Abstract
Ebolavirus causes sporadic outbreaks of lethal hemorrhagic fever in humans with no currently approved therapy. Cells take up Ebolavirus by macropinocytosis, followed by trafficking through endosomal vesicles. However, few factors controlling endosomal virus movement are known. Here we find that Ebolavirus entry into host cells requires the endosomal calcium channels called two pore channels (TPCs). Disrupting TPC function by gene knockout, small interfering RNAs or small molecule inhibitors halted virus trafficking and prevented infection. Tetrandrine, the most potent small molecule we tested, inhibited infection of human macrophages, the primary target of Ebolavirus in vivo, and also showed therapeutic efficacy in mice. Therefore, TPC proteins play a key role in Ebolavirus infection and may be effective targets for antiviral therapy.
Ebolaviruses (EBOV), together with Marburgvirus, are a highly diverse group of viruses that comprise the Filoviridae. Almost all of them, including the strain responsible for the latest outbreak in West Africa, cause a highly lethal, rapidly progressing hemorrhagic fever in humans, nonhuman primates and other forest animals (1, 2). However, there is currently no licensed drug treatment or broadly active vaccine (3), making them significant public health threats and potential biothreat agents. Since, like most viruses, EBOV depends on host cell factors to complete its life cycle (4), blocking such interactions may have a large impact on infection and disease outcome. Recent successes in cell culture and some animal models suggest that this approach holds promise for rapidly bringing new drugs to the clinic (5).
EBOV binds to several types of cell surface proteins to initiate host cell entry (6-8), after which it is internalized by macropinocytosis and follows an endosomal route to reach acidic compartments (9, 10). There, host proteases such as cathepsins cleave the viral glycoproteins (GPs) (11), which bind to the endosomal membrane protein, NPC1, and eventually facilitate the release of the viral core to the cell cytoplasm where replication begins (12, 13). Previously, we showed that host calcium signaling proteins were important for EBOV host cell entry but were unable to identify the functional mechanism nor address whether they could be therapeutic targets (14).
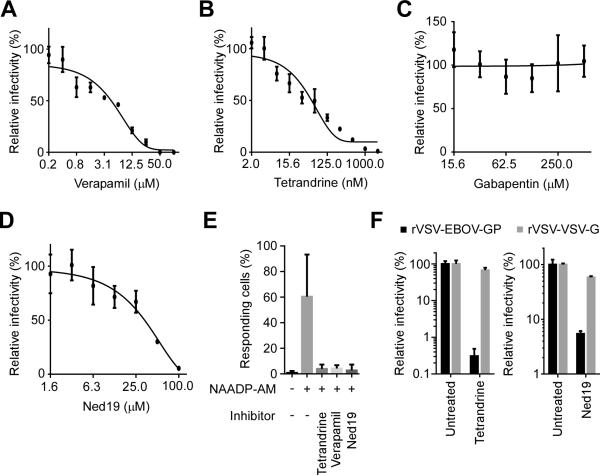
To identify and characterize upstream effectors regulating calcium signaling in the context of EBOV infection, the importance of calcium channels was tested using antagonists for each of the 4 common channel types (Fig. 1, A-C; and fig. S1). Only compounds blocking L-type channels inhibited EBOV infection in HeLa cells, which is consistent with previous reports (15, 16). Verapamil, an FDA-approved drug used to treat cardiovascular diseases, efficiently inhibited EBOV infection with an IC50 of 4 μM (Fig. 1A). Similarly, two other structurally distinct L-type channel antagonists, nimodipine and diltiazem, also reduced EBOV infection efficiency (fig. S1, A and B). Tetrandrine, originally isolated from Chinese and Japanese herbs but now produced synthetically, was especially potent, with an IC50 of 55 nM (Fig. 1B). By contrast, gabapentin, representing a fifth distinct class of L-type channel inhibitor, had no effect even at high concentrations (Fig. 1C). This finding suggested that classical L-type channels were not the upstream factor in EBOV calcium-channel dependence. Recently, verapamil, nimodipine and diltiazem were shown to also inhibit calcium signaling triggered by nicotinic acid adenine dinucleotide phosphate (NAADP) (17). NAADP is a highly potent intracellular calcium mobilizing agent and stimulates intracellular calcium channels to release Ca2+ from endosomes and lysosomes (18). This pathway is specifically blocked by the small molecule antagonist Ned19 (19). We found that Ned19 also blocked EBOV infection (Fig. 1D). All inhibitors tested showed no cytotoxicity at the highest concentration used (fig. S2). Like verapamil and Ned19, tetrandrine was also a potent inhibitor of NAADP-stimulated calcium release (Fig. 1E; and fig. S3). These results suggested a role for NAADP-stimulated calcium channels in EBOV infection and that tetrandrine could block this host factor.
Fig. 1. Inhibitors of NAADP signaling block EBOV infection.
Dose response curves for verapamil (A), tetrandrine (B), gabapentin (C) and Ned19 (D) were determined by pretreating HeLa cells with the indicated doses of each compound and then infecting with a recombinant EBOV encoding GFP as a marker of infection (EBOV-GFP). Infection efficiencies were calculated by dividing the numbers of GFP positive cells by those of total cells and normalizing the infectivity to untreated cells (mean ± SD, n = 3). Each data set is representative of three independent experiments. (E) The impact of the indicated compounds on NAADP-stimulated calcium release was measured by stimulating cells with 1 μM NAADP-AM (30, 31) or control DMSO and imaging cell fluorescence after addition of the calcium sensitive dye, Fluo-4. Cells showing Fmax/F0 > 2 (Fmax: maximum fluorescence intensity, F0: mean fluorescence intensity before stimulation) were counted as responsive cells. At least 800 cells were analyzed for each treatment and data averaged over 3 experiments ± SD. (F) Pseudotyped viruses bearing the glycoproteins of EBOV (rVSV-EBOV-GP) or VSV (rVSV-VSV-G) and encoding firefly luciferase as an infection marker were used to show entry dependence of EBOV on NAADP signaling. Cells were treated with tetrandrine (2 μM) or Ned19 (100 μM), and then infected with either pseudotyped virus. Luciferase activities were normalized to untreated controls.
NAADP has been suggested to regulate endosome maturation through vesicular fusion and trafficking (20). This would suggest a role in virus entry into cells, which was tested using pseudotyped viruses. Infection of cells by recombinant vesicular stomatitis virus bearing the glycoprotein of EBOV (rVSV-EBOV-GP) was highly sensitive to tetrandrine, verapamil or Ned19 (Fig. 1F; and fig. S4A). This suggests that NAADP-stimulated channel activity specifically affects the GP-mediated entry step of EBOV. Moreover, tetrandrine and verapamil potently inhibited infection of recombinant VSV bearing Marburgvirus glycoprotein, but only weakly for VSV, Lassa virus, Venezuelan equine encephalitis virus or Rabies virus (Fig. 1F; and fig. S4, B and C), suggesting filoviruses are much more dependent on this pathway than other virus types.
To gain further insight into the connection between the NAADP-mediated pathway and EBOV infection, we sought to identify the effector calcium channel required for the infection. Recent studies have shown that two pore channels (TPCs) are the major calcium channels activated by NAADP (21). They are also activated by the phosphoinositide PI(3,5)P2 and are highly conserved proteins with both TPC1 and TPC2 present in humans, mice and other animals (22). We found that mouse embryonic fibroblasts (MEFs) lacking TPC1 or TPC2 expression (Tpcn1−/− Tpcn2−/−) resisted EBOV infection (Fig. 2A). Overexpression of human TPCs in the mutant cells significantly recovered the infectivity (fig. S5), suggesting the specific effects of gene knockout. Similarly, even though suppression of TPC expression by small interfering RNAs (siRNAs) was incomplete (fig. S6), EBOV infection was reduced in HeLa cells transfected with either TPC1 or TPC2 siRNAs (Fig. 2B). In addition, overexpression of a dominant negative form of TPC2, which was reported to efficiently block NAADP-stimulated calcium release (23), inhibited EBOV infection (Fig. 2C). Furthermore, Ebola virus-like particles (VLPs) incubated with cells, localized to TPC1 and TPC2 positive endosomal compartments (Fig. 2D). Whole endolysosomal patch-clamp analyses showed that tetrandrine blocked both TPC1- and TPC2-mediated current elicited by PI(3,5)P2 as well as NAADP (Fig. 2, E and F; and fig. S7). In contrast, gabapentin, which did not inhibit virus infection, had no effect on TPC2 function. Together, our data showed that TPCs, the effector channels of NAADP and PI(3,5)P2-mediated signaling, are important for EBOV infection, probably while virus is inside endosomes. Calcium channel inhibitors targeted TPCs, with tetrandrine being the most potent.
Fig. 2. The endosomal calcium channels TPC1 and TPC2 are necessary for EBOV infection.
(A) Mouse embryonic fibroblasts (MEFs) from WT, Tpcn1−/− or Tpcn2−/− mice (25, 32) were infected with EBOV-GFP. The frequency of GFP positive cells in the total cell population was normalized to total cells. (B) HeLa cells were transfected with either two independent non-targeting, TPC1-specific or TPC2-specific siRNAs and infected with EBOV-GFP. The frequency of GFP positive cells in the total cell population was normalized to mock-transfected cells. (C) HeLa cells overexpressing a dominant negative form of TPC2 (L265P) tagged with GFP were infected with WT EBOV. Cells expressing GFP alone were used as control. Infected cells were detected using anti-EBOV GP antibody. The proportion of cells showing GFP fluorescence that were infected was calculated. All data for (A), (B) and (C) are mean ± SD (n = 3) and representative of three independent experiments. (D) Colocalization of Ebola VLPs with TPC1 or TPC2 was determined by incubating VLPs (red) for 2 h with cells transfected with TPC1 or TPC2 tagged with GFP (green). Colocalized particles were indicated by arrowheads. Scale bars, 10 μm. (E) Whole endolysosomal currents were recorded from TPC2-expressing HEK293T cells using modified conventional patch-clamp with PI(3,5)P2 (33-35). Current-voltage relations were recorded in the presence or absence of tetrandrine (500 nM). (F) Bar diagram summarizing data of current amplitude of TPC2 or TPC1 in the presence of gabapentin (100 μM), Ned19 (200 μM) or tetrandrine (500 nM), normalized to those before drug application. * indicates P < 0.001 using Student's t test, compared to current in the presence of gabapentin for TPC2 or without inhibitors for TPC1. Data are mean ± SEM.
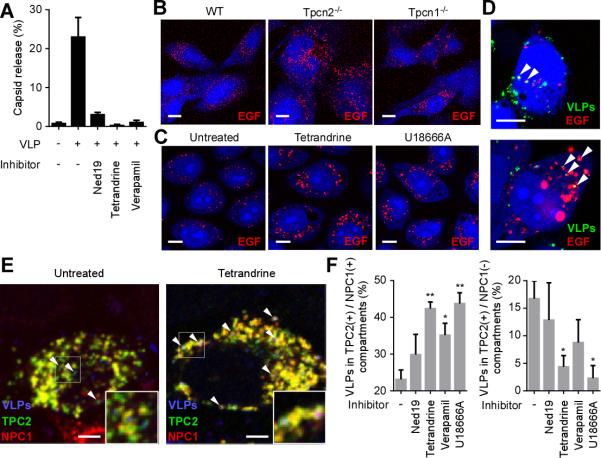
During host cell entry, EBOV is transported to acidic endosomes, which express LAMP1 (9). We found that VLPs still localized to LAMP1-positive vesicles in Tpcn1−/− and Tpcn2−/− MEFs as well as inhibitor-treated cells (fig. S8), indicating that this step was unaffected. The EBOV GP is then cleaved by endosomal cysteine proteases before virus-endosome membrane fusion can occur (11), and so we next examined if pre-cleaved GPs could overcome the action of the inhibitory drugs using rVSV-EBOV-GP pretreated with the protease thermolysin. Treatment with Ned19, tetrandrine or verapamil still efficiently blocked pre-cleaved virus infection, but a control cysteine protease inhibitor E-64-D did not (fig. S9), indicating that the calcium channel inhibitors impact a late entry step following GP proteolysis in endosomes. When membrane fusion was evaluated, using a virus contents release assay (24), these inhibitors significantly reduced the contents mixing signal (Fig. 3A; and fig. S10), indicating that virus-endosome membrane fusion and virus capsid release into the cell cytoplasm were arrested.
Fig. 3. Blocking TPC function impacts EBOV entry through endosomal compartments.
(A) VLPs loaded with β-lactamase were used to measure membrane fusion and virus capsid release into the cytoplasm after each treatment (as for fig. S10). The number of cells showing signal were divided by the number of total cells. (B) Evaluation of EGF trafficking in TPC knockout cells. Representative confocal images of WT, Tpcn2−/− and Tpcn1−/− MEFs incubated with AlexaFluor555-EGF. (C) Evaluation of EGF trafficking in tetrandrine or U18666A-treated cells. Representative confocal images of HeLa cells incubated with AlexaFluor555-EGF (red) in the presence or absence of tetrandrine or U18666A. (D) Colocalization of Ebola VLPs and EGF. HeLa cells were incubated with AlexaFluor555-EGF (red) for 30 min followed by Ebola VLPs (green) for 3.5 h in the presence of tetrandrine. VLPs were stained with a GP-specific antibody. Examples of colocalized particles are indicated by arrowheads. Scale bars, 10 μm for (B), (C) and (D). (E) Effect of tetrandrine on colocalization of Ebola VLPs with TPC2- and/or NPC1-positive endosomes was measured. HeLa cells overexpressing GFP-tagged TPC2 (green) and myc-tagged NPC1 (red) were pretreated with inhibitors and incubated with VLPs (blue) for 4 h. Insets show magnified areas of the image and arrowheads indicate examples of VLPs that are associated with the TPC2(+)/NPC1(−) compartment (left panel) or the TPC2(+)/NPC1(+) compartment (right panel). Scale bars, 5 μm. (F) In the presence of the indicated inhibitors, the ratio of VLPs colocalizing with the TPC2(+)/NPC1(+) compartment (left) or the TPC2 (+)/NPC1(−) compartment (right) was calculated. * and ** indicate P < 0.05 and P < 0.005, respectively, using unpaired Student's t-test to compare treated to untreated cells. Data are mean ± SEM (n = 3 or 4).
A recent study showed that blocking TPC2 function resulted in accumulation of epidermal growth factor (EGF) in LAMP1-positive endosomal compartments, suggesting a block of endosomal trafficking in the acidic compartments (25). We found that tetrandrine-treated HeLa cells showed a similar accumulation of EGF as well as Tpcn2−/− MEFs while Tpcn1−/− MEFs showed less EGF accumulation (Fig. 3, B and C). Moreover, Ebola VLPs and EGF colocalized in tetrandrine-treated cells (Fig. 3D), suggesting that both are using or converging upon a common endosomal trafficking route which is regulated by TPCs. Previously, EBOV entry was shown to be dependent on another endosomal protein, NPC1 (12, 13). The small molecule U18666A induces a phenotype that mimics NPC1 deficiency, leading to cholesterol accumulation in endosomes. When cells were treated with U18666A, the pattern of EGF accumulation was similar to that seen after treatment with tetrandrine (Fig. 3C). Moreover, treatment of rVSV-EBOV-GP-infected cells with verapamil or U18666A revealed similar inhibitory kinetics with each becoming ineffective when the drug was added 1.5-2 hours post-infection (fig. S11), suggesting that each impacted virus infection close to the same time. To further investigate this relationship and characterize the infection step affected by TPCs, viral colocalization with NPC1 or TPC2 was investigated (Fig. 3E). In untreated cells, VLPs were found in compartments containing both NPC1 and TPC2 as well as a distinct compartment containing only TPC2. However, treatment with tetrandrine significantly (and other channel inhibitors less potently) increased accumulation of VLPs in the TPC2(+)/NPC1(+) compartment while proportionately decreasing TPC2(+)/NPC1(−) compartment colocalization (Fig. 3F). These results suggest that disrupting endosomal trafficking with tetrandrine potently alters viral distribution such that VLPs are retained in the NPC1(+) compartment. Since decreased colocalization with the TPC2(+)/NPC1(−) compartment correlated with reduced infectivity, EBOV likely uses this compartment to enter host cells. U18666A again gave a similar outcome in VLP localization as calcium channel inhibitors (Fig. 3F). This may be explained by a recent report showing that U18666A treatment caused endosomal calcium depletion. Moreover, cells carrying a defective NPC1 were shown to have a loss of NAADP response, suggesting a close association of TPCs and NPC1 in host cells, which may affect EBOV infection (26).
Finally, we addressed whether TPC function could be targeted for anti-EBOV therapy. First, primary macrophages, an initial target of virus infection in humans and other animals were evaluated. Similar to HeLa cells, tetrandrine potently blocked EBOV infection in human monocyte-derived macrophages, with verapamil and Ned19 being effective but requiring high doses (Fig. 4A; and fig. S12) that did not show cytotoxicity. Of these, tetrandrine was the best candidate for animal testing because of its high potency and low cytotoxicity in culture. Moreover, the dose of tetrandrine needed to inhibit virus infection (IC50 = 55 nM) was at least 40 times less than safe plasma concentrations achieved in mice and was reported to have good pharmacological properties, being well tolerated and having a long circulatory time (27). We therefore assessed therapeutic efficacy in the mouse model of EBOV disease (28). Mice were challenged with mouse-adapted EBOV, and then given tetrandrine or saline every 2 days for 1 week. Starting tetrandrine treatment soon after infection significantly enhanced the survival of mice without any detectable side effects (Fig. 4B). Clinical scores in treated mice remained low compared to the control group (Fig. 4, C and D). Virus titers in sera measured at day 3 post-inoculation showed a 1000-fold decrease (Fig. 4E) and by day 9 virus was undetectable. Furthermore, when the treatment was started 1 day after virus challenge, half the mice survived (Fig. 4F). These results indicate that tetrandrine is highly effective against disease in mice.
Fig. 4. Tetrandrine inhibits EBOV infection both in vitro and in vivo.
(A) Macrophages were treated with tetrandrine (8 μM) and then infected with EBOV-GFP. After 48 h, the frequency of GFP positive cells was calculated and normalized to untreated controls. The data are mean ± SD (n = 3) and representative of two independent experiments. (B) Female Balb/c mice injected intraperitoneally with mouse-adapted EBOV were treated with 30 mg/kg of tetrandrine or control saline on days 0, 1, 3, 5 and 7 (n = 8 for each group). Survival curves are shown. * P = 0.0008 by log-rank (Mantel-Cox) test. (C and D) Clinical scores of EBOV-infected mice. Disease signs included weight loss, rough hair coat, squinty eyes, hunched back, moderate unresponsiveness, labored breathing and persistent prostration. Based on these criteria, a clinical score for each day was calculated and plotted (individually indicated by symbols) for the untreated animals (C) or tetrandrine-treated animals (D). (E) Virus titer in sera of infected mice was measured by plaque assays. * P = 0.006 by unpaired Student's t test. (F) Delayed treatment of EBOV-challenged mice. Female Balb/c mice injected intraperitoneally with mouse-adapted EBOV were treated with 30 mg/kg or 90 mg/kg of tetrandrine or control saline on days 1, 3, 5 and 7 (n = 7 for each group). Survival curves are shown * P = 0.04 by log-rank (Mantel-Cox) test comparing to untreated animals.
Taken together, we identified a role for TPCs in EBOV infection. These calcium channels appear responsible for controlling movement of endosomes containing virus particles. By disrupting TPC function we prevented EBOV from escaping the endosomal network into the cell cytoplasm, preventing infection. TPCs proved effective targets for existing drugs, with the bisbenzylisoquinoline alkaloid, tetrandrine, being the most potent. This may be due to its ability to block both TPC1 and TPC2, which regulate different stages of endosomal trafficking (22). Tetrandrine is one representative from this drug class with others being found in plants around the world (29) and these may also block EBOV infection. Since the entry of Marburgvirus, a distantly related filovirus, was also affected, it is likely that all filoviruses require TPC function to infect cells and that tetrandrine is a broad-spectrum filovirus inhibitor.
Supplementary Material
Acknowledgments
All BSL4 work was performed at Texas Biomedical Research Institute by YS and veterinary staff. For advice about preparation of NAADP-AM, we thank G. Churchill. For guidance and use of their HPLC, we thank A. Hayhurst and L. Sherwood. We thank D. Ren, University of Pennsylvania, for guidance in using the modified patch clamp. A. Reyes and J. Bentz provided technical support. O. Shtanko and M. Anantpadma gave helpful discussions and advice. We also thank all those cited in Material and Methods for reagents. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. This work was supported by NIH R01AI063513, DOD/DTRA HDTRA1-12-1-0002, Project FRBAA09-6H-2-0043, the Ewing Halsell Foundation, SFB TRR 152 TP04, TP05, TP06, and TP12.
Footnotes
Supporting online material.
Materials and methods
Figs. S1 to S13.
References and Notes
- 1.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011 Mar 5;377:849. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baize S, et al. Emergence of Zaire Ebola Virus Disease in Guinea - Preliminary Report. The New England journal of medicine. 2014 Apr 16; doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich BM, et al. Potential vaccines and post-exposure treatments for filovirus infections. Viruses. 2012 Sep;4:1619. doi: 10.3390/v4091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolnik O, Kolesnikova L, Becker S. Filoviruses: Interactions with the host cell. Cellular and molecular life sciences : CMLS. 2008 Mar;65:756. doi: 10.1007/s00018-007-7406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boguski MS, Mandl KD, Sukhatme VP. Drug discovery. Repurposing with a difference. Science. 2009 Jun 12;324:1394. doi: 10.1126/science.1169920. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez CP, et al. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. Journal of virology. 2002 Jul;76:6841. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takada A, et al. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. Journal of virology. 2004 Mar;78:2943. doi: 10.1128/JVI.78.6.2943-2947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondratowicz AS, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proceedings of the National Academy of Sciences of the United States of America. 2011 May 17;108:8426. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS pathogens. 2010;6:e1001110. doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanbo A, et al. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS pathogens. 2010;6:e1001121. doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005 Jun 10;308:1643. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cote M, et al. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011 Sep 15;477:344. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carette JE, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011 Sep 15;477:340. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolokoltsov AA, Saeed MF, Freiberg AN, Holbrook MR, Davey RA. Identification of novel cellular targets for therapeutic intervention against Ebola virus infection by siRNA screening. Drug development research. 2009 Jun 1;70:255. doi: 10.1002/ddr.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madrid PB, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PloS one. 2013;8:e60579. doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehring G, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. The Journal of antimicrobial chemotherapy. 2014 Apr 7; doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genazzani AA, et al. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. British journal of pharmacology. 1997 Aug;121:1489. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galione A. NAADP receptors. Cold Spring Harbor perspectives in biology. 2011 Jan;3:a004036. doi: 10.1101/cshperspect.a004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naylor E, et al. Identification of a chemical probe for NAADP by virtual screening. Nature chemical biology. 2009 Apr;5:220. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruas M, et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking. Current biology : CB. 2010 Apr 27;20:703. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009 May 28;459:596. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu MX, et al. Calcium signaling via two-pore channels: local or global, that is the question. American journal of physiology. Cell physiology. 2010 Mar;298:C430. doi: 10.1152/ajpcell.00475.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brailoiu E, et al. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. The Journal of biological chemistry. 2010 Dec 3;285:38511. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez O, et al. Zaire Ebola virus entry into human dendritic cells is insensitive to cathepsin L inhibition. Cellular microbiology. 2010 Feb;12:148. doi: 10.1111/j.1462-5822.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm C, et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nature communications. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Evans E, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nature medicine. 2008 Nov;14:1247. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 27.Dai CL, et al. Tetrandrine achieved plasma concentrations capable of reversing MDR in vitro and had no apparent effect on doxorubicin pharmacokinetics in mice. Cancer chemotherapy and pharmacology. 2007 Oct;60:741. doi: 10.1007/s00280-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 28.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. The Journal of infectious diseases. 1999 Feb;179(Suppl 1):S248. doi: 10.1086/514292. [DOI] [PubMed] [Google Scholar]
- 29.Kwan CY, Achike FI. Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action. Acta pharmacologica Sinica. 2002 Dec;23:1057. [PubMed] [Google Scholar]
- 30.Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. The Journal of biological chemistry. 1995 Dec 22;270:30327. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- 31.Parkesh R, et al. Cell-permeant NAADP: a novel chemical tool enabling the study of Ca2+ signalling in intact cells. Cell calcium. 2008 Jun;43:531. doi: 10.1016/j.ceca.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Arndt L, et al. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Molecular biology of the cell. 2014 Mar;25:948. doi: 10.1091/mbc.E13-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012 Oct 12;151:372. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cang C, et al. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013 Feb 14;152:778. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cang C, Bekele B, Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nature chemical biology. 2014 Jun;10:463. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.