Abstract
Objective
Reactive oxygen species (ROS) induced by exogenous toxicants are suggested to be involved in carcinogenesis by oxidative modification of DNA. 8-Hydroxyl-2-deoxyguanosine (8-OHdG) has been considered as a reliable biomarker for oxidative DNA damage both in vivo and in vitro studies. But the effect of smoking on oxidative damage has not yet been fully elucidated.
Methods
Wistar rats were exposed to cigarette smoke at concentrations of 20 and 60 % for 30 min, twice/day for 45 weeks. Then the histopathology of lung tissues, levels of ROS, 8-OHdG, and total antioxidant (T-AOC), expression of DNA repair enzymes, e.g. 8-oxyguaine DNA glycosylase (OGG1), and MutThomolog 1 (Oxidized Purine Nucleoside Triphosphatase, MTH1) were determined in urine, peripheral blood lymphocytes, and lung tissue.
Results
The results showed that long-term cigarette smoke exposure can cause obvious damages of lung tissue in rats. In addition, a significant and cigarette smoke concentration-dependent increase in ROS and 8-OHdG were observed compared with the non-exposed control rats. In contrast, the expression of OGG1 and MTH1, and T-AOC levels were obviously decreased after long-term exposure to cigarette smoke.
Conclusion
These findings indicate that long-term exposure to cigarette smoker increases ROS levels, decreases total antioxidant capacity, and interferes DNA repair capacity that eventually induces oxidative DNA damage, which appears to play an important role in cigarette smoke-induced lung injury in rats, and determination of 8-OHdG levels might be a useful method for monitoring oxidative damage in cigarette smokers.
Keywords: Cigarette smoke, Rats, 8-Hydroxy-deoxyguanosine, Reactive oxygen species, Oxidative stress
Introduction
Cigarette smoking has been identified as the second leading risk factor for death from any cause of the world [1]. It is widely acknowledged that cigarette smoking is also the most important risk factor for lung cancer which is considered as one of the most malignant tumors with the highest incidence and mortality among others [2]. However, the mechanism of carcinogenesis by cigarette smoking is still not fully understood. It is well known that cigarette smoke contains large amounts of reactive oxygen species (ROS) [3, 4], and ROS-induced oxidative DNA damage has been reported to play a major role in lung cancer [5].
8-Hydroxyl-2-deoxyguanosine (8-OHdG), a major by-product of hydroxyl radical attack on DNA, is considered as a reliable biomarker for oxidative damage, because it can be quantitated with high sensitivity [6–10]. Besides, numerous studies provide evidence showing that 8-OHdG is also a key biomarker relevant to carcinogenesis [11–13]. More importantly, significantly increased levels of 8-OHdG were observed in the urine of smokers [14]. But the relationship between 8-OHdG level and lung damage induced by cigarette smoke inhalation remains to be further investigated.
In the present study, rats were exposed to different concentrations of cigarette smoke and the levels of reactive oxygen species (ROS), 8-OHdG, expression of DNA repair enzymes, viz., 8-oxyguaine DNA glycosylase (OGG1), and Mut Thomolog 1 (MTHa), as well as total antioxidant (T-AOC) levels were determined in the urine, peripheral blood lymphocytes, and lung tissue to assess the extent of oxidative damage induced by long-term cigarette smoke exposure.
Materials and methods
Animals and chemicals
All animal handling procedures were reviewed and approved by the animal care/user ethical committee of Soochow University, Suzhou City, PR China. 8-week-old Wistar rats weighing 100 ± 10 g were (obtained from the Shanghai Laboratory Animal Center of the Chinese Academy of Medical Sciences, Shanghai, China) housed in a facility which maintained 22 ± 1 °C, 50 ± 5 % relative humidity and a 12 h light–dark cycle. All animals were provided with food and water ad libitum during the course of the experiment.
Cigarette smoke and exposure
A smoking apparatus (Model BT00-300; Changhe pump; China dynamic inhalation contamination equipment, Chinese Academy of Military Medical Sciences, Beijing, China) and commercially available cigarettes made by ‘Great Gate’ in China were used to generate the smoke which was pumped into the inhalation chambers (Chinese Academy of Military Medical Sciences, Beijing, China). The exposure method was performed as described previously [15]. Briefly, a smoke sensor was set inside the chamber to detect the smoke concentration and to transmit it through a computer. The rats were exposed inside the chamber twice daily for 30 min each with a 4-h interval between exposures, and each time, 4–5 cigarettes (low concentration group) or 9–10 cigarettes (high concentration group) with a declared content of 13 mg tar, 1.4 mg nicotine, and 14 mg CO were consumed. The smoke concentration in the chamber was controlled using computer software (Kingview 6.03, China) at 20 and 60 % (the ratio of smoke volume to whole air volume in the chamber) for the two smoking groups defined as the low- and high-concentration group. The oxygen ratio in the chamber was set as 21 %. The exposures were conducted from Monday to Friday every week and lasted for 45 weeks. The control animals were exposed in the same exposure setup for the same time but without smoke.
Sample collection
For each rat, 24-h urine samples were collected on the fifth day of 5, 15, 25, and 45 weeks using metabolic cages. At the end of exposure, all animals were euthanized using chloral hydrate (1 ml/100 g body weight), peripheral blood was collected, lymphocytes separated using Ficoll regent, and serum was separated from whole blood by centrifugation at 3000g for 5 min. The left lung was removed and lavaged thrice with 0.9 % NaCl. The obtained bronchoalveolar lavage fluid cells (BALF cells) were centrifuged at 800g for 5 min. The right lung was excised for pathological examination. All samples were stored at −80 °C until detection.
Histopathology of lung tissues
The right lung was excised and fixed in 4 % phosphate-buffered paraformaldehyde, embedded in paraffin wax, 10 micron sections were stained with hematoxylin and eosin (H&E) for pathological observation, and a modified version of Masson trichrome stain was used to assess the degree of fibrosis.
ROS detection
The ROS levels in BALF cells were determined by confocal laser scanning microscopy (CLSM) using 10 μmol/l 2′,7′-dichlorofluorescein-diacetate (DCFH-DA). In brief, cells were incubated at 37 °C with DCFH-DA for 90 min and washed with phosphate-buffered saline. Two hundred microliters of the cell suspension was used to determine ROS. The mean fluorescence intensity indicated the ROS levels.
Antioxidant capacity measurement
The total antioxidant capacity (T-AOC) in serum and BALF cells was determined using commercial T-AOC kits (Nanjing Jiancheng Biotechnology Inc., China) according to the manufacturer’s protocol.
Measurement of 8-OHdG in urine, lymphocytes, and lung tissue
For urine samples, a part of each urine sample was centrifuged at 4000 rpm for 20 min, the supernatants of urine samples were collected and examined for their concentration of 8-OHdG. For lymphocytes and lung tissue samples, whole DNA was extracted from 106 lymphocytes and from 100 mg of lung tissue. Approximately 500 ng of extracted DNA was digested with nuclease P1 (1 U) and acid phosphatase (1 U) in a 10-mM sodium acetate solution. After incubation at 37 °C for 90 min, the mixture was centrifuged twice at 10,000g for 15 min and the supernatant collected. All supernatants were used to measure the 8-OHdG level using 8-OHdG ELISA kit (Uscnlife Life Sciences, Inc.) according to the manufacturer’s protocols. The sensitivity limit of this ELISA system was 46.875 pg/ml of 8-OHdG, and its determination ranged from 78.125 to 5000 pg/ml. The creatinine level in urine samples was measured by a two-point assay and used for 8-OHdG correction. Each sample was measured in duplicate and the level of 8-OHdG in lymphocytes and lung tissue samples was represented in pg/mg DNA and ng/mg DNA, respectively.
OGG1 and MTH1 detection
Approximately 100 mg of left lung tissue was used for total RNA extraction following the instructions in a commercial kit (Trizol®, Invitrogen). The extracted RNA was recovered by isopropyl alcohol precipitation and resuspended in diethyl pyrocarbonate water (OD260/280, 1.75–1.8). The expression levels of OGG1 and MTH1 were determined from 50 ng total RNA using RT-PCR technique (MyCycler™ Thermal cycler; Bio-RadInc., USA). β-actin was used as the standard control. The sequences of the oligonucleotide primers used were as follows:
- OGG1 (356bp),
- Sense: AACATTGCTCGCATCACTGGC,
- Antisense: GATGTCCACAGGCACAGCCTG;
- MTH1 (165bp),
- Sense: AGCACCTCCAGGCTTTATAC,
- Antisense:ACTGAGGGCGCATTTCTTCA;
- β-actin (405bp),
- Sense: TCAGGTCATCACTATCGGCAAT,
- Antisense: AAAGAAAGGGTGTAAAACGCA
OGG1 RT-PCR was conducted for 5 min at 94 °C for reverse transcription, 35 cycles of 1 min at 94 °C, 1 min at 60 °C, and 1 min at 72 °C. MTH1 RT-PCR was conducted at 5 min at 94 °C for reverse transcription, followed by 30 cycles, for each cycle, 30 s at 94 °C, 1 min at 55 °C, and 2 min at 72 °C. β-actin RT-PCR was amplified as follows: 3 min at 94 °C for reverse transcription, 30 cycles of 30 s at 94 °C, 30 s at 55 °C, and 1 min at 72 °C. The amplified PCR products were visualized by GelRed staining and quantified with a gray scale scanner linked to software (Bandleader3.0, Magnitec Co.). The values were normalized against the β-actin standard, and the relative expression levels were calculated.
Statistical analysis
The t test and one-way ANOVA were performed using SPSS software (version 11.0, SPSS Inc., Chicago, IL, USA) to test the difference of the results among groups. The figures were constructed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA).
Results
Pathological observation of lung tissue in rats after exposure to cigarette smoke
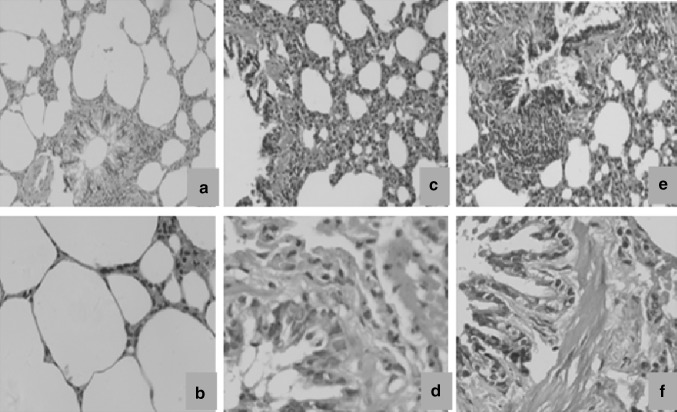
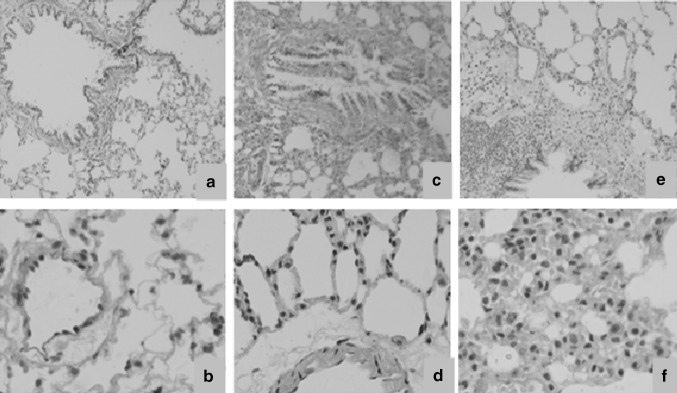
Photomicrographs of lung tissue sections of un-exposed control animals and rats exposed to low (20 %) and high (60 %) cigarette smoke concentrations stained using H&E and Masson trichrome are presented in Figs. 1 and 2. In H&E stained sections, large amount of vacuoles, goblet cell hyperplasia, mucus secretion, inter alveolar septum breakage, and inflamed lymphocytes in the alveolus interval were observed in lung tissue of rats exposed to low (20 %) concentrations of cigarette smoke (Fig. 1c, d). In lung tissue of rats exposed to high (60 %) concentrations of cigarette smoke, large amounts of inflammatory cells and vacuoles could be seen with enlarged and irregular cell nucleoli and collagen fibers filled in the interstitium of the alveolus. In addition, hyperemia in the alveolus interval and pulmonary edema were apparent, and the alveolar interstitium also became thicker (Fig. 1e, f). In Masson trichrome staining sections, compared with the control group, rats exposed to 20 and 60 % concentrations of cigarette smoke showed excessive collagen fibers (blue staining), filling in the alveolar interstitium, septal thickening, and peribronchial edema (Fig. 2c–f, respectively).
Fig. 1.
Photomicrographs of the lung tissues of the control rats (a, b) and those exposed to cigarette smoke (c–f). The tissue sections were stained with hematoxylin and eosin (H&E). a, c, e: magnification ×100; b, d f: magnification ×400
Fig. 2.
Histomorphology of the lung tissues of the control rats (a, b) and those exposed to cigarette smoke samples (c–f). Tissue sections were stained with Masson trichrome. a, c, e: magnification ×100; b, d, f: magnification ×400
Changes of ROS and T-AOC in rats after exposure to cigarette smoke
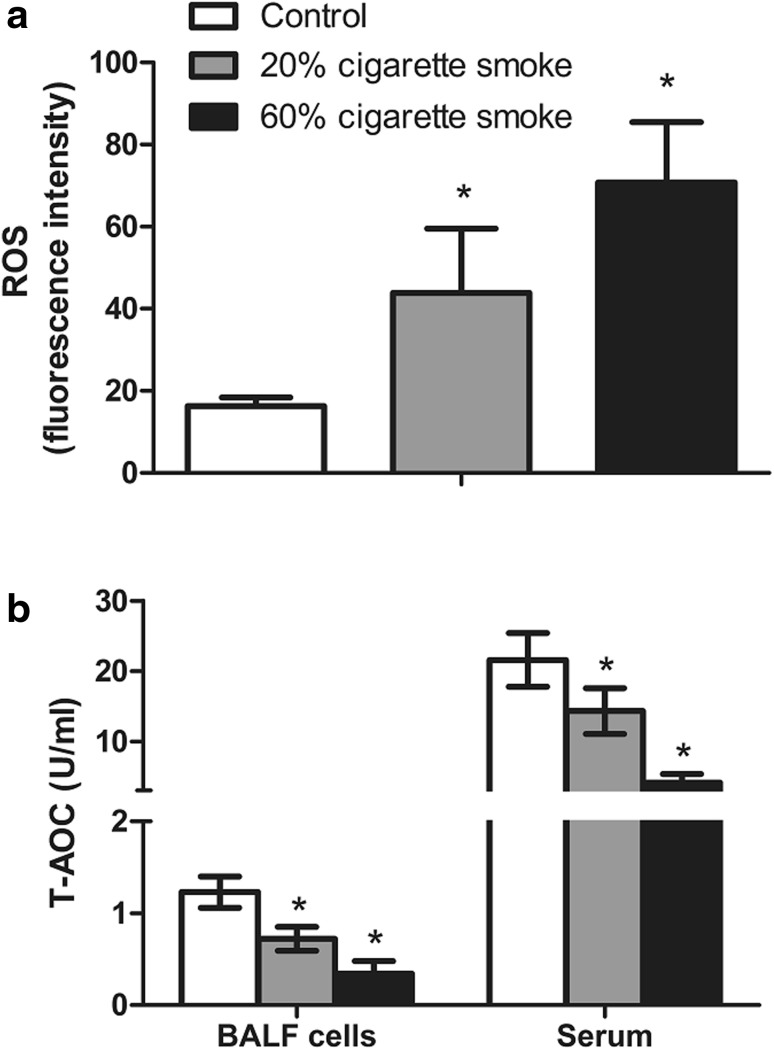
As shown in Fig. 3a, there was an obvious concentration-dependent increase in ROS levels (indicated by increased fluorescence intensity) in BALF cells of rats exposed to cigarette smoke compared with controls. On the contrary, the T-AOC activity showed a concentration-dependent decrease both in serum and BALF cells of rats exposed to low (20 %) and high (60 %) concentration of cigarette smoke compared with control group (Fig. 3b).
Fig. 3.
ROS levels a in broncho alveolar lavage fluid (BALF) cells in lung and T-AOC activity, b in broncho alveolar lavage fluid (BALF) cells in lung and serum of rats after exposure to cigarette smoke. *p < 0.05, compared with control group
8-OHdG levels in the urine, lymphocyte, and lung tissue
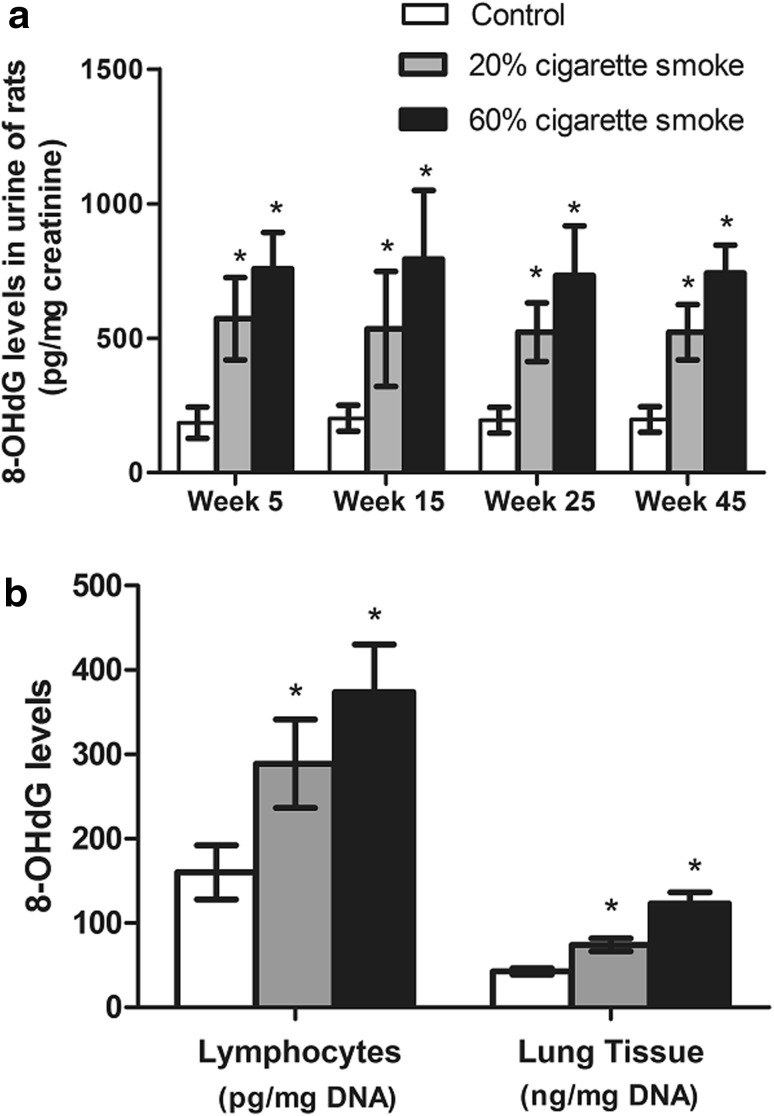
The results obtained on the 8-OHdG levels in the urine of rats exposed to low (20 %) and high (60 %) cigarette smoke concentrations are presented in Fig. 4a. Compared with un-exposed control rats, a significant and cigarette smoke concentration-dependent increase in 8-OHdG levels was observed after exposure for 5, 15, 25, and 45 weeks; however, no obvious time-dependent changes of 8-OHdG were found in rats after cigarette smoke exposure. Similar to urine results, the 8-OHdG levels in peripheral blood lymphocytes and lung tissue were also increased in a concentration-dependent manner after 45 weeks exposure to cigarette smoke (Fig. 4b).
Fig. 4.
Changes of 8-OHdG levels in rats after exposure to cigarette smoke. a 8-OHdG levels in urine of rats after exposure to cigarette smoke for different weeks. b 8-OHdG levels in lymphocytes and lung tissue of rats exposed to cigarette smoke. *p < 0.05, compared with control group
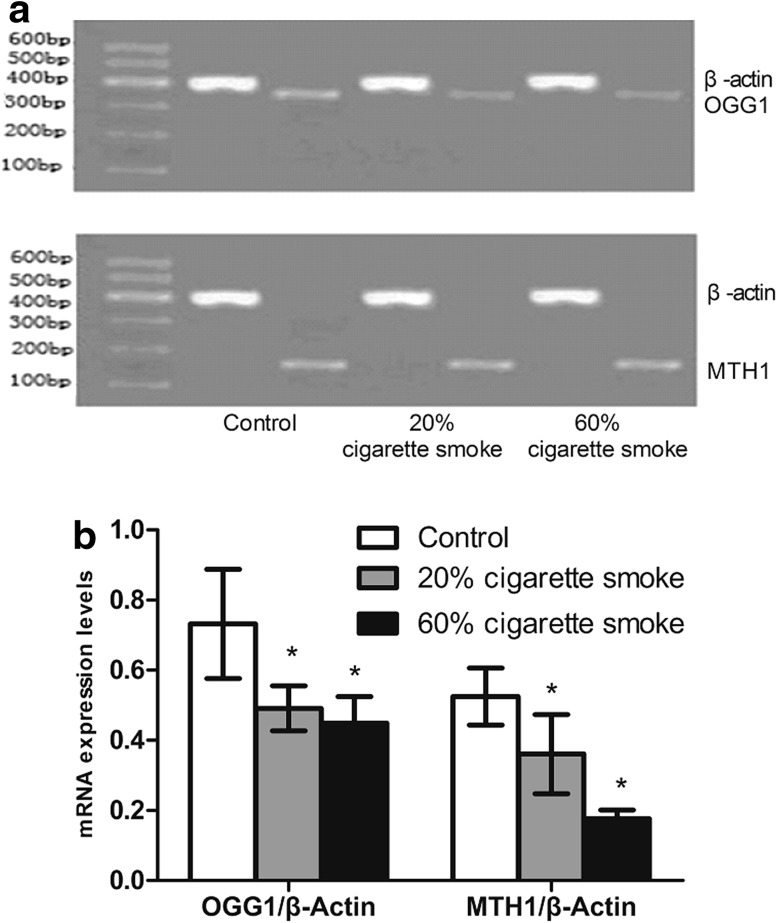
OGG1 and MTH1 mRNA expression in lung tissue
The expression levels of OGG1 and MTH1 measured by RT-PCR are presented in Fig. 5. The data indicated a significant down-regulation of OGG1 and MTH1 in rats exposed to low (20 %) and high (60 %) concentrations of cigarette smoke compared with unexposed control rats. The change of OGG1 was insignificantly different between low and high smoke concentration groups, whereas the MTH1 expression was significantly lower in high smoke concentration group (60 %) compared with low smoke concentration group (20 %).
Fig. 5.
mRNA levels of OGG1 and MTH1 in rats after exposure to cigarette smoke detected by RT-PCR. *p < 0.05, compared with control group
Discussion
It is well known that cigarette smoke contains thousands of different toxic substances such as free radicals, polycyclic aromatic hydrocarbons, aromatic amines, and tobacco-specific nitrosamines, etc., which are mainly metabolized in the lung and bronchus epithelium cells as an important step of lung carcinogenesis [16–18]. Epithelial cells in airway passage are directly exposed to cigarette smoke and hence are targets for cigarette smoke-induced damage. The pathological examination of right-lung tissue in rats exposed to cigarette smoke showed several abnormalities indicating smoke-induced lung injury.
Reactive oxygen species (ROS) generated in the smoke play an important role in carcinogenesis in lung cancer patients [5]. Carcinogenesis is a multi-step process involving initiation, promotion, and progression. Initiation is a step where increased ROS are produced exogenously by exposure to toxic chemicals/agents (or endogenously in normal physiological processes) which induces DNA oxidative damage in a normal cell, resulting in chromosome instability, mutations in critical genes, and ultimately carcinogenesis [19]. In the present study, ROS in the BALF cells of rats exposed to low and high concentrations of cigarette smoke were increased significantly with a corresponding decrease in T-AOC compared to that in un-exposed control rats. These results suggested that ROS in cigarette smoke might disturb the balance between oxidant and antioxidant status resulting in decreased anti-oxidative capability and eventually inducing DNA oxidative damage in cigarette smoke-exposed rats.
The relevance of 8-OHdG is considered as the best characterized oxidatively damaged DNA lesions and a reliable biomarker for oxidative stress. Over 100 of such adducts have been identified in an oxidatively stressed cell [20, 21]. Increased levels of 8-OHdG levels in blood and various tumor tissues have been reported in several animal carcinogenic studies [22, 23]. In the current study, when rats were exposed to low (20 %) and high (60 %) concentrations of cigarette smoke for 45 weeks, a significant and cigarette smoke concentration-dependent increase in 8-OHdG levels was observed in peripheral blood and lung tissues compared to those in unexposed control rats, which was consistent with those earlier reports [22, 23]. In addition, the same trend of variability was also found between 8-OHdG levels and ROS levels in serum and lung tissue of cigarette smoke-exposed rats. These observations further confirmed that oxidative DNA damage occurred in rats after long-term cigarette smoke exposure. It is worth mentioning that the 8-OHdG level in lung tissue was significantly higher than that in urine and peripheral blood lymphocytes, which may be due to that lung is a direct target organ for cigarettes smoke inhalation while urine and peripheral blood lymphocytes are exposed to cigarette smoke in an indirect way. It has been reported that most of the effects of smoking were observed in tissue or blood but poorly in urine. In those studies, little difference was found in urinary 8-OHdG between smokers and normal individuals [24]. Our studies demonstrated that 8-OHdG levels in urine showed cigarette smoke concentration-dependent increase but not a time-dependent manner, which indicated that the concentration of 8-OHdG in urine might have been saturated after continuous exposure to cigarette smoke for 5 weeks; therefore, urinary 8-OHdG may be a useful biomarker for short-term cigarette smoke exposure but not for long-term exposure. And this result suggested a new view in exploring smoke exposure.
DNA damage induced by exogenous toxicants could alter signaling pathways, leading to up- and/or down-regulation of several genes which encode key DNA repair proteins which maintain the integrity of DNA, such as OGG1, MTH1, and APE1. These genes are also reported to be associated with the carcinogenesis process [25–27]. In mammals, OGG1 is responsible for the removal of 8-OHdG adducts. Human mutT homologue (hMTH1), 8-hydroxy-2′-deoxyguanosine-5′-triphosphate pyrophos phohydrolase (8-OH-dGTPase) degrades 8-OH-dGTP to 8-OH-dGMP and pyrophosphate. 8-OH-dGTP can be formed from the direct oxidation of dGTP or the phosphorylation of 8-OH-dGDP [28]. 8-OH-dGTP can then be used as a substrate by DNA polymerases, which can pair with adenine and cytosine with equal efficiency to induce transversion mutations [28–31]. 8-OHdG accumulation in mammalian cells is prevented by the base excision repair enzymes OGG1, MYH and NEIL1, and by MTH1, an enzyme that removes 8-hydroxy-dGTP from the intracellular nucleotide pool [32]. In the present study, rats exposed to cigarette smoke had decreased levels of OGG1 and MTHa, indicating that cigarette smoke exposure may further enhance oxidative damage by interfering with DNA repair capacity.
In conclusion, the present study provides evidence suggesting that long-term exposure to cigarette smoke increases ROS levels, decreases total antioxidant capacity, and interferes DNA repair capacity that eventually induces oxidative DNA damage, which appears to play an important role in cigarette smoke-induced lung injury in rats, and determination of 8-OHdG levels might be a useful method for monitoring oxidative damage in cigarette smokers.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (81402626, 81172707 and 81473008), natural science fund for colleges and universities in Jiangsu Province (12KJB330005), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Postgraduate Innovation Project of Jiangsu province (CXZZ13_0841).
Conflict of interest
No potential conflicts of interest to disclose.
Footnotes
Z. Chen and D. Wang contributed equally to this work.
Contributor Information
Jihua Nie, Phone: 86-512-65880073, Email: niejihua@suda.edu.cn.
Jian Tong, Email: tongjian@suda.edu.cn.
References
- 1.Ahmedin J, Rebecca S, Ward EM. Global cancer facts & figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 2.Malyankar UM, MacDougall JR. Genome-scale analysis of lung cancer progression. Pharmacogenomics. 2004;4:169–176. doi: 10.2165/00129785-200404030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;130:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 6.Kasai H, Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984;12:21–37. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985;24:4476. doi: 10.1021/bi00337a032. [DOI] [PubMed] [Google Scholar]
- 8.Cho DH, Hong JT, Chin K, Cho TS, Lee BM. Organotropic formation and disappearance of 8-hydroxydeoxyguanosine in the kidney of Sprague–Dawley rats exposed to adriamycin and Br O3. Cancer Lett. 1993;74:141. doi: 10.1016/0304-3835(93)90235-2. [DOI] [PubMed] [Google Scholar]
- 9.Tagesson C, Kallberg M, Klintenberg C, Starkhammar H. Determination of urinary 8-hydroxydeoxyguanosine by automated coupled-column high performance liquid chromatography: a powerful technique for assaying in vivo oxidative DNA damage in cancer patients. Eur J Cancer. 1995;31A:934–940. doi: 10.1016/0959-8049(94)00490-0. [DOI] [PubMed] [Google Scholar]
- 10.Hond M, Yamada Y, Tomonaga M, Ichinose H, Kamihira S. Correlation of urinary 8-hydroxy-2%-deoxyguanosine (8-OHdG), a biomarker of oxidative DNA damage, and clinical features of hematological disorders: a pilot study. Leuk Res. 2000;24:461–468. doi: 10.1016/S0145-2126(00)00006-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi R, Hirano T, Asami S, Chung MH, Sugita A, Kasai H. Increased 8-hydroxyguanine levels in DNA and its repair activity in rat kidney after administration of carcinogen, ferric nitrilotriacetate. Carcinogenesis. 1996;17:2419–2422. doi: 10.1093/carcin/17.11.2419. [DOI] [PubMed] [Google Scholar]
- 12.Shimoda R, Nagashima M, Sakamoto M, Yamaguchi N, Hirohashi S, Yokota J, Kasai H. Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res. 1994;54:3171–3172. [PubMed] [Google Scholar]
- 13.Baik SH, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, et al. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996;56:1279–1282. [PubMed] [Google Scholar]
- 14.Yano T, Shoji F, Baba H, Koga T, Shiraishi T, Orita H, Kohno H. Significance of the urinary 8-OHdG level as an oxidative stress marker in lung cancer patients. Lung Cancer. 2009;63:111–114. doi: 10.1016/j.lungcan.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhang SP, Wu YW, Wu ZZ, Liu HY, Nie JH, Tong J. Up-regulation of rage and S100A6 in rats exposed to cigarette smoke. Environ Toxicol Pharmacol. 2009;28:259–264. doi: 10.1016/j.etap.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms andapproaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/S1470-2045(02)00815-X. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 19.Klaunig H, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Phrmacol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 20.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 21.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine poly-styrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharm. 2001;175:191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Fujita N, Sugimoto R, Urawa N, Horiike S, Kobayashi Y, et al. Hepatic oxidative DNA damage is associated with increased risk for hepatocellular carcinoma in chronic hepatitis C. Brit J Cancer. 2008;98:580–586. doi: 10.1038/sj.bjc.6604204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvilchuck JA, Pu X, Klaunig JE, Carlson GP. Indicators of oxidative stress and apoptosis in mouse whole lung and Clara cells following exposure to styrene and its metabolites. Toxicology. 2009;264:171–178. doi: 10.1016/j.tox.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Marnett LJ. Oxygen radicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 26.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 27.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Rehman A, Nourooz-Zadeh J, Möller W, Tritschler H, Pereira P, Halliwell B. Increased oxidative damage to all DNA bases in patients with type II diabetes mellitus. FEBS Lett. 1999;448:120–122. doi: 10.1016/S0014-5793(99)00339-7. [DOI] [PubMed] [Google Scholar]
- 29.Krapfenbauer K, Birnbacher R, Vierhapper H, Herkner K, Kampe D, Lubec G. Glycoxidation, and protein and DNA oxidation in patients with diabetes mellitus. Clin Sci. 1998;95:331–337. doi: 10.1042/cs0950331. [DOI] [PubMed] [Google Scholar]
- 30.Seidl R, Greber S, Schuller E, Bernert G, Cairns N, Lubec G. Evidence against increased oxidative DNA-damage in Down syndrome. Neurosci Lett. 1997;235:137–140. doi: 10.1016/S0304-3940(97)00748-9. [DOI] [PubMed] [Google Scholar]
- 31.Degan P, Bonassi S, De Caterina M, Korkina LG, Pinto L, Scopacasa F, et al. In vivo accumulation of 8-hydroxy-2′-deoxyguanosine in DNA correlates with release of reactive oxygen species in Fanconi’sanaemia families. Carcinogenesis. 1995;16:735–742. doi: 10.1093/carcin/16.4.735. [DOI] [PubMed] [Google Scholar]
- 32.Nakabeppu Y. Regulation of intracellular localization of humanMTH1, OGG1, and MYH proteins for repair of oxidativeDNA damage. Prog Nucleic Acid Res Mol Biol. 2001;68:75–94. doi: 10.1016/S0079-6603(01)68091-7. [DOI] [PubMed] [Google Scholar]