Abstract
The purpose of this study was to identify, at the voxel level, brain regions associated with the time to develop mild cognitive impairment (MCI) or Alzheimer’s disease (AD) from normal cognition. We analyzed incident MCI (n = 58) or AD (n = 151) in 292 cognitively normal participants in the Cardiovascular Health Study–Cognition Study (mean age = 79.2±3.6 years). We used segmented, modulated grey matter maps from 3D (spoiled gradient echo) MRI scans obtained in 1998/99 (with clinical follow-up through 2012) that were smoothed with a 3-D 4 mm Gaussian filter. We fit approximately 1.92 million voxel-level Cox proportional hazard models to examine the grey matter volume effect on time to event, adjusting for age, sex, and diabetes. We used the significance threshold of p < 0.005 with contiguity threshold of at least 68 voxels (false detection probability <2.5 × 10−8). Areas within the mesial temporal lobe (MTL), anterior temporal lobe, hippocampus, and posterior cingulate gyrus were associated with time to MCI or AD. The presence of white matter lesions (a marker of small vessel disease in the brain) was associated with the volumes of the MTL and precuneus; MRI-identified infarcts also predicted MTL volume. These findings are important because we identified critical brain regions that predict a person’s increased likelihood of developing MCI or AD over a decade prior to the onset of clinical symptoms; these critical brain regions were themselves affected by the presence of vascular disease.
Keywords: Alzheimer’s disease, Cox survival model, incidence, mild cognitive impairment, MRI
INTRODUCTION
The analysis of structural brain images, especially for predicting changes in neurodegenerative disease, generally falls into two camps. The first, a region of interest approach has the advantage of focused hypothesis testing to relate a specific set of regional brain measures to the disorder of interest (e.g., [1]). The region of interest approach has the additional advantage that volumes from preselected areas can then be used in traditional epidemiological analyses to predict, for example, the risk or hazard of an event—such as the development of mild cognitive impairment (MCI) or Alzheimer’s disease (AD)—from a state of normal cognition [2, 3]. The process is difficult, especially in the early stages of a neurodegenerative disease, if investigators do not select the appropriate or most critical brain regions of interest for analysis. The second major approach, whole brain analysis (or voxel-level analysis), has the advantage that there are no a priori selected regions: the entire brain, or segmented tissue types (e.g., grey or white matter) can be exhaustively searched to find brain regions (assessed at the voxel level) that are linked to the disease. However, this process is made difficult because the identified clusters of significant voxels may not fall neatly into clearly defined anatomical regions. However, as our understanding of the pathology of MCI and AD continues to evolve, especially in terms of the sequence of events leading to clinical disorder [4], the whole brain, voxel-level approach may be the least affected by prior expectations.
The purpose of this report is to describe the results of nearly 2 million survival models that were run to identify those brain regions, in cognitively normal elders, that were linked to future development of MCI or AD. To accomplish this goal we combined whole-brain, voxel-level analysis with Cox proportional hazard modeling using procedures similar to those described by Vemuri and colleagues[5]. We did this in the context of the Cardiovascular Health Study-Cognition Study (CHS-CS) which provided research-level diagnoses and up to 14 years of clinical follow-up. By combining the statistical strengths of Cox proportional hazards modeling with higher resolution magnetic resonance imaging data (i.e., 1 mm3 voxels), we identified areas of the brain that significantly predict the increased likelihood of developing MCI or AD from normal cognition—in some cases, over a decade before the presence of any cognitive behavioral symptoms.
MATERIALS AND METHODS
The Institutional Review Board of the University of Pittsburgh approved this study. Written informed consent was obtained from all participants before they underwent research procedures.
Subjects
The study sample was drawn from participants in the CHS-CS, which is nested within the larger Cardiovascular Health Study (CHS, http://www.chs-nhlbi.org/). The CHS was initiated in 1989–1990 as a prospective, population-based, longitudinal study of risk factors for coronary heart disease and stroke in adults aged 65 years and older. Subjects were recruited if they were over age 65, ambulatory, and non-institutionalized. The initial sample size was 5,888 persons.
The CHS-CS began in 1998–1999 and was designed to identify subjects who had developed dementia or MCI since 1992/1994 [3, 6]. The sample was limited to the 3,608 participants who had a brain magnetic resonance imaging (MRI) scan between 1991 and 1994; of this group, 2,005 study participants were classified as cognitively normal. In 2002, the remaining non-demented subjects in Pittsburgh (i.e., normal cognition or MCI) were enrolled in the CHS-CS and were followed annually through 2012. For those individuals who could not return to the clinic, home visits were completed. For those individuals who refused home visits, telephone interviews were conducted using the Telephone Interview of Cognitive Status [7], and the informants were interviewed using standard procedures, including the Dementia Questionnaire [8] and Informant Questionnaire on Cognitive Decline in the Elderly [9]. 349 participants were cognitively normal at the onset of the study and each had an MRI scan in 1998/99 as well as apolipoprotein E genotyping. Data from 57 individuals were excluded due to technical difficulties with their imaging data; with exception of a 2-point difference on the Modified Mini-Mental State Exam [10], there were no significant differences between the included and excluded subjects (see Table 1).
Table 1.
Characteristics of participants by inclusion status
| Included | Excluded | Effect size1 | |
|---|---|---|---|
| n | 292 | 57 | |
| Age | 79.2 (3.6) | 80.2 (4.5) | 0.20 |
| Education2 | 63.7 (186) | 50.9 (29) | 0.59 |
| APOE ε43 | 24.8 (68) | 22.4 (11) | 1.14 |
| Sex4 | 60.6 (177) | 63.2 (36) | 0.89 |
| Race3 | 83.2 (243) | 77.2 (44) | 0.68 |
| Estimated IQ | 118.6 (8.7) | 117.1 (8.4) | 0.13 |
| 3MSE5 | 96.2 (4.0) | 94.2 (6.9) | 0.33* |
| DSST6 | 47.0 (12.3) | 45.5 (10.7) | 0.09 |
| CES-D7 | 4.55 (4.0) | 4.80 (4.3) | 0.05 |
| White matter grade3 | 34.9 (102) | 31.6 (18) | 1.16 |
| Hypertension3 | 47.3 (138) | 50.9 (29) | 0.865 |
| Diabetes3 | 13.7 (40) | 8.9 (5) | 1.62 |
| Large Infarct3 | 26.0 (76) | 15.8 (9) | 1.88 |
Cohen’s d for continuous data; relative risk for categorical data.
Percent (n) Greater than High School.
Percent (n) Yes/Present.
Percent (n) Female.
Modified Mini Mental Status Examination [10].
Digit symbol substitution test [39].
Center for Epidemiological Studies–Depression scale [76].
Cognitive classification
The diagnoses of dementia and MCI were made by an Adjudication Committee that used all available cognitive and laboratory data from each participant [11, 12]. The first step in the diagnostic process was to determine the presence of dementia [13–16]) and the specific types of dementia (if present) (e.g., [13, 17–20]). MCI was classified following the CHS-CS diagnostic criteria [11]. Cognitive functions in MCI represented a decline from a previous level, but overall were within normal limits. Individuals with mild alterations on instrumental activities of daily living could be classified with MCI, and all had impairments (defined as performance >1.5 S.D. below age/education appropriate means) in one or more cognitive domains (i.e., two or more tests abnormal), or one abnormal test (which could be a memory test) in at least two separate domains, without sufficient severity or loss of IADLs to constitute dementia. The year of onset of dementia was set after review of all prior records, including the reports of informants (e.g., Informant Questionnaire on Cognitive Decline in the Elderly [21] and Dementia Questionnaire [22]). Once a patient was diagnosed with dementia, follow-up was limited to telephone contact and medical record review. Aclassification of MCI had no effect on the number or intensity of follow-up visits.
Brain imaging
Brain MRI scans were acquired using a single 1.5 Tesla GE scanner, as detailed elsewhere [23]. A 3-dimensional volumetric T1 weighted Spoiled Gradient Recall (SPGR) sequence was obtained (TE/TR =5/25, flip angle= 40°, NEX =1, slice thickness = 1.5 mm/0 mm interslice gap), with an in-plane acquisition matrix of 256 × 256 image elements and 124 slices, 250 × 250 mm field of view and an in-plane voxel size of 0.98 mm × 0.98 mm.
Voxel-based morphometry
The MRI data were processed with a non-parametric non-uniform intensity normalization [24] to reduce between-scan intensity differences in the images. This was followed by a bias correction to improve spatial registration.
We used the Pittsburgh Normal Elderly Template [25] for spatial normalization; it also provided the prior probabilities of each tissue class (i.e., grey, white, cerebrospinal fluid) for use in the segmentation routines. We then used the VBM5 script (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/) for normalization and segmentation of the data; as part of the normalization routine, the voxels were resized to 1 mm3. The resulting maps of grey matter (GM) were modulated to render the values in each voxel as a volume; the data were smoothed using a 4 mm isotropic Gaussian filter prior to analysis. We then applied a binary GM mask to reduce the number of voxels in the search space to 1,915,936.
Prior to the voxel-level analysis, we examined the time to develop MCI/AD using total brain GM volume (adjusted for total intracranial volume), age, sex, education, race, high blood pressure, heart disease, diabetes, and white matter hyperintensities as covariates. A backward stepwise regression analysis found that male sex (Hazard Ratio =1.10, 95% CI =1.05–1.15), older age (1.54, 1.03–2.3) and the presence of diabetes (1.59, 1.02–2.47) significantly predicted time to event. Therefore, we used these three variables in all of the voxel-level analyses described below.
We obtained from the main CHS-CS database measures of white matter hyperintensities and MRI-identified infarcts. These are standardized CHS visual ratings [23] that have been used as both an independent (e.g., [26, 27] and outcome (dependent) ([26, 27]). White matter lesions and infarcts were defined using CHS criteria [28, 29]. White matter lesions were the total volume of periventricular and subcortical white matter signal abnormality on spin density–weighted axial images compared with 8 standard scans, with the severity of the lesions increasing from barely detectable white matter changes (grade 1) to extensive, confluent changes (grade 8). Scans with no white matter changes received grade 0, and those with changes worse than grade 8 received grade 9. We dichotomized the white matter lesion severity using a cut-point of +3. Presumed infarcts in any region were classified as “small infarct-like lesions” if <3 mm in size and as “MRI infarcts” when ≥3 mm in size. Cerebral white matter lesions are common in older adults, and they are associated with cerebral and systemic small vessel disease (i.e., arteriosclerosis) [29–34] and hypertension [35–37]. MRI-identified infarcts have a more heterogeneous etiology, reflecting, in part, arterio- and athero-sclerosis, as well as being a consequence of atrial fibrillation (for example).
Voxel-level data analysis
We fit per-voxel Cox models to two longitudinal data sets: one (234 subjects: 83 cognitively normal, 151 developed dementia) examining the per voxel GM volume predicting onset of dementia, and the other (141 subjects: 83 cognitively normal and 58 developed MCI) examining the per voxel GM predicting the onset of MCI while adjusting for age, sex, and diabetes in both sets.
Hazard functions, h(t), are the probability of an event at time t, conditional on the survival up to that time. In our study, it is the probability of developing MCI or dementia at time t given that a subject was cognitively normal up to that time. The main assumptions of the Hazard model are (1) a participant’s being censored (dropping out of the study or dying) is not related to the underlying medical condition modeled (AD or MCI), and (2) the regression coefficients do not depend on time, which is usually referred to as the proportional hazard assumption. The first assumption is satisfied for our study by its design, and we checked that the second assumption was satisfied by performing the chi-square test for the proportional hazard assumption [38] for each fitted model. This was done using the cox.zph function of the survival package in R (http://www.R-project.org), which performs a test of the proportional hazard assumption for each of the covariates in the model as well as a global test.
One of the most commonly used models for the hazard function is:
hi(t) = exp(β1 xi1 + ⋯ + βk xik)
We fit the per voxel Cox model as:
log(hijxl(t)) =β1j GMVij + β2 agei + β3 sexk + β4 diabetesl
where: i= 1,… , Ns is the subject number; for the transition from being cognitively normal to developing dementia, we had a total of Ns = 234 subjects, and for the transition from being cognitively normal to developing MCI, we had a total of Ns = 141 subjects; j=1 … Nt, with Nt = 157 x 189 x 156 = 4,628,988 (total number of GM voxels in search space). We applied a conservative binary mask where a voxel was classified as grey matter if the GM volume (averaged across subjects) was greater than 3, which reduced the number of voxels to 1,915,936.
After fitting the models, we corrected for multiple comparisons for each voxel that had a significant β1j at α = 0.005 by (1) counting how many of its immediate 124 neighbors also had a significant coefficient and (2) classifying a voxel as statistically significant if it had at least 68/124 immediate neighbors that were also significant. This corresponds to a false detection probability of less than 2.5 × 10−8 for each voxel. All of the computations were carried out in R version 2.9.1 (http://www.R-project.org) and the survival package version 2.37–4.
RESULTS
We examined the characteristics of the study subjects in 1998/1999 as a function of their final study classification (see Table 2). All of the study subjects were cognitively normal in 1998/1999. The individuals who remained cognitively normal were less likely to be women, and the participants who developed dementia had lower scores on the Digit Symbol Substitution Task [39] (Least Significant Difference (LSD) test, p < 0.05). Otherwise, there were no significant differences between the groups of subjects at the beginning of the observation period as a function of clinical outcome by the end of the observation period.
Table 2.
Characteristics of study subjects in 1998/99 as a function of outcome
| Normal | MCI | AD | Effect size1 | |
|---|---|---|---|---|
| n | 83 | 55 | 151 | |
| Age | 78.7 (3.9) | 78.7 (3.4) | 79.7 (3.5) | 0.02 |
| Education2 | 68.7 (57) | 67.2 (39) | 59.6 (90) | 0.09 |
| Sex2 | 44.6 (37) | 63.8 (37) | 68.2 (103) | 0.21* |
| Race2 | 85.5 (71) | 79.3 (46) | 83.4 (126) | 0.06 |
| APOE ε42 | 23.1 (18) | 21.8 (12) | 27.0 (38) | 0.05 |
| Estimated IQ | 119.9 (8.3) | 120.3 (7.9) | 117.3 (9.0) | 0.03 |
| 3MSE3 | 96.6 (3.8) | 97.0 (3.6) | 95.8 (4.2) | 0.02 |
| DSST4 | 48.9 (11.5) | 50.6 (13.3) | 44.6 (12.0) | 0.04* |
| CES-D5 | 4.09 (3.6) | 4.01 (3.8) | 5.00 (4.2) | 0.01 |
| Hypertension2 | 45.8 (38) | 44.8 (26) | 49.0 (74) | 0.04 |
| Diabetes2 | 9.6 (8) | 12.1 (7) | 16.6 (25) | 0.09 |
| White matter hyperintensities2 | 32.5 (27) | 32.8 (19) | 37.1 (56) | 0.05 |
| MRI-identified infarcts2 | 25.3 (21) | 22.4 (13) | 27.8 (42) | 0.05 |
| Whole brain grey matter6 | 37.2 (1.1) | 37.4 (1.3) | 37.3 (1.2) | 0.01 |
Cohen’s f for continuous data, Cramer’s V for categorical data.
Percent (n) Present, Yes, Female, Caucasian, Greater than High School.
Modified Mini Mental Status Examination [10].
Digit symbol substitution test [39].
Center for Epidemiological Studies–Depression scale [76].
As a percentage of total intracranial volume.
p<0.05.
Transition to AD dementia
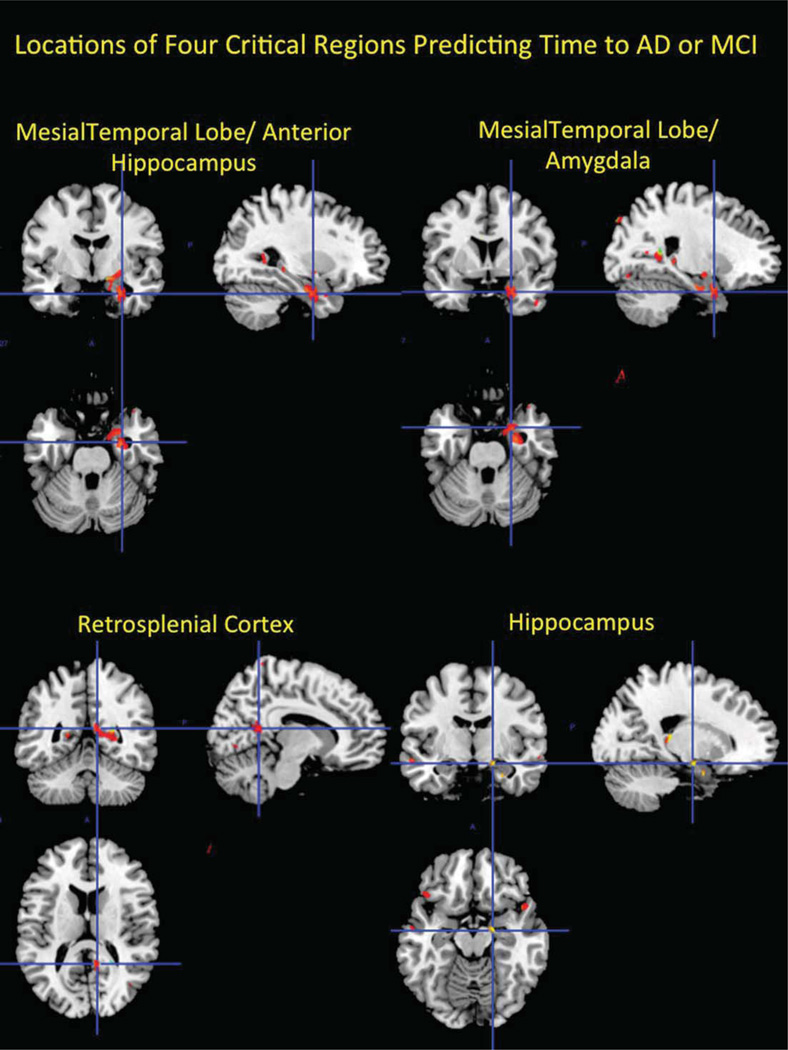
112,915 voxels (6.8% of analyzed voxels) were significant before adjusting for multiple comparisons; 6,683 were significant after adjusting for multiple comparisons using the 68 immediate neighbors threshold. Voxels located within three brain regions showed a significant association with time to dementia: the mesial temporal lobe including the anterior hippocampus extending into the amygdala, and the posterior cingulate gyrus (retrosplenial neocortex, see Fig. 1). In each case, voxels in these three regions were significantly associated with time to dementia after controlling for age, sex, and diabetes (locations of peak voxels are presented in Table 3), with smaller GM volume corresponding to an increased likelihood of developing the condition.
Fig. 1.
The three brain regions whose volumes were significantly associated with time to develop dementia from normal cognition (the mesial temporal lobe including the anterior hippocampus extending into the amygdala, and the retrosplenial neocortex), and the region of the hippocampus associated with time to mild cognitive impairment. The significant voxels are overlaid onto the Colin template [77] for ease of visualization.
Table 3.
Locations of regions associated with time to develop AD or MCI
| Region | Number of significant voxels |
Coordinates of Peak Hazard Ratio1 |
Hazard Ratio and 95% CI2 |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Mesial temporal lobe/hippocampus | 185 | −9 | 28 | −25 | 0.994 (0.990–0.998) |
| Anterior temporal lobe | 981 | 4 | 23 | −27 | 0.994 (0.991–0.998) |
| Precuneus | 204 | −49 | 11 | 11 | 0.989 (0.984–0.996) |
| Hippocampus | 542 | −7 | 18 | −15 | 0.980 (0.968–0.992) |
Coordinates are given relative to the MNI template brain.
Hazard Ratios at the peak voxel of the association between grey matter tissue volume and time to MCI or AD dementia. Values less than 1.00 mean that smaller volumes are associated with shorter time to event.
Transition to MCI
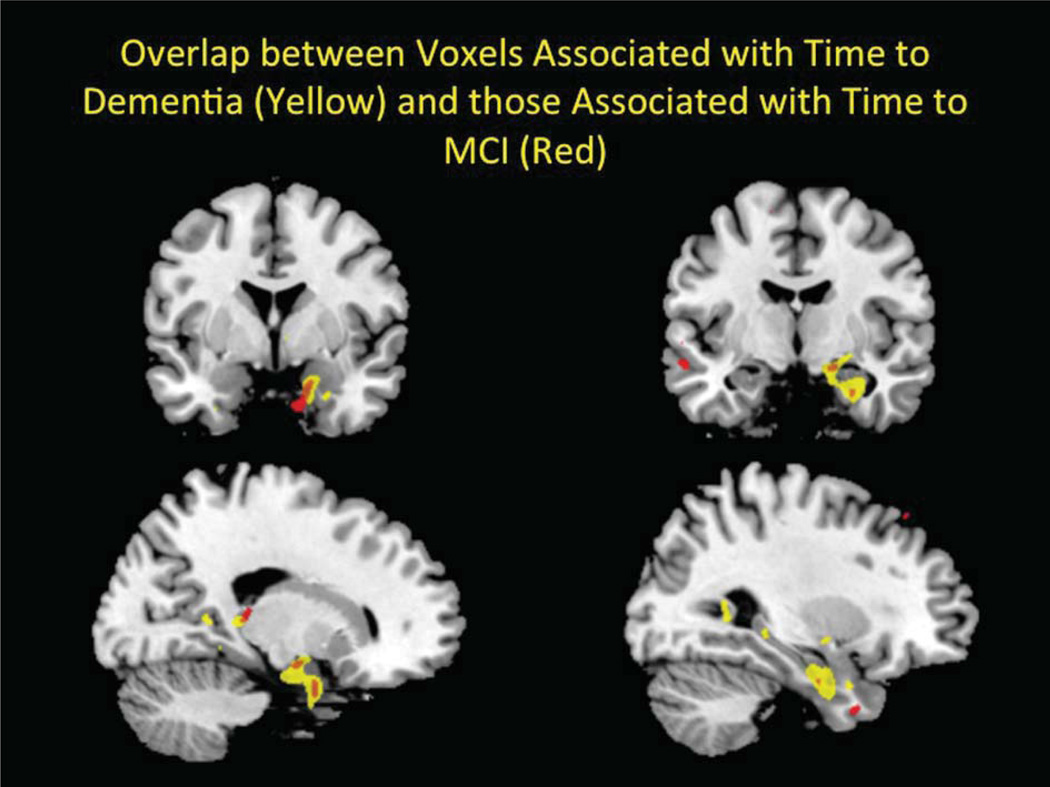
Before adjusting for multiple comparisons 104,908 voxels were significant (5.5% of analyzed voxels); 3,253 voxels were significant after the adjustment for multiple comparisons, with smaller GM volume corresponding to an increased likelihood of developing MCI. There was an area in the left medial temporal lobe whose volume was associated with time to MCI (see Fig. 1). In two areas, voxels associated with time to MCI overlapped with those associated with time to dementia (see Fig. 2). These two regions were in the anterior hippocampus/amygdala and were fully contained within the regions associated with time to dementia.
Fig. 2.
The two brain regions in the anterior hippocampus/amygdala related to time to MCI (in red) are shown overlapping with the regions associated with time to dementia (in yellow).
Predictors of regional volumes
We then computed the volume in each of these four critical brain regions for each subject. At study entry, there were significant differences between the volumes in the three AD-related regions as a function of study outcome (i.e., normal, AD, MCI; see Table 4). In these three regions, the volumes among the individuals who later developed dementia were significantly smaller than those of the individuals who remained cognitively normal (LSD test, p < 0.05). Finally, there were no significant differences in the volume of the region of the retrosplenial cortex that was related to time to develop MCI. However, there was a trend such that the volume of the normal controls was greater than that of the individuals who later developed dementia, which was greater than that of the individuals who later developed MCI.
Table 4.
Volumes of critical regions associated with time to AD or MCI (adjusting for total intracranial volume)
| Normal | Developed AD | Developed MCI | Cohen’s f | |
|---|---|---|---|---|
| MTL/anterior hippocampus | 113.4 (2.5) | 105.9 (2.3) | 112.4 (3.0) | 0.20* |
| Anterior temporal lobe | 144.6 (2.3) | 134.7 (1.7) | 136.2 (2.7) | 0.21* |
| Precuneus | 59.3 (2.0) | 52.7 (1.5) | 56.4 (2.4) | 0.20* |
| Hippocampus | 123.6 (2.7) | 119.7 (2.0) | 115.5 (3.3) | 0.11 |
MTL, Mesial temporal lobe.
p< 0.05.
We then regressed the total volumes in each of these four areas on total intracranial volume, age, female sex, non-Caucasian race, APOE ε4 allele (present), hypertension (present), heart disease (present), abnormal white matter, and the presence of MRI-identified infarcts. Age, Race and Sex were forced into the models in the first step, then the other predictors were tested as a group. Age was significantly associated with the volume of the two regions in the mesial temporal lobe, and was marginally associated with the volume of the anterior temporal lobe and the posterior cingulate gyrus. However, of the critical predictors, abnormal white matter (an index of small vessel disease [40–42]) was significantly associated with the volume in the mesial temporal lobe, and in the retrosplenial cortex. Large infarcts were only significantly associated with the area in the mesial temporal lobe (see Table 5).
Table 5.
Standardized regression coefficients of models of predictors of grey matter volume
| MTL/anterior hippocampus | Anterior temporal lobe | Precuneus | Hippocampus | |
|---|---|---|---|---|
| Age | −0.20* | −0.13* | −0.12 | −0.11 |
| Sex (female) | −0.02 | −0.25* | 0.08 | −0.02 |
| Race (non-Caucasian) | −0.04 | −0.03 | −0.14* | 0.04 |
| Hypertension | −0.18* | |||
| White matter lesions | −0.26* | −0.16* | ||
| Large infarcts | −0.20* | |||
| Model R2 | 0.22* | 0.05* | 0.09* | 0.05* |
p< 0.05.
MTL, Mesial temporal lobe.
Validation of model assumptions
We performed the chi-square test for the proportional hazard assumption [38] for each of the roughly 2 million fitted models for both transitions. We found no more violations of the proportional hazard assumption for any of the covariates or globally than would be expected by chance (5% of the time at the 0.05 significance level).
DISCUSSION
We report here the results of nearly 1.92 million survival analyses to identify—at the voxel-level—brain regions associated with time to MCI or AD [5] from a state of normal cognition. Critically, we demonstrated the presence of an association between GM volume and cognitive outcomes in cognitively normal individuals up to 13 years before the appearance of symptoms (as we were able to predict the increased likelihood of developing MCI/AD based on these GM volumes long before symptoms started), and the volumes of two of the regions were affected by cerebrovascular disease. These data are unique because for the first time we were able to: 1) study incident MCI and AD from a state of normal cognition; 2) use 14 years of follow-up from the time of the MRI scan with detailed clinical ascertainment; and 3) maintain the high precision of our voxel-level analyses by not down-sampling the data and using a minimal smoothing filter.
We found voxels in four specific regions in the mesial temporal lobe and posterior cingulate gyrus that predict time to MCI and AD; two of these regions were affected by cerebrovascular disease, as indicated by white matter lesions or MRI-identified infarcts. After adjusting for these critical covariates (i.e., white matter lesions, large infarcts), hypertension was associated with the volume of the hippocampus that predicted increased likelihood of developing MCI.
As this was only the second implementation of these procedures in the context of aging and dementia, we focused our analyses on the first clinical outcome in the cognitively normal individuals from 1998/99. Consequently, we did not consider a dementia outcome when it followed MCI, and thus the median time to develop dementia (5 years) was shorter than the median time to develop MCI (13 years). These findings confirm our earlier observations [43] that while the volume of the ventral striatum was already significantly atrophic among cognitively normal individuals who developed dementia (as atrophic as that of AD patients), it was the volume of the hippocampal formation that predicted fast versus slow conversion (within a 4–5 year range).
Like Vemuri and colleagues [5], we used proportional hazard models to determine whether voxel-level GM volumes predict the onset of AD and MCI. However, our implementation of the survival model differs in several important ways. First, we examined the transitions from normal cognition to both MCI and AD, rather than focusing on the transition from MCI to AD. This is important because MCI can exist for many years, and absence of information about duration of the syndrome can affect the outcome of survival models [2]. In addition, we did not downsample the pre-smoothed data, and used 1 mm3 voxels resulting in almost 2 million models. We used minimal Gaussian smoothing (4 mm isotropic) to make the data more amenable to parametric models and to account for errors in spatial normalization. We modulated the GM voxels prior to analysis rendering the data closer to volumes than to density values (i.e., unmodulated). This has the further advantage that the voxel-level values were not limited to the range of 0.00–1.00, so we did not encounter any modeling issues that might have necessitated a transformation of the data. As we tested a large number of models (1.92 million), we wanted to reduce Type I error. We therefore adopted a “height” threshold for each voxel of p < 0.005 with an “extent” threshold of 68 voxels, resulting in a false detection probability of 2.5 × 10−8 (see discussion by Johnson and colleagues [44]). Using this stringent threshold, and the very conservative false positive probability corresponding to it, allows us to detect only the most important voxels.
There are alternative methods that could be used to analyze these data, including the general linear model with time to onset as the covariate of interest. In our study, the predicted variable (time to onset) is effectively categorical. Furthermore, the hazard model is generally preferred because it models the probability of developing MCI or AD at time t given the subject was normal prior to time t. The probability varies continuously between 0 and 1, and in this case the hazard model is more powerful than a GLM.
Many studies have sought to define the early biological manifestations of AD, particularly those that precede clinical dementia [45–49]; the normal progression of the disorder includes a prodromal period in which the disease is present without clinical signs and this may extend decades [45, 50]. The prodromal period typically progresses insidiously to an MCI phase, the earliest symptomatic indicator of an evolving AD [51, 52], and then to the clinical dementia syndrome. In this context, our results are important because we identified alterations in brain structure in cognitively normal individuals, as many as 13 years before the onset of a clinical syndrome (in the case of MCI).
Much of the research on the prodromal phase of AD has focused on alterations in the hippocampus and related structures [53–56]. Hippocampal atrophy in presymptomatic individuals predicts AD with a specificity of 91% and a sensitivity of 89% [57]. There are both structural and functional abnormalities in the mesial temporal lobes and other heteromodal association cortices in MCI subjects who progress to AD [43, 54, 58–68], and in normal subjects at risk for AD [69]. Ventral striatum and hippocampal volumes are associated with incident AD over a 4.5-year follow-up period [43], and hippocampal volume and MRI-infarcts are independent predictors of incident dementia [67]. Ventricular expansion is faster in subjects who develop MCI [70], and these rates can be increased by diabetes mellitus and hypertension [71]. Our data are important in this context because we found that hypertension and the presence of markers of cerebrovascular disease were significant predictors of the volumes of the mesial temporal lobe and the precuneus. Thus, while there may be very early AD pathology in these individuals, it is becoming more certain that vascular factors affect brain structure and, ultimately time to MCI or AD.
We found that sex was associated with time to event at the whole brain level (and was thus included ion the voxel-level analyses), and was significantly associated with the volumes of the four regions identified by the Cox models. While it is tempting to ascribe biological significance to these associations, we take the more conservative view that in this case “sex” is, at least, an indicator variable for other factors. That is, there are sex and sex-by-age differences in the prevalence of cardio- and cerebro-vascular disease (and their clinical correlates) and these likely play a significant role in the clinical expression of MCI and AD dementia. In future studies we will work to model additional factors in the voxel-level analyses in order to disentangle the direct and indirect effects of sex on brain volumes and time to event.
Perhaps the most influential current model of the natural progression of AD includes a long asymptomatic phase, marked by the accumulation of amyloid-β, followed by brain structural changes, and ultimately MCI and dementia [72, 73]. However, this sequence of events is not as clear as once thought; for example, clinical dementia can develop relatively rapidly among individuals with low levels of amyloid [74]. Further, hippocampal atrophy is not necessarily a precondition for incident dementia [4]. Our data add to this discussion by showing that among cognitively normal individuals with an average age of 78 years, it is possible to detect associations between brain structural integrity and the development of MCI/AD as much as 13–14 years before the onset of symptoms. Obviously, the ideal data set would have included an in vivo measure of amyloid deposition (among other tools), but these techniques were not available in 1998/99. Hopefully, the longitudinal studies currently underway will be able to address this timing issue over the next 10–15 years.
This study advances current evidence of the important role of the structural integrity of the medial temporal lobe in the development of clinical dementia from a state of normal cognition. We tested nearly 2 million voxel-level survival models, giving us the power to examine time-to-event without a priori assumptions about which regions of interest are critical. Moving forward, the combination of these survival models (at the voxel level) using multiple imaging modalities simultaneously (e.g., in vivo amyloid, in vivo tau, magnetoencephalography [75]) may provide the best methods to test the relative merits of models of the temporal sequence of events associated with the transition from a state of brain health through clinical dementia (e.g., Jack and colleagues [72]).
ACKNOWLEDGMENTS
The research was supported in part by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through AG-023629, AG-15928, AG-20098, AG-027002, AG05133, and AG-027058 from the National Institute on Aging. L.E.Z. was supported by a National Science Foundation Graduate Research Fellowship. The funding sources had no role in the design, analysis, or reporting of the results of this study.
Footnotes
These results were reported, in part, at the 2014 Annual Meeting of the American Academy of Neurology (Philadelphia, PA, USA).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0047r1).
REFERENCES
- 1.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez OL, Becker JT, Chang YF, Sweet RA, Dekosky ST, Gach MH, Carmichael OT, McDade E, Kuller LH. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology. 2012;79:1599–1606. doi: 10.1212/WNL.0b013e31826e25f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 4.Lopez OL, Klunk WE, Mathis C, Coleman RL, Price J, Becker JT, Aizenstein HJ, Snitz B, Cohen A, Ikonomovic M, McDade E, DeKosky ST, Weissfeld L, Kuller LH. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83:1804–1811. doi: 10.1212/WNL.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vemuri P, Weigand SD, Knopman DS, Kantarci K, Boeve BF, Petersen RC, Jack CR., Jr Time-to-event voxel-based techniques to assess regional atrophy associated with MCI risk of progression to AD. Neuroimage. 2011;54:985–991. doi: 10.1016/j.neuroimage.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kuller LH, Ives D, Lopez OL, Jagust W, Breitner J, Jones B, Lyketsos C, Dulberg C. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 7.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiat Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 8.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the dementia questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 9.Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 10.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) xamination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 11.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson MC, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognitive Study Part 1. Arch Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 12.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman DA, Folstein MF, Katzman R, Price DL, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. DSM-IV: Diagnostic and Statistic Manual of Mental Disorders, Fourth Edition. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 16.WHO. The ICD-10 classification of mental and behavioral disorders: diagnostic criteria for research. Geneva: WHO; 1993. [Google Scholar]
- 17.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 18.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A. Vascular dementia: Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Loew J, Emre D, B’Brien JT, Feldman H, Cummings J, Dusa JE, Lippa C, Perry EK. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 20.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick A, Kuller L, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer’s disease and vascular dementia. J Neurol Sci. 2005;229–230:43–49. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Kawas CH, Corrada MM. Alzheimer’s and dementia in the oldest-old: A century of challenges. Curr Alzheimer Res. 2006;3:411–419. doi: 10.2174/156720506779025233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryan RN, Manolio TA, Scertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 24.Boyes RG, Gunter JL, Frost C, Janke AL, Yeatman T, Hill DL, Bernstein MA, Thompson PM, Weiner MW, Schuff N, Alexander GE, Killiany RJ, DeCarli C, Jack CR, Fox NC. Intensity non-uniformity correction using N3 on 3-T scanners with multichannel phased array coils. Neuroimage. 2008;39:1752–1762. doi: 10.1016/j.neuroimage.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spears JR, Greer PJ, Ziolko SK, Aizenstein HJ, Carmichael O, Becker JT, Meltzer CC. Evaluation of an age-specific neurological template. Presented at the Annual Meeting of the Organization of Human Brain Mapping; June 2005; Toronto Ontario, Canada. [Google Scholar]
- 26.Kuller LH, Lopez OL, Jagust W, Becker JT, Fitzpatrick A, Carlson M, Breitner J, Lyketsos C, Kawas C, DeKosky ST. Clinical and MRI factors for vascular dementia in the Cardiovascular Health Study Cognition study. Neurobiol Aging. 2004;25:S483. doi: 10.1212/01.WNL.0000159860.19413.C4. [DOI] [PubMed] [Google Scholar]
- 27.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R. Relationship between ApoE, MRI findings, and cognitive function in the cardiovascular health study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 28.Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Garden JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 29.Manolio TA, Burke GL, O’Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults : The Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 30.Longstreth WT, Arnold AM, Manolio T, Burke G, Bryan N, Jungreis CA, O’Laery D. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3301 elderly people. The cardiovascular health study. Neuroepidemiology. 2000;19:30–42. doi: 10.1159/000026235. [DOI] [PubMed] [Google Scholar]
- 31.Longstreth WT, Arnold AM, Beauchamp NJ, Manolino TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 32.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler M. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The Rotterdam scan study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 33.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 34.Mogi M, Horiuchi M. Clinical Interaction between Brain and Kidney in Small Vessel Disease. Cardiol Res Prac. 2011;2011:306189. doi: 10.4061/2011/306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 36.de Leeuw F-E, dr Groot JC, Oudkerk M, Witterman JCM, Hofman A, van Gjin J, Breteler MMB. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 37.Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities. The EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 38.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 39.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 40.Grafton ST, Sumi SM, Stimac GK, Alvord EC, Shaw C, Nochlin D. Comparison of postmortem magnetic resonance imaging and neuropsthologic findings in the cerebral white matter. Arch Neurol. 1991;48:293–298. doi: 10.1001/archneur.1991.00530150061019. [DOI] [PubMed] [Google Scholar]
- 41.Kobari M, Meyer JS, Ichijo M. Leuko-Araiosis, cerebral atrophy, and cerberal perfusion in normal aging. Arch Neurol. 1990;47:161–165. doi: 10.1001/archneur.1990.00530020061017. [DOI] [PubMed] [Google Scholar]
- 42.Scheltens P, Barkhof F, Leys D, Wolters E, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease. Neurology. 1995;45:883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- 43.Hall AM, Moore RY, Lopez OL, Kuller LH, Becker JT. Basal forebrain atrophy is a presymptomatic marker for Alzheimer’s disease. Alzheimers Dement. 2008;4:271–279. doi: 10.1016/j.jalz.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson VE. Revised standards for statistical evidence. Proc Natl Acad Sci U S A. 2013;110:19313–19317. doi: 10.1073/pnas.1313476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elias MF, Beiser A, Wolf PA, Au R, White RF, RB DA. The preclinical phase of Alzheimer disease. A 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 46.Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 47.Schott JM, Fox NC, Frost C, Scahill RI, Janssen JC, Chan D, Jenkins R, Rossor MN. Assessing the onset of structural changes in familial Alzheimer’s disease. Ann Neurol. 2003;53:181–188. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 48.Galvin JE, Pawlishta KK, Wilkins K, McKeel DW, Xiong G, Grant E, Storandt M, Morris JC. Predictors of pre-clinical Alzheimer disease and dementia. A clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 49.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 50.Desai AK, Grossberg GT. Diagnosis and treatment of Alzheimer’s disease. Neurology. 2005;64:S34–S39. doi: 10.1212/wnl.64.12_suppl_3.s34. [DOI] [PubMed] [Google Scholar]
- 51.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. [DOI] [PubMed] [Google Scholar]
- 52.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 53.Jack CR, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chetelat G, Baron JC. Early diagnosis of Alzheimer’s disease: Contribution of structural neuroimaging. Neuroimage. 2003;18:525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 57.Rusinek H, Endo Y, De Santi S, Frid D, Tsui WH, Segal S, Convit A, de Leon MJ. Atrophy rate in medial temporal lobe during progression of Alzheimer disease. Neurology. 2004;63:2354–2359. doi: 10.1212/01.wnl.0000148602.30175.ac. [DOI] [PubMed] [Google Scholar]
- 58.Berent S, Giordani B, Foster N, Minoshima S, Lajiness-O’Neil R, Koeppe R, Kuhl DE. Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimer’s disease. J Psychiatr Res. 1999;33:7–16. doi: 10.1016/s0022-3956(98)90048-6. [DOI] [PubMed] [Google Scholar]
- 59.De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J. Hippocampal formation, glucose metabolism, and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 60.Jagust WJ, Eberling JL, Wu CC, Finkbeiner A, Mungas D, Haan MN. Brain function and cognition in a community sample of elderly Latinos. Neurology. 2002;59:378–383. doi: 10.1212/wnl.59.3.378. [DOI] [PubMed] [Google Scholar]
- 61.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron J-C. Mild cognitive impairment. Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 62.de Leon MJ, Golomb J, George AE, Convit A, Tarshish CY, McRae T, DeSanti S, Smith G, Ferris SH, Noz M, Rusinek H. The radiologic prediction of Alzheimer disease: The atrophic hippocampal formation. AJNR. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 63.Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikanen M, Koivisto K, Reikkinen PJ. Volumetric MRI analysis of the amygdala and the hippocampua in subjects with age-associated memory impairment: Correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 64.Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Framer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Visser PJ, Scheltens P, Verhey FRJ, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 66.Becker JT, Davis SA, Hayaski KM, Meltzer CC, Thompson PM, Lopez OL, Toga AW. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- 67.Rosano R, Aizenstein HJ, Wu M, Newman A, Becker JT, Lopez OL, Kuller LH. Focal atrophy and cerebrovascular disease increase dementia risk among cognitively normal older adults. J Neuroimaging. 2007;17:148–155. doi: 10.1111/j.1552-6569.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 68.den Heijer T, Geerlings MJ, Hoebeek FE, Hofman A, Koudstaal PJ, Bereteler MB. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- 69.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 70.Carlson NE, Moore MM, Dame A, Howieson D, Silbert LC, Quinn JF, Kaye JA. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–833. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- 71.Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee SE, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT. Acceleration of cerebral ventricular expansion in the Cardiovascular Health Study. Neurbiol Aging. 2007;28:1316–1321. doi: 10.1016/j.neurobiolaging.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Boeve BF, Petersen RC. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zamrini E, Maestu F, Pekkonen E, Funke M, Makela J, Riley M, Bajo R, Sudre G, Fernandez A, Castellanos N, Del Pozo F, Stam CJ, van Dijk BW, Bagic A, Becker JT. Magnetoencephalography as a putative biomarker for Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:280289. doi: 10.4061/2011/280289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 77.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]