Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease of complex etiology in which B cells play a central role. An expanded number of B-1a cells have been consistently associated with murine lupus, and more recently with human SLE. We have identified CdKn2c, a gene that controls cell cycle progression, as a key regulator of B-1a cell numbers, and have associated Cdkn2c deficiency with autoimmune pathology, including the production of autoantibodies and the skewing of CD4+ T cells toward inflammatory effector functions. We review the genetic studies that have led to these findings, as well as the possible mechanisms by which B-1a cell expansion and Cdkn2c deficiency are related to SLE pathogenesis.
Keywords: systemic lupus erythematosus, B-1a cell, NZM2410 mice
Introduction
Systemic lupus erythematosus (SLE), an autoimmune disease of complex etiology that presents with considerable clinical heterogeneity and production of pathogenic autoantibodies that cause tissue damage and associated pathology. Several spontaneous and induced mouse models of SLE have been developed over the years.1 Although none of these models represents the full spectrum of clinical SLE, early studies have established that lupus-prone mice reproduce all the major components of SLE immunopathology, albeit with a reduced complexity of end-organ presentation.2 Moreover, lupus mice are excellent models of the genetic architecture of human SLE. This notion has been greatly strengthened by the convergence of three functional pathways, processing of apoptotic cells, B cell receptor signaling, and Toll-like receptor (TLR)/type I interferon signaling, that are defective in both human and murine lupus.3, 4 Within these pathways, variants in a number of genes, some identical and some in different positions in a same pathway, have now been shown to be directly associated with lupus in both species.5, 6
We have used the NZM2410 mouse model, which is a recombinant inbred strain from the classic (NZB x NZW)F1 (NZB/W) model.7 The NZM2410 strain is by far the model in which the genetic characterization is the most advanced,5, 8 with first the identification of three major genomic loci, Sle1, Sle2, and Sle3, that are necessary and sufficient in combination to induce clinical lupus, as well as the consequent identification of susceptibility genes within these three loci.7, 9 This review summarizes the studies that have characterized the genetic basis of the accumulation of B-1a cells in NZM2410 mice, as well as the role that these cells play in lupus pathogenesis.
B-1a cells and murine lupus
Pathogenic autoantibodies are the effector molecules responsible for tissue damage in SLE. This has led to a large body of lupus research conducted on B cells, including the regulation of B cell tolerance and differentiation to antibody producing cells.10 This central role of B cells in lupus has also placed these cells at the center of experimental targeted therapies. Specifically, clinical trials have been recently completed or are on-going to assess the therapeutic efficacy of B cell depletion with antibodies against CD20 or CD22, inhibition of B cell survival with BAFF blockade, or inhibition of B:T cell co-stimulation by blocking the CD40–CD40L, CD28–CD80/CD86, or ICOS–ICOSL pathways.11 Despite enormous effort, the results of B cell targeted therapies have been mixed at best,12 with anti-CD20 depletion with rituximab not meeting clinical trials end-points, anti-soluble BAFF (belimumab) receiving FDA approval but showing a small therapeutic effect, and soluble and membrane anti-BAFF tabalumab being discontinued from phase III clinical trials.13 One potential reason for these mixed results is that either anti-CD20 depletion or BAFF blockade are not equally effective against all types of B cells, and some of the spared subsets may strongly contribute to lupus pathogenesis. This includes the subset of B1-a cells, which rely on BAFF relatively less than conventional B cells for their survival.14 The efficacy of anti-CD20 treatment to deplete B-1a cells is unknown, but the predominate location of B-1a cells in serous cavities may protect them from the depleting antibodies.
An expanded number of B-1a cells has been consistently associated with murine lupus, with early studies showing that genetic or physical ablation of B-1a cells reduced disease severity in the NZB/W model (reviewed in Ref. 15). There are several mechanisms by which B-1a cells could contribute SLE pathogenesis, as summarized in Table 1. As detailed below, no formal causal relationship has been established yet between B-1a cells and lupus. The potential pathogenic mechanisms for lupus pathogenesis are therefore considered if they are either a part of the normal functional repertoire of B-1a cells and/or are enhanced in mouse models of lupus and lupus patients.
Table 1.
Proposed contributions of B-1a cells to lupus
B1-a cells are autoreactive
The B-1a cell repertoire is autoreactive;16 however, these cells produce low affinity IgM natural antibodies, while pathogenic lupus autoantibodies have a high affinity and are class-switched. The inflammatory environment present in lupus has been shown to change both B1-a cell location and their autoantibody repertoire. Indeed, NZB/W B-1a cells migrate to the sites of inflammation, notably the kidneys, where they class-switch and produce anti-dsDNA IgG, a hallmark of lupus autoantibodies.17 We have confirmed this finding in the NZM2410 model (unpublished results). The migration of B-1a cells to inflamed tissues is mediated by CXCL13,18 and this process has been shown to play a direct role in lupus nephritis in the MRL model.19 In addition, B-1a cells constitutively express plasma cell differentiation markers.20 Although this has not been formally shown for class-switched B-1a cells, it could result in an accelerated and less regulated production of autoantibodies.
Production of IL-10
B-1 cells produce large amount of IL-10,21 a cytokine that has been shown to both inhibit22 and exacerbate23 systemic autoimmune disease in mice as well as in SLE patients.24, 25 A specialized population of IL-10–producing B cells (B10 or Breg cells) has been characterized with regulatory functions.26 These Breg cells are both phenotypically and functionally different from B-1 cells, in spite of sharing the capacity to secrete IL-10.
Increased antigen presentation and costimulation
B-1a cells have a higher antigen presentation capacity and CD4+ T cell co-stimulatory ability than conventional B cells.27, 28 These functions are dependent on the expression of CD86 and PDL-2, two markers that are expressed at higher levels on the B1-a cells in lupus mice.29, 30 It should be noted, however, that these studies have been performed in vitro, and there is no (so far) direct evidence for B-1a:T cell interactions in vivo besides the expression of ligands on B-1a cells with corresponding receptors on T cells. In non-autoimmune mice, B-1a cells are most abundant limited to the peritoneal and thoracic cavities, in which T cells are infrequent. The antigen-presentation capacity of splenic B-1a cells has not been formally evaluated due their low numbers, but L2pB1 cells (B-1a cells that express PDL-2) are preferentially found in the peritoneal cavity (PerC).31 Therefore, interactions between B-1a cells and CD4+ T cells may not represent a major contribution to immune activation in non-inflammatory conditions. However, when inflamed B-1a cells migrate to tissues in which T cells are abundant, such as the blood, the thymus and the kidneys, the high co-stimulatory capacity of B-1a cells is most likely to amplify the activation of pathogenic T cells.
Increase in Th17 cell polarization
B-1a cells polarize CD4+ T cells to a Th17 effector phenotype, while conventional B cells skew T cell toward a regulatory phenotype.29, 32 These results were obtained in vitro with strong alloreactive stimulation. They are provocative, however, as increasing evidence suggests that Th17 cells contribute to SLE pathogenesis by providing help to autoreactive B cells in lupus mice33 and lupus patients,34 and by contributing to the inflammatory cascade in lupus nephritis.35, 36 As detailed below, we also have indirect evidence that B-1a cells skew T cells toward Th17 polarization in the NZM2410 model.37
B-1a cells and human SLE
On the basis of antibody repertoire and gene expression profile, human FCRL4+ CD21lo B cells have been proposed to be the equivalent of mouse B-1a cells,38 and this population of human B cells is expanded in the peripheral blood (PB) of SLE patients.39 More recently, human CD27+ CD43+ CD70− B cells have been identified as the functional equivalent to the murine B-1a cells on the basis of spontaneous IgM secretion, tonic B cell receptor signaling, and ability to activate T cells.40 A subset of these B1 cells expressing CD11b (which also express on murine PerC B-1a cells) is expanded in the PB of SLE patients and possesses a greatly enhanced T cell activation ability.41 This suggests that human B1 cells may contribute to SLE through their interaction with T cells rather than by the production of autoantibodies and, by extension, that this may be also the case for murine lupus. The absence of a single lineage marker for B-1 cells makes it impossible to selectively deplete B-1 cells in vivo, either in experimental models to assess directly their contribution to disease, or potentially as a therapeutic approach in lupus patients. For this reason, the role of B-1 cells in lupus (or any other disease) has been thus far assessed by association rather than causality.
The expansion of B-1a cells maps to the Sle2 locus in the NZM2410 mice
The characterization of congenic mice carrying each of the Sle1, Sle2, or Sle3 susceptibility loci on a non-autoimmune C57BL/6 (B6) background showed that the accumulation of B-1a cells mapped to Sle2.42 This locus is also linked with B cell hyperactivation and the production of polyreactive antibodies. The comparison of the bi-congenic to the triple congenic combinations of the Sle loci indicated that the addition of Sle2 to the Sle1/Sle3 combination doubled the incidence of fatal lupus nephritis.9 This demonstrated that although Sle2 is not pathogenic by itself, it contributes significantly to disease outcomes. This analysis also indicates that the role of Sle2 is to amplify immune dysfunctions induced by the combination of Sle1 and Sle3, rather than to initiate the autoimmune process.
The majority of B-1a cells are produced by fetal specific progenitors (B1P).43, 44 Mixed chimeras combining fetal livers originating from B6 and B6.Sle2 mice showed that the expansion of B-1a cells by Sle2 expression was cell intrinsic and that fetal B1P precursors expressing Sle2 provided, over time, a greater output of B-1a cells than B6 control B-1a cells.45 This could be due to either a higher number of B1Ps or a greater number of B-1a cells differentiated from each B1P, an issue that, to be answered, will require transplantation of a known number of B1Ps. We have also shown that bone marrow (BM) from adult B6.Sle2 mice gave rise to a subtantial number of B-1a cells after transplantation into a lethaly irradiated host, while control B6 BM yielded only conventional B cells, suggesting that either fetal B1Ps are maintained in the adult B6.Sle2 BM or B1Ps can be reprogrammed from adult BM in a lymphopenic setting (but still in competition with conventional B cell precursors). Finally, we have shown that B-1a cells from B6.Sle2 mice proliferate more spontaneouly in vivo and in vitro in response to LPS and were subject to lower rates of apoptosis, compared to control B-1a cells. Overall, these results sugested that the age-dependent accumulation of B-1a cells mediated by the lupus susceptibility locus Sle2 results from mutiple mechanisms: a greater output from fetal B1Ps, carry-over of B1Ps in adult BM, greater proliferation of adult B-1a cells, and reduced apoptosis, all resulting in the gradual expansion of PerC B-1a cells to the point that they become the dominating lymphocyte population in a percentage of aged B6.Sle2 mice.
Cyclin-dependent kinase inhibitor Cdkn2c regulates B-1a cell numbers
To identify the gene(s) responsible for the B-1a cell expansion in NZM2410 mice, we generated Sle2 congenic recombinants. From this we found that a telomeric region of Sle2, Sle2c, was the locus most strongly associated with the PerC B-1a cell expansion.45 Interestingly, this region is of NZB origin, and NZB was the strain in which B-1a cells were first reported to be largely expanded in number.46 Sle2c congenic recombinants were generated and screened for expanded numbers of PerC B-1a cells. The combination of high resolution mapping and the comparison of gene expression profiles between B6.Sle2c and B6 B-1a cells identified Cdkn2c as the most likely gene responsible for the increased number of B-1a cells.47 Cdkn2c expression was reduced in B6.Sle2c splenic (sB) cells and PerC B-1a cells to 5–35% of that found in B6 B cells. We identified a novel −74 C/T SNP in a highly conserved region of the Cdkn2c promoter, and luciferase assays demonstrated that the T allele led to decreased Cdkn2c expression, compared to the C allele.47 While the −74 C → T polymorphism results in the loss of a binding site for the transcription factor NRF2, it also creates a binding site for YY-1 that is adjacent to an already existing YY-1 site. YY-1 siRNA eliminated the difference in promoter activity between the two alleles, indicating that YY-1 expression was responsible for the difference in Cdkn2c expression, which was confirmed by ChIP assays.48 Thus, YY-1 is a direct regulator of Cdkn2c expression in an allele-dependent fashion that is consistent with the lupus-associated T allele inducing a lower p18 transcriptional activity by increasing YY-1 binding. We have therefore established that the −74 C/T SNP is the causal variant for the low Cdkn2c expression in Sle2c1 B cells.
Cdkn2c encodes for p18INK4c (p18), one of the four cyclin-dependent kinase inhibitors that regulate cell cycle in the G1-to-S phase; p16INK4a, p15INK4b, p18INK4c, and p19INKd prevent Cdk4 and Cdk6 from binding to the D cyclins and thereby block cell cycle progression at the restriction point (R). p18 is essential for G1 arrest in mouse and human B cells and thus for promoting their maturation into plasma cells.49, 50 Consequently, p18−/− mice have defective immune responses due to a greatly reduced immunoglobulin production by plasma cells.51 Low p18 expression has also been implicated in the development of human B cells tumors.52 p18 promotes B cell differentiation from hematopoietic stem cells53 and regulates B cell homeostasis in opposition to BAFF by preventing early cell cycle entry by binding to cyclin D2–Cdk4 complexes.54
B-1a and B2 cells have different cell cycle regulation mechanisms,55 with B-1a cells maintained by self-renewal while B2 cells proliferate in response to B cell receptor signaling. Comparing cell cycle regulation of these two B cell populations has established that they have different requirements for and kinetics of the cyclin D2 or D3-Cdk4 complexes.55 Briefly, cyclin D2 deficiency leads to a selective ablation of B-1a cells,56 and D2-Cdk4 complexes accumulate early in B-1a, but not in B2 cells stimulated with phorbol myristate acetate (PMA).57 Cyclin D3–deficient mice have reduced numbers and impaired function of B2 cells, but normal B-1a cells, owing to the compensatory role of cyclin D2.58 In normal B-1a cells, however, disruption of D3–Cdk4/6 complexes blocks B-1a cell proliferation, demonstrating a complex, non-overlapping role for these two cyclins. The role of cyclin-dependent kinase inhibitors has not been investigated in this process. Consistent with these results, however, we found defective G1 cell cycle arrest in Sle2c sB cells, and increased spontaneous proliferation and IgM production of Sle2c PerC B-1a cells. We have also found, as predicted by the model, that cyclin D2 deficiency eliminated the accumulation of B-1a cells in B6.Sle2c mice.59 Therefore, our results are consistent with p18 being a major regulator of B-1a cell homeostasis, with decreased p18 levels resulting in increased cyclin D2–dependent cell cycle activation in these cells and, consequently, accumulation of B-1a cells. Our results also predict, because of p18 function in stem cells, that B1Ps expressing low p18 levels would lead to differentiation to B-1a cells, which is consistent with our BM transfer experiments.
p18 deficiency results in preferential B-1a cell expansion and autoantibody production
To test the hypothesis that low expression of Cdkn2c is responsible for the Sle2c1 phenotypes, we compared B6.Sle2c to Cdkn2c-deficient (B6.p18−/−)49 mice. B6.p18−/− mice showed an early B-1a cell expansion, which corresponded to preferential homeostatic expansion of these cells, compared to B2 cells, after transfer into lymphopenic B6.Rag1−/− mice. p18−/− B-1a cells produced more IgM, either spontaneously or after either LPS or PMA stimulation, than wild-type B6 B-1a cells. Furthermore, a majority of B6.p18−/− mice produced anti-dsDNA IgG and antinuclear autoantibodies by 6–8 months of age. The magnitude of these phenotypes was greater in B6.p18−/− than in B6.Sle2c1 mice that produce ANA but not anti-dsDNA IgG, demonstrating that p18 limits the number of B-1a cells and their functions in a dose-dependent manner. It also suggests that, by itself, a large expansion of B-1a cell numbers is sufficient for production of lupus-associated autoantibodies. This occurs, however, only in aged mice, and cannot therefore play any role in the initiation of disease, which is consistent with the amplification role of Sle2.
Deficiency of p18 may affect the function of immune cells other than B-1a cells, thus contributing to the autoimmune phenotype. In particular, it is known that p18 sets an inhibitory threshold for T cell proliferation, and that one function of CD28 co-stimulation is to counteract p18 binding on cyclin D2/3–Cdk6 complexes60. Foxp3+ CD4+ regulatory T cells (Treg cells) require cell cycle arrest, which is at least partially controlled by an increased p27kip expression, which blocks the activation of cyclins E and A by Cdk261. A requirement for an early G1 cell cycle arrest in human Treg cells62 suggests the possibility that p18 plays a role in this process. In support of this hypothesis, p18 expression in CD4+ T cells is reduced by about 2-fold in young B6.Sle2c mice, compared to wild-type B6 (unpublished results). B6.p18−/− T cells spontaneously proliferate more than B6 T cells in vivo, which is consistent with the higher number of B6.p18−/− T cells. These unpublished results suggest that p18 deficiency increases T cell spontaneous proliferation, which may result in chronic activation independently (or not) from B-1a cell involvement. Accordingly, B6.Sle2c mice have fewer Treg cells than B6 mice,37 and both B6.Sle2c and B6.p18−/− CD4+ T cells secrete lower amounts of IL-2 than B6 cells in response to receptor stimulation (unpublished results), which may be responsible for the reduced number of Treg cells. In contrast, normal IL-2 production has been reported for p18−/− total LN cells;60 the discrepancy between this and our results could be accounted for by a number of factors, including differences in stimulatory conditions. Therefore, a formal exclusion of T cells for being involved in the production of autoantibodies in B6.p18−/− mice would require a B cell–specific deletion of p18. Overall, however, the above data demonstrate that p18-regulation of early cell cycle entry plays a key role in B-1a cell homeostasis, and in the production of autoantibodies.
Cdkn2c has not been thur far linked to autoimmunity. However, other CDK inhibitors have been implicated in SLE, including CDKN1B (p27KIP1), which functionally partners with CDKN2C (p18INK4c). 53, 54 CDKN1A (p21WAF1/CIP1), as well as CDKN1B polymorphisms are associated with SLE susceptibility in humans.63, 64, 65 The decreased expression of CDKN1A and CDKN1B in the lymphocytes of lupus patients has been associated with increased activation through AKT phosphorylation.66 In the mouse, p21-deficiency results in a lupus-like phenotype with the production of activated/memory T cells;67 p21 also accumulates in G1 cell cycle-arrested memory T cells, and its deficiency prevents disease in the NZB/W model68. In addition, B cell homeostasis is regulated by the RAPL-mediated translocation of Cdkn1b to the nucleus, and the forced sequestration of CDKN1B in the cytoplasm leads to a lupus-like phenotype69.
Sle2c and p18 deficiency contribute to lupus
To investigate further how Sle2 contributes to lupus pathogenesis, we characterized the phenotypes of B6.Sle2.lpr mice, a strain in which Sle2 is co-expressed with the lpr mutation (Fas deficiency), a well-characterized lupus-susceptibility factor.70 Sle2 dramatically increased lpr-induced autoimmune pathogenesis by preferentially expanding T cell numbers, including CD4−CD8− double negative (DN) T cells, leading to enhanced polarization to Th17 cells and infiltration of Th17 cells into the kidneys and the skin37. This synergy between Sle2 and lpr mapped to Sle2c. The reduction of Treg cell numbers seen in B6.Sle2c mice was further accentuated by Fas deficiency, but the skewed Th17 polarization was observed only in combination with lpr,37 suggesting that the IL-2 and Treg cell deficiencies are primary defects induced by Sle2c expression, and that Sle2c enhances Th17 polarization that is induced by lpr itself.36
To determine whether p18-deficiency reproduced the synergy between Sle2c and lpr, we compared immune phenotypes between B6.p18−/−.lpr, B6.Sle2c1.lpr, and B6.lpr mice.59 B6.p18−/−.lpr mice showed severe lymphadenopathy resulting in significantly shorter survival compared to B6.Sle2c1.lpr mice; IL-17 production was equivalent in CD4+ and DN CD3+ T cells of B6.p18−/−.lpr and B6.Sle2c1.lpr mice; however, the renal pathology of B6.p18−/−.lpr mice was intermediate between that of B6.lpr and B6.Sle2c1.lpr mice. The production of additional Sle2c congenic recombinants crossed to B6.lpr demonstrated the presence of at least one additional closely linked locus that promotes renal and skin pathology.71 It should be noted that the level of autoantibodies found in either B6.p18−/−.lpr or B6.Sle2c1.lpr mice was equivalent to that of B6.lpr mice, indicating that the enhancement of autoimmune pathology was not antibody-mediated, suggesting a T cell polarization mechanism.
Conclusion
The results above demonstrate that, in addition to regulating the size of the B-1a cell compartment, Cdkn2c contributes to immunopathology. The mechanism by which Cdkn2c does this is open to interpretation; thus far we have found associations, but no direct causal effects. Our study combining either Sle2c- or p18-deficiency with Lpr-deficiency is the first to associate in vivo B-1a cell number increases with T cell polarization toward inflammation-associated phenotypes. One complicating factor in the interpretation of this study is that the lpr mutation is associated with a reduction of the B-1a cell compartment.72 We have verified this finding in both B6.p18−/−.lpr and B6.Sle2c1.lpr mice, which have a low number of B-1a cells, equivalent to B6.lpr (unpublished results). It is still possible that B-1a cells expressing low or no p18 in these mice are more pathogenic than those expressing normal levels of p18. On the other hand, the more inflammatory T cell phenotypes observed in these mice could be T cell intrinsic. One may also argue that the autoantibody production observed in p18-deficient mice is not B cell intrinsic.
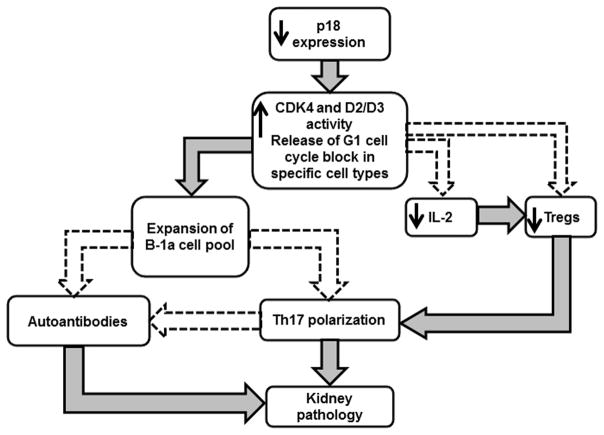
Together, our studies have identified a gene, Cdkn2c, that plays a major role in regulating the size of the B1-a cell compartment, and we have shown that deficiency in Cdkn2c leads to autoimmune pathology, especially in combination with the lpr mutation. What we have not established yet is whether the expansion of B-1a cells is a causal factor in the process. The proposed model shown in Figure 1 summarizes these findings.
Figure 1.
Proposed model for the p18 regulation of autoimmune pathogenesis in the NZM2410 model of lupus. Boxes show processes that we have observed in the experiments reviewed here. Filled arrows shown causal links established by our studies or that of others. Dashed arrows show potential causal links based on either in vitro studies and/or associations.
Acknowledgments
This work was supported by NIH R01-AI068965 grant to LM.
References
- 1.Sang A, et al. Animal models of molecular pathology: Systemic Lupus Erythematosus. In: Conn PM, editor. Progress in Molecular Biology and Translational Science. Vol. 105. Academic Press; 2012. pp. 321–370. [DOI] [PubMed] [Google Scholar]
- 2.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 3.Wakeland EK, et al. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 4.Harley IT, et al. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morel L. Genetics of SLE: evidence from mouse models. Nat Rev Rheumatol. 2010;6:348–357. doi: 10.1038/nrrheum.2010.63. [DOI] [PubMed] [Google Scholar]
- 6.Vaughn SE, et al. Genetic susceptibility to lupus: the biological basis of genetic risk found in B cell signaling pathways. J Leukoc Biol. 2012;92:577–591. doi: 10.1189/jlb.0212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morel L, et al. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 8.Morel L. Mapping lupus susceptibility genes in the NZM2410 mouse model. Adv Immunol. 2012;115:113–139. doi: 10.1016/B978-0-12-394299-9.00004-7. [DOI] [PubMed] [Google Scholar]
- 9.Morel L, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 11.Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97–107. doi: 10.1038/nrrheum.2013.157. [DOI] [PubMed] [Google Scholar]
- 12.Kamal A. The efficacy of novel B cell biologics as the future of SLE treatment: A review. Autoimm Rev. Aug 20; doi: 10.1016/j.autrev.2014.08.020. pii: S1568–9972(14)00164–5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.MacGregor JS. Lilly To Discontinue Development Of Tabalumab Based On Efficacy Results In Phase 3 Lupus Studies. In FierceBiotech 2014 [Google Scholar]
- 14.Casola S. Control of peripheral B-cell development. Curr Opin Immunol. 2007;19:143–149. doi: 10.1016/j.coi.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Ann Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 17.Enghard P, et al. Class switching and consecutive loss of dsDNA reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur J Immunol. 2010;40:1809–1818. doi: 10.1002/eji.200940050. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, et al. Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J Immunol. 2004;172:3628–3634. doi: 10.4049/jimmunol.172.6.3628. [DOI] [PubMed] [Google Scholar]
- 19.Moreth K, et al. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumang JR, et al. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- 21.O’Garra A, Howard M. IL-10 production by CD5 B cells. Ann N Y Acad Sci. 1992;651:182–199. doi: 10.1111/j.1749-6632.1992.tb24615.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishida H, et al. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–310. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Z, et al. IL-10 regulates murine lupus. J Immunol. 2002;169:2148–2155. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 24.Gunnarsson I, et al. Development of lupus-related side-effects in patients with early RA during sulphasalazine treatment-the role of IL-10 and HLA. Rheumatology (Oxford) 2000;39:886–893. doi: 10.1093/rheumatology/39.8.886. [DOI] [PubMed] [Google Scholar]
- 25.Tyrrell-Price J, Lydyard PM, Isenberg DA. The effect of interleukin-10 and of interleukin-12 on the in vitro production of anti-double-stranded DNA antibodies from patients with systemic lupus erythematosus. Clin Exp Immunol. 2001;124:118–125. doi: 10.1046/j.1365-2249.2001.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan C, et al. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 1998;41:1652–1662. doi: 10.1002/1529-0131(199809)41:9<1652::AID-ART17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, et al. Aberrant B1 cell migration into the thymus results in activation of CD4 T cells through its potent antigen-presenting activity in the development of murine lupus. Eur J Immunol. 2004;34:3346–3358. doi: 10.1002/eji.200425373. [DOI] [PubMed] [Google Scholar]
- 29.Zhong X, et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 30.Zhong X, et al. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60:3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, et al. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin Exp Med. 2011;11:75–80. doi: 10.1007/s10238-010-0115-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Rothstein TL. Induction of Th17 cell differentiation by B-1 cells. Front Immunol. 2012;3:281. doi: 10.3389/fimmu.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 34.Doreau A, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyttaris VC, et al. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z, et al. The NZM2410-derived lupus susceptibility locus Sle2c1 increases TH17 polarization and induces nephritis in Fas-deficient mice. Arthritis Rheum. 2011;63:764–774. doi: 10.1002/art.30146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakhmanov M, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wehr C, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113:161–171. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2566–2569. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan C, et al. Genetic dissection of systemic lupus erythematosus pathogenesis - Sle2 on murine chromosome 4 leads to B cell hyperactivity. J Immunol. 1997;159:454–465. [PubMed] [Google Scholar]
- 43.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 44.Ghosn EE, et al. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, et al. Genetic dissection of the murine lupus susceptibility locus Sle2 : contributions to increased peritoneal B-1a cells and lupus nephritis map to different loci. J Immunol. 2005;175:936–943. doi: 10.4049/jimmunol.175.2.936. [DOI] [PubMed] [Google Scholar]
- 46.Hayakawa K, et al. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z, et al. Cyclin-dependent kinase inhibitor Cdkn2c regulates B cell homeostasis and function in the NZM2410-derived murine lupus susceptibility locus Sle2c1. J Immunol. 2011;186:6673–6682. doi: 10.4049/jimmunol.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potula HH, Morel L. Genetic variation at an YY-1 response site regulates the transcription of cyclin-dependent kinase inhibitor p18 INK4C transcript in lupus-prone mice. J Immunol. 2012;188:4992–5002. doi: 10.4049/jimmunol.1101992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morse L, et al. Induction of cell cycle arrest and B cell terminal differentiation by CDK inhibitor p18 INK4c and IL-6. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 50.Schrantz N, et al. The expression of p18INK4 and p27kip1 cyclin-dependent kinase inhibitors is regulated differently during human B cell differentiation. J Immunol. 2000;165:4346–4352. doi: 10.4049/jimmunol.165.8.4346. [DOI] [PubMed] [Google Scholar]
- 51.Tourigny MR, et al. CDK inhibitor p18(INK4c) is required for the generation of functional plasma cells. Immunity. 2002;17:179–189. doi: 10.1016/s1074-7613(02)00364-3. [DOI] [PubMed] [Google Scholar]
- 52.Leone PE, et al. Deletions of CDKN2C in multiple myeloma: biological and clinical implications. Clin Cancer Res. 2008;14:6033–6041. doi: 10.1158/1078-0432.CCR-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang YY, et al. RNA interference reveals a requirement for both p18 INK4c and p27 Kip1 in B lymphopoiesis. J Mol Cell Biol. 2010;2:209–216. doi: 10.1093/jmcb/mjq013. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, et al. Homeostatic cell-cycle control by BLyS: Induction of cell-cycle entry but not G1/S transition in opposition to p18INK4c and p27Kip1. Proc Natl Acad Sci U S A. 2004;101:17789–17794. doi: 10.1073/pnas.0406111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piatelli MJ, et al. Cell cycle control mechanisms in B-1 and B-2 lymphoid subsets. Immunol Res. 2003;27:31–51. doi: 10.1385/IR:27:1:31. [DOI] [PubMed] [Google Scholar]
- 56.Solvason N, et al. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Interntl Immunol. 2000;12:631–638. doi: 10.1093/intimm/12.5.631. [DOI] [PubMed] [Google Scholar]
- 57.Tanguay DA, et al. Early induction of cyclin D2 expression in phorbol ester-responsive B-1 lymphocytes. J Exp Med. 1999;189:1685–1690. doi: 10.1084/jem.189.11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mataraza JM, et al. Disruption of cyclin D3 blocks proliferation of normal B-1a cells, but loss of cyclin D3 is compensated by cyclin D2 in cyclin D3-deficient mice. J Immunol. 2006;177:787–795. doi: 10.4049/jimmunol.177.2.787. [DOI] [PubMed] [Google Scholar]
- 59.Potula HH, et al. Cyclin-dependent kinase inhibitor Cdkn2c deficiency promotes B1a cell expansion and autoimmunity in a mouse model of lupus. J Immunol. 2012;189:2931–2940. doi: 10.4049/jimmunol.1200556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovalev GI, et al. An important role of CDK inhibitor p18(INK4c) in modulating antigen receptor-mediated T cell proliferation. J Immunol. 2001;167:3285–3292. doi: 10.4049/jimmunol.167.6.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L, et al. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson P, Gonzalez-Rey E. Vasoactive intestinal peptide induces cell cycle arrest and regulatory functions in human T cells at multiple levels. Mol Cell Biol. 2010;30:2537–2551. doi: 10.1128/MCB.01282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim K, et al. A regulatory SNP at position -899 in CDKN1A is associated with systemic lupus erythematosus and lupus nephritis. Genes Immun. 2009;10:482–486. doi: 10.1038/gene.2009.5. [DOI] [PubMed] [Google Scholar]
- 64.Kong EK, et al. p21 gene polymorphisms in systemic lupus erythematosus. Rheumatology (Oxford) 2007;46:220–226. doi: 10.1093/rheumatology/kel210. [DOI] [PubMed] [Google Scholar]
- 65.Yang W, et al. Meta-analysis followed by replication identifies loci in or near CDKN1B, TET3, CD80, DRAM1, and ARID5B as associated with systemic lupus erythematosus in Asians. Amer J Hum Genet. 2013;92:41–51. doi: 10.1016/j.ajhg.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang H, et al. Abnormal activation of the Akt-GSK3β signaling pathway in peripheral blood T cells from patients with systemic lupus erythematosus. Cell Cycle. 2009;8:2789–2793. doi: 10.4161/cc.8.17.9446. [DOI] [PubMed] [Google Scholar]
- 67.Arias CF, et al. p21CIP1/WAF1 controls proliferation of activated/memory T cells and affects homeostasis and memory T cell responses. J Immunol. 2007;178:2296–2306. doi: 10.4049/jimmunol.178.4.2296. [DOI] [PubMed] [Google Scholar]
- 68.Lawson BR, et al. Deficiency of the cyclin kinase inhibitor p21(WAF-1/CIP-1) promotes apoptosis of activated/memory T cells and inhibits spontaneous systemic autoimmunity. J Exp Med. 2004;199:547–557. doi: 10.1084/jem.20031685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katagiri K, et al. Deficiency of Rap1-binding protein RAPL causes lymphoproliferative disorders through mislocalization of p27kip1. Immunity. 2011;34:24–38. doi: 10.1016/j.immuni.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 70.Cohen PL, Eisenberg RA. The Lpr and Gld genes in systemic autoimmunity - Life and death in the Fas lane. Immunol Today. 1992;13:427–428. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- 71.Xu Z, Croker BP, Morel L. The combination of two Sle2 lupus-susceptibility loci and Cdkn2c deficiency leads to T-cell-mediated pathology in B6.Fas mice. Genes Immun. 2013;14:373–379. doi: 10.1038/gene.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reap EA, et al. Conventional B cells, not B-1 cells, are responsible for producing autoantibodies in lpr mice. J Exp Med. 1993;177:69–78. doi: 10.1084/jem.177.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]