responses to DNA damage, spermatocytes were immunolabeled to detect DNA damage response-related proteins, RAD51 (Fig. 9a-b), ATR (Fig. 9c-d), BRCA1 (Fig. 9e-f), and MDC1 (Fig. 9g-h). In wild type pachytene spermatocytes, signals for RAD51, ATR, BRCA1, and MDC1 localized only in sex chromatin domains, whereas in the mutant spermatocytes, persistent autosomal localization of RAD51, ATR, BRCA1, and MDC1 was always observed. Taken together, these data are evidence for aberrant and/or delayed DNA DSB repair in Prdm9-mutant spermatocytes.
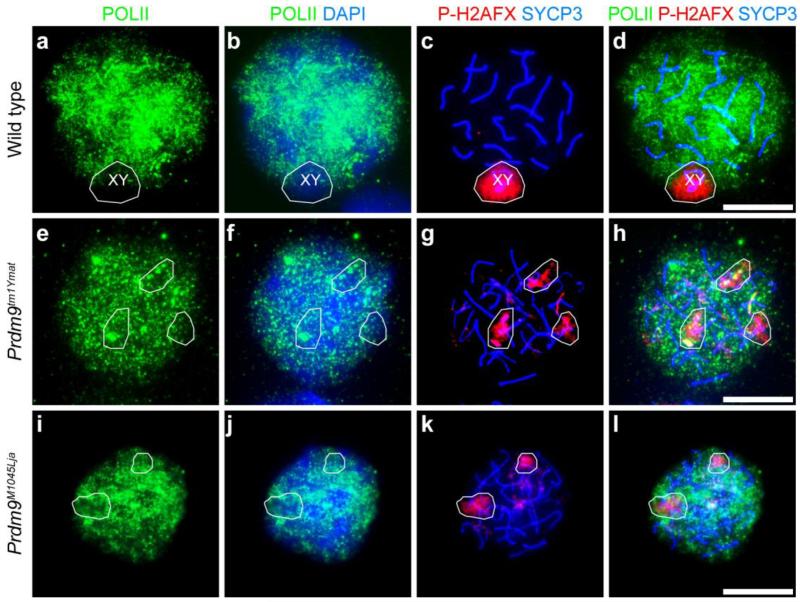
Unrepaired DSBs are frequently associated with aberrant transcription, and is known that P-H2AFX plays a key role in triggering meiotic silencing of unsynapsed chromatin (MSUC) as well as meiotic silencing of sex chromosomes (MSCI) (Turner 2007). To determine if autosomal transcriptional silencing occurs in Prdm9-mutant spermatocytes, we labeled the spermatocytes with POLII antibody to mark transcriptionally active chromatin. In wild type pachytene spermatocytes, sex chromosomes are transcriptionally inactive, resulting in a “hole” in the otherwise pervasive POLII signal that co-localizes with P-H2AFX (Turner 2007) (Fig. 10a-d). In Prdm9-mutant spermatocytes, the co-localized POLII “holes” and patches of P-H2AFX were not limited to the sex chromosomes, but frequently associated with autosomes (Fig. 10e-l). Spermatocytes isolated from wild type and Prdm9-deficient mutants were also labeled with antibody to SUMO1, also a marker of transcriptional silencing (Rogers, et al. 2004). Similar to POLII localization, this also provided evidence for abnormal transcriptional silencing in mutant spermatocytes (Supplemental Fig. 3a-b). Together, these findings on abnormal pairing (Fig. 8), aberrant DSB repair (Figs. 7 and 9) and autosomal transcriptional silencing (Fig. 10) provide evidence for meiotic silencing of unsynapsed chromatin, which might contribute to apoptosis and germ-cell loss in PRDM9-deficient spermatocytes.
Fig. 10.
Abnormal meiotic silencing of chromatin in Prdm9-mutant spermatocytes. Genotypes are designated for each horizontal row. Chromatin spreads prepared from spermatocytes isolated from wild type (a-d) and Prdm9-deficient (e-l) testes were immunolabeled with anti-POLII (green) to detect ongoing RNA synthesis, anti-SYCP3 (blue in c-d, g-h and k-l) to mark the SC, and anti-P-H2AFX in red. DNA was counterstained with DAPI (blue in b, f and j). In wild type spermatocytes, transcriptional silencing is restricted to the XY body (a-d). The white circles denote overlapping P-H2AFX and POLII “holes,” which indicate abnormal transcriptional silencing in the mutant autosomal chromatin (e-l). Scale bars = 20 μm
Discussion
Following meiotic commitment of differentiated spermatogonia, the earliest events of meiosis include DNA synthesis during the pre-meiotic S phase, and chromatin assembly around the AEs to form the chromosome configurations typical of the leptotene and zygotene sub-stages. Here we show that the period of nuclear occupancy by PRDM9 is brief, encompassing the pre-leptotene to zygotene stages, placing PRDM9 in the right place at the right time to be a player in any of the early or later events of meiosis I prophase: chromatin remodeling, chromosome axis formation, homologous pairing and synapsis. Chromatin-associated PRDM9 is not required for concurrent deposition of meiosis-specific cohesin complexes, assembly of the lateral chromosome elements, or accumulation of the DSB-recognizing protein RAD51. However, PRDM9 is required, either directly or indirectly, for effective and stable pairing and synapsis of homologs. Moreover, absence of PRDM9 leads to delays and aberrations of both DNA damage repair and global aspects of spermatocyte transcription.
PRDM9 and developmental onset of meiosis and recombination
In spite of our knowledge about PRDM9 for over a decade, there has been little knowledge of its intracellular localization and trafficking. In part this is due to the challenges inherent in producing specific antibodies against PRDM9, a protein with highly conserved domains. The N-terminal domain-recognizing antibody we produced recognizes a specific band of the expected size on western blot that is absent in the mutants. Nonetheless, in immunofluorescence with a variety of germ cell preparations, the antibody produces a cytoplasmic signal inferred to be non-specific, as it is present in both Prdm9-null as well as wild type spermatocytes. This antibody is thus useful for determining nuclear localization of PRDM9, but not for assessing trafficking of the protein between the nucleus and cytoplasm.
Cytological protein co-localization analyses revealed no nuclear PRDM9 in spermatogenic cells positive for KIT, a mark of differentiating spermatogonia. This observation suggests that meiotic recombination sites are not selected prior to the pre-leptotene S-phase, in spite of recent evidence that overall rate of crossover recombination might be established in spermatogonial stem cells (Vrooman, et al. 2015). In contrast, nuclear localization of PRDM9 was evident in germ cells positive for STRA8, a marker of differentiated spermatogonia and pre-leptotene to leptotene spermatocytes. In mammals, meiotic initiation is prompted by the stimulated-by-retinoic acid (STRA8) protein (Anderson, et al. 2008). In the absence of STRA8, mutant testes lack cells with morphological characteristics of meiosis prophase I and express dramatically reduced levels of transcripts for several proteins characteristic of recombination, such as Spo11 and Dmc1 (Anderson, et al. 2008), although STRA8 expression is not required for Rec8 expression (Koubova, et al. 2014). Our observations suggest that PRDM9 does not appear in germ cells until after STRA8-induced initiation of meiosis, and thus it is part of a meiotic program of protein expression, rather than a pre-existing spermatogonial program. The presence of PRDM9 in STRA8-positive and EdU-labeled cells suggests that it could be active as early as during the pre-leptotene S phase, but it is not known if Prdm9 gene and/or protein expression specifically require STRA8 (like the expression of Spo11 and Dmc1) or are independent of STRA8 (like Rec8 expression). The temporal pattern of nuclear localization of PRDM9 raises the interesting possibility that STRA8 may regulate meiotic initiation in part by controlling expression and/or nuclear localization of PRDM9 protein, possibly by establishing a specific cohort of nuclear importin proteins active as meiosis I prophase is initiated (Major, et al. 2011).
After transient localization in the nuclei of early meiosis prophase I spermatocytes, PRDM9 is no longer detectable in nuclei beyond the late zygotene stage and in early pachynema, when homologous synapsis is established and intact axes of both SYCP3 and SYCP1 are apparent. Additionally, the PRDM9-dependent histone trimethylation marks on several well characterized hotspots are not detected in enriched pachytene spermatocyte cell populations. These observations support a hypothesis that PRDM9 at specific hotspots is displaced by proteins involved in creating and repairing meiotic DSBs. However, the mechanisms by which PRDM9 and its methyl marks are removed and/or replaced are not known. This could be dependent on protein stability and time (progress of the meiotic cell cycle), or it could be related to active removal during formation of DSBs or synapsis. Assays of histone modifications on specific hotspots cannot currently be done at a single-cell level, and spermatocyte population-level assays do not allow marks at a specific hotspot be related to meiotic substage or synapsis. Moreover, it is not clear how PRDM9 is removed from the nucleus by the late zygotene stage, and whether it is modified or specifically exported. Another important issue to be resolved is whether or not PRDM9 or its histone methyl marks are present in regions not participating in homologous synapsis (e.g., in a variety of meiotic mutants as well as infertile inter-specific hybrids).
Localization data reported here place PRDM9 in spermatocyte nuclei during the period when meiotic recombination is initiated by formation of DSBs, mediated by SPO11, after which single-stranded DNA becomes coated by RAD51. RAD51 foci are first seen cytologically in leptotene nuclei, where they gradually accumulate into tracks or fibers of foci, which then co-localize with the forming LEs of the SC (Ashley, et al. 1995). Subsequently, the number of RAD51 foci declines as spermatocytes achieve full synapsis through late zygonema to the onset of pachynema. We extended previous results (Hayashi, et al. 2005) by showing that RAD51 foci form on chromatin in Prdm9-mutant spermatocytes, and that there are no major or consistent differences in the number of cytologically visible RAD51 foci in Prdm9-mutant and -heterozygous spermatocytes compared to wild type controls. Thus the number of DSBs being processed by the RAD51-mediated pathway is apparently not crucially dependent on dose of PRDM9. This inference is in interesting contrast to findings that numbers of RAD51 foci are related to SPO11 dose (Kauppi, et al. 2013), and implies that the number of RAD51 foci is a reflection of the actual number of SPO11-induced DSBs and not the number of PRDM9-activated sequences. Indeed, it is known that there are a greater number of PRDM9-dependent sites of histone H3K4 trimethylation than DSBs (Baker, et al. 2014), and that DSBs occur in sites other than the PRDM9-dependent H3K4 trimethylation sites in Prdm9-mutant spermatocytes, such as promoter sequences (Brick, et al. 2012). The latter finding suggests that synapsis and other defects in Prdm9-mutant spermatocytes may be attributed to aberrant sites of DSBs in mutants, which perhaps lead to inefficient and/or imperfect synapsis. In spite of no obvious Prdm9-dose dependency, we did observe somewhat greater between animal and cell-to-cell variability in number of RAD51 foci in Prdm9-mutants (but not in heterozygotes). The reason for this is not known, but the observation suggests the possibility of developmental delay in progress through meiotic prophase in the absence of PRDM9 binding and activation of specific target recombination hotspot sequences, reinforcing the inference that ectopic DSBs lead to meiotic disturbances.
PRDM9 and meiosis I prophase chromatin dynamics
PRDM9 is present in spermatocyte nuclei during the time period when chromosomal axes are elaborated, homologous pairing is established and synapsis is initiated. One of the earliest events of meiosis, probably concurrent with the pre-meiotic (pre-leptotene) S phase, is the elaboration of the chromosomal AEs of sister chromatids and deposition of meiosis-specific subunits of the cohesin complexes. The ring-shaped SMC1/3 cohesin complex is fundamental for the formation of a chromosomal axis. In spermatocytes, there is a meiosis-specific SMC1 paralog, known as SMC1B; male mice with a mutated Smc1b gene are infertile with arrest of meiosis and abnormal structure of the SC (Revenkova and Jessberger 2006). Early meiosis I prophase is also the time when meiosis-specific non-SMC cohesin complex subunits, REC8, RAD21L, and STAG3, are assembled into chromosomal axes. Mutant phenotypes for all three of these cohesin subunits include arrest of meiosis, abnormal SC morphology, disrupted homolog pairing and synapsis, and impaired DSB processing. Notably, not all of these proteins co-localize within the same cohesin complexes, i.e., there are several different meiosis-specific cohesin complexes, with separate functions not yet fully understood (Revenkova and Jessberger 2006). Specific single (Stag3) or double (Rec8-Rad21l) cohesin subunit mutants exhibit very early meiotic prophase arrest, in the leptotene stage (Fukuda, et al. 2014; Hopkins, et al. 2014; Llano, et al. 2014; Llano, et al. 2012), providing evidence that these proteins are essential for setting up a chromosomal architecture favoring recombination and homology pairing. Interestingly, the temporal setting for establishing the cohesin-mediated meiotic chromosome AEs encompasses the time period when PRDM9 is first detected in male germ-cell nuclei. In fact, the temporal overlap is such that it has not been possible to discern what comes first, PRDM9 localization or localization of meiosis-specific cohesin complexes. However, the data presented here reveal that PRDM9 is not required for either expression or correct localization of meiosis-specific cohesin subunits. Not only can REC8, RAD21L be detected within spermatocytes bearing any of several mutant Prdm9 alleles, but also the chromosomal AEs appear to be assembled normally in the mutant spermatocytes, an important point because it is known that cohesins are required for assembly of the axis and the SC (Herran, et al. 2011; Llano, et al. 2012; Novak, et al. 2008; Revenkova and Jessberger 2006). Moreover, the meiotic cohesins have been postulated to be involved in homologous recombination template bias; by establishing proximity of non-sister chromatids, they appear to promote repair of meiotic DSBs by a non-sister chromatid (Jin, et al. 2009; Xu, et al. 2005). Thus, an interesting question for future resolution is whether the establishment of the AEs is required for PRDM9 activation of hotspots and concurrent histone methylation.
Although the SC is assembled with apparently normal morphology in Prdm9 mutants, homology recognition and synapsis are not normal in the mutants, with many of the same defects apparent in mutants for the various meiosis-specific cohesin proteins. It is unlikely that PRDM9 is directly involved in homology recognition; however, it may recruit proteins and/or set up a chromatin structure that facilitates or is a prerequisite for translocation of recombining sequences to the chromosomal axis and the homology search. It is known that in the absence of PRDM9, meiotic DSBs are directed to ectopic promoter sites (Brick, et al. 2012), and possibly these are less effective as sites to promote homology recognition. The fact that some homologous synapsis does occur in Prdm9-mutant spermatocytes suggests that the process depends only in part on PRDM9-induced chromatin features. However, if abnormal pairing/synapsis were the sole cause of arrest of Prdm9-mutant spermatocytes, we would expect that some of the normal-appearing pachytene spermatocytes should be positive for MLH1 (or H1T); however, this was not found. Thus, abnormal placement of meiotic DSBs, or some other direct or downstream function of PRDM9 must determine the early pachytene arrest point.
PRDM9, regulation of meiotic progress and infertility
Other downstream events precipitated by absence of PRDM9 include aberrant transcriptional effects and eventual germ-cell death by apoptosis, which likely eliminates cells with abnormal synapsis. These may well follow from a synapsis surveillance mechanism that is thought to involve the HORMAD1 protein (Daniel, et al. 2011), which is present in Prdm9-mutant spermatocytes (Fig. 8). Interestingly, Prdm9 alleles strongly influence synapsis and homologous chromosome pairing also in interspecific sterile hybrids (Bhattacharyya, et al. 2013; Bhattacharyya, et al. 2014), perhaps from hybrid incompatibility with other genes that may be in the same pathway. In the mouse, although meiotic recombination initiates prior to and independently of synapsis (Mahadevaiah, et al. 2001), synapsis is required for recombination sites to repair DSBs via development into meiotic crossovers (de Vries, et al. 2005). Although arrest of Prdm9-mutant spermatocytes is at a more advanced stage than arrest of most single or double mutants for meiosis-specific cohesins (Fukuda, et al. 2014; Hopkins, et al. 2014; Llano, et al. 2014; Llano, et al. 2012), Prdm9-mutant spermatocytes exhibit synapsis defects that are in common with those described for spermatocytes mutant for other proteins playing early roles in the recombination pathway, e.g., SPO11 and DMC1 (Bannister, et al. 2007; Baudat, et al. 2000; Pittman, et al. 1998; Romanienko and Camerini-Otero 2000). These include delayed and/or inefficient repair of DNA DSBs, persistent RAD51 foci and patches of P-H2AFX (this study; Hayashi et al., 2005). Characteristically, unpaired homologs undergo transcriptional silencing of genes within unsynapsed chromatin (MSUC) (Baarends, et al. 2005; Turner, et al. 2005), and the cytological marks of this are typically associated with unpaired chromatin in many different recombination pathway mutants with incomplete aberrant synapsis (Burgoyne, et al. 2009), as well as in Prdm9-mutant spermatocytes. Microarray analysis of gene expression in Prdm9-mutant testes is consistent with the cytological observations, revealing autosomal candidate genes with downregulated expression in mutants (Hayashi and Matsui 2006).
By establishing the time window during which PRDM9 is localized in meiotic nuclei, these findings place PRDM9 in a pathway unfolding independently of specification of the chromosomal axis, and via the PRDM9-dependent activation of recombination hotspots by histone trimethylation. Arrest and elimination of spermatocytes at similar stages can be caused by distinctly different mechanisms and pathways (Barchi, et al. 2005). It is thus likely that the phenotypes of mutant Prdm9 alleles, namely aberrant synapsis, MSUC, germ-cell death and infertility, are indirect consequences of failed and/or misplaced recombination and shared in common with mutant phenotypes of other genes in the early recombination pathway. Together these reflect the inextricable link between faithful meiotic recombination and gametogenic success.
Materials and Methods
Mice
All mice were obtained from the Jackson Laboratory (JAX) and were bred and maintained in the research colony of the authors at JAX. The protocols for their care and use at suitable ages were approved by JAX Institutional Animal Care and Use Committee (IACUC).
Histological and cytological procedures
Testes were fixed in Bouin’s or 4% paraformaldehyde in PBS overnight and paraffin-embedded. 5 μM sections were stained with Periodic Acid Schiff (PAS) by standard procedures. A NanoZoomer 2.0HT, (Hamamatsu, Japan) was used to acquire images.
Whole mount seminiferous tubule labeling
Whole mounts of seminiferous tubules were prepared as described (Buaas, et al. 2004; Rosen and Beddington 1993) with minor modifications. Tubules from 8-day-old mice were fixed in 4% PFA at 4°C for 2 hours, washed twice with PBST (PBS containing 0.1% Tween 20) and permeabilized with 0.2% NP-40 in PBS for 20 min at room temperature. Tubules were then incubated in 1% BSA in PBST for 1 h at room temperature. The tubules were stained with different antibodies (Table 1) in PBS containing 0.5% BSA at room temperature overnight, washed three times in PBS for 5 min each, and incubated with suitable secondary antibody (1:1,000) for 1 h at room temperature. To obtain seminiferous tubules at specified stages, tubules were isolated from adult mice following procedure previously described (Parvinen, et al. 1993). In brief, the testes were detunicated in cold PBS, and the stage-specific seminiferous tubules were isolated under a dissection microscope according to the differential light transmission patterns of seminiferous tubules. Due to the presence of step 16 spermatids with highly condensed chromatin, Stage VII-VIII tubules are darker than tubules at any other stages. In contrast, tubules at stages IX-X are paler due to the presence of less highly condensed step 9 and step 10 spermatids. After identification of specific stages, 1-3 mm segments were collected into a 4-well dish containing cold PBS, and fixed and stained in the same manner as tubules from 8-day-old mice. Tubules were washed as before and mounted in Vectashield with DAPI (Vector Laboratories). To confirm the stage of isolated adult seminiferous tubules, some were fixed in Bouins solution for 1 h followed by ethanol washes, then embedded and subjected to PAS staining. In some experiments, after isolation of specifically staged seminiferous tubules, a squash method was used for the cytological preparation. Squash preparations of germ cells were prepared based on previously described methods (Fujiwara, et al. 2013; Kotaja, et al. 2004), with some modifications. Briefly, mouse testes were placed in PBS and detunicated. Seminiferous tubules at stages VII-XI were selected based on light absorption pattern, and cut into segments about 1 mm in length. The segments were placed in 15 μl of 100 mM sucrose on a glass slide, and squashed with a coverslip to allow the cells to flow from the tubule. The slide was quickly dipped in liquid nitrogen, and the coverslip was removed using a scalpel. The slides were then fixed with 95% ethanol for 2-5 min and subsequently with 1% PFA for 2-3 h at RT, followed by 4% PFA for 15 min at RT. The cells were then permeabilized with 0.15% Triton X-100 for 10 min at RT. After washing three time in PBS and blocking with ADB, the slides were incubated with primary antibodies and the secondary antibodies (Table 1) in 10% ADB. The nuclei were counterstained with DAPI, then mounted using glycerol and observed as described using a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Table 1. Primary Antibodies Used in this Study*.
| Antibody | Host | Producer | Cat. Number/Reference |
Dilution |
|
|---|---|---|---|---|---|
| IF | WB | ||||
| PRDM9 | G. Pig | Custom-made | N/A | 1:100 | 1:5000 |
| FLAG | Rabbit | Sigma | F7425 | 1:200 | n/a |
| KIT | Rabbit | Millipore | CBL1360 | 1:100 | n/a |
| STRA8 | Rabbit | Abcam | Ab49405 | 1:200 | n/a |
| SMC1A | Mouse | Watanabe Lab | Ishiguro et al. 2011 | 1:500 | n/a |
| RAD21L | Rabbit | Watanabe Lab | Ishiguro et al. 2011 | 1:500 | n/a |
| REC8 | Rabbit | Watanabe Lab | Ishiguro et al. 2011 | 1:500 | n/a |
| BRCA1 | Rabbit | Namekawa Lab | Ichijima et al. 2011 | 1:100 | n/a |
| ATR | Rabbit | GeneTec | GTX74211 | 1:200 | n/a |
| MDC1 | Sheep | SeroTex | AHP799 | 1:1000 | n/a |
| SYCP1 | Rabbit | Novus | NB 300-229 | 1:100 | n/a |
| SUMO1 | Rabbit | Cell Signaling | 4390 | 1:100 | n/a |
| SYCP3 | Rat | Handel Lab | Eaker et al. 2001 | 1:1000 | n/a |
| HIST1H1T | G. Pig | Handel Lab | Cobb et al. 1999 | 1:500 | n/a |
| RAD51 | Rabbit | CalBiochem | PC130 | 1: 200 | n/a |
| P-H2AFX | Rabbit | Millipore | 07-146 | 1:200 | n/a |
| HORMAD1 | Rabbit | GeneTex | GTX119236 | 1:100 | n/a |
| TEX12 | Rabbit | PTG | 17068-1-AP | 1:200 | n/a |
| H3K4me3 | Rabbit | Millipore | 07-743 | n/a | n/a |
| MLH1 | Mouse | BD Pharmingen | 51-1327GR | 1:50 | n/a |
| POLII | Mouse | Abcam | Ab817 | 1:200 | n/a |
References: (Cobb, et al. 1999; Eaker, et al. 2001; Ichijima, et al. 2011; Ishiguro, et al. 2011)
EdU labeling
Methods for labeling proliferating cells with 5-ethynyl-2′-deoxyuridine (EdU) were modified from those previously published (Boateng, et al. 2013). Mice at age of 8 dpp were intraperitoneally injected with EdU at the dose of 50 mg/kg bodyweight. 6 h after injection, testes were removed from abdomen and put into cold PBS on ice. After removal of tunica albuginea, seminiferous tubules were washed with cold PBS. The tubules were then fixed with 4% PFA in PBS. The tubules were permeablelized with 0.2% NP-40 in PBS. After blocking, the incorporated EdU was detected by Click-iT EdU Imaging Kit (Invitrogen) following the manufacturer’s procedure. For the combination of immunofluorescence analysis, after the Click-iT reaction, the preparation was incubated in 1% BSA in PBS for 15 min and then in primary antibodies overnight. After washes, the preparation was incubated in secondary antibodies followed by series of washes. Images were acquired with a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Surface-spread chromatin preparations
Two methods were used to prepare surface-spread chromatin. Spermatocytes were collected by centrifugation, and cells were surface-spread in wells of multi-spot slides (Shandon, Pittsburgh, PA, USA) and fixed as previously described (Cobb, et al. 1999; Cobb, et al. 1997; Sun and Handel 2008). An alternative method (Peters, et al. 1997) was used with some modifications. Briefly, the testes were detunicated in PBS, and the seminiferous tubules were washed and immersed in ice-cold hypotonic extraction buffer (30 mM Tris, 50 mM sucrose, 17 mM trisodium citrate dihydrate, 5 mM ethylenediamineteraacetic acid (EDTA), 0.5 mM dithiothreitol (DTT), and 0.5 mM phenylmethyl-sulphonyl fluoride (PMSF), pH 8.2) for 1 h. Segments of the tubules were minced in 100 mM sucrose solution (pH 8.2), and the cell suspension collected was spread onto a slide glass, previously immersed in 1% paraformaldehyde containing 0.15% Triton X-100; the slide was placed in a humidified box for 3 h. The slide was washed twice for 2 min with PBS with 0.04% Photo-Flo 200 (Kodak, Rochester, NY, USA). After blocking with 5% dried milk in PBS, the slides were incubated with primary antibodies diluted in the blocking buffer for 3 h at RT. Primary antibodies (Table 1) were used as previously described (Sun and Handel 2008). Secondary antibodies conjugated with Alexa 594 or 488 or 647 (Molecular Probes, Invitrogen, Carlsbad, CA, USA) were used at 1:1000 dilution. The slides were then mounted with VECTASHIELD® Mounting Medium with DAPI (H-1200, Vector Laboratories Inc.), or mounted with SlowFade® Antifade Kit (Life Technologies) after counterstaining with DAPI. Images were acquired with a Zeiss AxioImager.Z2 epifluorescence microscope with a Zeiss AxioCam MRm CCD camera (Carl Zeiss, USA) or a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Antibodies
Primary antibodies are detailed in Table 1. Antibody recognizing PRDM9 was custom-produced in guinea pig against a peptide encompassing amino acids 101-170 of mouse PRDM9, spanning the region between the KRAB domain and the SSXRD domain (Figure 1a). This peptide was expressed in E. coli and purified to homogeneity using affinity chromatograph and fast protein liquid chromatography (FPLC). Crude guinea pig sera were affinity-purified on antigen-conjugated resin. The titer of the purified antibodies was over 1:100,000 as measured by ELISA.
Quantitation of RAD51
Spread chromatin and immunostaining were performed as described as above on testicular cell suspensions from male mice (WT, heterozygotes and homozygous Prdm9tm1Ymat mutants), age 12-16 dpp. Cells from each preparation were scored by cytological criteria of SYCP3 labeling as late leptotene, early zygotene and late zygotene. After being photographed, these cells were subjected to counting RAD51 foci using Fiji (Schindelin, et al. 2012); only clearly visible RAD51 foci associated with SYCP3 were counted. The statistical significance of differences in RAD51 counts among cells and genotypes was assessed by a Mann-Whitney test.
Germ cell isolation and enrichment
Mice at suitable ages were killed by cervical dislocation. Testes were removed, detunicated and digested in 0.5 mg/ml collagenase (Sigma) in Krebs-Ringer bicarbonate solution (KRB) [120.1 mM NaCl, 4.8 mM KCl, 25.2 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 1.3 mM CaCl2, 11 mM glucose, 1 × essential amino acids, 1 × nonessential amino acids] at 32°C for 20 min, followed by digestion in 0.5 mg/ml trypsin (Sigma) containing 20 mg/ml DNase I in KRB at 32°C for 13 min. After digestion, the cell suspension was filtered through an 80-μm mesh filter and washed three times in KRB. Germ cells were then processed as described below. Pachytene spermatocytes from adult mice were enriched by velocity sedimentation as previously described (Baker, et al. 2014; La Salle, et al. 2009).
Chromatin immunoprecipitation and quantitative PCR (ChIP-qPCR)
Cross-linking, chromatin immunoprecipitation to identify sequences exhibiting histone H3K4 trimethylation and qPCR amplification of specific sequences were performed as previously described (Baker, et al. 2014). The primer sequences are listed in Table 2.
Table 2. Primer Sequences Used in this Study.
| Locus | Genomic Position | Sequence | Usage |
|---|---|---|---|
| Gapdh | Chr6-128,203(kb) | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ Reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
qRT-PCR |
| Prdm9 | Chr17-15,690(kb) | Forward: 5′-GTGGGGAAGCAAGATGAAGA-3′ Reverse: 5′-GCTGGCCTTGTCATTTTTGT-3′ |
qRT-PCR |
| “Pbx1”* | Chr1-170,450(kb) | Forward: 5′-ATACAGCTGGCTTGCTTGGT-3′ Reverse: 5′-CCCCCTTCCCCATAATACTG-3′ |
ChIP-qPCR |
| “Armc9-1”* | Chr1-88,114(kb) | Forward: 5′-CCCACAGTTTCCTTCCGTCTT--3′ Reverse: 5′-CCTCTGTATTTCCTAGAACATG--3′ |
ChIP-qPCR |
| “Fcgr4”* | Chr1-172,970(kb) | Forward: 5′-CAAGGTGCATTCTTAGGAGAGA-3′ Reverse: 5′-TTAATGCTTGCCTCACGTTC--3′ |
ChIP-qPCR |
| “Hlx1”* | Chr1-186,567(kb) | Forward: 5′-ACCACCCAGATCATCTTTGC-3′ Reverse: 5′-AGTGTCCCTGCTTCTCTGGA-3′ |
ChIP-qPCR |
| Sycp1 | Chr3-102,740(kb) | Forward: 5′-TCTACCAAGTCTGCGCTCAA-3′ Reverse: 5′-GAAAGCACTGAGACGCCTTT-3′ |
ChIP-qPCR |
| Gapdh (ChIP) | Chr6-125,116(kb) | Forward: 5′-GAGGAGTCCTTGGAGTGTGC-3′ Reverse: 5′-CAGGGAGACCCACACTTCTC-3′ |
ChIP-qPCR |
For ease of referral, recombination hotspots are designated by the symbol for an associated gene (in quotes and not italicized, because the hotspot is not the actual gene sequence); the actual hotspot is defined by the genomic position.
RNA extraction and quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from whole testis using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and 1 μg RNA was reverse transcribed using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA), according the manufacturer’s instructions. Quantitative RT-PCR (qRT-PCR) was performed on the Applied Biosystems 7500 Real-Time PCR System (Foster City, CA) using the QuantiTect SYBR Green® RT-PCR kit (Qiagen). Gene-specific primers were used to determine the overall relative expression levels of Prdm9 according to the standard curve method (Bustin 2000). qRT-PCR results were normalized to their corresponding Gapdh content. Data are reported as mean ± SD. The primer sequences are listed in Table 2.
Western blot analysis
Total protein was extracted from testes using RIPA buffer (Santa Cruz, Santa Cruz, CA, USA) containing protease inhibitor cocktail (Santa Cruz, Santa Cruz, CA, USA). Protein concentration was measured by the BCA method (Pierce Biotechnology, Rockford, IL, USA). 40 μg protein from each group was boiled for 5 min, and proteins were separated by electrophoresis using 10% SDS-PAGE. Proteins were transferred onto PVDF membrane (Millipore, Temecula, CA, USA). The membranes were blocked overnight at 4°C with 5% dried milk in Tris-buffered saline with 0.1% Tween-20, and then probed with antibodies at concentrations specified in Table 1. The blots were incubated for 1 h with primary antibody at room temperature, and then incubated with horseradish peroxidase-conjugated secondary antibodies made from mouse or guinea pig (Invitrogen, South San Francisco, CA, USA) for 45 min at room temperature. Proteins were detected using Pierce ECL Plus Western Blotting substrate (Thermo Scientific, IL, USA).
Cell culture and transfection
HEK293 cells were cultured in DMEM (Gibco, Life Technologies) supplemented with 10% FBS (Gibco) at 37 °C and 5% CO2. 24 h prior to transfection, cells were seeded at with 10 ml 2.5·105 cell/ml in 10 cM plates with tissue culture treated glass slides. Cells were transfected using FuGENE HD (Promega, Madison, WI, USA) transfection reagent following manufacturer’s protocol and 10 ng of plasmid DNA. Full length PRDM9 was cloned from mouse testis cDNA library into the pCEP4 expression vector (Invitrogen). A 3X-FLAG tag was inserted in frame replacing the 5′ start methionine using yeast based homologous recombination technology to allow detection of expressed protein.
Supplementary Material
Supplementary Fig. 1 Expression Prdm9 and nuclear localization of PRDM9 protein. a: qRT-PCR showing the developmental pattern of Prdm9 transcript expression during the juvenile onset of spermatogenesis. b: Western blot showing developmental pattern of expression of PRDM9 protein in testis during the juvenile onset of spermatogenesis. TUBA1A is the loading control. c: Nuclear localization of PRDM9 was visualized in HEK293 cells transfected with FLAG-tagged Prdm9 after immunolabeling with preimmune serum (left panel), with anti-PRDM9 antibody in green (middle panel), and with anti-FLAG antibody in green (right panel). DNA was counterstained with DAPI (blue). The colocalization of PRDM9 with nuclear DAPI signal suggests inherent nuclear localization of PRDM9 protein, even in a heterologous system. Scale bar = 20 μm
Supplementary Fig. 2 PRDM9 in the nuclei of germ cells during early meiotic prophase I. a-b: PRDM9 in germ cells at early meiosis I prophase is revealed by immunolabeling of fibrin clot-embedded germ cells with a combination of anti-SYCP3 (red) to mark the SC and anti-PRDM9 (green). PRDM9 was not detected in pachytene spermatocytes (arrows in a), but was detected in germ cells with the patchy SYCP3 labeling typical of preleptotene/leptotene spermatocytes (arrows in b). c-f: PRDM9 is detected in the nuclei of spermatocytes co-labeled with EdU during the pre-meiotic S-phase. Juvenile males (8 dpp) were injected with EdU for a 6-hr pulse, followed by removal of testes for immunolabeling. Whole-mounted seminiferous tubules were immunolabeled with PRDM9 antibody (green), the incorporation of EdU was detected with Alexa Fluor®594 (red), and nuclei were counterstained with DAPI (blue in c and f). Localization of PRDM9 in EdU-positive germ cells provides further evidence that PRDM9 is initially expressed in S-phase/post-S-phase preleptotene/leptotene spermatocytes (arrow in e-f). Scale bars = 20 μm
Supplementary Fig. 3 Abnormal silencing of unsynapsed chromatin in Prdm9-deficient spermatocytes. Spread chromatin preparations from wild type (a) and Prdm9M1045Lja-mutant (b) spermatocytes were labeled with antibodies recognizing SUMO1 (green), a marker of transcriptional silencing, SYCP3 (blue) to mark the SC, P-H2AFX (red) antibodies. The colocalization of SUMO1 and P-H2AFX (arrows in b) provides evidence for transcriptional silencing. Scale bar = 20 μm
Acknowledgements
We are indebted to members of the Handel and Paigen laboratories for discussion of this work in progress, to Kristina Palmer and Sabrina Petri for animal care and technical assistance, to Dr. G. Carter for statistical consultation, and to Drs. G. Carter, J. Eppig and S. Handel for critical comments on the manuscript. We are grateful to Y. Watanabe and S. Namekawa for providing antibodies. This work was supported by P01 grants from the NIH (HD42137 and GM99640) and by fellowships to YF from the Japan Society for the Promotion of Science (JSPS) and the Strategic Young Researcher Oversea Visits Program for Accelerating Brain Research. Research reported in this publication was also partially supported by the National Cancer Institute under award number P30 CA034196; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley T, Plug AW, Xu JH, Solari AJ, Reddy G, Golub EI, Ward DC. Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma. 1995;104:19–28. doi: 10.1007/BF00352222. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers HJ, de Boer P, Grootegoed JA. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol. 2005;25:1041–1053. doi: 10.1128/MCB.25.3.1041-1053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Walker M, Kajita S, Petkov PM, Paigen K. PRDM9 binding organizes hotspot nucleosomes and limits Holliday junction migration. Genome Res. 2014;24:724–732. doi: 10.1101/gr.170167.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister L, Pezza R, Donaldson J, de Rooij DG, Schimenti K, Camerini-Otero RD, Schimenti JC. Male-specific sterility in mice carrying a dominant, recombination-defective allele of the RecA homolog Dmc1. PLoS Biol. 2007;5:e105. doi: 10.1371/journal.pbio.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, de Rooij DG, Burgoyne PS, Jasin M, Keeney S. Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol. 2005;25:7203–7215. doi: 10.1128/MCB.25.16.7203-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Buard J, Fledel-Alon A, Ober C, Przeworski M, Coop G, de Massy B. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science. 2010;327:836–840. doi: 10.1126/science.1183439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 2013;14:794–806. doi: 10.1038/nrg3573. [DOI] [PubMed] [Google Scholar]
- Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya T, Gregorova S, Mihola O, Anger M, Sebestova J, Denny P, Simecek P, Forejt J. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc Natl Acad Sci USA. 2013;110:E468–477. doi: 10.1073/pnas.1219126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T, Reifova R, Gregorova S, Simecek P, Gergelits V, Mistrik M, Martincova I, Pialek J, Forejt J. X chromosome control of meiotic chromosome synapsis in mouse inter-subspecific hybrids. PLoS Genet. 2014;10:e1004088. doi: 10.1371/journal.pgen.1004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings T, Parvanov ED, Baker CL, Walker M, Paigen K, Petkov PM. DNA binding specificities of the long zinc-finger recombination protein PRDM9. Genome Biol. 2013;14:R35. doi: 10.1186/gb-2013-14-4-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng KA, Bellani MA, Gregoretti IV, Pratto F, Camerini-Otero RD. Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev Cell. 2013;24:196–205. doi: 10.1016/j.devcel.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Schimenti JC. Genetics of meiosis and recombination in mice. Int Rev Cell Mol Biol. 2012;298:179–227. doi: 10.1016/B978-0-12-394309-5.00005-5. [DOI] [PubMed] [Google Scholar]
- Borde V, de Massy B. Programmed induction of DNA double strand breaks during meiosis: setting up communication between DNA and the chromosome structure. Curr Opin Genet Dev. 2013;23:147–155. doi: 10.1016/j.gde.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Brick K, Smagulova F, Khil P, Camerini-Otero RD, Petukhova GV. Genetic recombination is directed away from functional genomic elements in mice. Nature. 2012;485:642–645. doi: 10.1038/nature11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10:207–216. doi: 10.1038/nrg2505. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Cobb J, Cargile B, Handel MA. Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol. 1999;205:49–64. doi: 10.1006/dbio.1998.9101. [DOI] [PubMed] [Google Scholar]
- Cobb J, Reddy RK, Park C, Handel MA. Analysis of expression and function of topoisomerase I and II during meiosis in male mice. Mol Reprod Dev. 1997;46:489–498. doi: 10.1002/(SICI)1098-2795(199704)46:4<489::AID-MRD7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Pollack SE, Pollard JW. Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocr Rev. 2006;27:398–426. doi: 10.1210/er.2005-0017. [DOI] [PubMed] [Google Scholar]
- Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, Cooke HJ, Stewart AF, Wassmann K, Jasin M, Keeney S, Toth A. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol. 2011;13:599–610. doi: 10.1038/ncb2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B. Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu Rev Genet. 2013;47:563–599. doi: 10.1146/annurev-genet-110711-155423. [DOI] [PubMed] [Google Scholar]
- de Vries FA, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, Liu JG, van Zeeland AA, Heyting C, Pastink A. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005;19:1376–1389. doi: 10.1101/gad.329705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker S, Pyle A, Cobb J, Handel MA. Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J Cell Sci. 2001;114:2953–2965. doi: 10.1242/jcs.114.16.2953. [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1 beta and SMC3. J Cell Biol. 2003;160:657–670. doi: 10.1083/jcb.200212080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Ogonuki N, Inoue K, Ogura A, Handel MA, Noguchi J, Kunieda T. t-SNARE Syntaxin2 (STX2) is implicated in intracellular transport of sulfoglycolipids during meiotic prophase in mouse spermatogenesis. Biol Reprod. 2013;88:141. doi: 10.1095/biolreprod.112.107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Fukuda N, Agostinho A, Hernandez-Hernandez A, Kouznetsova A, Hoog C. STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 2014;33:1243–1255. doi: 10.1002/embj.201387329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Caballero C, Herran Y, Sanchez-Martin M, Suja JA, Barbero JL, Llano E, Pendas AM. Identification and molecular characterization of the mammalian alpha-kleisin RAD21L. Cell Cycle. 2011;10:1477–1487. doi: 10.4161/cc.10.9.15515. [DOI] [PubMed] [Google Scholar]
- Handel MA, Lessard C, Schimenti JC, Eppig JJ. Mutagenesis as an unbiased approach to identify novel contraceptive targets. Mol Cell Endo. 2006;250:201–205. doi: 10.1016/j.mce.2005.12.046. L. R. [DOI] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Matsui Y. Meisetz, a novel histone tri-methyltransferase, regulates meiosis-specific epigenesis. Cell Cycle. 2006;5:615–620. doi: 10.4161/cc.5.6.2572. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–378. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- Herran Y, Gutierrez-Caballero C, Sanchez-Martin M, Hernandez T, Viera A, Barbero JL, de Alava E, de Rooij DG, Suja JA, Llano E, Pendas AM. The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J. 2011;30:3091–3105. doi: 10.1038/emboj.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J, Hwang G, Jacob J, Sapp N, Bedigian R, Oka K, Overbeek P, Murray S, Jordan PW. Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet. 2014;10:e1004413. doi: 10.1371/journal.pgen.1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Camerini-Otero RD, Chen J, Andreassen PR, Namekawa SH. MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev. 2011;25:959–971. doi: 10.1101/gad.2030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y. A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep. 2011;12:267–275. doi: 10.1038/embor.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Guacci V, Yu HG. Pds5 is required for homologue pairing and inhibits synapsis of sister chromatids during yeast meiosis. J Cell Biol. 2009;186:713–725. doi: 10.1083/jcb.200810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi L, Barchi M, Lange J, Baudat F, Jasin M, Keeney S. Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev. 2013;27:873–886. doi: 10.1101/gad.213652.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja N, Kimmins S, Brancorsini S, Hentsch D, Vonesch J-L, Davidson I, Parvinen M, Sassone-Corsi P. Preparation, isolation and characterization of stage-specific spermatogenic cells for cellular and molecular analysis. Nat Methods. 2004;1:249–254. doi: 10.1038/nmeth1204-249. [DOI] [PubMed] [Google Scholar]
- Koubova J, Hu YC, Bhattacharyya T, Soh YQ, Gill ME, Goodheart ML, Hogarth CA, Griswold MD, Page DC. Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet. 2014;10:e1004541. doi: 10.1371/journal.pgen.1004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Salle S, Sun F, Handel MA. Isolation and short-tem culture of mouse spermatocytes for analysis of meiosis. In: Keeney S, editor. Methods in Molecular Biology, Molecular Medicine and Biotechnology: Meiosis Protocols. Humana Press; 2009. pp. 279–297. [DOI] [PubMed] [Google Scholar]
- Llano E, Gomez HL, Garcia-Tunon I, Sanchez-Martin M, Caburet S, Barbero JL, Schimenti JC, Veitia RA, Pendas AM. STAG3 is a strong candidate gene for male infertility. Hum Mol Genet. 2014;23:3421–3431. doi: 10.1093/hmg/ddu051. [DOI] [PubMed] [Google Scholar]
- Llano E, Herran Y, Garcia-Tunon I, Gutierrez-Caballero C, de Alava E, Barbero JL, Schimenti J, de Rooij DG, Sanchez-Martin M, Pendas AM. Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol. 2012;197:877–885. doi: 10.1083/jcb.201201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JMA, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Major AT, Whiley PA, Loveland KL. Expression of nucleocytoplasmic transport machinery: clues to regulation of spermatogenic development. Biochim Biophys Acta. 2011;1813:1668–1688. doi: 10.1016/j.bbamcr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Novak I, Wang H, Revenkova E, Jessberger R, Scherthan H, Hoog C. Cohesin Smc1beta determines meiotic chromatin axis loop organization. J Cell Biol. 2008;180:83–90. doi: 10.1083/jcb.200706136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K, Petkov P. Mammalian recombination hot spots: properties, control and evolution. Nat Rev Genet. 2010;11:221–233. doi: 10.1038/nrg2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvanov ED, Petkov PM, Paigen K. Prdm9 controls activation of mammalian recombination hotspots. Science. 2010;327:835. doi: 10.1126/science.1181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen M, Toppari J, Lahdetie J. Transillumination phase contrast microscope techniques for evaluation of male germ cell toxicity and mutagenicity. In: Heindel JJ, editor. Methods Toxicol. Academic Press; San Diego: 1993. pp. 142–165. [Google Scholar]
- Peters AHFM, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997;5:66–71. doi: 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
- Pezzi N, Prieto I, Kremer L, Jurado LAP, Valero C, Del Mazo J, Martinez C, Barbero JL. STAG3, a novel gene encoding a protein involved in meiotic chromosome pairing and location of STAG3-related genes flanking the Williams-Beuren syndrome deletion. FASEB J. 2000;14:581–592. doi: 10.1096/fasebj.14.3.581. [DOI] [PubMed] [Google Scholar]
- Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- Prieto I, Suja JA, Pezzi N, Kremer L, Martinez C, Rufas JS, Barbero JL. Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol. 2001;3:761–766. doi: 10.1038/35087082. [DOI] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, Gross B, Jessberger R. Novel meiosis-specific isoform of mammalian SMC1. Mol Cell Biol. 2001;21:6984–6998. doi: 10.1128/MCB.21.20.6984-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Jessberger R. Shaping meiotic prophase chromosomes: cohesins and synaptonemal complex proteins. Chromosoma. 2006;115:235–240. doi: 10.1007/s00412-006-0060-x. [DOI] [PubMed] [Google Scholar]
- Rogers RS, Inselman A, Handel MA, Matunis MJ. SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma. 2004;113:233–243. doi: 10.1007/s00412-004-0311-7. [DOI] [PubMed] [Google Scholar]
- Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Rosen B, Beddington RS. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet. 1993;9:162–167. doi: 10.1016/0168-9525(93)90162-b. [DOI] [PubMed] [Google Scholar]
- Scherthan H. A bouquet makes ends meet. Nat Rev Mol Cell Biol. 2001;2:621–627. doi: 10.1038/35085086. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Handel MA. Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes. Chromosoma. 2008;117:471–485. doi: 10.1007/s00412-008-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM. Meiotic sex chromosome inactivation. Development. 2007;134:1823–1831. doi: 10.1242/dev.000018. [DOI] [PubMed] [Google Scholar]
- Turner JMA, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng C-X, Burgoyne PS. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet. 2005;37:41–47. doi: 10.1038/ng1484. [DOI] [PubMed] [Google Scholar]
- Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet. 2015;11:e1004949. doi: 10.1371/journal.pgen.1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Hurley LA, Harris RM, Finlayson C, Tong M, Fisher LA, Moran JL, Beier DR, Mason C, Jameson JL. ENU mutagenesis in mice identifies candidate genes for hypogonadism. Mammalian Genome. 2012;23:346–355. doi: 10.1007/s00335-011-9388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005;8:949–961. doi: 10.1016/j.devcel.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S-I, Kunisada T, Fujimoto T, Nishikawa S-I. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik DJ, Banasik B, McCarrey JR, Small C, Griswold MD. Expression of Stimulated by retinoic acid gene 8 (Stra8) and mauration of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod. 2008;78:537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Expression Prdm9 and nuclear localization of PRDM9 protein. a: qRT-PCR showing the developmental pattern of Prdm9 transcript expression during the juvenile onset of spermatogenesis. b: Western blot showing developmental pattern of expression of PRDM9 protein in testis during the juvenile onset of spermatogenesis. TUBA1A is the loading control. c: Nuclear localization of PRDM9 was visualized in HEK293 cells transfected with FLAG-tagged Prdm9 after immunolabeling with preimmune serum (left panel), with anti-PRDM9 antibody in green (middle panel), and with anti-FLAG antibody in green (right panel). DNA was counterstained with DAPI (blue). The colocalization of PRDM9 with nuclear DAPI signal suggests inherent nuclear localization of PRDM9 protein, even in a heterologous system. Scale bar = 20 μm
Supplementary Fig. 2 PRDM9 in the nuclei of germ cells during early meiotic prophase I. a-b: PRDM9 in germ cells at early meiosis I prophase is revealed by immunolabeling of fibrin clot-embedded germ cells with a combination of anti-SYCP3 (red) to mark the SC and anti-PRDM9 (green). PRDM9 was not detected in pachytene spermatocytes (arrows in a), but was detected in germ cells with the patchy SYCP3 labeling typical of preleptotene/leptotene spermatocytes (arrows in b). c-f: PRDM9 is detected in the nuclei of spermatocytes co-labeled with EdU during the pre-meiotic S-phase. Juvenile males (8 dpp) were injected with EdU for a 6-hr pulse, followed by removal of testes for immunolabeling. Whole-mounted seminiferous tubules were immunolabeled with PRDM9 antibody (green), the incorporation of EdU was detected with Alexa Fluor®594 (red), and nuclei were counterstained with DAPI (blue in c and f). Localization of PRDM9 in EdU-positive germ cells provides further evidence that PRDM9 is initially expressed in S-phase/post-S-phase preleptotene/leptotene spermatocytes (arrow in e-f). Scale bars = 20 μm
Supplementary Fig. 3 Abnormal silencing of unsynapsed chromatin in Prdm9-deficient spermatocytes. Spread chromatin preparations from wild type (a) and Prdm9M1045Lja-mutant (b) spermatocytes were labeled with antibodies recognizing SUMO1 (green), a marker of transcriptional silencing, SYCP3 (blue) to mark the SC, P-H2AFX (red) antibodies. The colocalization of SUMO1 and P-H2AFX (arrows in b) provides evidence for transcriptional silencing. Scale bar = 20 μm