Abstract
1,3-Butadiene is an important industrial and environmental carcinogen present in cigarette smoke, automobile exhaust, and urban air. The major urinary metabolites of BD in humans are 2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene/1-(N-acetyl-L-cystein-S-yl)-2-hydroxybut-3-ene (MHBMA), 4-(N-acetyl-L-cystein-S-yl)-1,2-dihydroxybutane (DHBMA), and 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutyl mercapturic acid (THBMA), which are formed from the electrophilic metabolites of BD, 3,4-epoxy-1-butene (EB), hydroxymethyl vinyl ketone (HMVK), and 3,4-epoxy-1,2-diol (EBD), respectively. In the present work, a sensitive high-throughput HPLC-ESI−MS/MS method was developed for simultaneous quantification of MHBMA and DHBMA in small volumes of human urine (200 µl). The method employs a 96 well Oasis HLB SPE enrichment step, followed by isotope dilution HPLC-ESI−-MS/MS analysis on a triple quadrupole mass spectrometer. The validated method was used to quantify MHBMA and DHBMA in urine of workers from a BD monomer and styrene-butadiene rubber production facility (40 controls and 32 occupationally exposed to BD). Urinary THBMA concentrations were also determined in the same samples. The concentrations of all three BD-mercapturic acids and the metabolic ratio (MHBMA/ (MHBMA+DHBMA+THBMA)) were significantly higher in the occupationally exposed group as compared to controls and correlated with BD exposure, with each other, and with BD-hemoglobin biomarkers. This improved high throughput methodology for MHBMA and DHBMA will be useful for future epidemiological studies in smokers and in occupationally exposed workers.
Keywords: 1,3-butadiene; urinary metabolites; occupational exposure; metabolism; quantitative analysis
1. Introduction
1,3-butadiene (BD) is a high volume industrial chemical widely used as a monomer in the production of synthetic rubber and plastics and as an intermediate in the manufacture of other industrial chemicals.1 BD is also a common environmental pollutant due to its widespread presence in automobile exhaust, forest fires, urban air, and cigarette smoke.1 Inhalation studies in laboratory mice and rats have shown that BD induces tumors in multiple tissues including the lung, heart, liver, and the mammary gland.2;3 Epidemiological studies in rubber industry and BD production workers have revealed an association between BD exposure and an increased risk of leukemia in highly exposed SBR workers4–6 and non-Hodgkin's lymphoma among butadiene monomer workers,7 leading to BD classification as a human carcinogen.1
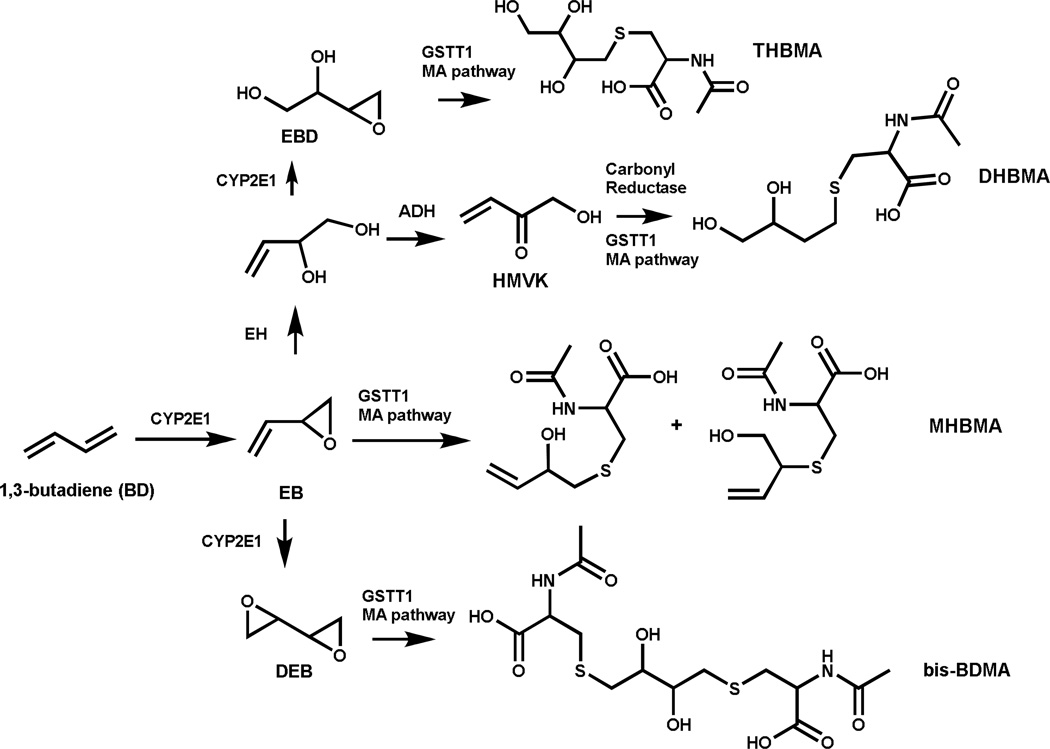
BD requires metabolic activation to electrophilic species for its mutagenic activity.8;9 Cytochrome P450 monooxygenases CYP2E1 and CYP2A6 catalyze BD oxidation to (R)- and (S)-3,4-epoxy-1-butene (EB) (Scheme 1).8;10 EB can undergo further oxidation to 1,2,3,4-diepoxybutane (DEB) or epoxide hydrolysis to 3-butene-1,2-diol (EB-diol), which can be further metabolized to hydroxymethyl vinylketone (HMVK).11–13 Alternatively, EB-diol can be oxidized by CYP2E1 to form 3,4-epoxy-1,2-butanediol (EBD).8;14 If not detoxified by epoxide hydrolase (EH) or glutathione S-transferase (GST), EB, EBD, HMVK, and DEB can alkylate DNA to form promutagenic nucleobase adducts, which are thought to be responsible for the adverse biological effects of BD.8;15–17 EB, HMVK, EBD and DEB undergo GST-mediated conjugation with glutathione and are ultimately excreted in urine as the corresponding mercapturic acids 2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene/1-(N-acetyl-L-cystein-S-yl)-2-hydroxybut-3-ene (MHBMA), 4-(N-acetyl-L-cystein-S-yl)-1,2-dihydroxybutane (DHBMA), 4-(N-acetyl-L-cystein-Syl)-1,2,3-trihydroxy-butane (THBMA), and 1,4-bis-(N-acetyl-L-cystein-S-yl)butane-2,3-diol (bis-BDMA), respectively (Scheme 1).18–20
Scheme 1.
Formation of urinary BD-mercapturic acids.
Because they can be derived from toxic and carcinogenic metabolites of BD, urinary MHBMA, DHBMA, THBMA, and bis-BDMA are useful biomarkers of BD exposure and bioactivation.21–23 Among these, MHBMA is regarded as a specific biomarker of BD exposure from tobacco smoke because urinary MHBMA concentrations decrease by more than 90% upon smoking cessation.24 In contrast, DHBMA and THBMA are present in urine of both smokers and non-smokers, suggesting that they are at least partially formed from an unidentified endogenous source.18;24;25 bis-BDMA, a specific biomarker of DEB, is found in urine of BD-exposed laboratory rats and mice, but is not detectable in urine of smokers and workers occupationally exposed to BD, probably because of the inefficient formation of DEB in humans.26 Several mass spectrometry-based methods have been developed for quantification of MHBMA and DHBMA in human urine.20;23–25;27–32 However, many of these methods require a large sample volume (> 0.5 ml) and are not amenable to high throughput analysis (Table S-1).
In the present study, a sensitive isotope dilution HPLC-ESI−-MS/MS method was developed for simultaneous quantification of MHBMA and DHBMA in human urine. The method requires only 200 µl of urine and employs a 96 well plate SPE sample cleanup step to enable simultaneous processing of multiple samples at the same time. The new method has a high sensitivity, with LOQ values of 0.5 and 10 ng/ml for MHBMA and DHBMA, respectively. This method was applied to urine samples from workers employed at a BD monomer and styrene-butadiene rubber manufacturing facility in Czech Republic.22 THBMA concentrations were also determined in the same urine samples using HPLC-ESI−-MS/MS methodology recently developed in our laboratory.18;33 We found that MHBMA, DHBMA, THBMA, and the metabolic ratio MHBMA/(MHBMA+DHBMA+THBMA) were correlated with BD exposure concentrations. Furthermore, significant associations were also observed between BD urinary mercapturic acids and their corresponding hemoglobin adducts, N, N-(2,3-dihydroxy-1,4-butadiyl)-valine (pyr-Val) and 2,3,4-trihydroxybutyl-valine (THB-Val).34;35 Our results confirm that BD urinary mercapturic acids are sensitive, non-invasive biomarkers of human exposure to BD and establish an accurate and rapid methodology for their analyses in future large epidemiological studies.
2. Materials and Methods
2.1 Materials
MHBMA, DHBMA, 2H6-MHBMA and 2H7-DHBMA were purchased from Toronto Research Chemicals (Toronto, Canada). THBMA and 2H3-THBMA were synthesized in our laboratory as described previously.18 HPLC grade methanol, LC-MS grade formic acid, and acetonitrile were obtained from Fisher Scientific (Pittsburgh, PA). All other reagents and chemicals were obtained from Sigma Aldrich (St. Louis, MO). Oasis HLB SPE 96 well plates (30 mg) were purchased from Waters Corporation (Milford, MA), and Isolute ENV+ 96 well plates (50 mg) were obtained from Biotage (Charlotte, NC).
2.2 Sample preparation
Urine samples (200 µl) were diluted with 200 µl of water and acidified with hydrochloric acid (1M solution, 20 µl). 2H6-MHBMA and 2H7-DHBMA internal standards (60 ng each) were added, and the spiked samples were mixed by vortexing and subsequently centrifuged. The supernatants were loaded onto an Oasis HLB 96 well plate (30 mg) preconditioned with 1 ml methanol and 1 ml of water. The SPE wells were washed with 1 ml of 5% methanol. MHBMA, DHBMA, 2H6-MHBMA and 2H7-DHBMA were eluted into a 96 well elution plate with 75% methanol. The eluates were dried under vacuum and reconstituted with 30 µl of 0.1% formic acid for analysis by HPLC-ESI−-MS/MS methodology as described below.
For analysis of THBMA, 100 µl aliquots of human urine were processed by SPE on Isolute ENV+ 96 well plates (50 mg). Briefly, 100 µl aliquots of urine were acidified with 100 µl of 50 mM ammonium formate buffer (pH 2.5) and 10 µL of formic acid. Following the addition of 2H3-THBMA internal standard (60 ng), samples were thoroughly mixed by vortexing. Following centrifugation, the supernatants were loaded onto an Isolute ENV+ 96 well plate pre-conditioned with 3 ml each of methanol and 0.3% formic acid. The well plates were washed with 1.5 mL of 0.3% formic acid and further with 0.75 mL of 5% aqueous methanol containing 0.3% formic acid. The SPE wells were dried completely under vacuum, and the analyte and its internal standard were eluted with 1.2 ml of 2% formic acid in methanol into a 96 well elution plate. The elution plates were dried under vacuum, and the eluates were reconstituted in 30 µl of water. THBMA concentrations were determined by isotope dilution HPLC-ESI−-MS/MS as described below.
2.3 HPLC-ESI−-MS/MS method for quantification of MHBMA and DHBMA
The HPLC-ESI-MS/MS system utilized in this study consisted of an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA) equipped with a 96 well plate autosampler and interfaced to Thermo TSQ Vantage mass spectrometer (Thermo Scientific Corp., Waltham, MA). Samples (10 µl) were injected onto an Agilent Pursuit 3 Diphenyl column (2.0×150 mm, 3 µm) fitted with an Agilent Metaguard Pursuit 3 DP guard column. The column was eluted at a flow rate of 150 µl/min with a gradient of aqueous 0.1% formic acid (A) and 0.1% formic acid in acetonitrile (B). The column was maintained at 5 °C. A linear gradient program was employed (time, % of solvent B): 0–12 min, 3 to 9% B; 12–14 min, 9 to 50% B; 14–16 min, isocratic at 50% B; 16–18 min, 50 to 3% B; 18–30 min, column equilibration at 3% B.
The mass spectrometer was operated in the ESI− mode. Typical MS instrument settings were as follows: spray voltage, − 3000 V; sheath gas pressure, 65 psi; capillary temperature, 250 ° C; collision energy, 14 (MHBMA) or 24 (DHBMA); S-lens, 75; source CID, 8 V; collision gas pressure, 1.1 mTorr; Q1 (fwhm), 0.4; Q3 (fwhm), 0.7; scan width, 0.4 m/z; and scan time, 0.3 s. MHBMA and DHBMA were quantified in the SRM mode using isotope dilution with deuterated internal standards (2H6-MHBMA and 2H7-DHBMA, respectively). The SRM transitions used for quantitative analyses were m/z 238.1 → 109.1 (2H6-MHBMA), 232.1 → 103.1 (MHBMA), 257.1 → 78.1 (2H7-DHBMA) and 250.1 → 75.1 (DHBMA). Quantitation was conducted by comparing the HPLC-ESI−MS/MS peak areas corresponding to the analytes and the corresponding internal standards using calibration curves obtained with spiked matrix samples (see below).
2.4 HPLC-ESI−-MS/MS method for quantification of THBMA
THBMA was quantified by the HPLC-ESI−-MS/MS methodology reported previously.18;36 In brief, samples (10 µl) were injected onto a SIELC Primesep D column (2.1×100 mm, 5 µm particle size) connected to a guard column (Primesep D; 2.1×10 mm). The HPLC-MS/MS system consisted of an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA) interfaced to a Thermo-Finnigan TSQ Quantum Discovery mass spectrometer (Thermo Scientific Corp., Waltham, MA). The column was eluted with a gradient of water (A) and 49.5:49.5:1 water: ACN: formic acid (B). THBMA concentrations were determined by isotope dilution with the corresponding internal standard (2H3-THBMA). The mass spectrometer was operated in the selected reaction monitoring (SRM) mode by the following MS/MS transitions m/z 269.1 → 137.1 (2H3-THBMA) and 266.1 → 137.1 (THBMA), respectively.
2.5 Method Validation
Non-smoker urine samples (200 µl) were spiked with increasing amounts of MHBMA (0.1–40 ng), DHBMA (2.0–1000 ng), and 60 ng of each of 2H6-MHBMA and 2H7-DHBMA, in triplicate. The samples were processed on Oasis HLB 96 SPE well plates as described above, and analyte concentrations were determined by isotope dilution HPLC-ESI−-MS/MS described above. Method validation curves were constructed separately for MHBMA and DHBMA to determine the correlation between the theoretical analyte/internal standard amount ratios and the corresponding peak area ratios observed experimentally upon HPLC-ESI− -MS/MS analysis of spiked samples.
2.6 LOD and LOQ determination
Synthetic urine (200 µl) was spiked with MHBMA (20–100 pg) and DHBMA (0.2–2.0 ng) and 60 ng each of 2H6-MHBMA and 2H7-DHBMA (internal standards for quantitation). Synthetic urine rather than nonsmoker urine was employed in this case due to the presence of endogenous MHBMA and DHBMA in all human samples. Spiked samples were subjected to SPE enrichment on Oasis HLB 96 well plates (30 mg) and subsequently analyzed by HPLC-ESI−-MS/MS as described above. Analyte amounts at which the signal-to-noise ratios (S/N) were greater than 10 and % CV < 15% were designated as the limit of quantification (LOQ). Similarly, the lowest analyte amount at which the signal-to-noise ratios (S/N) were greater than 3 were defined as the limit of detection (LOD).
2.7 Intraday and Interday Precision
Nine 200 µL urine aliquots from a confirmed smoker were processed by SPE as described above. The reconstituted SPE eluates were pooled into a single sample, and the pooled sample was injected three times per day on three consecutive days. Intra-day and inter-day accuracy and precision were determined by calculating the relative standard deviations (% RSD) between these 9 measurements.
2.8 Method Accuracy
Aliquots of non-smoker urine (N = 5) were spiked with 10 ng/ml of MHBMA, 250 ng/ml of DHBMA, and 60 ng each 2H6-MHBMA and 2H7-DHBMA. The aliquots were processed by SPE and subsequently analyzed by HPLC-MS/MS as described above. Background concentrations of MHBMA and DHBMA in the same non-smoker urine sample were determined by analyzing 200 µl aliquots of the non-spiked nonsmoker urine by HPLC-ESI−-MS/MS (in triplicate) and subtracted from the observed analyte concentration to determine method accuracy.
2.9 Analyte Recovery
Three aliquots of non-smoker urine (200 µl each) were spiked with MHBMA (10 ng/ml) and DHBMA (250 ng/ml) and processed by SPE as described above. 2H6-MHBMA and 2H7-DHBMA (60 ng) were added to the SPE eluates, and the samples were analyzed by HPLC-ESI−-MS/MS methodology as described above. SPE recoveries of MHBMA and DHBMA were determined by comparing the observed concentrations with the theoretical concentrations after adjusting for endogenous MHBMA and DHBMA (determined by analyzing unspiked samples).
2.10 Quantification of BD urinary metabolites in workers employed at BD monomer and styrene-butadiene rubber plant
Spot urine samples were obtained from 72 workers employed at a BD monomer and styrene-butadiene rubber (SBR) production facility near Prague, Czech Republic.22;37 Of the 72 subjects selected for analysis, 40 were administrative workers (21 male and 19 female) not occupationally exposed to BD (BD exposure < 0.03 mg/m3), while 32 were workers in the production unit (16 male and 16 female; BD exposure, 0.05–1.5 mg/m3). Complete details of the study population have been reported.22;37 The present investigation was conducted in a blind fashion, e.g. subject information was not revealed until the urinary BD mercapturic acid concentrations were determined. Following the completion of HPLC-ESIMS/ MS analysis, the information about BD exposure concentrations, subject age, gender, protein biomarkers, mutation frequencies, and smoking status of each subject were made available for data analysis.
2.11 Statistical Analysis
Multiple regression analyses were conducted to determine the associations between urinary BD-mercapturic acids and occupational BD exposure concentrations, gender, and smoking status. Additionally, Pearson partial correlation coefficients were computed to determine the correlation between MHBMA, DHBMA, THBMA, THB-Val, pyr-Val and hprt mutation frequencies. A two-way ANOVA test was also performed to identify any differences in urinary MHBMA, DHBMA, THBMA concentrations between the controls and the occupationally exposed groups.
3. Results
3.1 SPE and HPLC-MS/MS method development for MHBMA and DHBMA
We chose solid phase extraction (SPE) for isolation of BD-mercapturic acids from human urine because of its ability to remove the bulk of the interferences with minimal analyte loss. Because typical urinary concentrations of MHBMA in smokers are 50–100 fold lower than those of DHBMA,29;30 MHBMA recovery was of main concern. Multiple SPE stationary phases were tested, including reversed phase, anion exchange, weak anion exchange, and mixed mode phases. Among these, Waters Oasis hydrophilic-lipophilic balanced stationary phase (HLB)23 afforded the best MHBMA recovery. We incorporated a 5% methanol wash to remove additional interferences and conducted the final elution step with 75% methanol. SPE recoveries for MHBMA and DHBMA were estimated as 92 and 18%, respectively. The relatively low recovery for DHBMA (18%) did not interfere with analyses because of high concentrations of DHBMA in human urine28 and the use of isotopically labeled internal standards to account for analyte losses.24;30
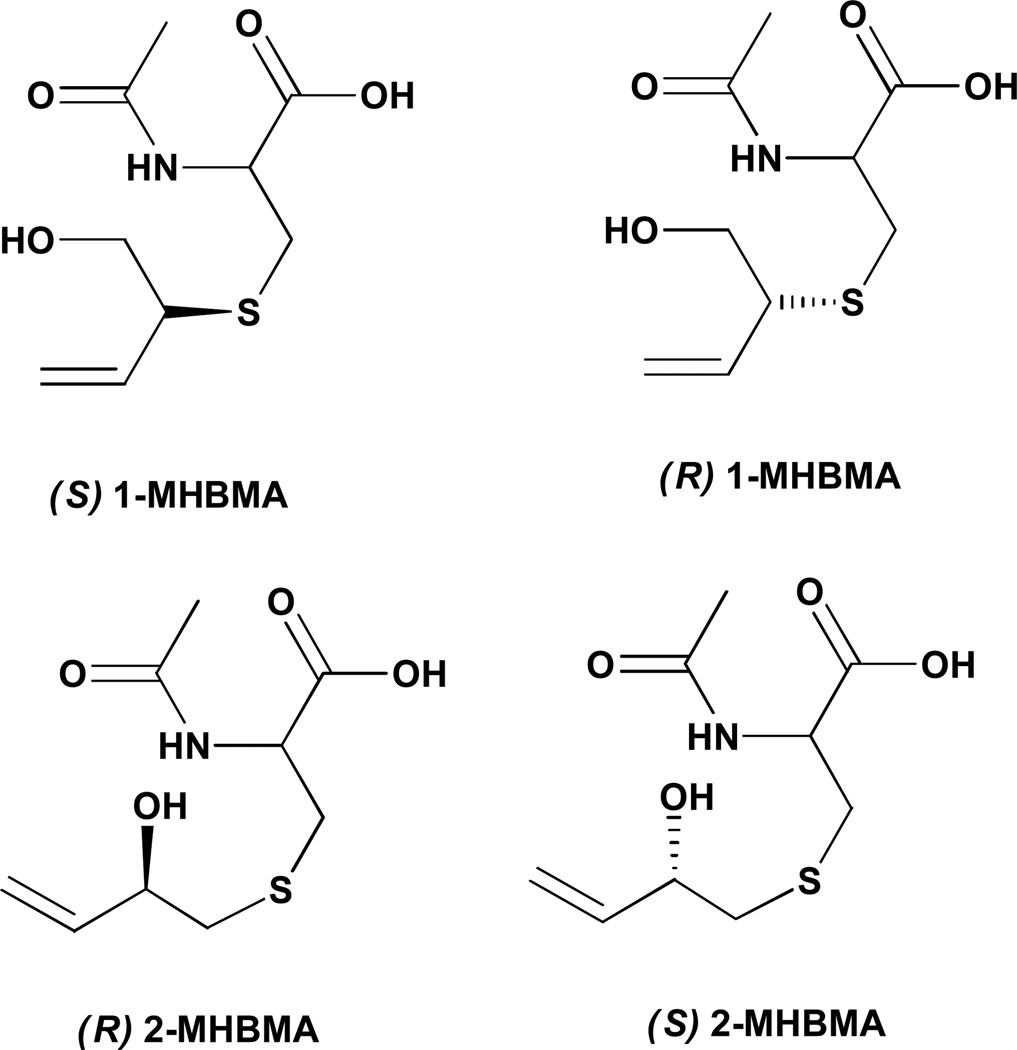
HPLC-ESI−-MS/MS method development for MHBMA and DHBMA was complicated by their poor retention on reverse phase HPLC columns. Both BD-mercapturic acids are highly polar metabolites with hydroxyl and carboxylic acid functionalities in their structure (Scheme 1). Furthermore, MHBMA is a mixture of two regioisomers (1-MHBMA and 2-MHBMA) and two stereoisomers (Scheme 2), leading to peak broadening/poor chromatography Multiple HPLC stationary phases were evaluated in an attempt to identify a set of conditions that can resolve MHBMA and 2H6-MHBMA isomers from co-eluting interferences present in human urine.
Scheme 2.
MHBMA isomers.
Capillary HPLC columns (0.5 mm i.d.) were tested in the initial stages of method development in an attempt to improve HPLC-ESI−MS/MS sensitivity. However, despite promising results for pure standards, poor results were obtained for “real” human samples due to sample-dependent retention time shifts (not shown). To minimize retention time shifts, we turned our attention to narrow bore (2.1 mm i.d.) columns. The use of ion pairing reagents such as N,N-dimethyl hexylamine and triethylamine allowed for good analyte retention, but required long equilibration times and resulted in a severe ESI MS signal suppression for subsequent users of the instrument. Therefore, we have focused on specialized HPLC phases capable of retaining polar analytes in the absence of ion pairing agents.
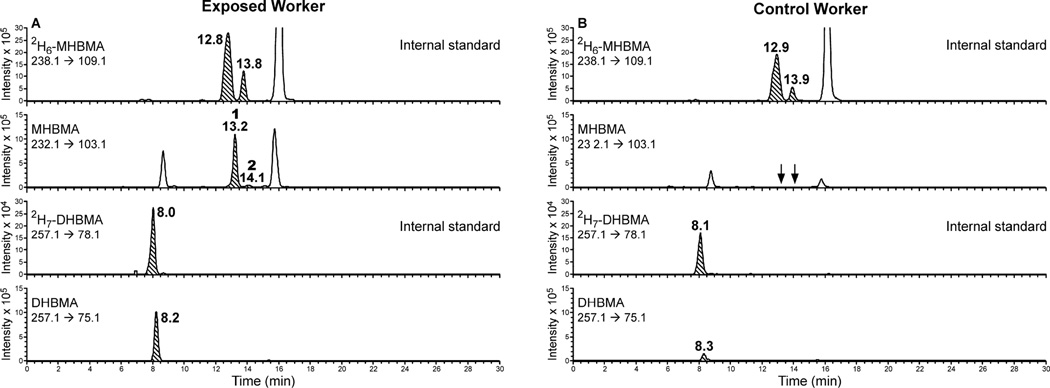
Good analyte retention in the absence of ion-pairing agents was achieved on Synergi MAX-RP (Phenomenex), Luna C18 (Phenomenex), Xterra MS-C18 (Waters), Primesep B2 (Sielc), Primesep D (Sielc), Zorbax SB-C18 (Agilent) and Hypersil Gold (Thermo). However, these columns were unable to resolve MHBMA from co-eluting interferences present in human urine. The best results in terms of HPLC retention and analyte separation from the matrix was achieved with an Agilent Pursuit Diphenyl column (2.1 × 150 mm, 3 µ) (Agilent Technologies). In order to maximize analyte retention, the carboxylate group of the analyte (pKa ~4.5) was protonated by employing an acidic mobile phase (0.1% formic acid, pH ~2.5). Although higher sensitivity was achieved with 0.5% acetic acid, MHBMA separation from a co-eluting peak was not satisfactory with this mobile phase. A linear gradient of acetonitrile in 0.1% aqueous formic acid has afforded a good HPLC resolution and an improved MS sensitivity for MHBMA. The HPLC column was maintained at 5 °C in order to maximize the separation of MHBMA isomers (Peaks at 13.2 and 14.1 min, Figure 1) from an interfering impurity present in human samples (peak at 15.8 min, Figure 1).
Figure 1.
Representative traces for HPLC-ESI−-MS/MS analysis of MHBMA and DHBMA in urine of a BD-exposed worker (A) and a control worker (B).
The best MS sensitivity for MHBMA and DHBMA was achieved in the negative ion ESI mode. The major ESI− MS/MS fragmentation pathways for MHBMA and DHBMA correspond to the cleavage of the C-S bond, leading to major MS/MS fragments at m/z 103.1 (MHBMA) and 121.1 (DHBMA) (Supplemental Figure S-1). Our initial method development efforts have employed MS/MS transitions 238.1 → 109.1 (2H6-MHBMA), 232.1 → 103.1 (MHBMA), 257.1 → 128.1 (2H7-DHBMA) and 250.1 → 121.1 (DHBMA) (Supplemental Figure S-1). However, since experiments with spiked urine samples have revealed a co-eluting impurity in the major SRM transition for DHBMA (m/z 250.1 → 121.1), an alternative MS/MS transition (m/z 250.1 → 75.1) was selected for DHBMA and its 2H7-internal standard (m/z 257.1 → 78.1). Under our HPLC conditions, MHBMA isomers eluted as two HPLC-ESI−-MS/MS peaks at 13.2 and 14.1 min, while the corresponding isomers of 2H6-MHBMA elute at 12.8 and 13.8 min, respectively (Figure 1A, top panel). The peak at 12.8 min contains (R) and (S) 2-MHBMA as well as one of the stereoisomers of 1-MHBMA, while the second peak corresponds to the other stereoisomer of 1-MHBMA (Scheme 2). In the same samples, DHBMA signal was observed at 8.2–8.3 min, while 2H7-DHBMA internal standard eluted slightly earlier at 8.0–8.1 min (Figure 1, bottom panels). THMBA was analyzed using a similar HPLC-ESI−-MS/MS approach (Figure 2).
Figure 2.
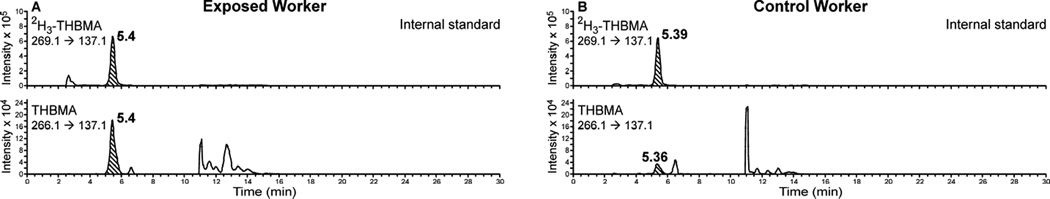
Representative traces for HPLC-ESI−-MS/MS analysis of THBMA in urine of a BDexposed worker (A) and a control worker (B).
3.2 Method Validation
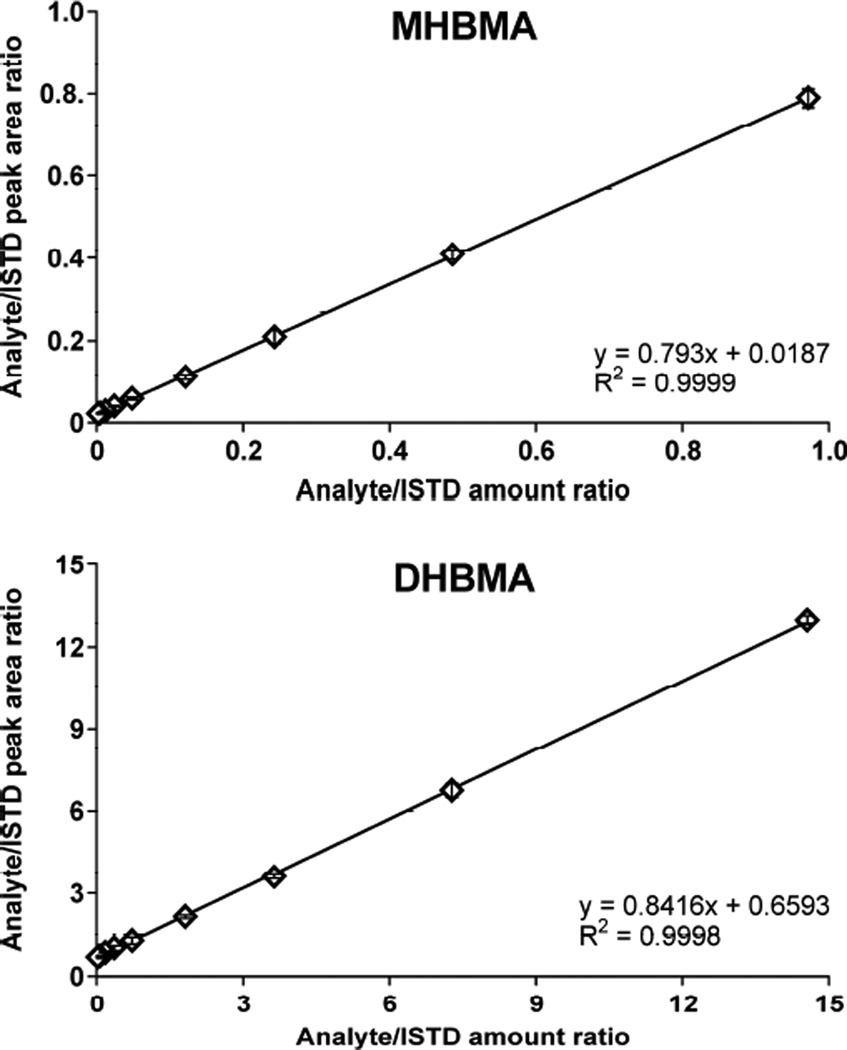
The quantitative HPLC-ESI−-MS/MS method for MHBMA and DHBMA was validated by analyzing non-smoker urine spiked with increasing concentrations of MHBMA and DHBMA and fixed amounts of the corresponding internal standards. As shown in Figure 3, linear correlation curves were obtained for both analytes (R2 > 0.9998). The HPLC-ESI−-MS/MS response was linear between 0.5–200 ng/ml urine for MHBMA and 10–5000 ng/ml for DHBMA (Table 1). The yintercepts in both method validation curves were above zero (Figure 3) due to the presence of background amounts of BD-mercapturic acids in non-smoker urine (MHBMA, 3.8 ng/ml; DHBMA, 229 ng/ml). The LOD and LOQ values for MHBMA were 0.2 and 0.5 ng/ml, respectively, while the corresponding values for DHBMA were 5 and 10 ng/ml (Table 1). The LOD and LOQ values for DHBMA are relatively high because minor SRM transitions were chosen for quantification of this analyte (see above). However, this did not interfere with accurate analysis of DHBMA due to its relatively high concentrations in human urine.
Figure 3.
Method validation curves for MHBMA and DHBMA spiked into non-smoker urine (200 µl).
Table 1.
HPLC-ESI--MS/MS method validation parameters for quantification of MHBMA and DHBMA in human urine.
| Analyte | Range (ng/ml) |
LOD (ng/ml) |
LOQ (ng/ml) |
Precision (% RSD) | Accuracy (%) |
Recovery (%) |
|
|---|---|---|---|---|---|---|---|
| intra-day | inter-day | ||||||
| MHBMA | 0.5–200 | 0.2 | 0.5 | 4.1 | 4.5 | 100.5 ± 5.1 | 92 |
| DHBMA | 10.0–5000 | 5 | 10 | 2.4 | 3.0 | 102.7 ± 3.2 | 18 |
Intra-day and inter-day precision was determined by repeated analysis of a smoker urine sample (three times per day on three consecutive days). The calculated % RSD values for both analytes were below 5% (Table 1), demonstrating the reproducibility of our HPLC-ESI−-MS/MS method for BD-mercapturic acids. The accuracy of the HPLC-ESI−-MS/MS method for MHBMA and DHBMA was determined by spiking known amounts of each analyte (MHBMA, 10 ng/ml and DHBMA, 250 ng/ml) into a non-smoker urine sample. We found that the accuracy range for MHBMA was 100.5 ± 5.1, while the corresponding value for DHBMA was 102.7 ± 3.2. Complete validation parameters for both analytes are presented in Table 1. Overall, the new method is highly accurate and reproducible (Table 1) and is characterized by an improved sensitivity as compared to reported methodologies (Table S-1).
3.3 Quantification of BD metabolites in urine of occupationally exposed workers and the corresponding controls
In order to test the applicability of the new method to human biomonitoring, the concentrations of MHBMA, DHBMA and THBMA were determined in after-work urine samples of 72 workers from a BD monomer/styrene-butadiene rubber production facility in Czech Republic. Each worker’s individual exposure to BD was monitored separately during their work shifts with diffusive solid sorbent tubes.22 Among 72 workers participating in the study, 40 were administrative workers (21 male and 19 female) with minimal exposure to BD (<0.03 mg/m3) and 32 were workers in a BD production unit (16 male and 16 female, BD exposure 0.05–1.5 mg/m3). BD exposure concentrations shown in Table 2 are an average of 10 different days over a 4-month interval. Average BD exposure concentrations were 0.007 ± 0.005 mg/m3 for control workers and 0.55 ± 0.50 mg/m3 for exposed workers (Table 2).
Table 2.
Mean urinary MHBMA, DHBMA, THBMA concentrations and metabolic ratios in exposed and control workers by sex
| Concentrations (ng/ml) | ||||||
|---|---|---|---|---|---|---|
| N | BD exposure (mg/m3) |
MHBMA* | DHBMA* | THBMA* | Metabolic Ratio* | |
| Males | ||||||
| Controls | 21 | 0.007 ± 0.005a | 9.9 ± 11.2a,b | 1480.6 ± 968.5a,b | 57.1 ± 33.5a,b | 0.007 ± 0.008a |
| Exposed | 16 | 0.68 ± 0.41a,c | 95.9 ± 111.4a,c (176.5 ± 228.3)e | 3136.1 ± 2560.3a,c (5922.7 ± 4737.5) | 139.3 ± 104.7a,c (254.1 ± 152.1) | 0.027 ± 0.026a (0.07 ± 0.10) |
| Females | ||||||
| Controls | 19 | 0.007 ± 0.005d | 3.1 ± 4.8b,d | 561.2 ± 531.5b | 24.2 ± 16.6b | 0.006 ± 0.007d |
| Exposed | 16 | 0.32 ± 0.34c,d | 8.3 ± 8.1c,d (44.3 ± 45.4)e | 716.1 ± 830.7c (3251.3 ± 2907.5) | 47.4 ± 70.9c (232.2 ± 243.2) | 0.017 ± 0.012d (0.11 ± 0.14) |
Values in parenthesis ( ) are per unit dose of BD
Male exposed significantly greater than male controls (Two way ANOVA test, p-value <0.01)
Male controls significantly greater than Female controls (Two way ANOVA test, p-value <0.05)
Male exposed significantly greater than Female exposed (Two way ANOVA test, p-value <0.01)
Female exposed significantly greater than Female controls (Two way ANOVA test, p-value <0.05)
Male exposed significantly greater than Female exposed (Two way ANOVA test, p-value <0.01)
A summary of urinary concentrations of MHBMA, DHBMA and THBMA and their metabolic ratio MHBMA/(MHBMA +DHBMA+THBMA) are given in Table 2. Mean MHBMA concentrations in urine of BD-exposed female workers (8.3 ± 8.1 ng/ml) were significantly higher than those in control female workers (3.1 ± 4.8 ng/ml) (p < 0.05) (Table 2). An even greater difference was seen in male workers, with mean MHBMA concentrations of 95.9 ± 111.4 ng/ml (exposed) and 9.9 ± 11.2 ng/ml (controls) (p = 0.001). This is consistent with the differences in exposure concentrations. Interestingly, urinary MHBMA concentrations in males were significantly greater than in females both in controls (p < 0.02) and in occupationally exposed workers (p = 0.005) (Table 2).
Urinary concentrations of DHBMA (561.2 ± 531.5 ng/ml in control female workers and 716.1 ± 830.7 ng/ml in occupationally exposed females) were 20–30 times greater than those of MHBMA amounts in the same samples (Table 2). Female workers occupationally exposed to BD excreted higher DHBMA concentrations than controls (716±830.7 vs 561 ± 531.5 ng/ml urine), but this difference was not statistically significant (p > 0.05). Mean DHBMA concentrations in exposed males (3136.1 ± 2560.3 ng/ml) were significantly greater than in control males (1480.6 ± 968.5 ng/ml) (p = 0.01). Similar to our results for MHBMA, urinary DHBMA concentrations were higher in males as compared to females for both control groups (p < 0.001) and the occupationally exposed groups (p = 0.002).
Mean THBMA concentrations in control and occupationally exposed females were 24.2 ± 16.6 ng/ml and 47.4 ± 70.9 ng/ml, respectively, this difference did not reach a statistical significance (p>0.05, Table 2). The corresponding values in males were 57.1 ± 33.5 ng/ml (controls) and 139.3 ± 104.7 ng/ml (exposed) (p = 0.002). Furthermore, THBMA concentrations in males were higher than in females for both control (p < 0.001) and BD-exposed group (p < 0.01). Metabolic ratio calculated as MHBMA/(MHBMA+DHBMA+THBMA) is an indicator of the fraction of EB that is not detoxified via hydrolysis and is available for binding to cellular biomolecules. Mean metabolic ratio in male and female exposed workers were 0.027 ± 0.026 and 0.017 ± 0.012, respectively, while the ratios in control workers were 0.007 ± 0.008 and 0.006 ± 0.007, respectively. The metabolic ratio was significantly higher in occupationally exposed groups than in control groups (p < 0.005), but did not differ between male and female subjects.
3.4 Correlation studies
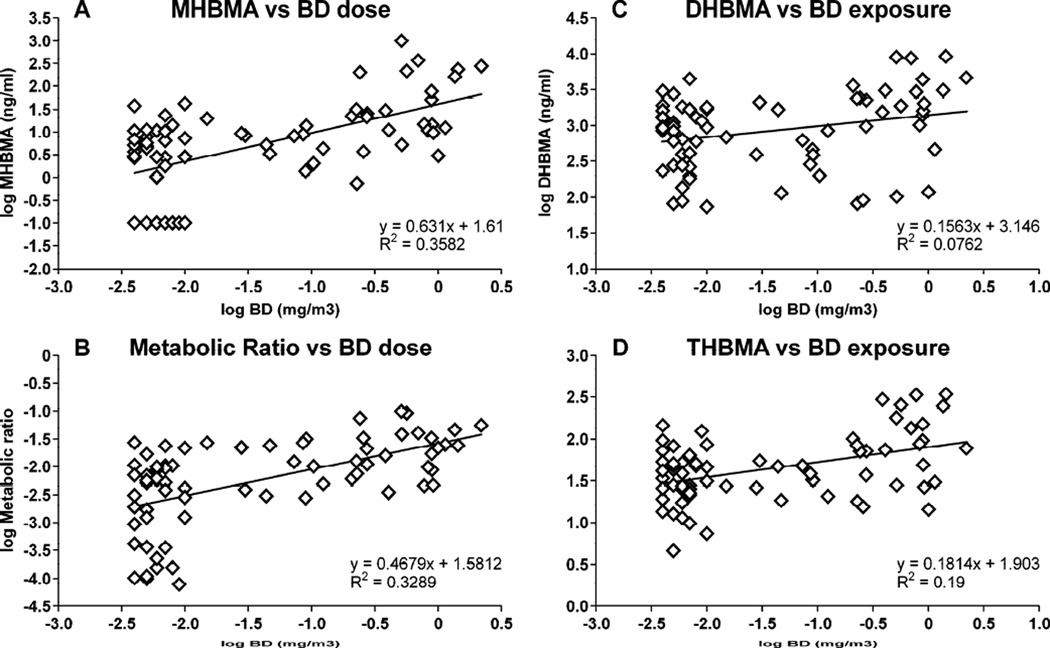
Multiple regression analyses were conducted to reveal associations between urinary MHBMA, DHBMA, THBMA concentrations and BD exposure concentration, smoking status, gender, and other biomarkers of exposure to BD. Among these, a significant correlation was found between urinary BD-mercapturic acids (MHBMA, DHBMA and THBMA) and BD exposure (p < 0.005, see Table 3). We found that urinary MHBMA and DHBMA concentrations were associated with smoking status (p < 0.05). Linear regression analyses were conducted to further understand the association between urinary BD-mercapturic acids and exposure to BD. We found that urinary MHBMA, DHBMA and THBMA concentrations and metabolic ratio in individual workers significantly correlated with BD exposure (Figure 4 and Supplemental Figure S-2)
Table 3.
Association between and among all BD biomarkers determined by Pearson correlation analysis (p-values)
| MHBMA | DHBMA | THBMA | THB-Val | pyr-Val |
hprt mutation Frequency |
|
|---|---|---|---|---|---|---|
| MHBMA | NA | <0.001 | <0.001 | 0.007 | <0.001 | None |
| DHBMA | <0.001 | NA | <0.001 | <0.001 | <0.001 | None |
| THBMA | <0.001 | <0.001 | NA | <0.001 | 0.07 | None |
NA: Not Applicable
None: No significant association observed
Figure 4.
Associations between urinary MHBMA, DHBMA, THBMA, and their metabolic ratio and BD exposure concentrations.
In order to assess the relationship between various measures of BD exposure, we examined the correlations among individual BD mercapturic acids (MHBMA, DHBMA and THBMA), between BD-mercapturic acids and the corresponding BD-hemoglobin adducts (THBVal, pyr-Val), and between BD-mercapturic acids and hprt mutation frequency. THB-Val, pyr-Val, and hprt mutation frequencies for individual workers were available from an earlier study by Albertini et al.22 Significant correlations were observed between urinary MHBMA and DHBMA (r = 0.742, p < 0.001), MHBMA and THBMA (r = 0.419, p < 0.001) (Table 3, Supplemental Figure S-2), and between DHBMA and THBMA (r=0.594, p<0.001) (Table 3, Supplemental Figure S-2). In addition, MHBMA and DHBMA concentrations were also significantly correlated with both THB-Val and pyr-Val concentrations, while THBMA was strongly associated with THB-Val (Table 3, Supplemental Figure S-2). In contrast, the correlation between THBMA and pyr-Val was not significant (p=0.07). Overall, THBMA was significantly correlated with MHBMA, DHBMA, and THB-Val (Table 3 and Supplemental Figure S-2). However, no correlation was found between BD-urinary mercapturic acids and hprt mutation frequencies (Table 3).
4. Discussion
BD is an important environmental and industrial chemical classified as a human carcinogen.4;6 The availability of sensitive, robust, high throughput methodologies for monitoring human exposure to butadiene (BD) is essential for molecular epidemiology studies of human cancer risk and susceptibility. Although multiple HPLC-ESI−MS/MS methods have been previously described for quantification of BD-mercapturic acids in humans (Table S-1),23–25;27–32 many of these methods require at least 0.5 ml of urine, and thus are not applicable to studies where the sample volume is limited (< 0.2 ml). Furthermore, large epidemiological studies typically involve hundreds or even thousands of human subjects, necessitating high throughput analytical strategies. In the present work, a high throughput HPLC-ESI−-MS/MS method was developed for simultaneous quantification of two BD-mercapturic acids (MHBMA and DHBMA) in human urine. The new method requires only 200 µl of urine and measures both metabolites simultaneously. The method involves sample cleanup in a high throughput format on 96 well Oasis HLB SPE plates followed by HPLC-ESI−-MS/MS analysis. The current approach is characterized by an improved sensitivity as compared to reported methodologies (Table S-1), with LOD values of 0.2 ng/ml urine (MHBMA) and 5 ng/ml urine (DHBMA), and is highly accurate and reproducible (Table 1). The intra-day precision (%RSD) values of this new method for MHBMA and DHBMA were 4.1% and 2.4 %, respectively, while the corresponding inter-day values were 4.5% and 3%. Method accuracy values for MHBMA and DHBMA were 100.5 and 102.7%, respectively.
The new method was employed for the quantification of MHBMA and DHBMA in urine samples from workers employed at a styrene-butadiene rubber production plant (40 controls and 32 exposed).22 In addition to MHBMA and DHBMA, another BD-mercapturic acid (THBMA, Scheme 1) was also quantified in the same samples. THBMA is a recently discovered urinary metabolite of BD that is formed from 3,4-epoxy-1,2-diol (Scheme 1).18 To the best of our knowledge, this is the first report for the detection and quantification of this metabolite in occupationally exposed workers. Among the three BD-mercapturic acids, the order of urinary concentrations was DHBMA > THBMA > MHBMA, consistent with previous report in smokers.38 Occupationally exposed workers were exposed to significantly higher concentrations of BD (0.55 ± 0.50 mg/m3) than control workers (0.007 ± 0.005 mg/m3) (Table 2) and hence the concentrations of all three urinary BD-mercapturic acids were increased in occupationally exposed workers as compared to controls (Table 2).
The metabolic ratio MHBMA/(MHBMA+DHBMA) and its complementary ratio DHBMA/(MHBMA+DHBMA) have been widely used in the literature as a measure of BD bioactivation and detoxification via hydrolysis, because MHBMA is formed by the reaction of the EB with glutathione, while DHBMA is produced from EB hydrolysis product, 3-butene-1,2-diol (Scheme 1). Sabourin et al. reported that the metabolic ratio DHBMA/(DHBMA + MHBMA) increased from mice (0.2) to rats (0.25–0.4) to hamsters (0.4) to monkeys (0.9),39 which parallels the order of epoxide hydrolase activity in these species. Since the analyses described here have included another important BD-mercapturic acid (THBMA, which is formed from EBD – see Scheme 1), we have calculated the metabolic ratio MHBMA/(MHBMA+DHBMA+THBMA) as a fraction of EB that is not detoxified via hydrolysis and is available for binding to cellular biomolecules. Our results shown in Table 2 indicate that in humans, less than 3% of EB (metabolic ratio <0.03) is detoxified by conjugation with glutathione, and >97% of EB formed is hydrolyzed by epoxide hydrolase to EB-diol (Table 2). This is consistent with previous results which indicate the predominance of hydrolytic pathway of BD metabolism in humans as compared to laboratory rats and mice.20;40 Similar to BD-mercapturic acids, the metabolic ratio was also observed to be significantly higher in occupationally exposed groups than in control groups (p < 0.005).
Statistical analyses have revealed that the concentrations of MHBMA, DHBMA, and THBMA in human urine are strongly associated with BD exposure (Figure 4) (p < 0.001). This can be explained by an increased formation of EB, HMVK and EBD in exposed individuals, which are excreted as MHBMA, DHBMA and THBMA, respectively (Scheme 1). In addition to occupational sources, BD is also present in cigarette smoke (16–75 µg/cigarette in the mainstream smoke and 205–361 µg/cigarette in the sidestream smoke).41 We therefore examined a possible association between BD-mercapturic acids concentrations and smoking status. Both BD-mercapturic acids were associated with smoking status, which is consistent with an earlier study by Roethig et. al. who found correlation between MHBMA/DHBMA concentrations in urine and nicotine equivalents (a measure of smoking status).30 In contrast, there was no association between BD mercapturic acids and hprt mutation frequency in the same individuals (Table 3).
Significant association were observed between and among individual BD-mercapturic acids MHBMA, DHBMA and THBMA (p<0.001) (Table 3). These results are not unexpected because all three metabolites can originate from BD exposure. Additionally, the three BDmercapturic acids were also strongly associated (p<0.001) with BD-hemoglobin adducts (THB Val and pyr-Val) except for MHBMA vs THB-Val (good correlation, p=0.007) and THBMA vs pyr-Val (weak correlation, p=0.07). These results are interesting, especially since no such correlation studies with THBMA were previously done. Overall our results indicate significant association between and among all major BD biomarkers (BD-mercapturic acids and BD-hemoglobin adducts).
Our results suggest that males excrete higher concentrations of MHBMA, DHBMA and THBMA in their urine than females (Table 2). This is only partially explained by the fact that females are exposed to relatively lower BD concentrations (0.32 mg/m3) as compared to males (0.68 mg/m3), because gender differences in urinary MHBMA levels remained after metabolite concentrations were normalized to BD exposures (Table 2, values in parenthesis). Females formed significantly lower MHBMA amounts per unit dose of BD than did males (p < 0.05). In contrast, DHBMA and THBMA concentrations per unit of BD were not significantly different among the two genders (Table 2, values in parenthesis). Overall, our results are suggestive of gender differences in BD metabolism, with males converting a greater portion of BD to EB, which is subsequently excreted as MHBMA. Alternatively, the observed differences in urinary MHBMA may be a result of variable GST activity.
5. Conclusions
In conclusion, we report a robust, sensitive, high throughput HPLC-ESI−-MS/MS method for quantification of BD-mercapturic acids in human urine as biomarkers of exposure to BD. The applicability of the new method was demonstrated by analyzing the two biomarkers in urine samples from workers employed at a BD-SBR production facility. We found that urinary MHBMA concentrations were strongly associated with BD exposure. Furthermore, MHBMA concentrations (adjusted per unit of BD exposure) were significantly higher in males as compared to females, suggesting that there might be gender differences in metabolism of BD in humans. All three metabolites were detected in subjects with no known exposure to BD, suggesting that there are other sources of MHBMA, DHBMA, and THBMA in humans. This novel method is now being applied to a large multiethnic cohort study aimed to identify ethnic/racial differences in metabolism of BD.
Supplementary Material
Highlights.
New HPLC-ESI−-MS/MS method was developed for urinary metabolites of 1,3-butadiene.
BD-mercapturic acids were quantified in occupationally exposed workers and controls.
BD-mercapturic acids levels were increased in BD exposed workers.
Urinary BD-mercapturic acids were associated with BD-hemoglobin adducts in blood.
Acknowledgements
We thank Cullin Bachmeier, Chromtech and Gerald Nagatani, Phenomenex for providing technical assistance on SPE method development and HPLC column selection. We are also thankful to Gregory Janis (Medtox Laboratories) and Steve Carmella (University of Minnesota Cancer Center) for their helpful advice during method development and Robert Carlson for preparing graphics for this paper. SK was partially supported by a doctoral dissertation fellowship from the University of Minnesota Graduate School. All the mass spectrometry work in this paper was performed at the Analytical Biomarkers facility at the Masonic Cancer Center, University of Minnesota. This research was supported by grants from the NCI (CA-138338) and the American Chemistry Council Olefins Panel. Urine samples from occupationally exposed workers were generously provided by Professor Richard J. Albertini (University of Vermont) and the repository maintained by the American Chemistry Council Olefins Panel.
Abbreviations
- BD
1,3-butadiene
- EB
3,4-epoxy-1-butene
- HMVK
hydroxymethylvinylketone
- EBD
3,4-epoxy-1,2-diol
- DEB
1,2,3,4-diepoxybutane
- MHBMA
monohydroxybutenyl mercapturic acid
- DHBMA
dihydroxybutyl mercapturic acid
- THBMA
trihydroxybutyl mercapturic acid
- THB-Val
1,2,3-trihydroxybutyl-valine
- Pyr-Val
N,N-(2,3-dihydroxy-1,4-butadiyl)-valine
- EH
epoxide hydrolase
- GST
glutathione S-transferase
- HPLC-ESI−-MS/MS
high performance liquid chromatography-electrospray ionization tandem mass spectrometry
- SPE
solid phase extraction
- LOD
limit of detection
- LOQ
limit of quantification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information Available
Summary of quantitative HPLC-MS/MS methods, MS/MS spectra of d6-MHBMA, MHBMA, d7-MHBMA, and DHBMA, plots of association between urinary DHBMA and THBMA and THBMA and THB-Val, and plots of correlation between urinary mercapturic acids MHBMA and DHBMA and MHBMA and THBMA. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.National Toxicology Program. Rep Carcinog. 2011;12:75–77. [Google Scholar]
- 2.Melnick RL, Huff J, Chou BJ, Miller RA. Cancer Res. 1990;50:6592–6599. [PubMed] [Google Scholar]
- 3.Owen PE, Glaister JR, Gaunt IF, Pullinger DH. Am.Ind.Hyg.Assoc.J. 1987;48:407–413. doi: 10.1080/15298668791384959. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Sathiakumar N, Graff J, Matthews R, Delzell E. Chem.Biol.Interact. 2007;166:15–24. doi: 10.1016/j.cbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Delzell E, Sathiakumar N, Hovinga M, Macaluso M, Julian J, Larson R, Cole P, Muir DC. Toxicology. 1996;113:182–189. doi: 10.1016/0300-483x(96)03443-9. [DOI] [PubMed] [Google Scholar]
- 6.Macaluso M, Larson R, Delzell E, Sathiakumar N, Hovinga M, Julian J, Muir D, Cole P. Toxicology. 1996;113:190–202. doi: 10.1016/0300-483x(96)03444-0. [DOI] [PubMed] [Google Scholar]
- 7.Divine BJ, Hartman CM. Chem.Biol.Interact. 2001;135–136:535–553. doi: 10.1016/s0009-2797(01)00212-5. [DOI] [PubMed] [Google Scholar]
- 8.Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Crit Rev.Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- 9.Kirman CR, Albertini RA, Gargas ML. Crit Rev.Toxicol. 2010;40(Suppl 1):74–92. doi: 10.3109/10408444.2010.507183. [DOI] [PubMed] [Google Scholar]
- 10.Duescher RJ, Elfarra AA. Arch.Biochem.Biophys. 1994;311:342–349. doi: 10.1006/abbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- 11.Krause RJ, Elfarra AA. Arch.Biochem.Biophys. 1997;337:176–184. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- 12.Krause RJ, Sharer JE, Elfarra AA. Drug Metab Dispos. 1997;25:1013–1015. [PubMed] [Google Scholar]
- 13.Sprague CL, Elfarra AA. Chem.Res.Toxicol. 2004;17:819–826. doi: 10.1021/tx049949f. [DOI] [PubMed] [Google Scholar]
- 14.Malvoisin E, Roberfroid M. Xenobiotica. 1982;12:137–144. doi: 10.3109/00498258209046787. [DOI] [PubMed] [Google Scholar]
- 15.Swenberg JA, Bordeerat NK, Boysen G, Carro S, Georgieva NI, Nakamura J, Troutman JM, Upton PB, Albertini RJ, Vacek PM, Walker VE, Sram RJ, Goggin M, Tretyakova N. Chem.Biol.Interact. 2011;192:150–154. doi: 10.1016/j.cbi.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goggin M, Sangaraju D, Walker VE, Wickliffe J, Swenberg JA, Tretyakova N. Chem.Res.Toxicol. 2011;24:809–817. doi: 10.1021/tx200009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangaraju D, Villalta P, Goggin M, Agunsoye MO, Campbell C, Tretyakova N. Chem.Res.Toxicol. 2013 doi: 10.1021/tx400213m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotapati S, Matter BA, Grant AL, Tretyakova NY. Chem.Res.Toxicol. 2011;24:1516–1526. doi: 10.1021/tx2001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotapati S, Sangaraju D, Esades A, Hallberg L, Walker VE, Swenberg JA, Tretyakova NY. Carcinogenesis. 2014;35:1371–1378. doi: 10.1093/carcin/bgu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Toxicol.Sci. 2000;56:189–202. doi: 10.1093/toxsci/56.1.189. [DOI] [PubMed] [Google Scholar]
- 21.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Wright M, Nicklas JA, Boogaard PJ, Henderson RF, Swenberg JA, Tates AD, Ward JB., Jr Chem.Biol.Interact. 2001;135–136:429–453. doi: 10.1016/s0009-2797(01)00181-8. [DOI] [PubMed] [Google Scholar]
- 22.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Rossner P, Nicklas JA, McDonald JD, Boysen G, Georgieva N, Swenberg JA. Chem.Biol.Interact. 2007;166:63–77. doi: 10.1016/j.cbi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Sapkota A, Halden RU, Dominici F, Groopman JD, Buckley TJ. Chem.Biol.Interact. 2006;160:70–79. doi: 10.1016/j.cbi.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Chem.Res.Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urban M, Gilch G, Schepers G, van Miert E, Scherer G. J Chromatogr.B Analyt.Technol.Biomed.Life Sci. 2003;796:131–140. doi: 10.1016/j.jchromb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Kotapati S, Sangaraju D, Esades A, Hallberg L, Walker VE, Swenberg JA, Tretyakova NY. Carcinogenesis. 2014;35:1371–1378. doi: 10.1093/carcin/bgu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald JD, Bechtold WE, Krone JR, Blackwell WB, Kracko DA, Henderson RF. J Anal.Toxicol. 2004;28:168–173. doi: 10.1093/jat/28.3.168. [DOI] [PubMed] [Google Scholar]
- 28.Eckert E, Drexler H, Goen T. J Chromatogr.B Analyt.Technol.Biomed.Life Sci. 2010;878:2506–2514. doi: 10.1016/j.jchromb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Schettgen T, Musiol A, Alt A, Ochsmann E, Kraus T. Anal.Bioanal.Chem. 2009;393:969–981. doi: 10.1007/s00216-008-2510-1. [DOI] [PubMed] [Google Scholar]
- 30.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, Mendes PE. Nicotine.Tob.Res. 2009;11:1216–1225. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 31.Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL. Chem.Res.Toxicol. 2009;22:1018–1025. doi: 10.1021/tx800468w. [DOI] [PubMed] [Google Scholar]
- 32.Sterz K, Scherer G, Krumsiek J, Theis FJ, Ecker J. Chem.Res.Toxicol. 2012;25:1565–1567. doi: 10.1021/tx3002862. [DOI] [PubMed] [Google Scholar]
- 33.Kotapati S, Sangaraju D, Esades A, Hallberg L, Walker VE, Swenberg JA, Tretyakova NY. Carcinogenesis. 2014;35:1371–1378. doi: 10.1093/carcin/bgu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boysen G, Georgieva NI, Upton PB, Walker VE, Swenberg JA. Chem.Biol.Interact. 2007;166:84–92. doi: 10.1016/j.cbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Boysen G, Georgieva NI, Bordeerat NK, Sram RJ, Vacek P, Albertini RJ, Swenberg JA. Toxicol.Sci. 2012;125:30–40. doi: 10.1093/toxsci/kfr272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotapati S, Sangaraju D, Esades A, Hallberg L, Walker VE, Swenberg JA, Tretyakova NY. Carcinogenesis. 2014;35:1371–1378. doi: 10.1093/carcin/bgu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Nicklas JA, van Sittert NJ, Boogaard PJ, Henderson RF, Swenberg JA, Tates AD, Ward JB, Jr, Wright M, Ammenheuser MM, Binkova B, Blackwell W, de Zwart FA, Krako D, Krone J, Megens H, Musilova P, Rajska G, Ranasinghe A, Rosenblatt JI, Rossner P, Rubes J, Sullivan L, Upton P, Zwinderman AH. Res.Rep.Health Eff.Inst. 2003:1–141. [PubMed] [Google Scholar]
- 38.Kotapati S, Sangaraju D, Esades A, Hallberg L, Walker VE, Swenberg JA, Tretyakova NY. Carcinogenesis. 2014;35:1371–1378. doi: 10.1093/carcin/bgu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabourin PJ, Burka LT, Bechtold WE, Dahl AR, Hoover MD, Chang IY, Henderson RF. Carcinogenesis. 1992;13:1633–1638. doi: 10.1093/carcin/13.9.1633. [DOI] [PubMed] [Google Scholar]
- 40.Bechtold WE, Strunk MR, Chang IY, Ward JB, Jr, Henderson RF. Toxicol.Appl.Pharmacol. 1994;127:44–49. doi: 10.1006/taap.1994.1137. [DOI] [PubMed] [Google Scholar]
- 41.Brunnemann KD, Kagan MR, Cox JE, Hoffmann D. Carcinogenesis. 1990;11:1863–1868. doi: 10.1093/carcin/11.10.1863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.