Abstract
Primary intracranial and sellar squamous cell carcinoma is an extremely rare entity, usually caused by malignant transformation of epidermoid cysts, or very rarely other non-malignant epithelial cysts. Malignant transformation of a Rathke’s cleft cyst has never been described. We present a 49-year-old male patient who presented with a 3-month history of progressive frontotemporal headaches. Imaging revealed a 1.2 cm cystic pituitary mass consistent with a hemorrhagic Rathke’s cleft cyst. The patient underwent trans-sphenoidal resection of the pituitary cyst, and pathologic analysis revealed a squamous cell carcinoma lining a Rathke’s cleft cyst. Extensive imaging and otorhinolaryngologic evaluation revealed no primary source for metastasis. We feel this represents the first case of a patient with a pituitary lesion in which presentation and MRI imaging were consistent with Rathke’s cleft cyst, yet histology revealed squamous cell carcinoma in situ.
Keywords: Pituitary, Cancer
INTRODUCTION
Intracranial squamous cell carcinomas are typically manifestations of metastatic lesions from a primary focus outside the brain, or result from direct extension from the cranial base (secondary SCC). The third most common causes, albeit much rarer, is malignant transformation of a benign cyst (primary SCC) [1]. Those cysts are most commonly dermoid or epidermoid cysts, with extremely rare cases of malignant transformation of epithelial cysts, neuroepithelial cysts, endodermal cysts and craniopharyngiomas [1–4].
To the authors’ best knowledge there have been no reports of squamous cell carcinoma within a Rathke’s cleft cyst. We present a very striking and atypical case of squamous cell carcinoma within a Rathke’s cleft cyst with no evidence of metastatic disease in an otherwise healthy patient. The patient was informed that data regarding his case would be submitted for publication.
CASE REPORT
A 49-year-old man presented to the hospital for worsening headache of two months duration. It was located frontally and did not abate with anti-allergic medications or two courses of antibiotics. After his headache worsened, a brain MRI revealed a 1.2 cm cystic pituitary mass consistent with a Rathke’s cleft cyst (RCC) versus hemorrhagic adenoma (Figure 1). The patient was evaluated by neurosurgery, neurology, and endocrinology. Visual fields were full to both confrontation and formal testing, and pituitary hormonal evaluation revealed only hypogonadotrophic hypogonadism in the setting of narcotics for headache (Table 1). A CT scan showed no calcifications to suggest craniopharyngioma [5].
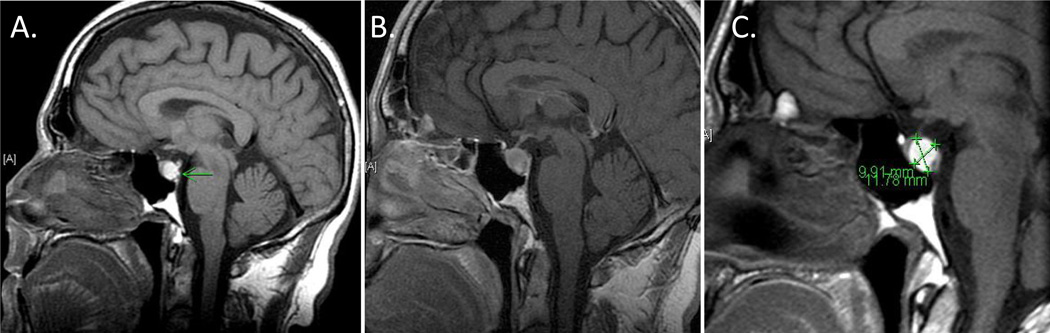
Figure 1. Initial MRI of the Pituitary.
Pre-contrast (A) and post-contrast (B) MRI of the pituitary on presentation reveals mildly enlarged pituitary gland, likely due to hemorrhagic microadenoma or Rathke's cleft cyst. Follow-up MRI 2 months later (C) reveals unchanged pituitary MRI with appearance indicating Rathke’s cleft cyst.
Table 1.
Laboratory values on presentation
| Test | Result | Normal range |
|---|---|---|
| Prolactin | 5.3 | (4–15) |
| Cortisol (7 AM) | 17.9 | (2–20) |
| TSH | 3.0 | (0.27–4.2) |
| Total T4 | 8.6 | (4.6–12) |
| FSH | 3.4 | (2–12) |
| LH | 1.1 | (2–10) |
| Testosterone | 87 | (280–800) |
| Free Testosterone | 26 | (60–85) |
| IGF-1 | 263 | (50–303) |
| Alpha-subunit | <0.3 | (<0.6) |
The patient was subsequently discharged with a plan to control his pain and pursue surgical resection after resolution of pituitary hemorrhage. Over the next 3 months he had ongoing, unremitting headaches, and a repeat MRI confirmed an unchanged cystic lesion of the pituitary with proteinaceous contents. Four months after his initial presentation, the patient underwent uncomplicated trans-sphenoidal surgery to decompress the lesion and thereby treat his unremitting headache [6, 7]. Dissection of the pituitary revealed clear hemorrhagic fluid and a large portion of granular necrotic debris. Intraoperative fresh frozen sections revealed spindle cells and necrotic debris without obvious pituitary tissue.
On histopathologic analysis of the surgical specimen, (Figure 2) there was loose myxoid fibrous tissue with extensive inflammation, including lymphocytes, histiocytes, and eosinophils. Fragments were focally lined by atypical squamous cells, which formed aggregates. Immunohistochemical staining of the lining epithelial cells was positive for p63, CK7, and cytokeratin cocktail and negative for chromogranin, MART-1, HMB 45, CK20, and TTF-1, thus establishing the diagnosis of a squamous cell carcinoma (SCC) lining the cyst. It could not be determined by pathologic appearance whether this squamous lesion represented a primary SCC or a metastatic SCC with subsequent cystic change.
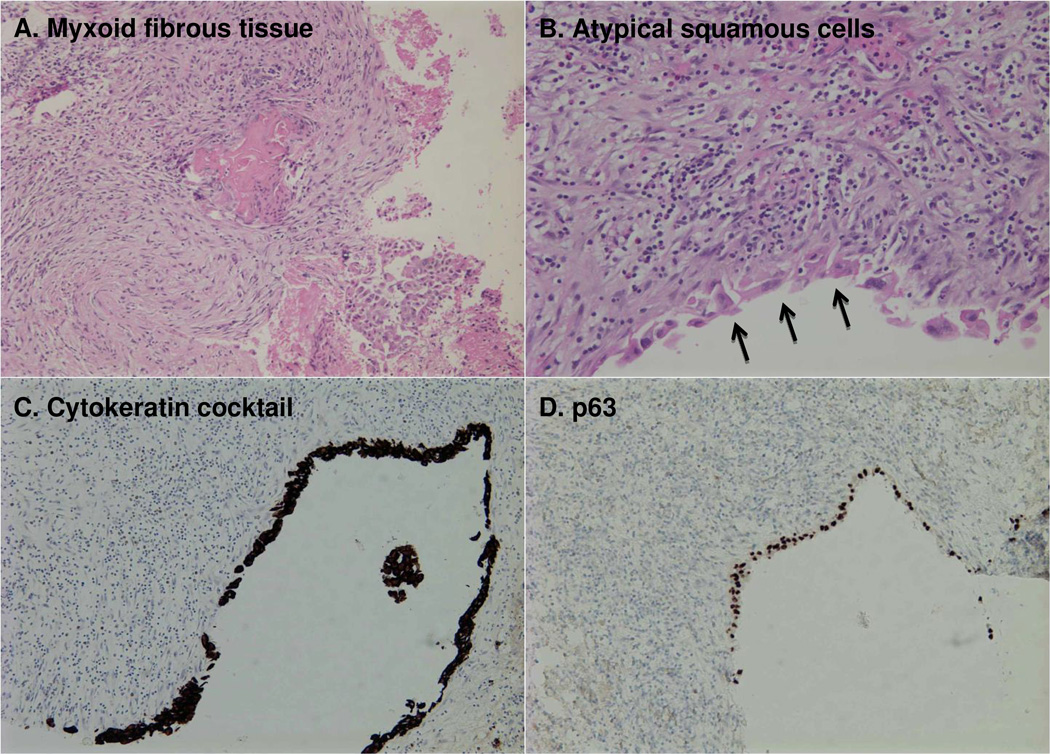
Figure 2. Pathologic report: Squamous Cell Carcinoma (at least in-situ).
Loose myxoid fibrous tissue with extensive inflammation, including lymphocytes, histiocytes, and eosinophils (A). Fragments are focally lined by atypical squamous cells, which form aggregates (arrows) (B). Immunohistochemical staining of the lining epithelial cells is positive for cytokeratin cocktail (C) and p63 (D). It could not be determined by pathologic appearance whether this squamous lesion represented a primary SCC or a metastatic SCC with subsequent cystic change.
The patient was referred to neuro- and radiation-oncology. An MRI of the C-, T-, and L-spine spine showed no evidence of metastasis. A PET-CT scan showed FDG-avidity around the surgical bed and the pharyngeal tonsils which, when inspected by ENT showed no squamous cell lesions. A 6-week course of focal stereotactic radiation therapy to the pituitary was recommended and performed without any immediate complications. Follow-up MRIs at 3 and 6 months showed no evidence of recurrence. Additionally, a 1-year follow-up PET-CT and repeat MRI (Figure 3), showed no evidence of recurrent disease. His hypogonadotrophic hypogonadism and his headache have resolved off narcotics. Despite being initially hormonally intact, he has subsequently developed central hypogonadism and hypothyroidism, which can be attributed to the adjuvant radiation. The patient remains otherwise asymptomatic and neurologically intact.
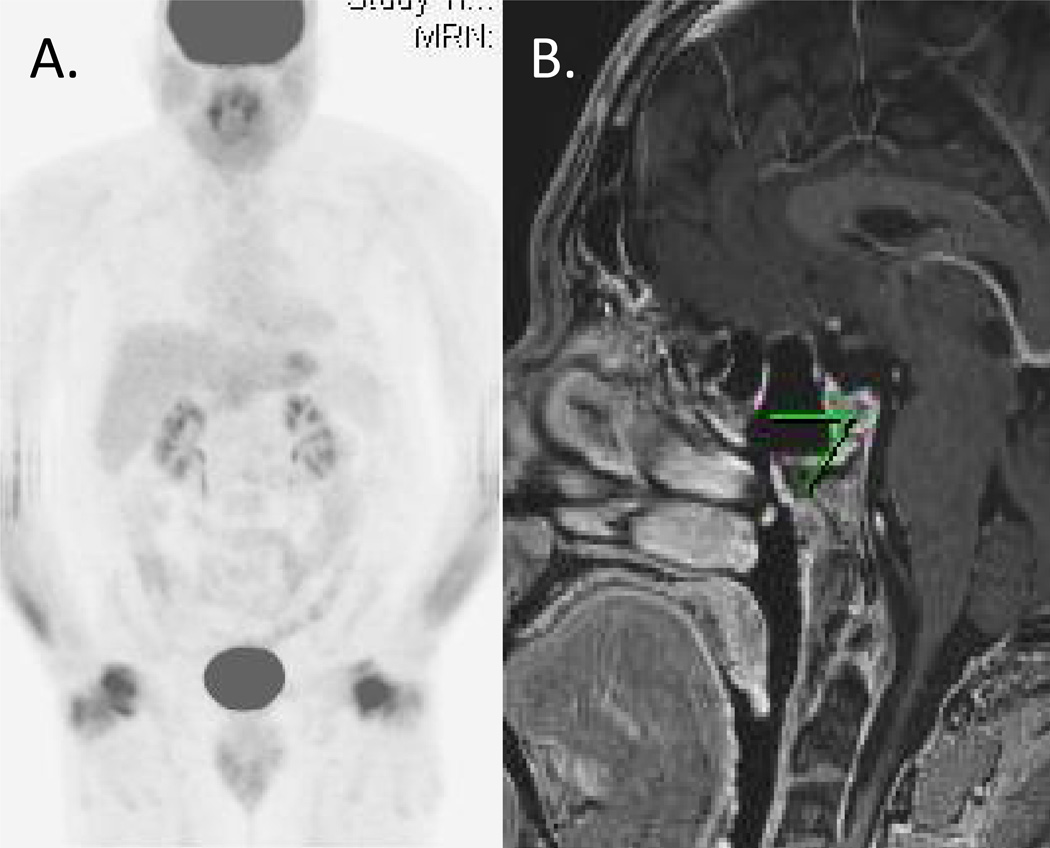
Figure 3.
PET-CT (A) and brain MRI (B) at 1-year after surgical resection without evidence of recurrent disease.
DISCUSSION
Rathke’s cleft cysts (RCC) originate from the embryonic remnants of Rathke’s pouch [8]. They are benign cystic lesions, located in the sellar and/or suprasellar region of the brain [9]. They are usually asymptomatic and found most commonly in autopsies, but an increasing number is found incidentally on brain imaging (CT or MRI) due to better availability and improvement in imaging techniques [10]. There is an unexplained female preponderance (M:F up to 1:3) and most cases are detected in patients in their 4th to 6th decade of life. Typically, RCC is asymptomatic although the clinical presentation of a symptomatic RCC can vary from simple hormonal abnormalities to headache or even vision problems [11–15]. Along the spectrum of sellar epithelial cysts, RCC is the most benign, behind craniopharyngiomas and dermoid/epidermoid cysts [16].
RCC is most often lined by simple cuboidal or columnar epithelium, with or without mucous-secreting goblet cells, and it is usually filled with a thick, mucoid material consisting of cholesterol and protein [8–10, 12]. Squamous metaplasia has been described in as many as 9–39% of lesions and is associated with inflammation and cyst recurrence [16]. At higher risk for recurrence are also a minority of RCCs which are pathologically distinct by stratified squamous epithelium [7]. It has been proposed that this squamous metaplasia is caused by acute and chronic inflammation likely due to repeated cyst leakage or microhemorrhage [16, 17].
There have been several case reports of primary intracranial squamous cell carcinomas, but there has never been a case of SCC originating from a RCC. The most common cause of intracranial SCC is by virtue of metastasizing from a cutaneous or nasopharyngeal primary carcinoma, or by direct extension of a carcinoma from the cranial base. A less common cause of intracranial primary SCC is via malignant transformation of a benign cyst [1]. Garcia et. al. have proposed the following criteria for this malignant transformation [18]. The tumor has to be restricted to the intracranial, intra-dural compartment without extension beyond the dura, cranial orifices, or connection with the middle ear, air sinuses, or sella turcica. There should be no evidence of a nasopharyngeal tumor. Hamlat et. al. added two additional criteria [1]: There must be evidence of a benign squamous cell epithelium within the malignant tumor mass. In addition, a nasopharyngeal tumor has to be ruled out. Hamlat et. al. have also proposed classification of malignant transformation to these carcinomas. These five groups include initial malignant transformation of an epidermoid cyst, malignant transformation from a remnant epidermoid cyst, malignant transformation with leptomeningeal carcinomatosis, squamous cell carcinoma arising from other benign cysts, other malignancies arising from benign cysts. Transformation of an epidermoid cyst has been described in the vast majority of such cases [19, 20]. There have been only few case reports of de novo intra-cranial SCC [21, 22] and malignant transformation of other non-neoplastic epithelial cysts. Those cysts include most commonly dermoid cysts, or more rarely epithelial cysts, neuroepithelial cysts, endodermal cysts and a craniopharyngioma [1, 3, 23–26]. It seems that most cases arise at the cerebello-pontine angle, which is also the preferential site for epidermoid cysts.
In the present case, the presentation was subacute with unremitting headaches for a few months, and there was no clinical evidence of pituitary pathology. MRI findings were consistent with pituitary hemorrhage or hemorrhagic microadenoma or RCC. At the time of the surgery 3 months later, dissection of the pituitary revealed hemorrhagic fluid and a large portion of granular necrotic debris. As mentioned above, squamous metaplasia of a RCC has been described in multiple cases, with suggested mechanism of acute and chronic inflammation likely due to repeated cyst leakage or microhemorrhage [16, 17]. It is certainly possible that the patient had a RCC that underwent squamous metaplasia and subsequently malignant transformation to SCC. No prior imaging is available to confirm this hypothesis. On histopathologic examination, the tumor did not extend beyond the lining of the RCC. There was no evidence of a nasopharyngeal tumor by ENT inspection, despite FDG-avidity around the tonsils and the surgical bed on a PET-CT of the head and torso. There was also no evidence of another primary focus on an MRI of the C-, T- and L-spine at the time of diagnosis. Thus, the lesion was to the best of our observation not metastatic, though a metastatic cancer of unknown primary source cannot be ruled out. However an alternative primary SCC seems highly unlikely given that there is only one site involved in this patient and follow-up surveillance with yearly MRI of the head and spine, as well as PET-CT and nasopharyngeal inspection, for 3 years has not revealed any recurrent cancer or other source. Based on this evaluation, the tumor met the criteria by Garcia et al. for malignant transformation of a benign cyst.
Treatment of a symptomatic RCC is usually surgical, with the goal of the draining its content, and removal of the capsule [8]. Radiotherapy or radiosurgery has been used in a few cases of cyst recurrence with promising results. As malignant transformation of a RCC has never been described, there is no proposed treatment algorithm. However, it is well known that intracranial SCC has poor prognosis. More specifically, Hamlat et al reviewed 52 cases of SCC due to malignant transformation of epidermoid cysts, and most of the patients died within 12 months of diagnosis, with 12 patients being alive at the time of the review article and only one being alive after 5 years from diagnosis [1]. Although not statistically significant, Hamlat et al proposed that radiation therapy is likely beneficial, given a median survival of 26 months for the patients of their cohort who received radiation postoperatively, as compared to a median survival of 3 months for the patients who did not (p=0.077). Based on this evidence, our patient received a 6-week course of focused radiation therapy to the pituitary. Subsequent 1- and 3-year follow-up PET-CT and MRI showed no evidence of recurrent disease.
More recently, Nagasawa et al reviewed 58 cases of malignant transformation of epidermoid cysts and compared different treatment options, including surgery alone, surgery plus multiple adjuvant treatments, stereotactic radiosurgery (SRS) and chemotherapy [27]. There was no statistical significance in terms of the overall survival among SRS, chemotherapy or surgery plus multiple adjuvant treatments (at least 2), but there was statistically significant improved overall survival when comparing each of these treatment modalities with surgery alone. This review further supports the use of radiation as one possible treatment adjunct to surgery as in cases of malignant transformation of epidermoid cysts, and the present case suggests potential benefit from similar treatment modalities for rare cases of malignant transformation of RCCs.
CONCLUSION
Intracranial squamous cell carcinomas are rare tumors with a poor prognosis, and require a high index of suspicion and ultimately histopathological confirmation, as they do not have specific characteristics on imaging. Treatment of intracranial SCC is controversial due to the lack of outcome data which is due to insufficient cases reported in the literature, - but there is sufficient evidence that adjuvant treatments to surgery should be used [27]. The authors suggest that the treatment of non-metastatic SCC within a Rathke’s cleft cyst can be similar to the treatment of other types of non-metastatic intracranial squamous cell carcinomas.
Acknowledgements
BTO was supported by an NIH T32 training grant (T32DK007260) and by a K08 training award (K08DK100543). We would like to thank Dr. Matthew Anderson from the Department of Pathology at Beth Israel Deaconess Medical Center and Dr. William C. Faquin from the Department of Pathology at Massachusetts General Hospital for assistance with the diagnosis.
Footnotes
Conflict of interest. The authors declare no conflict of interest related to this manuscript.
REFERENCES
- 1.Hamlat A, et al. Malignant transformation of intra-cranial epithelial cysts: systematic article review. J Neurooncol. 2005;74(2):187–194. doi: 10.1007/s11060-004-5175-4. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, et al. Primary intracranial squamous cell carcinoma arising in an epidermoid cyst--a case report and review of literature. Clin Neurol Neurosurg. 2007;109(10):888–891. doi: 10.1016/j.clineuro.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Gluszcz A. A cancer arising in a dermoid of the brain. A case report. J Neuropathol Exp Neurol. 1962;21:383–387. doi: 10.1097/00005072-196207000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Salyer D, Carter D. Squamous carcinoma arising in the pituitary gland. Cancer. 1973;31(3):713–718. doi: 10.1002/1097-0142(197303)31:3<713::aid-cncr2820310334>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopharyngioma. Pituitary. 2013;16(1):46–55. doi: 10.1007/s11102-012-0428-2. [DOI] [PubMed] [Google Scholar]
- 6.Madhok R, et al. Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg. 2010;112(6):1333–1339. doi: 10.3171/2009.10.JNS09348. [DOI] [PubMed] [Google Scholar]
- 7.Kim JE, et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 2004;100(1):33–40. doi: 10.3171/jns.2004.100.1.0033. [DOI] [PubMed] [Google Scholar]
- 8.Trifanescu R, et al. Rathke's cleft cysts. Clin Endocrinol (Oxf) 2012;76(2):151–160. doi: 10.1111/j.1365-2265.2011.04235.x. [DOI] [PubMed] [Google Scholar]
- 9.Karavitaki N, Wass JA. Non-adenomatous pituitary tumours. Best Pract Res Clin Endocrinol Metab. 2009;23(5):651–665. doi: 10.1016/j.beem.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Ross DA, Norman D, Wilson CB. Radiologic characteristics and results of surgical management of Rathke's cysts in 43 patients. Neurosurgery. 1992;30(2):173–178. doi: 10.1227/00006123-199202000-00004. discussion 178-9. [DOI] [PubMed] [Google Scholar]
- 11.Billeci D, et al. Symptomatic Rathke's Cleft Cysts: A Radiological, Surgical and Pathological Review. Pituitary. 2005;7(3):131–137. doi: 10.1007/s11102-005-1755-3. [DOI] [PubMed] [Google Scholar]
- 12.Midha R, Jay V, Smyth HS. Transsphenoidal management of Rathke's cleft cysts. A clinicopathological review of 10 cases. Surg Neurol. 1991;35(6):446–454. doi: 10.1016/0090-3019(91)90178-c. [DOI] [PubMed] [Google Scholar]
- 13.Nishioka H, et al. Headaches associated with Rathke's cleft cyst. Headache. 2006;46(10):1580–1586. doi: 10.1111/j.1526-4610.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 14.Raper DM, Besser M. Clinical features, management and recurrence of symptomatic Rathke's cleft cyst. J Clin Neurosci. 2009;16(3):385–389. doi: 10.1016/j.jocn.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Voelker JL, Campbell RL, Muller J. Clinical, radiographic, and pathological features of symptomatic Rathke's cleft cysts. J Neurosurg. 1991;74(4):535–544. doi: 10.3171/jns.1991.74.4.0535. [DOI] [PubMed] [Google Scholar]
- 16.Zada G, et al. Craniopharyngioma and other cystic epithelial lesions of the sellar region: a review of clinical, imaging, and histopathological relationships. Neurosurg Focus. 2010;28(4):E4. doi: 10.3171/2010.2.FOCUS09318. [DOI] [PubMed] [Google Scholar]
- 17.Asa SL, Kovacs K, Bilbao JM. The pars tuberalis of the human pituitary. A histologic, immunohistochemical, ultrastructural and immunoelectron microscopic analysis. Virchows Arch A Pathol Anat Histopathol. 1983;399(1):49–59. doi: 10.1007/BF00666218. [DOI] [PubMed] [Google Scholar]
- 18.Garcia CA, McGarry PA, Rodriguez F. Primary intracranial squamous cell carcinoma of the right cerebellopontine angle. J Neurosurg. 1981;54(6):824–828. doi: 10.3171/jns.1981.54.6.0824. [DOI] [PubMed] [Google Scholar]
- 19.Nosaka Y, et al. Primary intracranial epidermoid carcinoma. Case report. J Neurosurg. 1979;50(6):830–833. doi: 10.3171/jns.1979.50.6.0830. [DOI] [PubMed] [Google Scholar]
- 20.Wong SW, Ducker TB, Powers JM. Fulminating parapontine epidermoid carcinoma in a four-year-old boy. Cancer. 1976;37(3):1525–1531. doi: 10.1002/1097-0142(197603)37:3<1525::aid-cncr2820370341>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Ogata N, et al. Total removal of a primary intracranial squamous cell carcinoma invading the brain stem. Surg Neurol. 1996;46(5):477–480. doi: 10.1016/s0090-3019(96)00222-4. [DOI] [PubMed] [Google Scholar]
- 22.Jain R, et al. Imaging findings associated with childhood primary intracranial squamous cell carcinoma. AJNR Am J Neuroradiol. 2003;24(1):109–111. [PMC free article] [PubMed] [Google Scholar]
- 23.Ho LC, et al. Well-differentiated papillary adenocarcinoma arising in a supratentorial enterogenous cyst: case report. Neurosurgery. 1998;43(6):1474–1477. doi: 10.1097/00006123-199812000-00131. [DOI] [PubMed] [Google Scholar]
- 24.Monaco R, et al. Intraepithelial carcinoma arising in an endodermal cyst of the posterior fossa. Neuropathology. 2003;23(3):219–224. doi: 10.1046/j.1440-1789.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 25.Oertel J, et al. Posterior fossa squamous cell carcinoma due to dedifferentiation of a dermoid cyst in Klippel-Feil syndrome case illustration. J Neurosurg. 2002;97(5):1244. doi: 10.3171/jns.2002.97.5.1244. [DOI] [PubMed] [Google Scholar]
- 26.Sahara Y, et al. Recurrence of a neurenteric cyst with malignant transformation in the foramen magnum after total resection. Case report. J Neurosurg. 2001;95(2):341–345. doi: 10.3171/jns.2001.95.2.0341. [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa DT, et al. An analysis of intracranial epidermoid tumors with malignant transformation: treatment and outcomes. Clin Neurol Neurosurg. 2013;115(7):1071–1078. doi: 10.1016/j.clineuro.2012.10.026. [DOI] [PubMed] [Google Scholar]