Abstract
Background and Objectives
Posttraumatic stress disorder (PTSD) and Major Depressive Disorder (MDD) are associated with high disease burden. Pathways by which PTSD and MDD contribute to disease burden are not understood.
Design
Path analysis was used to examine pathways between PTSD symptoms, MDD symptoms, and disease burden among 251 low-income heart failure patients.
Methods
In Model 1, we explored the independent relationship between PTSD and MDD symptoms on disease burden. In Model 2, we examined the association of PTSD symptoms and disease burden on MDD symptoms. We also examined indirect associations of PTSD symptoms on MDD symptoms, mediated by disease burden, and of PTSD symptoms on disease burden mediated by MDD symptoms.
Results
Disease burden correlated with PTSD symptoms (r = .41; p < .001) and MDD symptoms (r = .43; p < .001) symptoms. Both models fit the data well and displayed comparable fit. MDD symptoms did not mediate the association of PTSD symptoms with disease burden. Disease burden did mediate the relationship between PTSD symptoms and MDD symptoms.
Conclusions
Results support the importance of detection of PTSD in individuals with disease. Results also provide preliminary models for testing longitudinal data in future studies.
Keywords: Posttraumatic stress disorder, depression, chronic disease burden, congestive heart failure, path analysis
Introduction
The association between exposure to traumatic stress and poor physical health is well supported (Boscarino, 2004; Dutton et al., 2006; Keyes et al., 2013). Individuals experiencing adverse childhood experiences are at greater risk for liver disease (Dong, Dube, Felitti, Giles, & Anda, 2003), heart disease (Dong et al., 2004), stroke, renal disease, arthritis, and lung diseases (Goodwin & Stein, 2004; Spitzer et al., 2009). In addition, poor physical health has been found in adults who were exposed to traumatic stress related to combat or war (O'Toole & Catts, 2008), natural disasters (Armenian, Melkonian, & Hovanesian, 1998; Escobar, Canino, Rubio-Stipec, & Bravo, 1992; Phifer, 1990), and sexual assault (Clum, Calhoun, & Kimerling, 2000; Leserman, 2005). Most of this research focuses on specific disease outcomes and not on the overall disease profile of affected individuals. Moreover, little research has explored the relative influences of posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) on these associations. This is critical because traumatic events lead to both PTSD and MDD, both of which are modifiable with treatment.
Approximately 18 to 24% of individuals who experience trauma will develop PTSD (Breslau, Davis, Andreski, & Peterson, 1991; Breslau, Davis, Peterson, & Schultz, 2000; Shalev et al., 1998) and approximately 14 to 19% will develop MDD (Shalev, et al., 1998). PTSD and MDD are also frequently comorbid, with approximately 44 to 49% of those with PTSD receiving a diagnosis of comorbid MDD (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Shalev, et al., 1998). Although PTSD and MDD co-occur with high frequency, each has distinct physiological and psychosocial correlates that may differentially contribute to disease outcomes (Schulkin, Gold, & McEwen, 1998; Yehuda, Teicher, Trestman, Levengood, & Siever, 1996).
Because disease comorbidity adds to the severity of any existing medical condition, researchers often use a measure of chronic disease burden, or a summary measure of individuals’ comorbid medical conditions to examine the interactions between disease and psychopathology. PTSD and MDD are associated with chronic disease burden through a number of potential pathways. First, physical bodily damage that occurs as a direct result of the traumatic stress exposure, as in the case of motor vehicle accidents, physical assault, or military combat, can contribute to tissue or organ damage. Secondly, PTSD and MDD can influence health behaviors that increase risk for multiple chronic diseases. For instance, individuals with PTSD or MDD are more likely to smoke tobacco, abuse substances, have decreased physical activity (Katon, 2003; McNutt, Carlson, Persaud, & Postmus, 2002; Schnurr & Green, 2004), and are less adherent to medical recommendations (DiMatteo, Lepper, & Croghan, 2000; Shemesh Rudnick Kalusk, Milovanov, Salah et alet al, 2001). Third, repeated traumatic stress exposure may be a more general reflection of an unsafe or negligent environment in which repeated, low grade, chronic stress, poor nutrition and difficulty accessing health care influence multiple disease processes, especially in low income, inner city populations where traumatic stress exposure and poor health behaviors are high and resources are low (Lantz, House, Lepkowski, Williams, Mero & Chen, 1998.)
Evidence regarding the independent or combined impact of PTSD and MDD on chronic disease burden is mixed. A number of studies support the contribution of PTSD over and above MDD. For instance, male veterans with symptoms of PTSD have an increased risk of early onset chronic disease (Andersen, Wade, Possemato, & Ouimette, 2010) and higher rates of heart disease-related mortality (Boscarino, 2008), after controlling for depression symptoms. Female veterans with PTSD have more physical health problems than those without a diagnosis of PTSD, independent of depression symptomatology (Beckham et al., 1998; Frayne et al., 2004). Women with PTSD symptoms related to a sexual assault had higher rates of physical health complaints after controlling for depressive symptoms (Clum, et al., 2000). Other studies find that the combination of PTSD with MDD may be more strongly associated with worse physical health outcomes than either diagnosis alone. Seng and colleagues (Seng, Clark, McCarthy, & Ronis, 2006) found that women with PTSD and depression symptoms were at greater risk for specific health conditions compared to those with PTSD alone. In contrast, Kinder and colleagues (Kinder et al., 2008) found that veterans with depressive symptoms and not PTSD had an increased risk of mortality compared to patients with PTSD alone. Still other evidence suggests that individuals with PTSD and MDD do not differ from those with PTSD alone in terms of total number of health complaints (Calhoun, Wiley, Dennis, & Beckham, 2009).
Chronic disease burden and PTSD symptoms may have individual direct associations on MDD. Specifically, PTSD may contribute to MDD rather than occurring concurrently. Indeed, a growing literature suggests that PTSD and MDD are distinct disorders that arise from different factors (Blanchard, Buckley, Hickling, & Taylor, 1998; Chiu et al., 2011), with PTSD more often preceding MDD than the opposite (Chiu, et al., 2011; Ginzburg, Ein-Dor, & Solomon, 2010; Nickerson et al., 2013). Chronic disease burden may cause or worsen depressive symptoms through functional limitations, pain, and reduced productivity (Gureje, Simon, & Von Korff, 2001; Prince, Harwood, Thomas, & Mann, 1998). If PTSD contributes to chronic disease burden, which also contributes to depression, chronic disease burden would serve as a mediating factor between PTSD and depression.
In the current study, we examined the relationships among PTSD symptoms, MDD, symptoms and chronic disease burden. Based upon prior research, two models were hypothesized and tested using path analysis (Figure 1). Path analysis allows for modeling of theory-driven, hypothesized causal pathways using cross sectional data (Lleras, 2005). Hypothesized models may then guide future modeling with prospective data. We also examined indirect pathways within these models to test 1) depressive symptoms as a mediator of PTSD symptoms on chronic disease burden [Figure 1(a)] and 2) chronic disease burden as a mediator of PTSD symptoms on depressive symptoms [Figure 1(b)]. We hypothesized that both of the prior mentioned models would co-occur, but that the second model, of PTSD having a direct association with chronic disease burden and an indirect association with MDD through disease burden, would have more support.
Figure 1.
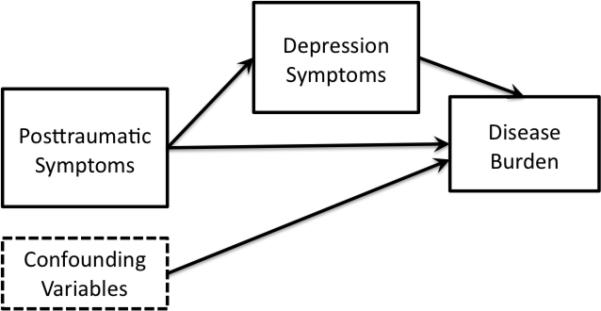
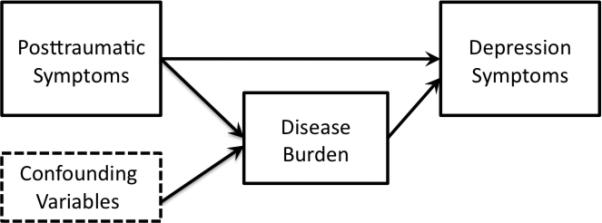
Hypothesized models
1a. Hypothesized model
1 1b. Hypothesized model 2
Method
Participants and Procedure
Data were from the baseline assessment of participants in the Congestive Heart failure Adherence Redesign Trial (CHART), which is an ongoing, prospective, multi-site study in a major, highly segregated, United States metropolitan area aimed at reducing repeated hospitalizations in low-income, congestive heart failure (CHF) patients. The sample was chosen due to expected vulnerability to PTSD and multiple physical illnesses. Eligibility for the CHART trial included being 18 years or older, English or Spanish speaking, diagnosed with heart failure, earning less than $30,000 per year, having had at least one hospitalization for heart failure during the prior 6 months, and having had physical evidence of systolic dysfunction, defined by an ejection of less than 50 according to an echocardiography, radiographic contrast ventriculography, or nuclear ventriculography within the past 12 months. Patients were not eligible if they had a significant psychiatric co-morbidity, such as dementia, schizophrenia, alcohol or drug dependence, were listed for imminent cardiac transplant, had an advanced directive of “do not resuscitate,” or otherwise had an uncertain 12-month prognosis according to the study cardiologist (author, J.C.). Subsequent to the approval and enrollment of their heart failure physician, patients were assigned to receive either the “Enhanced Training” or “Enhanced Education” intervention (Figure 2). The institutional review board at Rush University Medical Center approved this study.
Figure 2.
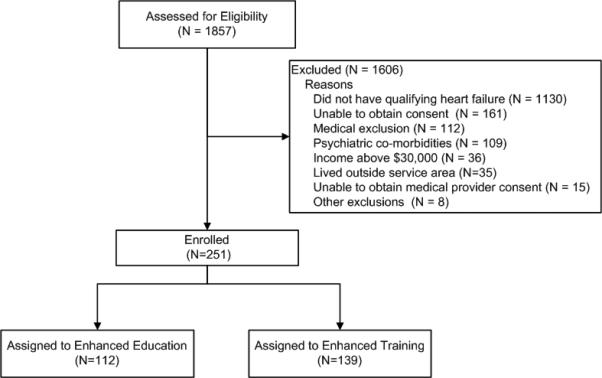
Study recruitment chart
Measures
Lifetime Trauma Exposure
Participants responded to 11 yes-or-no items on the Composite International Diagnostic Interview PTSD module (Kessler, Andrews, Mroczek, Ustun, & Wittchen, 1998) evaluating exposure to traumatic events. Events included physical assault, sexual assault, childhood sexual abuse, accidents, natural disasters, and other life threatening events (excluding those related to heart failure). Only individuals experiencing at least one traumatic incident were including in the model.
Posttraumatic Stress Disorder
Participants respond to the six-item Short Form of the PTSD Checklist—Civilian Version (PCL-SF; Lang & Stein, 2005) by rating the severity of a symptom from 1 (not at all) to 5 (extremely). The measure is composed of two items each from the re-experiencing, avoidance, and hyperarousal symptom clusters. Example items are, “Repeated, disturbing memories, thoughts, or images of a stressful experience?”, and “Avoiding activities or situations because they reminded you of a stressful experience?” Internal consistency for the six-item measure was good in the current sample (Cronbach's α = .85). Individuals’ symptoms are often the result of multiple events, and the events referred to were related to physical assault, sexual assault, childhood sexual abuse, accidents, natural disasters, and other life threatening events (excluding those related to heart failure).
Depression
Adult participants respond to the nine-item Patient Health Questionnaire–9 (PHQ-9; Kroenke, Spitzer, & Williams, 2001) by rating the frequency of a depression symptom from 1 (not at all) to 4 (nearly every day) for the two weeks prior to the administration of the measure. Example items are “Little interest or pleasure in doing things” and “Feeling low, depressed or hopeless.” Internal consistency for the nine-item measure was good in the current sample (Cronbach's α = .82).
Chronic Disease Burden
Medical history was assessed in eligible patients via interview and questionnaire. Patients provided personal medical histories via a paper questionnaire. Patients were asked if a physician had ever given them a diagnosis of a number of health conditions, including asthma, diabetes, chronic obstructive pulmonary disease (COPD) and other chronic diseases. Participants were not asked to specify the dates or sequences of onset for each of the diagnoses. Although there are multiple methodologies for determining disease burden (de Groot, Beckerman, Lankhorst, & Bouter, 2003) we chose to define burden as the summed total number of chronic diseases endorsed, excluding factors contributing to or resulting from congestive heart failure. Several studies have found total disease count to be as predictive of outcomes as more complex indexes (Farley, Harley, & Devine, 2006; Monami et al., 2007).
Statistical Analyses
Descriptive statistics, bivariate analyses, and correlations were conducted using IBM SPSS 19 for Windows. Measured variables path analysis, a form of structural equation modeling that estimates the relationships between observed variables, was used to estimate direct and indirect associations of PTSD symptoms, MDD symptoms and disease burden. Missing data were imputed using Maximum Likelihood Estimation (Enders & Bandalos, 2001).
Models were estimated using Analysis of Moment Structure (AMOS) version 7.0 (Arbuckle, 2006). Confounding variables were chosen based on their relationship to disease burden in the research literature. Comparative fit index (CFI), Tucker and Lewis Index (TLI; Tucker & Lewis, 1973), standardized root mean square residual (SRMR), root mean square error of approximation (RMSEA) and Akaike information criterion (AIC; Akaike, 1974; Bentler, 1990; McDonald & Ho, 2002) were examined as indices of goodness of fit for each model. A model with a CFI and TLI value of 0.90 or higher, SRMR of .08 or less, and a RMSEA value of less than .07 is considered a good fit (Hu & Bentler, 1995; Steiger, 2007). AIC was used to compare the fit of the final model to the initial conceptual model; models with smaller absolute values indicate a better fit with the data (Kline, 2011).
The bootstrap method was used to test whether depression symptoms mediated the relationship between PTSD symptoms and chronic disease and to examine the alternate hypothesis that chronic disease mediated the relationship between PTSD symptoms and depression (Figure 1). Briefly, statistical mediation refers to a process in which a significant proportion of the relationship between the independent variable (IV) and dependent variable (DV) is explained by indirect associations with a third mediating variable (M) (Baron & Kenny, 1986). This analysis is useful for exploring mechanisms of association in complex biobehavioral processes. Because of the complex reciprocal and interactive nature of biobehavioral processes, mediation analysis often relies on the integration of theoretical assumptions about potential mechanisms with analysis of empirical data. The current study tested plausible mediation models in which the association between PTSD and depression symptoms were explained in part by an indirect association through disease burden, and a competing model in which the association between PTSD symptoms and disease burden was explained by depression symptoms. The bootstrap method is used to estimate indirect associations in simple mediation and moderation models (Preacher & Hayes, 2004) and is more powerful than the three-step multiple regression approach (Baron & Kenny, 1986) and the Sobel test (Sobel, 1982). We used the recommended minimum of 1000 repetitions of the bootstrap process. Evidence of significant mediation or indirect association is indicated by a 95% confidence interval that does not include zero.
Results
Participants were primarily African American (88%) and single (52%) with 57% males and 43% females. Sixty-one percent of individuals reported a yearly household income of less than $10,000. Current tobacco and alcohol use occurred in 20% and 24% of participants, respectively. Thirty-five percent of the sample did not complete high school, 42% completed high school or a GED, 4% held a vocational degree, 13% completed some college coursework. 4% completed college, and 1% held advanced degrees. Fifty-three percent of the sample had at least one youth son or daughter living in their residence, and 40% had at least one adult son or daughter living in their residence. Of the 251 participants, 111 (43%) reported exposure to at least one traumatic incident. Thirty-one percent of the 111 exposed to trauma reported two or more traumas. The mean reported PTSD symptoms score was 8.45 (SD = 1.76). Based on the suggested clinical cut-off of 14 (Lang & Stein, 2005), 10% reported probable PTSD symptoms. The mean reported depression score was 4.95 (SD = 4.87). Seventeen percent of the sample reported at least moderate symptoms of depression according to the recommended cut-off of 10 or greater (Kroenke, Spitzer, & Williams, 2001). On average, participants reported 3.56 (SD = 1.91) total diseases in addition to congestive heart failure (CHF). Only 17% of respondents reported no comorbid diseases. Approximately 68% reported three or more chronic diseases in addition to CHF. Diabetes was the most commonly reported comorbid disease, occurring in approximately 46% of respondents, followed by arthritis (39%) and asthma (34%). Chronic disease burden was significantly correlated with PTSD symptoms and MDD symptoms (r = .41 and .43, p < .01, respectively).
Comparisons of Model Fit
Path loadings for both models are presented in Figure 3. The final model for Model 1 showed good fit to the observed data χ2 = 11.05, p = .14, CFI = .98, TLI = .95, SRMR = .08, and RMSEA = .05 (Table 2). The final model for Model 2 also showed good fit, χ2 = 12.77, p = .12, CFI = .97, TLI = .95, SRMR = .08, RMSEA = .05 (Table 2). In terms of our original hypotheses comparing goodness of fit of both models, the difference between A1C values for each model was not significant, suggesting that neither model showed a better fit to the data (Hilbe, 2014). Interestingly, the path from depression to chronic disease burden, adjusting for PTSD symptoms and other factors in the model, was quite small, indicating a small association with chronic disease burden in Model 1.
Figure 3.
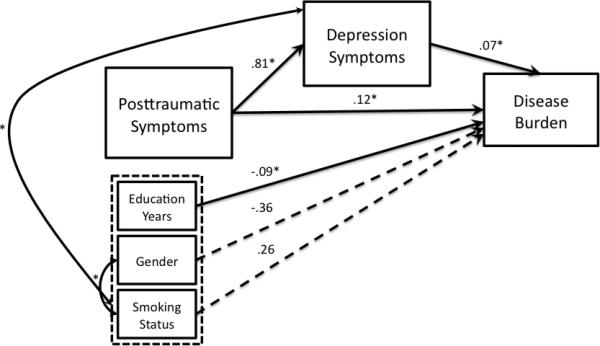
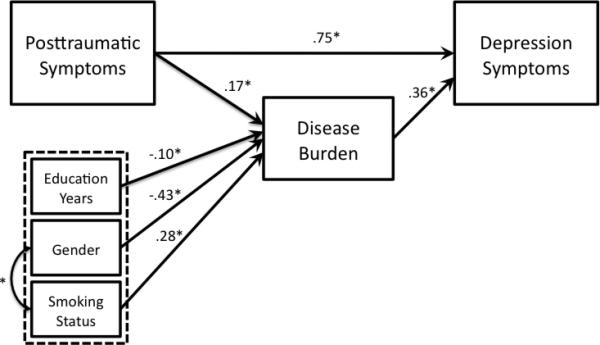
Models with path coefficients
3a. Model 1 with path coefficients
3b. Model 2 with path coefficients
Table 2.
Fit indices and direct, total and indirect effects and model fit indices for Models.
| Fit Indices | χ2 (df) | CFI | TLI | SRMR | RMSEA | AIC |
|---|---|---|---|---|---|---|
| Model 1 | 11.05 (7) | .98 | .95 | .08 | .05 | 51.05 |
| Model 2 | 12.77 (8) | .97 | .95 | .08 | .05 | 50.77 |
| Model 1 | β | B | SE | BC p-value |
|---|---|---|---|---|
| Direct | .24 | .12 | .11 | .04 |
| Indirect | .09 | .05 | .05 | .08 |
| Total | .33 | .17 | .08 | .001 |
| Model 2 | β | B | SE | BC p-value |
|---|---|---|---|---|
| Direct | .58 | .75 | .05 | .002 |
| Indirect | .05 | .06 | .02 | .01 |
| Total | .63 | .82 | .04 | .002 |
β: standardized beta weight; B: unstandardized beta weight; SE: standard error of the weight; BC: Bias-corrected; χ2: chi-squared; df: degrees of freedom; CFI: comparison fit index; TLI: Tucker-Lewis index; SRMR: standardized root mean square residual; RMSEA: root mean square error of approximation; AIC: Akaike information criterion.
Test of Mediation
Total, direct, and indirect associations in both models are presented in Table 3. In these analyses, mediation is significant if the 95% bias corrected and accelerated confidence intervals for the indirect association do not include zero (Preacher, Rucker, & Hayes, 2007; Preacher & Hayes, 2004). Results of Model 1 (Figure 3a) indicated that although the total and direct associations of PTSD symptoms with disease burden were significant (total association; TA = .33, p = .001 and direct association; DA = .24, p = .04, respectively), the indirect association (IA) was not (IA = .09, p = .08). Depression symptoms, therefore, did not mediate the relationship between PTSD symptoms and disease burden (IA lower 95% bias corrected confidence interval; CI = .006, upper 95% CI = .18). Results of Model 2 (Figure 3b) indicated that the total (TA = .63, p = .002), direct (DA = .58, p = .002), and indirect associations (IA = .05, p = .01) of PTSD symptoms with depression were all significant. The indirect association of PTSD symptoms with depression was significantly different from zero at (IA lower 95% CI = .01, upper 95% CI = .09, p = .01), indicating that chronic disease burden mediated the relationship between PTSD symptoms and depression symptoms.
Discussion
Traumatic stress exposure was relatively high in the present population of low-income, primarily African-American, congestive heart failure (CHF) patients. About 43% of patients had experienced at least one traumatic stressor. At the same time, given the high rates of violence and crime in the urban Chicago neighborhoods in which participants reside, all participants likely live amidst the real threat of violence. Chronic disease burden was high, with 83% of patients reporting one or more comorbid chronic diseases in addition to their CHF. As with prior research, we found strong correlations between PTSD symptoms and MDD symptoms and with chronic disease burden (Frayne, et al., 2004; Boscarino, 2004).
Results of path analyses were consistent with several hypotheses for the often-observed comorbidity between PTSD symptoms, MDD symptoms, and chronic diseases. Because both models demonstrated good fit to the data, it is most likely the case that multiple pathways contribute to chronic disease burden. Results support the individual contributions of both PTSD symptoms and MDD symptoms on chronic disease burden. Our results also support chronic disease burden as a mediator between PTSD symptoms and MDD symptoms. This finding is consistent with a sequence in which PTSD symptoms influences chronic disease and chronic disease burden then affects depression symptoms. In contrast, depression symptoms did not mediate the relationship between PTSD symptoms and chronic disease burden. It is notable that we are using continuous scale data and not diagnosis, as this suggests that it is not necessary to achieve diagnostic levels of disorder for these influences to occur. Results may also be especially pertinent to the mostly Black sample, who are living for a large part in poverty, and who have experienced both racial discrimination and neighborhood social, economic, and violent stressors (Guyll, Matthews, & Bromberger, 2001; Mays, Cochran, & Barnes, 2007).
The pathways between PTSD symptoms, MDD symptoms, and chronic disease burden hold important practical and clinical implications, which again may be especially relevant to African Americans who are disproportionally ill compared to middle class and White Western populations in the U.S. and elsewhere (Centers for Disease Control and Prevention, 2005; Mays Cochran, & Barnes, 2007). Although PTSD is often associated with increased utilization of health care (Fogarty, Sharma, Chetty, & Culpepper, 2008; Stein & Barrett-Connor, 2000), physicians are poor at identifying those with PTSD (Liebschutz et al., 2007; Taubman-Ben-Ari, Rabinowitz, Feldman, & Vaturi, 2001). Moreover, efforts aimed at increasing detection of mental health disorders in primary care settings have overwhelmingly focused on MDD symptoms. Individuals with PTSD symptoms may be more likely to present in primary care or hospital settings as opposed to mental health settings (Samson, Bensen, Beck, Price, & Nimmer, 1999) and this especially appears to be the case in samples of primarily African-American patients (Rosenheck & Fontana, 1994). The increased utilization of medical services among patients with PTSD symptoms supports the need for increased detection and treatment of PTSD in medical settings. Given that comorbidity of chronic disease with heart failure results in greater hospitalizations and mortality (Braunstein, et al., 2003; Brown & Cleland, 1998), the detection and treatment of PTSD symptomatology is especially important for patients with cardiovascular disease.
The current study is limited in establishing causality by use of cross sectional data. Age of exposure to traumatic stressors was not collected. Therefore, the present results cannot establish a causal link between MDD and PTSD diagnoses with chronic disease burden. However, the current models do provide a guide for future modeling with longitudinal studies (Lleras, 2005). Our study is also limited by the use of brief self-report measures of PTSD and MDD symptoms. Such measures decrease the study burden for participants with multiple chronic diseases but limit definitive diagnoses. Studies find the scales highly correlated with more intensive interviews, so they are indicative of diagnosis (Hobfoll, Canetti, Hall, Brom, Palmieri, Johnson, Pat-Horenczyk,, & Galea, 2011), but caution in extrapolating to diagnosis is called for. The over-representation of African Americans of very low income is both a limitation as well as a definite strength, as this population carries a disproportionate disease burden in both human and financial terms (Centers for Disease Control and Prevention, 2005). Lastly, generalizability of results may be limited due to inclusion of only those individuals with CHF, which represents a particularly burdensome and severe form of chronic illness with multiple health implications.
Comparisons of model fit relied on the AIC statistic, and follow-up tests of significant difference of model fit are not possible (Kline, 2011). Therefore, replication of these findings with longitudinal data is needed to evaluate potential causal processes at work with traumatic stress and chronic disease burden (Duckworth, Tsukayam, & May, 2010). Further, although PTSD and MDD symptoms have large overlap, the models were robust and reveal several patterns of interest. Clearly, however, prospective data is required in order to deconstruct the pathways and feedback loops that occur as PTSD, MDD and disease burden exist across a multi-decade course in these people's lives, and we only view a snapshot of these associations here.
Longitudinal treatment studies are also needed to determine whether treatment of PTSD and MDD can improve medical outcomes or whether mental health treatment would simply lessen psychological distress. It is likely that mental health treatment would at least improve quality of life among those with PTSD and high chronic disease burden. Our findings also underscore that intervention might be called for even for those who have some symptoms of PTSD, even if they do not currently meet criteria for diagnosis. Currently, there are few such interventions developed, as progress has mainly been made on intervening with those with frank PTSD. Future longitudinal studies with more thorough diagnostic interviews are needed to determine the pathways between PTSD, MDD, and chronic disease burden. Our results suggest that PTSD, MDD, and disease burden interact, and that in some cases, disease burden may actually mediate the relationship between PTSD and MDD. Taken together, our results highlight the importance of identifying and treating PTSD symptoms, which are substantive in individuals from underserved backgrounds with high disease burden.
Table 1.
Spearman's rho correlation coefficients of variables included in the models.
| Age | Education Years | Chronic Disease Burden | PTSD Symptoms | |
|---|---|---|---|---|
| Age | ||||
| Education Years | −.25* | |||
| Chronic Disease Burden | .18 | −.15 | ||
| PTSD Symptoms | .03 | −.03 | .41** | |
| MDD Symptoms | .09 | −.16 | .43** | .67** |
p < .01; p < .001
PTSD: posttraumatic stress disorder symptoms as measured by the Posttraumatic Checklist-Short Form (PCL-SF); MDD: major depressive disorder as measured by the Patient Health Questionnaire-9-item Depression module (PHQ-9).
Acknowledgments
This research is supported by the Charles J. and Margaret Roberts Fund and a grant from the NIH-NHLBI 1P50HL105189 Rush Center for Urban Health Equity.
Contributor Information
April Taylor-Clift, Behavioral Sciences Department, Rush University Medical Center, 1645 West Jackson Boulevard, Suite 400, Chicago, IL 60612.
Stevan E Hobfoll, Behavioral Sciences Department, Rush University Medical Center, 1645 West Jackson Boulevard, Suite 400, Chicago, IL 60612.
James I Gerhart, Behavioral Sciences Department, Rush University Medical Center, 1645 West Jackson Boulevard, Suite 400, Chicago, IL 60612.
DeJuran Richardson, Lake Forest College, Young Hall 125, 555 Sheridan Road, Lake Forest, IL 60045 and Rush University Medical Center, Preventive Medicine Department, 1700 West Van Buren Street, Suite 470, Chicago, IL 60612.
James E Calvin, Rush University Medical Center, Preventive Medicine Department, 1700 West Van Buren Street, Suite 470, Chicago, IL 60612.
Lynda H Powell, Rush University Medical Center, Preventive Medicine Department, 1700 West Van Buren Street, Suite 470, Chicago, IL 60612.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Andersen J, Wade M, Possemato K, Ouimette P. Association between posttraumatic stress disorder and primary care provider-diagnosed disease among Iraq and Afghanistan veterans. Psychosomatic Medicine. 2010;72(5):498–504. doi: 10.1097/PSY.0b013e3181d969a1. doi: 10.1097/PSY.0b013e3181d969a1. [DOI] [PubMed] [Google Scholar]
- Arbuckle J. Amos (Version 7.0) SPSS; Chicago: 2006. [Google Scholar]
- Armenian HK, Melkonian AK, Hovanesian AP. Long term mortality and morbidity related to degree of damage following the 1998 earthquake in Armenia. American Journal of Epidemiology. 1998;148(11):1077–1084. doi: 10.1093/oxfordjournals.aje.a009585. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Moore SD, Feldman ME, Hertzberg MA, Kirby AC, Fairbank JA. Health status, somatization, and severity of posttraumatic stress disorder in Vietnam combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1998;155(11):1565–1569. doi: 10.1176/ajp.155.11.1565. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Buckley TC, Hickling EJ, Taylor AE. Posttraumatic stress disorder and comorbid major depression: Is the correlation an illusion? Journal of Anxiety Disorders. 1998;12(1):21–37. doi: 10.1016/s0887-6185(97)00047-9. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and physical illness: Results from clinical and epidemiologic studies. Annals of the New York Academy of Sciences. 2004;1032:141–153. doi: 10.1196/annals.1314.011. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosomatic Medicine. 2008;70(6):668–676. doi: 10.1097/PSY.0b013e31817bccaf. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. Journal of the American College of Cardiology. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of General Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder-major depression connection. Biological Psychiatry. 2000;48(9):902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Brown AM, Cleland JG. Influence of concomitant disease on patterns of hospitalization in patients with heart failure discharged from Scottish hospitals in 1995. European Heart Journal. 1998;19(7):1063–1069. [PubMed] [Google Scholar]
- Calhoun KS, Wiley M, Dennis MF, Beckham JC. Self-reported health and physician diagnosed illnesses in women with posttraumatic stress disorder and major depressive disorder. Journal of Trauma Stress. 2009;22(2):122–130. doi: 10.1002/jts.20400. doi: 10.1002/jts.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Health disparities experienced by black or African Americans--United States. MMWR: Morbidity and Mortality Weekly Report. 2005;54:1–3. [PubMed] [Google Scholar]
- Chiu S, Niles JK, Webber MP, Zeig-Owens R, Gustave J, Lee R, Prezant DJ. Evaluating risk factors and possible mediation effects in posttraumatic depression and posttraumatic stress disorder comorbidity. Public Health Report. 2011;126(2):201–209. doi: 10.1177/003335491112600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clum GA, Calhoun KS, Kimerling R. Associations among symptoms of depression and posttraumatic stress disorder and self-reported health in sexually assaulted women. Journal of Nervous and Mental Disease. 2000;188(10):671–678. doi: 10.1097/00005053-200010000-00005. [DOI] [PubMed] [Google Scholar]
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: A critical review of available methods. Journal of Clinical Epidemiology. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dong M, Dube SR, Felitti VJ, Giles WH, Anda RF. Adverse childhood experiences and self-reported liver disease: new insights into the causal pathway. Archives of Internal Medicine. 2003;163(16):1949–1956. doi: 10.1001/archinte.163.16.1949. doi: 10.1001/archinte.163.16.1949. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Tsukayama E, May H. Establishing causality using longitudinal hierarchical linear modeling: An illustration predicting achievement from self-control. Social psychological and personality science. 2010;1:311–317. doi: 10.1177/1948550609359707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton MA, Green BL, Kaltman SI, Roesch DM, Zeffiro TA, Krause ED. Journal of Interpersonal Violence. 2006;Intimate partner violence, PTSD, and adverse health outcomes.21(7):955–968. doi: 10.1177/0886260506289178. doi: 10.1177/0886260506289178. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8(3):430–457. [Google Scholar]
- Escobar JI, Canino G, Rubio-Stipec M, Bravo M. Somatic symptoms after a natural disaster: A prospective study. American Journal of Psychiatry. 1992;149(7):965–967. doi: 10.1176/ajp.149.7.965. [DOI] [PubMed] [Google Scholar]
- Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. American Journal of Managed Care. 2006;12(2):110–119. [PubMed] [Google Scholar]
- Fogarty CT, Sharma S, Chetty VK, Culpepper L. Mental health conditions are associated with increased health care utilization among urban family medicine patients. Journal of the American Board of Family Medicine. 2008;21(5):398–407. doi: 10.3122/jabfm.2008.05.070082. doi: 10.3122/jabfm.2008.05.070082. [DOI] [PubMed] [Google Scholar]
- Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro A, Parker VA, Skinner KM. Burden of medical illness in women with depression and posttraumatic stress disorder. Archives of Internal Medicine. 2004;164(12):1306–1312. doi: 10.1001/archinte.164.12.1306. doi: 10.1001/archinte.164.12.1306. [DOI] [PubMed] [Google Scholar]
- Ginzburg K, Ein-Dor T, Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: A 20-year longitudinal study of war veterans. Journal of Affective Disorders. 2010;123(1-3):249–257. doi: 10.1016/j.jad.2009.08.006. doi: 10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92(1-2):195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- Guyll M, Matthews KA, Bromberger JT. Discrimination and unfair treatment: relationship to cardiovascular reactivity among African American and European American women. Health Psychology. 2001;20(5):315. doi: 10.1037//0278-6133.20.5.315. [DOI] [PubMed] [Google Scholar]
- Hilbe Joseph M. Modeling Count Data. Cambridge University Press; Cambridge, UK: 2014. [Google Scholar]
- Hobfoll SE, Canetti D, Hall BJ, Brom D, Palmieri PA, Johnson RJ, Pat-Horenczyk R, Galea S. Are community studies of psychological trauma's impact accurate? A study among Jews and Palestinians. Psychological Assessment. 2011;23:599–605. doi: 10.1037/a0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler P. Evaluating Model Fit. Sage; Thousand Oaks, CA: 1995. [Google Scholar]
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biological Psychiatry. 2003;54(3):216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun TB, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF). International Journal of Methods in Psychiatric Research. 1998;7(4):171–185. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Keyes KM, McLaughlin KA, Demmer RT, Cerdá M, Koenen KC, Uddin M, Galea S. Potentially traumatic events and the risk of six physical health conditions in a population-based sample. Depression and Anxiety. 2013;30(5):451–460. doi: 10.1002/da.22090. doi: 10.1002/da.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder LS, Bradley KA, Katon WJ, Ludman E, McDonell MB, Bryson CL. Depression, posttraumatic stress disorder, and mortality. Psychosomatic Medicine. 2008;70(1):20–26. doi: 10.1097/PSY.0b013e31815aac93. doi: 10.1097/PSY.0b013e31815aac93. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Guilford press; New York: 2011. [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AJ, Stein MB. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behaviour Research and Therapy. 2005;43(5):585–594. doi: 10.1016/j.brat.2004.04.005. doi: 10.1016/j.brat.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: Results from a nationally representative prospective study of US adults. Journal of the American Medical Association. 1998;279:1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- Leserman J. Sexual abuse history: Prevalence, health effects, mediators, and psychological treatment. Psychosomatic Medicine. 2005;67(6):906–915. doi: 10.1097/01.psy.0000188405.54425.20. doi: 10.1097/01.psy.0000188405.54425.20. [DOI] [PubMed] [Google Scholar]
- Liebschutz J, Saitz R, Brower V, Keane TM, Lloyd-Travaglini C, Averbuch T, Samet JH. PTSD in urban primary care: High prevalence and low physician recognition. Journal of General Internal Medicine. 2007;22(6):719–726. doi: 10.1007/s11606-007-0161-0. doi: 10.1007/s11606-007-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleras C. Path Analysis. In: Kempf-Leonard K, editor. Encyclopedia of Social Measurement. Vol. 3. Academic Press; San Diego: 2005. pp. 25–30. [Google Scholar]
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RP, Ho MH. Principles and practice in reporting structural equation analyses. Psychological Methods. 2002;7(1):64–82. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- McNutt LA, Carlson BE, Persaud M, Postmus J. Cumulative abuse experiences, physical health and health behaviors. Annals of Epidemiology. 2002;12(2):123–130. doi: 10.1016/s1047-2797(01)00243-5. [DOI] [PubMed] [Google Scholar]
- Monami M, Lambertucci L, Lamanna C, Lotti E, Marsili A, Masotti G, Mannucci E. Are comorbidity indices useful in predicting all-cause mortality in Type 2 diabetic patients? Comparison between Charlson index and disease count. Aging Clinical and Experimental Research. 2007;19(6):492–496. doi: 10.1007/BF03324736. [DOI] [PubMed] [Google Scholar]
- Nickerson A, Steenkamp M, Aerka IM, Salters-Pedneault K, Carper TL, Barnes JB, Litz BT. Prospective investigation of mental health following sexual assault. Depression and Anxiety. 2013;30(5):444–450. doi: 10.1002/da.22023. doi: 10.1002/da.22023. [DOI] [PubMed] [Google Scholar]
- O'Toole BI, Catts SV. Trauma, PTSD and physical health: An epidemiological study of Australian Vietnam veterans. Journal of Psychosomatic Research. 2008;64(1):33–40. doi: 10.1016/j.jpsychores.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Phifer JF. Psychological distress and somatic symptoms after natural disaster: differential vulnerability among older adults. Psychology of Aging. 1990;5(3):412–420. doi: 10.1037//0882-7974.5.3.412. [DOI] [PubMed] [Google Scholar]
- Preacher K, Rucker D, Hayes A. Addressing moderated mediation hypotheses: Theory, methods and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods Instruments and Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Prince MJ, Harwood RH, Thomas A, Mann AH. A prospective population-based cohort study of the effects of disablement and social milieu on the onset and maintenance of late-life depression: The Gospel Oak Project VII. Psychological Medicine. 1998;28(2):337–350. doi: 10.1017/s0033291797006478. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Fontana A. Utilization of mental health services by minority veterans of the Vietnam era. Journal of Nervous and Mental Disease. 1994;182(12):685–691. doi: 10.1097/00005053-199412000-00002. [DOI] [PubMed] [Google Scholar]
- Samson AY, Bensen S, Beck A, Price D, Nimmer C. Posttraumatic stress disorder in primary care. Journal of Family Practice. 1999;48(3):222–227. [PubMed] [Google Scholar]
- Schnurr PP, Green BL. Understanding relationships among trauma, post-tramatic stress disorder, and health outcomes. Advances in Mind Body Medicine. 2004;20(1):18–29. [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: Implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23(3):219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Seng JS, Clark MK, McCarthy AM, Ronis DL. PTSD and physical comorbidity among women receiving Medicaid: Results from service-use data. Journal of Traumatic Stress. 2006;19(1):45–56. doi: 10.1002/jts.20097. doi: 10.1002/jts.20097. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. American Journal of Psychiatry. 1998;155(5):630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- Shemesh E, Rudnick A, Kaluski E, Milovanov O, Salah A, et al. A prospective study of posttraumatic stress symptoms and nonadherence in survivors of a myocardial infarction (MI). General Hospital Psychiatry. 2001;23:215–222. doi: 10.1016/s0163-8343(01)00150-5. [DOI] [PubMed] [Google Scholar]
- Sobel M. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociological Methodology. 1982;13:290–312. [Google Scholar]
- Spitzer C, Barnow S, Volzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosomatic Medicine. 2009;71(9):1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- Steiger J. Understanding the limitations of global fit assessment in structural equation modeling. Personality and Individual Differences. 2007;42(5):893–898. [Google Scholar]
- Stein MB, Barrett-Connor E. Sexual assault and physical health: Findings from a population-based study of older adults. Psychosomatic Medicine. 2000;62(6):838–843. doi: 10.1097/00006842-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Taubman-Ben-Ari O, Rabinowitz J, Feldman D, Vaturi R. Post-traumatic stress disorder in primary-care settings: Prevalence and physicians' detection. Psychological Medicine. 2001;31(3):555–560. doi: 10.1017/s0033291701003658. [DOI] [PubMed] [Google Scholar]
- Tucker L, Lewis C. The reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. 38. [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]


