Abstract
Background
Approaches to antiretroviral therapy (ART) in HIV-infected pregnant women have changed considerably in recent years, but there are few comparative data on the implementation of different models of service delivery.
Methods
Using routine clinic records we examined ART initiation in pregnant women attending a large antenatal care (ANC) facility between January 2010 and December 2013 in Cape Town, South Africa. Over this time six different service delivery models were implemented sequentially to provide ART in pregnancy, including the integration of ART into ANC, use of point-of-care CD4 cell count testing, and universal ART initiation for all HIV-infected pregnant women.
Results
During the study period 19,432 women sought ANC, levels of HIV testing were high (98%) and 30% of pregnant women tested HIV-positive. Integration of ART into ANC was associated with significant increases in the proportion of eligible women initiating treatment before delivery compared to referral to a separate ART clinic (p<0.001). When CD4 cell counts were used to determine ART eligibility, point-of-care testing was associated with decreased delays to ART initiation compared to laboratory-based testing (p<0.001). The strategy of universal ART led to the highest levels of ART initiation (with 92% of women starting before delivery) and the shortest delays, with 82% of women starting ART on the day of the first ANC visit.
Conclusion
Developments in service delivery models, most notably service integration and universal ART for pregnant women, have improved antenatal ART initiation dramatically in this setting. Further research is needed into how strategies for antenatal ART initiation impact maternal and child health over the long-term.
Keywords: antiretroviral therapy, mother-to-child transmission, pregnancy, antenatal care, service integration, point-of-care CD4, South Africa
Introduction
Over the last decade there have been major advances in the prevention of mother-to-child transmission (PMTCT) of HIV infection across sub-Saharan Africa [1,2]. Increasingly complex antiretroviral regimens have been promulgated for prophylaxis along with the expansion of eligibility of pregnant women for triple-drug, lifelong antiretroviral therapy (ART) [3-5]. Yet implementation of PMTCT services remains suboptimal in many settings, with PMTCT coverage limited by health systems and clinical barriers to ART initiation in pregnancy [6-8].
The functional separation of PMTCT and adult ART services within health systems is a potential hurdle to PMTCT implementation [9]. In many settings, PMTCT services operate within antenatal care (ANC), separate from ART services, and ART-eligible pregnant women are referred out of ANC for treatment initiation. There is evidence that a substantial proportion of pregnant women do not complete this referral [10,11] and strategies such as use of ‘patient navigators’ have been suggested as an intervention to better link women from ANC/PMTCT services to ART clinics [12,13]. Another strategy to overcome this functional separation is the integration of ART services into ANC/PMTCT programmes. This approach typically sees providers working in ANC/PMTCT conducting pre-initiation assessments (including clinical examination, counseling and laboratory testing)and initiating ART in eligible pregnant women, eliminating the need for referral from ANC/PMTCT for ART [14-16]. However data are mixed on whether such integration increases antenatal ART initiation in practice [15,17].
Specific clinical and immunologic eligibility criteria for treatment may introduce further barriers to ART initiation in pregnancy. Historically, guidelines have called for ART initiation in pregnant women based on WHO staging or CD4 cell count thresholds used for non-pregnant adults [18, 19]. But as laboratory access is usually required for CD4 cell enumeration, CD4-based criteria for ART eligibility can introduce significant delays related to laboratory logistics [20, 21]. Provision of point-of-care (POC) CD4 testing is one approach to overcome these logistical barriers and may be particularly helpful in facilitating the rapid identification of ART-eligible pregnant women [22,23], but there are few data on the impact of POC CD4 testing within ANC/PMTCT services. More recently, the 2013 WHO guidelines recommended universal ART for all HIV-infected pregnant women regardless of CD4 cell count or WHO stage, either to be continued for life (“Option B+”) or to be stopped at the end of breastfeeding for women with relatively high CD4 cell counts (“Option B”) [5]. While these approaches for universal ART initiation have gained widespread endorsement there are few data on their practical implementation, and insights into how these compare with approaches to ART initiation in pregnancy that rely on CD4-based eligibility criteria are required [24-26].
Many of these strategies to facilitate ART initiation in pregnancy have been discussed individually, yet little is known about the implementation of different interventions in practice. To address this, we examined the evolution of ART services for HIV-infected pregnant women over a four-year period in Cape Town, South Africa. Specifically, we described how different service delivery models for providing ART to HIV-infected pregnant women influenced the uptake of ART in pregnant women before delivery.
Methods
We used routine health care records to construct a retrospective cohort of all HIV-infected pregnant women seeking ANC between 1 January 2010 and 31 December 2013 at a large ANC facility and nearby ART services in the community of Gugulethu in Cape Town, South Africa. The facility serves an estimated population of 350 000 and levels of poverty are high, with 60% of the community living in informal housing.
ANC and PMTCT services
Approximately 4800 pregnant women seek ANC annually at the facility, which provides basic antenatal and obstetric services with hospital referral available for more complex care. PMTCT services are provided within the antenatal clinic (ANC/PMTCT) based on South African national guidelines. At the first ANC visit, all women receive HIV counseling and testing (HCT) with group and individual pre-test counseling and individual post-test counseling. All HIV-infected women receive additional counseling, phlebotomy for CD4 enumeration, and further intervention as appropriate. This ANC service is nested within a community health centre that provides a range of primary health care services, including ART, on a campus of adjacent buildings; while the ANC is staffed by nurse-midwives and counselors (with limited support from medical officers and obstetricians) the facility governance and oversight is through the community health centre. Levels of ANC coverage in this community are high, with >95% of pregnant women attending antenatal care before delivery. Partner testing is encouraged by the ANC/PMTCT service but was uncommon during the study period; separate HCT services for non-pregnant individuals are widely available in the community, including in the community health centre.
Routine ART services
General adult ART services are provided at a dedicated primary care facility located 100 metres from the ANC/PMTCT facility. The operation and outcomes of the adult ART program have been described previously [27]. Briefly, patients identified as ART-eligible based on CD4 cell count or WHO staging are referred to these services for pre-initiation assessments that include clinical examination, counseling and laboratory investigations; ART initiation takes places 2-4 weeks later following review of laboratory testing and completion of adherence counseling. At the start of the study period, there were approximately 3000 adults on ART, and over the four-year period an additional 4400 non-pregnant adults and children initiated therapy from the surrounding community.
Models of care for ART initiation in pregnancy
Six service delivery models were examined in sequential phases during the 48-month study period (Table 1).
During a first pre-intervention period (January 2010 to October 2010) ANC/PMTCT services referred all eligible pregnant women based on 2006 WHO guidelines to the ART clinic [18]. ART eligibility was determined using laboratory-based CD4, and women with CD4 ≤200 cells/μL were provided with a standardized referral letter and counselling to attend the ART service. Women who completed this referral underwent pre-initiation assessment and subsequent ART initiation as described above.
In a second pre-intervention period, services (November 2010 to July 2011) followed the same approach but the CD4 threshold for ART eligibility was increased to CD4 ≤350 cells/μL[19].
Third, an Enhanced Linkage intervention (August 2011 to January 2012) used lay PMTCT counselors as ‘patient navigators’ to support referrals to the ART service. Lay counsellors accompanied eligible women, based on CD4 ≤350 cells/μL, from the ANC/PMTCT service to the ART clinic, assisting with the appointment process and the first clinic visit while providing PMTCT and ART-related counselling.
Fourth, an Integrated ART intervention period (January 2012 to August 2012) shifted the site of ART initiation from the ART clinic to the ANC/PMTCT service, removing the need to refer women between services. The Integrated ART service was delivered by ART-trained midwives working in the ANC using clinical and monitoring protocols identical to those used in the ART clinic. Throughout this period, ART-eligibility was determined with laboratory-based CD4 ≤350 cells/μL.
During the fifth phase, On-site CD4 testing, (July 2012 to June 2013) CD4 testing was added to the Integrated ART service using the Alere Pima POC device. While we found good agreement between POC and laboratory-based testing [28], all specimens tested on the POC machine during this phase were also sent for laboratory testing, and women deemed eligible for ART based on CD4≤350 cells/μL on either platform. The on-site CD4 test resulted in a slightly higher proportion of women being ART-eligible based on CD4≤350 cells/μL compared to the laboratory platform [28]. During the On-site CD4 testing phase, approaches to counseling ART-eligible pregnant women emphasized rapid ART initiation [29,30].
Finally, during the sixth phase, Universal ART (July 2013-December 2013), changes in provincial PMTCT policy to “Option B+” meant that all HIV-infected pregnant women were eligible for lifelong therapy [31]. With this shift to universal ART provided as part of PMTCT/ANC, CD4 cell counts were no longer used to determine eligibility but were still performed using laboratory testing to determine nadir CD4.
Table 1. Key features of service delivery models implemented during the study period, January 2010 – December 2013.
| Pre-intervention, CD4≤200* | Pre-intervention, CD4≤350** | Enhanced linkage** | Integrated ART** | On-site CD4 testing ** | Universal ART*** | |
|---|---|---|---|---|---|---|
| Calendar period | January 2010-October 2010 | November 2010-July 2011 | August 2011-January 2012 | January 2012-June 2012 | July 2012- June 2013 | July 2013-December 2013 |
| ART eligibility criteria | CD4 ≤200 cells/μL & WHO stage III/IV | CD4 ≤350 cells/μL & WHO stage III/IV | CD4 ≤350 cells/μL & WHO stage III/IV | CD4 ≤350 cells/μL & WHO stage III/IV | CD4 ≤350 cells/μL & WHO stage III/IV | All HIV-infected pregnant women |
| Location of CD4 cell count testing for ART eligibility | Off-site laboratory | Off-site laboratory | Off-site laboratory | Off-site laboratory | Point-of-care testing and off-site laboratory | N/A – CD4 not used to determine eligibility |
| Site of ART initiation | ART services separate from ANC/PMTCT services**** | ART services separate from PMTCT/ANC services**** | Adult ART separate from PMTCT/ANC services**** | ART provided within PMTCT/ANC services | ART provided within PMTCT/ANC services | ART provided within PMTCT/ANC services |
| Support for referrals between ANC & ART service | None | None | Patient navigators assisting referral of pregnant women | N/A (no referral in integrated service) | N/A (no referral in integrated service) | N/A (no referral in integrated service) |
PMTCT services and ART eligibility based on South African national policies following 2006 WHO guidelines [18]
PMTCT services and ART eligibility based on South African national policies following 2010 WHO guidelines [19]
PMTCT services and ART eligibility based on South African national policies following 2013 WHO guidelines [5]
Referral to separate ART services located on the same community health centre campus 100 metres from the ANC/PMTCT services
Following national guidelines, all women initiating ART during the study period received two nucleoside reverse transcriptase inhibitors (usually tenofovir and lamivudine) and a non-nucleoside reverse transcriptase inhibitor (nevirapine up to 2011, and efavirenz thereafter). Throughout the observation period, both the routine ART service and integrated ART service employed the same clinical and laboratory monitoring protocols, the same laboratory service (with the exception of the POC CD4 technology used during the on-site CD4 phase), and ART and PMTCT counselors were overseen by the same local non-governmental organization. As part of the service delivery models for On-site CD4 testing and Universal ART, there was an effort to expedite ART initiation including minimizing delays to ART initiation due to counseling, however the counselors working in the service, the patient education materials and the counseling messages were not changed with the service delivery models.
Data sources and measures
Data were drawn from two routinely collected data sources. First, the PMTCT register maintained at the ANC was used to extract the number of women making their first ANC visit, consenting to HCT, testing HIV positive, and CD4 cell counts. Second, records of all patients seen at either the ART service or the PMTCT/ANC service were accessed to abstract the dates of pre-initiation assessment and ART initiation, as well as socio-demographic and clinical information. All patient data were de-identified prior to analysis, and permission to use routine clinical data for the purposes of research was provided by the University of Cape Town Health Research Ethics Committee.
Data analysis
Data were analysed using Stata version 12.0 (Stata Corporation, USA). The proportions of women completing different steps within the PMTCT ‘cascade’, including accepting HCT, testing HIV-positive, with CD4 cell counts results, ART eligibility, and ART initiation were estimated with exact 95% confidence intervals (CI); proportions were compared across service delivery models using chi-squared tests. All women who were deemed ART-eligible according to the criteria used in each phase (as listed above) were included in analyses of ART eligibility; in instances of multiple pregnancies involving the same woman during the four-year period, analyses of ART-eligibility were restricted to the woman's first visit at which she was ART-naïve and -eligible. Delays between the dates of the first antenatal visit, pre-initiation assessment and ART initiation (for women starting ART) were summarized using medians with interquartile ranges (IQR); comparisons employed non-parametric tests for trend [32]. We used the product-limit method to estimate the proportion of women initiating ART from the date of the first ANC visit, with censoring at the time of delivery, presented as ‘failure’ plots for different periods. Proportional hazards regression was used to compare the frequency of antenatal ART initiation between periods after accounting for covariation in participant demographic and clinical characteristics; results are presented as hazard ratios (HR) with 95% CI. Throughout, the primary analyses included all women making their first ANC visit during each intervention period; in sensitivity analyses we (i) excluded the first two months of observation under each service delivery model to allow for transitioning from the previous model and gradual optimization of service routines and then (ii) limited analyses to the first 6 months of each period (based on the shortest duration of the observation periods) to limit the possibility of service enhancements that make take place after six months of implementation.
Results
Between January 2010 and December 2013, 19,432 women made their first ANC visit and were included in analysis. Table 2 describes the proportion of women engaged in different aspects of ANC/PMTCT and ART care during each service delivery model. Uptake of HCT was high (96%-100%) with the antenatal HIV seroprevalence approximately 30% throughout the study period. Among HIV-infected women the proportion of women with available CD4 results increased over time but was not significantly different in the integrated ART service that used laboratory-based testing compared to the same service after the introduction of POC CD4 testing (p=0.98). The proportion of HIV-infected women who entered ANC already on ART increased over time, from less than 10% in the first period to more than 40% under universal ART (p<0.001).
Table 2. Description of antenatal care (ANC) and PMTCT services during the study period, by service delivery model.
| Pre-intervention, CD4≤200 | Pre-intervention, CD4≤350 | Enhanced linkage | Integrated ART | On-site CD4 testing | Universal ART | Total | |
|---|---|---|---|---|---|---|---|
| Duration of period, in months | 10 | 9 | 6 | 6 | 11 | 6 | 48 |
| Number women (N) seeking ANC | 4329 | 3692 | 2444 | 2568 | 4419 | 2070 | 19432 |
| per month | 434 | 410 | 407 | 428 | 401 | 345 | 404 |
| N (%) consenting to HCT | 4165 (96) | 3682 (99) | 2274 (93) | 2491 (97) | 4399 (99) | 2068 (100) | 19079 (98) |
| N (%) HIV-positive | 1223 (29) | 1140 (31) | 580 (26) | 789 (32) | 1309 (30) | 688 (33) | 5736 (30) |
| per month | 122 | 127 | 97 | 132 | 119 | 115 | 120 |
| N (%) already on ART at time of ANC | 84 (7) | 136 (12) | 98 (17) | 229 (29) | 445 (34) | 319 (46) | 1311 (23) |
| N (%) not already on ART at time of ANC | 1139 (93) | 1004 (88) | 492 (83) | 560 (71) | 864 (66) | 369 (54) | 4425 (77) |
| N (%) CD4 available * | 974 (86) | 883 (88) | 449 (91) | 552 (99) | 853 (99) | 348 (94) | 4065 (92) |
| Median CD4,not on ART | 365 | 400 | 400 | 392 | 413 | 390 | 395 |
| Median CD4, on ART | 288 | 360 | 335 | 341 | 324 | 391 | 340 |
| N (%) not on ART, CD4≤200 | 176 (18) | 121 (14) | 53 (11) | 79 (14) | 93 (11) | 51 (15) | 576 (14) |
| N (%) not on ART, CD4≤350 | 469 (48) | 344 (39) | 177 (40) | 232 (42) | 317 (37) | 141 (41) | 1681 (41) |
| N (%) women eligible for ART ** | 176 (18) | 344 (39) | 177 (40) | 232 (42) | 317 (37) | 369 (100) | 1615 (35) |
| NART-eligible women per month | 18 | 38 | 30 | 39 | 29 | 62 | 35 |
| N initiated ART | 82 | 83 | 88 | 190 | 268 | 339 | 1050 |
| N Initiated ART before delivery | 59 | 60 | 77 | 182 | 264 | 339 | 981 |
| % initiating, of CD4≤200 | 18% | 17% | 45% | 80% | 87% | 92% | 47% |
| % initiating, of CD4≤350 | 12% | 16% | 41% | 76% | 80% | 94% | 44% |
| % initiating, of ART-eligible | 34% | 17% | 44% | 78% | 83% | 92% | 65% |
| % initiating, of all HIV-infectedwomen | 5% | 6% | 16% | 33% | 31% | 92% | 22% |
| Initiated ART after delivery | 23 | 23 | 11 | 8 | 4 | 0 | 69 |
Among women not already on ART;
ART eligibility as defined by the service delivery model
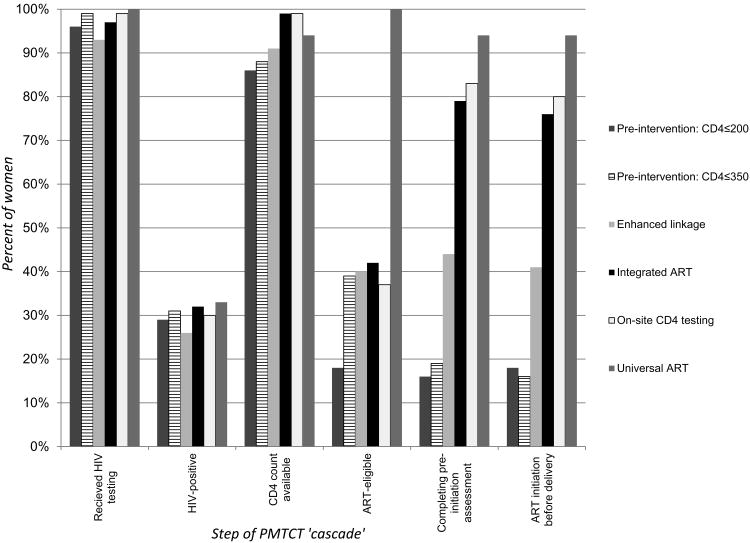
Figure 1 shows the ART initiation ‘cascade’ across service delivery models. The proportion of all HIV-infected pregnant women not already on ART who were identified as eligible increased from 18% during the first phase when eligibility was based on CD4≤200 cells/μl, to approximately 40% during subsequent phases when eligibility criteria broadened to CD4≤350 cells/μl, to 100% under the Universal ART approach. The absolute numbers of women requiring ART per month increased in parallel.
Figure 1. PMTCT ‘cascade’ during the study period, by service delivery model.
Among ART-eligible women, the proportions women undergoing pre-initiation assessment and initiating ART before delivery were significantly different across service delivery models (Table 2 and Figure 1). Compared to the preceding period of separate ART and ANC/PMTCT services, the proportion of ART-eligible women initiating treatment under the Enhanced Linkage model increased by 27% (from 17% to 44%), and subsequently the proportion of women initiating ART before delivery in the Integrated ART service model increased a further 34% (from 44% to 78%; p<0.001 for both comparisons). The inclusion of POC CD4 testing in the On-site CD4 testing model did not increase this proportion further however (78% vs 83%, p=0.64). The highest levels of ART initiation were observed under the Universal ART model with 92% of all HIV-infected women initiating ART during pregnancy, and 94% of women with CD4≤350 cells/μl, compared to 76-80% of women initiated under the Integrated ART model using CD4-based eligibility criteria.
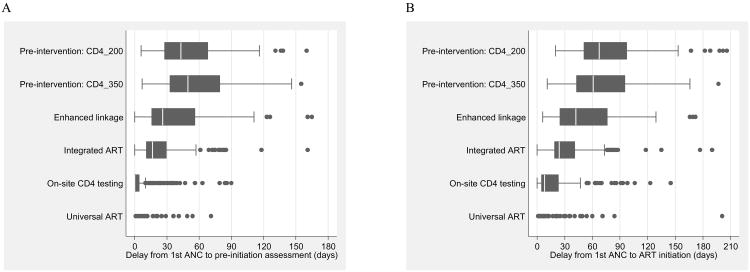
In the subset of women initiating ART during pregnancy, the distribution of delays from the time of first ANC attendance to pre-initiation assessment and ART initiation are shown in Figure 2. The median delay from first ANC attendance to pre-initiation assessment (Figure 2a) decreased from more than 40 days during the two pre-intervention phases (IQR, 19-80 days), to 17 days (IQR, 11-31 days) in the Integrated ART model, to 0 days (ie, pre-initiation assessments were carried out on the same day as the first ANC visit for the majority of women) under both the On-site CD4 testing and Universal ART models (p-value for trend, <0.001). In parallel, the median delay from first ANC attendance to ART initiation decreased from more than 60 days during the pre-intervention periods (IQR, 34-105 days), to 42 (IQR, 23-76 days), 23 (IQR, 18-42 days), 8 (IQR, 5-23 days)and 0 days under the Enhanced Linkage, Integrated ART, On-site CD4 testing and Universal ART models, respectively (p-value for trend <0.001). Under the Universal ART model of “Option B+”, 82% of women who initiated ART did so on the date of their first ANC visit.
Figure 2. Box plots of distribution of delays in days from first antenatal care (ANC) visit to (a) date of pre-initiation assessment among eligible women* and (b) date of ART initiation, by service delivery model.
A. Delay from first ANC visit to pre-initiation assessment
B. Delay from first ANC visit to ART initiation
* ART eligibility as defined by the service delivery model
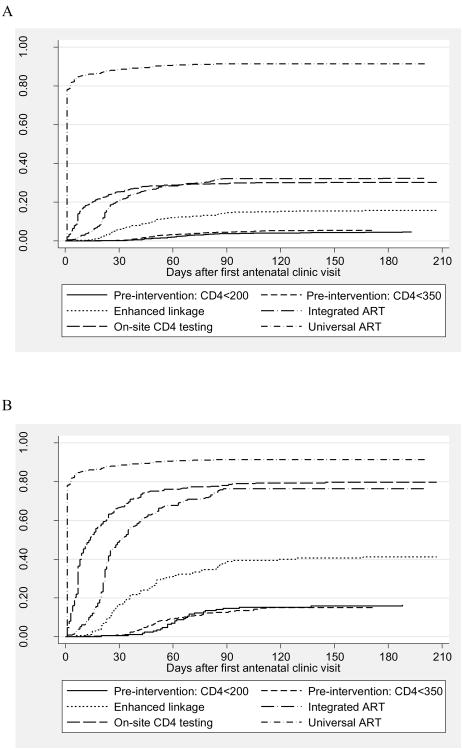
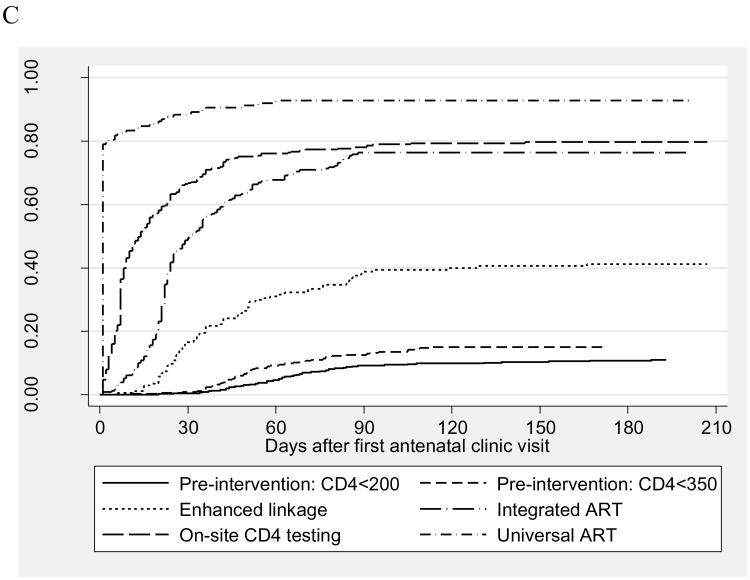
Figure 3 shows the proportions of women starting ART during pregnancy over time from the first ANC visit under different models of service delivery, including analysis of all HIV-infected women (Figure 3a) as well as restricted to women determined to be ART-eligible under the respective service model (Figure 3b) and women with CD4 cell counts <350 cells/μL (Figure 3c). In all analyses, the proportion of women initiating therapy before delivery was lowest in the pre-intervention phases, increased under the Integrated ART model, and was highest in the Universal ART model. When the analysis was restricted to women identified as ART-eligible as per the different service delivery models (Figure 3b), the proportion of women initiating ART before delivery under CD4-based eligibility criteria did not exceed 80% regardless of the model of care. When using an ART initiation CD4 threshold of 350 cells/μl (Figure 3c), the proportion of women initiating therapy over time under On-site CD4 testing model was significantly higher than was observed under the Integrated ART model with laboratory-based CD4 cell counts only (log-rank p<0.001). In sensitivity analyses (Supplemental Digital Content - Appendix 1), larger differences were observed between the models when the first 2 months of observation under each model were excluded to allow for service optimization, however the findings did not vary substantively when analyses were restricted to the first six months of each service delivery period.
Figure 3.
Kaplan-Meier “failure” plots of time from the start of antenatal care to ART initiation before delivery, by service delivery model, for (A) all HIV-infected pregnant women, (B) for ART-eligible women (as defined by the service delivery model), and (C) for women with CD4 cell counts ≤350 cells/μl.
A. All HIV-infected pregnant women
B. ART-eligible women, as defined by the service delivery model
C. Women with CD4 ≤350 cells/μl
In proportional hazards regression (Table 3), the differences in ART initiation between service delivery models persisted after adjustment for women's age, gestation in weeks at the start of ANC, and CD4 cell count. Compared to the pre-intervention model with ART eligibility based on a CD4≤350 cells/μl, women were approximately 7 times more likely to initiate ART before delivery under the Integrated ART model, and more than 20 times as likely under the Universal ART model. When the model was restricted to women with CD4≤350 cells/μl, antenatal ART initiation was 30 times more likely under the Universal ART model, compared to the pre-intervention phases (HR, 32.09; 95% CI, 22.95-44.85).
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) from proportional hazards models predicting antenatal ART initiation during the study period (a) for all HIV-infected women making their first antenatal clinic visit, (b) for HIV-infected women determined to be eligible for ART as defined by the service delivery model and (c) for all women with CD4 cell counts≤350 cells/μL. Models include all covariates shown.
| (A) All HIV-infected women | (B) ART-eligible women * | (C) Women with CD4 ≤350 cells/μL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Model of care: | |||||||||
| Pre-intervention, CD4≤200 | 0.83 | 0.55-1.25 | <0.001 | 1.06 | 0.65-1.71 | <0.001 | 0.75 | 0.50-1.12 | <0.001 |
| Pre-intervention, CD4≤350 | 1.0 | (reference) | 1.0 | (reference) | 1.0 | (reference) | |||
| Enhanced linkage | 3.07 | 2.13-4.44 | 3.05 | 2.11-4.40 | 3.20 | 2.21-4.63 | |||
| Integrated ART | 7.12 | 5.20-9.76 | 7.60 | 5.54-10.44 | 8.18 | 5.95-11.23 | |||
| On-site CD4 testing | 8.53 | 6.29-11.57 | 10.66 | 7.84-14.48 | 12.11 | 8.90-16.47 | |||
| Universal ART | 57.07 | 42.00-77.54 | 27.74 | 20.22-38.07 | 32.09 | 22.95-44.85 | |||
| Age (years) | 1.00 | 0.99-1.01 | 0.836 | 1.00 | 0.98-1.01 | 0.774 | 0.99 | 0.98-1.01 | 0.400 |
| Gestation at first antenatal visit (weeks) | 0.97 | 0.96-0.98 | <0.001 | 0.82 | 0.97-1.00 | 0.006 | 0.98 | 0.67-0.99 | 0.004 |
| CD4 cell count (50-unit change) | 0.80 | 0.78-0.81 | <0.001 | 0.97 | 0.85-0.99 | 0.023 | 0.91 | 0.87-0.95 | <0.001 |
ART eligibility as defined by the service delivery model
Discussion
This analysis documents the rapid evolution of services for ART initiation in HIV-infected pregnant women in Cape Town, South Africa. Over four years, implementation of new models of service delivery were accompanied by increases in both the numbers of pregnant women who were ART-eligible and the proportion of women starting ART before delivery, as well as significant decreases in the delays to antenatal treatment initiation.
Integration of ART into ANC services has been discussed widely, yet empirical data on the effects of integration on ART initiation are surprisingly mixed [14,15,17]. Here, the shift to integrated ART within ANC services, compared to the preceding period of referral of ART-eligible women from ANC to separate ART facilities, was associated with the single greatest absolute increase in the proportion of ART-eligible women initiating treatment before delivery. Integrated ART services were also associated with significant reductions in the delay from first ANC visit to ART initiation. As with any health service intervention, the effects of integration of ART into ANC services are likely to depend heavily on local contexts. In this setting the system of referral of ART-eligible pregnant women to separate services for treatment initiation was clearly suboptimal, resulting in only 20-50% of women initiating before delivery [10,33]. While other health systems across sub-Saharan Africa may have stronger ANC-ART referral systems, these data suggest that in settings where referral of pregnant women for ART initiation functions poorly, the integration of services can result in significant advances.
We found that the inclusion of POC CD4 testing did not increase the proportion of women initiating ART before delivery compared to laboratory-based testing systems. However these observations are from a context of relatively good laboratory access, where more than 80% of women had CD4 counts available through laboratory testing. In settings where laboratory access is limited or non-existent, POC technologies are likely to play an important role in provision of timely test results. This may be particularly important in settings where CD4 cell counts are used to determine ART eligibility among pregnant women [34,35]. In addition, we found that POC testing was associated with a significant reduction in the delays from the first antenatal care visit to treatment initiation. Minimizing delays to antenatal ART initiation is likely to be associated with improvement in PMTCT outcomes, as increasing the duration of ART received before delivery is associated with significant reductions in vertical transmission risk in utero, intrapartum and when breastfeeding [36,37].
These are the first data from the Western Cape province of South Africa on the implementation of universal ART for all HIV-infected pregnant women. The findings are in broad keeping with previous data on “Option B+” implementation from Malawi [38], demonstrating substantial increases in the initiation of triple-drug regimens during pregnancy when CD4-based ART eligibility criteria are removed. The absolute increases observed here under “Option B+” appear somewhat more modest than those reported from Malawi, however, likely due to the relatively low levels of ART initiation in pregnancy in Malawi before the implementation of “Option B+” [38]. Importantly, while the services implemented here called for lifelong ART for all HIV-infected pregnant women regardless of CD4 cell count (“Option B+”), this analysis focused specifically on ART initiation in pregnancy, and the results observed are unlikely to have been substantially different under a policy of universal ART for all pregnant and breastfeeding women, to be continued as lifelong treatment only for women with relatively low CD4 cell counts (“Option B”) [5]. Also of note, local PMTCT guidelines in this setting call for ongoing use of pre-ART CD4 enumeration in HIV-infected pregnant women despite universal ART eligibility; this approach is thought to be of possible value in clinical management on women on lifelong ART. However empirical data on the clinical utility and programmatic cost-effectiveness of CD4 cell counts under “Option B+” are sparse and this aspect of “Option B+” requires further consideration.
These data point to two important features of “Option B+” implementation when compared with the preceding policies of CD4-based ART eligibility in pregnant women (“Option A”). First, the removal of CD4-based eligibility criteria is associated with significant increases in antenatal ART initiation in the subgroup of women with low CD4 cell counts who are at greatest risk of vertical transmission as well as morbidity and mortality [39]. Here, the proportion of women with CD4≤350 cells/μL who started ART before delivery under “Option A” ranged from 16% in the pre-intervention period to 80% with the POC CD4 intervention, which may represent the optimal implementation of the WHO's “Option A” approach; under “Option B+” this increased to 92% of all women.
Second, delays to antenatal ART initiation were largely eliminated by the removal of CD4-based ART eligibility criteria under “Option B+”, with approximately 80% of women initiating ART on the day of their first ANC visit. Reducing delays to treatment initiation may have significant transmission benefits by increasing the duration of antenatal ART exposure [40] however the putative advantages of same-day ART initiation must be weighed against the possible limitations of this approach from a patient perspective. Not all women may be willing to start lifelong therapy immediately, and the frequency of same-day initiation has been posited as one reason for the relatively high levels of loss to follow-up observed under “Option B+” in Malawi [41]. Here, we do not have data on long-term maternal retention on ART under “Option B+”, and how same-day treatment initiation may influence adherence and retention outcomes requires urgent research attention [42,26].
This analysis is subject to several limitations. As routine health systems data, the results are representative of real-world ANC/PMTCT and ART services but detailed psychosocial and clinical measures are not available. In particular we do not have data on the NNRTI used in ART regimens. Although the transition from NVP to EFV took place during 2011, we are unable to disaggregate the specific impact of different NNRTIs on the timing of antenatal ART initiation. From our experience, the shift to widespread EFV use in pregnancy may have contributed to the reductions in time to ART initiation, as the use of liver function testing before NVP initiation (which was routine in local ART protocols) was removed. In addition, we cannot rule out health system factors influencing ART initiation in pregnancy over time that may have coincided with the service delivery changes described here, though we note that available HIV-related indicators such as HCT uptake within the PMTCT service appeared remarkably constant during the four-year study period. Similarly the limited availability of patient-level demographic and psychosocial measures that may influence uptake of ART in pregnancy [6] hinders our ability to examine possible residual confounding, although the plausibility of patient-level factors changing appreciably over time to explain the observed trends is unclear.
The improvements in service delivery observed in this analysis took place over relatively short periods, with new interventions implemented and optimized within several months of changes in service policy. While the drivers of this phenomenon require additional attention, we hypothesize that the rapidity of implementation may be attributed to the combination of a robust underlying service delivery platform in the existing ANC/PMTCT service; a strong local ART service with dedicated providers (including counselors, nurses and doctors) within the community health centre to support integration and optimization of ART into ANC/PMTCT; and focused training and support to nurse-midwives working within the ANC/PMTCT to assist in implementing each change in service delivery. More broadly, the developments documented here took place in the context of an urban South African health service with a high burden of HIV and relatively functional health system; while the findings may be broadly applicable to urban centres across the region, any extrapolation should proceed with caution.
While this analysis focuses on ART initiation in pregnancy, it is important to note that retaining mothers adherent to ART, and preventing mother-to-child transmission, are the ultimate goals of effective PMTCT services; understanding how PMTCT policies, most notably universal ART strategies, impact on these outcomes requires further research. In particular, while this analysis focuses on models of service delivery for providing ART during the antenatal period, there has been little attention to optimal models of care for mothers on ART and their infants, and health systems and services that support maternal ART during the postnatal period are required.
In summary, these data demonstrate significant advances in ART initiation during pregnancy over the past 4 years in this setting. Integration of ART into ANC/PMTCT services was associated with substantial increases in antenatal ART initiation. Recent developments calling for universal ART initiation for all HIV-infected pregnant women appear associated with significant increases in the proportion of women starting ART before delivery as well as concomitant decreases in the delays to starting ART. Further investigations are required into how policy developments for ART initiation in pregnancy may influence long-term maternal and child health outcomes, including vertical HIV transmission, maternal ART adherence, and long-term retention in care.
Supplementary Material
Acknowledgments
Sources of support: This research was funded by the Elizabeth Glaser Pediatric AIDS Foundation.
Contributor Information
Landon Myer, Division of Epidemiology & Biostatistics, School of Public Health & Family Medicine, University of Cape Town, South Africa; Desmond Tutu HIV Centre, Institute of Infectious Diseases & Molecular Medicine, University of Cape Town, South Africa.
Tamsin Phillips, Division of Epidemiology & Biostatistics, School of Public Health & Family Medicine, University of Cape Town, South Africa.
Victoria Manuelli, Department of Obstetrics, Gynecology & Reproductive Sciences, University of California, San Francisco, USA.
James McIntyre, Anova Health Institute, Johannesburg, South Africa; School of Public Health & Family Medicine, University of Cape Town, South Africa.
Linda-Gail Bekker, Desmond Tutu HIV Centre, Institute of Infectious Diseases & Molecular Medicine, University of Cape Town, South Africa.
Elaine J. Abrams, ICAP at Columbia University and Department of Epidemiology, Mailman School of Public Health, New York, NY, USA; College of Physicians & Surgeons, Columbia University, New York, NY, USA
References
- 1.Abrams EJ, Myer L. Can we achieve an AIDS-free generation? Perspectives on the global campaign to eliminate new pediatric HIV infections. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S208–212. doi: 10.1097/QAI.0b013e3182986f55. [DOI] [PubMed] [Google Scholar]
- 2.Barron P, Pillay Y, Doherty T, et al. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ. 2013;91(1):70–74. doi: 10.2471/BLT.12.106807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi BH, Stringer JS, Moodley D. Antiretroviral drug regimens to prevent mother-to-child transmission of HIV: a review of scientific, program, and policy advances for sub-Saharan Africa. Curr HIV/AIDS Rep. 2013;10:124–133. doi: 10.1007/s11904-013-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre J. Use of antiretrovirals during pregnancy and breastfeeding in low-income and middle-income countries. Curr Opin HIV AIDS. 2010;5:48–53. doi: 10.1097/COH.0b013e328333b8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 6.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16(1):18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNICEF. Towards and AIDS-free generation: Children & AIDS, 6th stocktaking report. New York: UNICEF; 2013. [Google Scholar]
- 8.Sprague C, Chersich MF, Black V. Health system weaknesses constrain access to PMTCT and maternal HIV services in South Africa: a qualitative enquiry. AIDS Res Ther. 2011;8:10. doi: 10.1186/1742-6405-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong'ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17(5):564–80. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 10.Stinson K, Jennings K, Myer L. Integration of antiretroviral therapy services into antenatal care increases treatment initiation during pregnancy: a cohort study. PLoS One. 2013;8(5):e63328. doi: 10.1371/journal.pone.0063328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson-Jones D, Balira R, Ross DA, Weiss HA, Mabey D. Missed opportunities: poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One. 2012;7(7):e40091. doi: 10.1371/journal.pone.0040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Msellati P. Improving mothers' access to PMTCT programs in West Africa: a public health perspective. Soc Sci Med. 2009;69(6):807–12. doi: 10.1016/j.socscimed.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Kim MH, Ahmed S, Buck WC, Preidis GA, Hosseinipour MC, Bhalakia A, Nanthuru D, Kazembe PN, Chimbwandira F, Giordano TP, Chiao EY, Schutze GE, Kline MW. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV cascade of services in Malawi. J Int AIDS Soc. 2012;15(Suppl 2):17389. doi: 10.7448/IAS.15.4.17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killam WP, Tambatamba BC, Chintu N, Rouse D, Stringer E, Bweupe M, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24(1):85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- 15.Suthar AB, Hoos D, Beqiri A, Lorenz-Dehne K, McClure C, Duncombe C. Integrating antiretroviral therapy into antenatal care and maternal and child health settings: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(1):46–56. doi: 10.2471/BLT.12.107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Merwe K, Chersich MF, Technau K, Umurungi Y, Conradie F, Coovadia A. Integration of antiretroviral treatment within antenatal care in Gauteng Province, South Africa. J Acquir Immune Defic Syndr. 2006;43(5):577–81. doi: 10.1097/01.qai.0000243099.72770.d2. [DOI] [PubMed] [Google Scholar]
- 17.Tudor Car L, Van Velthoven MH, Brusamento S, Elmoniry H, Car J, Majeed A, Tugwell P, Welch V, Marusic A, Atun R. Integrating prevention of mother-to-child HIV transmission programs to improve uptake: a systematic review. PLoS One. 2012;7(4):e35268. doi: 10.1371/journal.pone.0035268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Towards universal access. Geneva: WHO; 2006. [Google Scholar]
- 19.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Recommendations for a public health approach. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 20.Weigel R, Hosseinipour MC, Feldacker C, Gareta D, Tweya H, Chiwoko J, Gumulira J, Kalulu M, Mofolo I, Kamanga E, Mwale G, Kadzakumanja A, Jere E, Phiri S. Ensuring HIV-infected pregnant women start antiretroviral treatment: an operational cohort study from Lilongwe, Malawi. Trop Med Int Health. 2012;17(6):751–9. doi: 10.1111/j.1365-3156.2012.02980.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen JY, Ogwu AC, Svab P, Lockman S, Moffat HJ, Gaolathe T, et al. Antiretroviral Treatment Initiation Among HIV-Infected Pregnant Women with Low CD4 + Cell Counts in Gaborone, Botswana. J Acquir Immune Defic Syndr. 2010;54(1):102–6. doi: 10.1097/QAI.0b013e3181c080bf. [DOI] [PubMed] [Google Scholar]
- 22.De Schacht C, Lucas C, Sitoe N, Manuel I, Tobaiwa O, Ramanlal N, Quevedo J, Kassaye S, Sixpence JC, Jani IV. Point of care CD4 testing leads to increased uptake of antiretroviral therapy among pregnant women in Gaza Province, Mozambique; 19th Conference on Retroviruses and Opportunistic Infections; Seattle, USA. 2012. Abstract THPE079. [Google Scholar]
- 23.Mnyani CN, McIntyre JA, Myer L. The reliability of point-of-care CD4 testing in identifying HIV-infected pregnant women eligible for antiretroviral therapy. J Acquir Immune DeficSyndr. 2012;60(3):260–4. doi: 10.1097/QAI.0b013e318256b651. [DOI] [PubMed] [Google Scholar]
- 24.Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, et al. Prevention of mother-to-child transmission of HIV and the health-related millennium development goals: time for a public health approach. Lancet. 2011;378(9787):282–4. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS. 2013;8:474–489. doi: 10.1097/COH.0b013e328363a8f2. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer N, Abrams EJ, Becquet R. Option B+ for prevention of mother-to-child transmission of HIV in resource-constrained settings: great promise but some early caution. AIDS. 2014;28(4):599–601. doi: 10.1097/QAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 27.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 28.Myer L, Daskilewicz K, McIntyre J, Bekker LG. Comparison of point-of-care versus laboratory-based CD4 cell enumeration in HIV-positive pregnant women. J Int AIDS Soc. 2013;16(1):18649. doi: 10.7448/IAS.16.1.18649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myer L, Zulliger R, Black S, Pienaar D, Bekker LG. Pilot programme for the rapid initiation of antiretroviral therapy in pregnancy in Cape Town, South Africa. AIDS Care. 2012;24(8):986–992. doi: 10.1080/09540121.2012.668173. [DOI] [PubMed] [Google Scholar]
- 30.Zulliger R, Black S, Holtgrave DR, Ciaranello AL, Bekker LG, Myer L. Cost-effectiveness of a package of interventions for expedited antiretroviral therapy initiation during pregnancy in Cape Town, South Africa. AIDS and Behavior. 2014;18(4):697–705. doi: 10.1007/s10461-013-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provincial Government of the Western Cape. PMTCT clinical guidelines update, May 2013. Cape Town: PGWC; 2013. [Google Scholar]
- 32.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 33.Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15(7):825–32. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 34.Hussain A, Moodley D, Naidoo S, Esterhuizen TM. Pregnant women's access to PMTCT and ART services in South Africa and implications for universal antiretroviral treatment. PLoS One. 2011;6(12):e27907. doi: 10.1371/journal.pone.0027907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muchedzi A, Chandisarewa W, Keatinge J, Stranix-Chibanda L, Woelk G, Mbizvo E, Shetty AK. Factors associated with access to HIV care and treatment in a prevention of mother to child transmission programme in urban Zimbabwe. J Int AIDS Soc. 2010;13:38. doi: 10.1186/1758-2652-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chibwesha CJ, Giganti MJ, Putta N, Chintu N, Mulindwa J, Dorton BJ, Chi BH, Stringer JS, Stringer EM. Optimal time on HAART for prevention of mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;58(2):224–8. doi: 10.1097/QAI.0b013e318229147e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, Taylor GP, Peckham CS, Tookey PA. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28(7):1049–57. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) Impact of an innovative approach to prevent mother-to-child transmission of HIV--Malawi, July 2011-September 2012. MMWR Morb Mortal Wkly Rep. 2013;62(8):148–51. [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to-child HIV transmission. AIDS. 2010;24(9):1374–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Myer L. Initiating antiretroviral therapy in pregnancy: the importance of timing. J Acquir Immune Defic Syndr. 2011;58(2):125–6. doi: 10.1097/QAI.0b013e31822ad573. [DOI] [PubMed] [Google Scholar]
- 41.Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, Chirwa Z, Ng'ambi W, Bakali A, Phiri S, Myer L, Valeri F, Zwahlen M, Wandeler G, Keiser O Ministry of Health in Malawi and IeDEA Southern Africa. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28(4):589–98. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutsoudis A, Goga A, Desmond C, Barron P, Black V, Coovadia H. Is Option B+ the best choice? Lancet. 2013;381:269–271. doi: 10.1016/S0140-6736(12)61807-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.