Abstract
Bacterial biomass collected from sheath-forming bacteria of the genera Sphaerotilus and Leptothrix was collected from a high-mountain natural stream water source. The elemental constitution and oxide phases of the products after selective cultivation of the bacteria on two different elective media using neutron activation analysis (NAA), electron microscopy (SEM, TEM), and X-ray diffraction (XRD) were studied. A high enrichment level of iron was revealed by the NAA technique in cultivated isolates as compared to the reference sample from nature. Three types of iron oxide compounds were established after cultivation in Adler’s medium: lepidocrocite (γ-FeOOH), magnetite (Fe3O4), and goethite (α-FeOOH). The cultivation in the Isolation medium yielded a single phase, that of goethite, excluding one sample with a distinguishable amount of lepidocrocite. XRD and EM investigations show that the biogenic oxides are nanosized. Our study exemplifies the possibilities of the biotechnology approach for obtaining, under artificial conditions, large quantities of iron-containing by-products that could be of further used in appropriate nano- and biotechnologies.
Keywords: Sheath-forming bacteria, Biogenic iron oxides, Neutron activation analysis, Nanotechnologies
Introduction
During the 1830s, iron bacteria were among the first groups of microorganisms to be recognized for carrying out a fundamental geological process—the oxidation of iron at neutral pH. Two types of bacteria that produce biogenic iron oxides have attracted the attention of researchers over the past decade: magnetotactic bacteria [1] and bacteria from the genera Sphaerotilus and Leptothrix [2]. In the first case, magnetite was found in specific inclusions—magnetosomes in the bacterial cell. For the second group, biogenic iron oxides are deposited on the surface of the bacterial cell where specific structures are formed. Biogenic iron oxides are formed during the cultivation of the bacteria at neutral pH on selective nutrient media. Delivery of the biogenic iron oxides from the bacteria mentioned above is a widespread process in nature, making them attractive in establishing a biotechnological method for voluminous production of such materials.
Iron bacteria are known for the unique morphology structures they produce [3], such as powders, sheaths, or stalks that act as organic matrices upon which the deposition of hydrous ferric oxides can occur. The bacteria from genera Sphaerotilus and Leptothrix are typical β-proteobacteria capable of oxidizing Fe2+ and Mn2+ at neutral pH and are commonly found in aquatic environments [3, 4]. They grow as chains of cells into filaments 0.4–7 μm in width. As a result of their metabolism, the bacteria form an extracellular material of iron oxides/(oxy)hydroxides accumulated in their “sheaths”. Leptothrix ochracea forms short and mostly empty sheaths in contrast to the relatively long sheaths, partly filled with cells, of the typical sewage bacteria Sphaerotilus natans. The presence of the sheaths has nutritional and ecological consequences for this group of bacteria. This concerns particularly their growth in slowly running waters low in nutrients, where the presence of the sheath helps the bacteria to attach themselves to solid surfaces. In aquatic environments, Leptothrix bacteria produce uniquely shaped hollow microtubules composed of aquatic inorganic and bacterium-derived organic hybrids, and the wall structure of these formations consists of nanoparticles with spherical forms [4].
Due to the contradictory theoretical explanations of their metabolism, studies of Fe-oxidizing bacteria at neutral pH have lagged in comparison to other bacteria, oxidizing iron at acidophilic conditions. On the other hand, these microorganisms offer new opportunities for studying fundamental biological processes that can be of practical importance. Numerous investigations have already been performed and provided information about the possibilities of their important applications as pigments, catalysts, adsorbents, etc. [4].
The main objective of the present study is to examine the iron oxides as products of bacterial metabolism by means of instrumental neutron activation analysis, X-ray diffraction, and electron microscopy. Also of interest in this study are the bacterial cultivation and the degree of crystal structure formation of the iron oxides. Attention is also paid to the optimization of the parameters of cultivation directed to development of an optimized scheme for isolation and enrichment of the sheath-forming Leptothrix bacteria with possible implications for nanotechnology.
The material investigated was obtained in powder form in small amounts and we applied k0-NAA for determination of the elemental constitution of the biogenic products. k0-NAA was chosen instead of inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy (AAS), or other sensitive analytical methods because the NAA technique is non-destructive and allows analysis of the solid material without sample preparation and standardization problems. Introduction of a solid sample in ICP-MS by electrothermal vaporization or laser ablation entails problematic standardization and accurate and precise analyses are only possible after thorough optimization for each individual variety of biogenic material.
Experimental
Materials and cultivation
The source of bacterial biomass was a natural stream flowing in Vitosha Mountain at an altitude of 1,783 m where deposits with specific brown-red color due to iron oxides are observed (Fig. 1). The samples, filtered and homogenized, were used as inoculation for isolation of the iron bacteria on seven elective nutrient media. The bacteria were cultivated under static and dynamic conditions at 20 °C for different periods of time at conditions close to those of the natural environment (pH, aeration, temperature). The cultivation was performed both in Erlenmeyer flasks by shaking at 70 rpm and in a specially constructed bioreactor with additional aeration. Periodically, samples were taken and were analyzed by light and electron microscopy. Typically, enriched cultures were obtained after 14 days of cultivation.
Fig. 1.

Typical deposits in natural water flow in Vitosha Mountain
From the media tested, the Leptothrix bacteria were selectively grown successfully on two elective media: Adler’s and Isolation medium. The Adler’s medium (AM) (pH 7.0) [7] contained sodium lactate (NaC3H5O3) 40 mg, yeast extract 1 g, ascorbic acid (C6H8O6) 0.1 g, MgSO4.7H2O 0.2 g, K2HPO4 0.01 g, (NH4)2Fe(SO4)2 · 6H2O 0.1 g and 1,000 ml of distilled water.
The Isolation medium (IM) (pH 7.0) [8] contained glucose (C6H12O6) 0.150 g, (NH4)2SO4 0.500 g, Ca(NO3)2 0.010 g, K2HPO4 0.050 g, MgSO4 · 7H2O 0.050 g, KCl 0.050 g, CaCO3 0.100 g, cyanocobalamin (vitamin B12) 0.00001 g, thiamine (vitamin B1) 0.0004 g and 1,000 ml of distilled water.
As a source of Fe, iron cuttings (10% in volume) were added.
Both media were used for isolation of the pure cultures as well as for receiving relatively large amounts of iron-containing material.
The identification of the pure cultures obtained from enriched culture was performed according to the taxonomic scheme of the Bergey Manual of Determinative Bacteriology [5] including morphological, physiological, and biochemical characteristics. Confirmation of the taxonomic status of the isolates was done by PCR detection assay. The published sequence of mofA gene (GenBank № Z25774.3) was chosen as a specific target for PCR detection of Leptothrix sp. The specific primers were constructed with Primer-Blast Software. F1_ thrix is 5′- TGT TCG AGC CGG T TCG GC - 3′ and R1_ thrix is 5′- GAA TCG ATC GCG AC C GGC GT - 3′ (LKB GmbH).
Several different samples were investigated:
Two samples denoted ‘2 Adler-DE’ and ‘2 Adler-DP’ were products after cultivation of the bacteria in AM with ammonium iron (II) at different periods of cultivation: Adler-DE – 56 days cultivation in Erlenmeyer flasks of Leptothrix sp, Adler-DP – 88 days cultivation in Roux flasks, enriched culture.
Three samples denoted ‘1 Isolation-МР’, ‘1 Isolation-МY’ and ‘1 Isolation-МF’ were products from bacterial action of the bacteria in IM with (NH4)2Fe(SO4)2 · 6H2O: sample 1MP – 103 days cultivation on Isolation medium in Roux flasks; sample 1MY – 103 days cultivation in bioreactor; sample 1MF – 103 days cultivation on Isolation medium in Fernbach flask.
-
‘Natural-A’ – a mat taken from a natural source in Vitosha Mountain (Fig. 1).
All the samples were filtered, dried at 20 °C and the resulting powder was subjected to analyses as further described.
Methods
Structural characterization of the biogenic iron oxides/(oxy)hydroxides was carried out by laboratory X-ray diffraction using a Bruker D8 Advanced powder diffractometer in reflection mode with Bragg-Brentano geometry. Topas-4.2 software was used to analyze the diffraction patterns collected with Cu-Kα radiation (λ = 1.5418 Å) and a LynxEye PSD detector at 20 °C in the angular range from 5 to 80° with a constant step of 0.2°. Phase identification was made with Diffracplus EVA software using the International Centre for Diffraction Data (ICDD) PDF-2 database. For the crystallite size, Topas reported both the size based on IB (integral breadth) and FWHM (full width at half maximum).
The electron microscope JEOL JSM-5510LV (JEOL, JAPAN) with a tungsten filament operating between 0.5 and 30 kV and commercial software provided by the producer were used for SEM image manipulations. The specimens were prepared by the dropping of bacterial suspension on cover glasses for light microscopy and drying. Transmission electron microscopy images were obtained with a TEM JEOL 2100 operated at 200-kV accelerating voltage. The specimens were dispersed in ethanol by ultrasonic treatment for 5 min and the suspensions were dripped on standard holey carbon/Cu grids.
The k0-based NAA technique was applied for multi-element determination in the biogenic products irradiated in vertical channels of the Budapest Research Reactor [8]. To the best of our knowledge this analytical method was implemented for the first time on such a biogenic matrix and hereafter the accumulated experience is briefly described.
For k0-standardization in neutron activation analysis (k0-NAA) the determinations of the neutron flux parameters α (epithermal flux distribution parameter) and f (thermal-to-epithermal neutron flux ratio) have to be performed [6]. The neutron flux parameters were measured with the “Bare Triple-Monitor” method using Zr and Al-0.1%Au alloyed foils [10]. Sample irradiations were performed in the vertical channels of the reactor with various time intervals (12 h for long and 2 min for short irradiations in general).
Short-term irradiations of iron bacteria samples and monitors were performed using a fast pneumatic transfer system. At the irradiation position, a thermal neutron flux of 4.45 × 1013 n/(cm2 · s), a thermal-to-epithermal neutron flux ratio (f) of 34.8, and an α = 0.029 were available. Within a maximum decay period of 120 s, the samples were packed into inactive polyethylene capsules and measured for 10 min and later for 20–30 min. Elements such as Ca, Mn, and S were analyzed.
Long-term irradiation was used for the determination of elements producing medium- or long-lived isotopes (T1/2 ≥ 6 h). Samples together with flux monitors were irradiated for 12 h in one of the vertical rotating irradiation channels (No. 17) of the reactor at a thermal neutron flux density of 1.86 × 1013 n/(cm2 · s), a thermal-to-epithermal flux ratio (f) of 42 and an α = 0.031. Each sample was measured three times. After a typical decay time of 2–3 days, the radionuclides 76As, 47Ca, 42 K, and 24Na were counted for a measuring time of 1,800 s. To improve the detection limit for several radionuclides, a second measurement was made after 7–12 days (when the 24Na isotope had decayed). After a decay period of about 20–30 days (when the 82Br isotope decayed), the samples were counted for 5–15 h and the radionuclide 59Fe was measured.
A Canberra gamma radiation spectrometer was used for determination of specific activities of gamma-ray emitting radionuclides. Detection of gamma rays was performed at a pre-calibrated sample-detector distance with a p-type Canberra HPGe detector, with an energy resolution of 1.82 keV and relative efficiency of 36% for the 1,332.5 keV 60Co line. Counting losses were corrected with a Westphal-type Loss-Free Counting (LFC) module with a dual spectrum storage option providing full compensation.
The in-house developed processing software for quantitative evaluation of measured spectral data involves the following quantities and features: absolute activity, alpha value, element concentration, detector efficiency, isotope identification, thermal and fast neutron flux and flux ratio (f), nuclear data library, specific activity computation.
The interferences caused by neutron-induced reaction can be important sources of bias in NAA. The interferences due to fast-neutron-induced threshold reactions i.e., (n,p) or (n,α), can be accurately calculated and corrections can be accomplished based on the measurement of neutron flux parameters (thermal and fast neutron flux and flux ratio) [9]. Interference from the 56Fe (n,p)56Mn reaction was calculated and the Mn content of the samples was corrected on the basis of the Fe content of the samples. The combined uncertainty (±1σ) of the results of this analysis is calculated as the square root of the quadratic summation of the statistical counting error and the estimated systematic uncertainty due to all fundamental parameters involved in k0-NAA [9, 10].
Results and discussion
Electron microscopy analyses of samples from the area of the sampling showed that the iron bacteria are stable components of the microbial community in the selected natural habitat. The electron microscopy images indicated the presence of bacteria from genera Sphaerotilus and Leptothrix with the expected morphology and the typical sheaths that they formed. The isolated pure cultures grown in the above-described specific elective media (AM and IM) were identified as strains of the Leptothrix genus (Figs. 2 and 3).
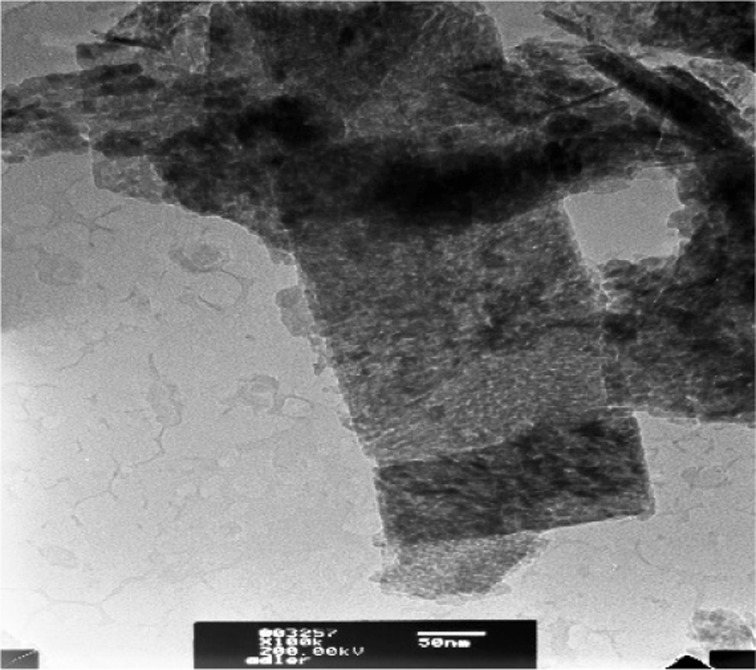
Fig. 2.
TEM image: bacterial isolates on Adler’s medium
Fig. 3.
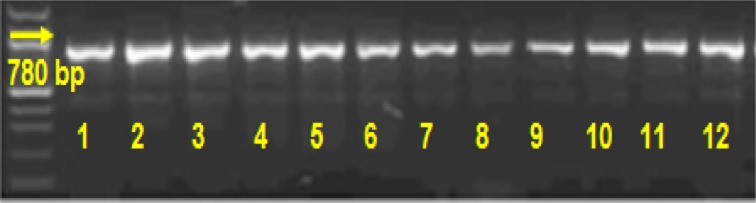
Amplification profile of mofA – PCR
Altogether, 30 elements and their radionuclides were reliably determined in the samples originating from high-mountain or cultivated biomaterial. Most of these elements had approximately the same trace amounts as in the nutrient medium or changed very weakly in the measured solid samples. They were left out of the scope of the present discussion.
Table 1 summarizes the NAA results for iron and manganese and illustrates well the possibilities of the biotechnology approach for obtaining significant quantities of target iron byproducts under non-natural conditions.
Table 1.
INAA results for sample 2 Adler-D and sample 1 Isolation-M
| Elem./unit | Reference sample (Vitosha) | 2DE** 56 days | 2DP* 88 days | 1MP* 56 days | 1MF* 88 Days | 1MY* 103 days |
|---|---|---|---|---|---|---|
| Fe, g/kg | 93 ± 4 | 375 ± 17 | 354 ± 16 | 457 ± 26 | 486 ± 22 | 689 ± 40 |
| Mn, mg/kg | 1,280 ± 90 | 2,280 ± 140 | – | 19.8 ± 1.5 | 108 ± 7 | 39.6 ± 2.8 |
*Enriched; **Pure
A high enrichment level of iron was found in all cultivated samples in comparison with the reference. The strong increase in Fe content depends on the culture medium and is superior in IM. Comparing the Mn concentration with that of the reference sample (product of nature), where it was in large concentrations, a higher enrichment level of manganese was established in the sample grown in AM under dynamic conditions. The SEM-EDS analysis confirmed the small quantity of manganese in the sample Adler 2DE pure. Table 2 illustrates the sharing of elements in that sample. The deviations could be connected with the iron cuttings’ batch in the media used.
Table 2.
SEM-EDS of the sample Adler 2DE pure
| Element | App Conc. | Intensity Corrn. | Weight% | Weight% sigma | Atomic% |
|---|---|---|---|---|---|
| Mn | 1.60 | 0.9005 | 1.40 | 0.24 | 0.71 |
| Fe | 82.63 | 1.0227 | 63.91 | 0.90 | 31.68 |
Table 3 shows the major impurities established in the biogenic products in comparison with the reference sample. A somewhat higher concentration of arsenic was found in two samples. This is most probably due to the presence of a small quantity of As processing bacteria, which will be the subject of further experiments. Basic elements from the nutrient media except Ca are also present.
Table 3.
Impurities of the chemicals used for the media Adler 2D and 1IM
| Elem./unit | Reference sample (Vitosha) | 2DE** 56 days | 2DP* 88 days | 1MP** /103 days | 1MF* /103 days | 1MY* /103 days |
|---|---|---|---|---|---|---|
| As, mg/kg | 18.3 ± 0.9 | 5.5 ± 0.5 | 17.4 ± 0.8 | 11.7 ± 0.9 | 25.7 ± 2.0 | 23.9 ± 1.6 |
| Ca, g/kg | 9.4 ± 2.3 | – | – | – | – | – |
| K, mg/kg | 3080 ± 204 | 604 ± 35 | 940 ± 164 | 1270 ± 90 | 947 ± 54 | 254 ± 163 |
| Na, mg/kg | 4600 ± 300 | 309 ± 14 | 438 ± 20 | 401 ± 31 | 123 ± 12 | 102 ± 6 |
| S, mg/kg | – | – | – | 13.1 ± 1.4 | 47.6 ± 4.1 | 40.5 ± 3.3 |
*Enriched; **Pure
As can be seen from the tables, some small amount of manganese was also in the sample IM. The presence of manganese is most probably connected with the impurities of the raw ingredients used for preparation of the media.
Figures 4 and 5 illustrate the determination of the oxides present in the biogenic matrix by powder X-ray diffraction (XRD). The analyses of XRD patterns provided as well the relative amounts and particle sizes of the different substances building the material under study. Generally, most particle sizes were evaluated as being significantly below 30 nm.
Fig. 4.
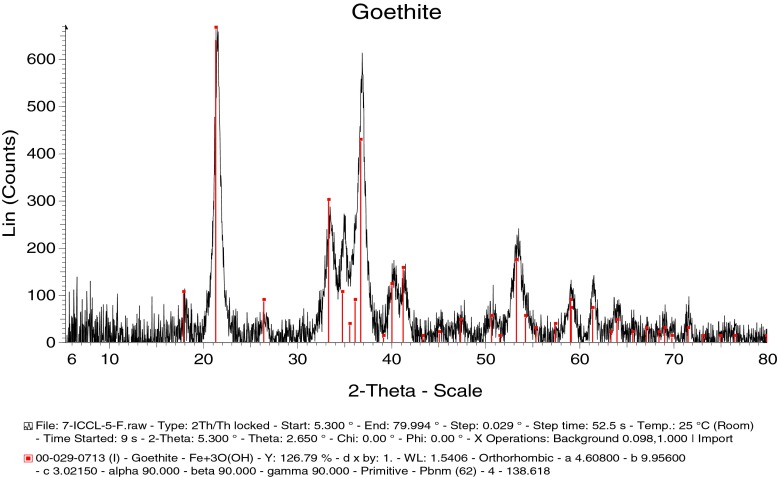
XRD phase analysis of sample Isolation-MF (biogenic goethite, 6.2 nm)
Fig. 5.
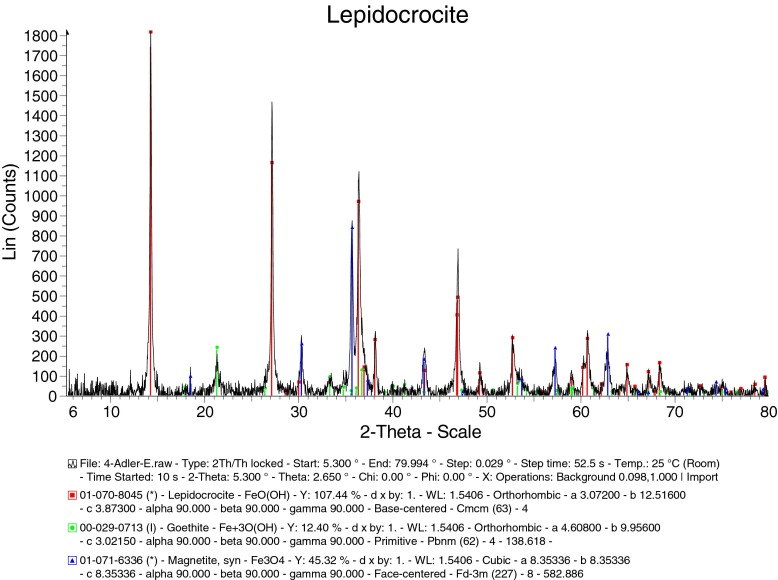
XRD phase analysis of sample 2DE: lepidocrocite 59.67%/30 nm, magnetite 21.56%/24 nm, goethite 18.77%/12 nm
In the 1 Isolation-M samples, the fractions and the size of the constituents were: 1 Isolation-MP: goethite 77.03%/2.0 nm, lepidocrocite 22.97%/4.2 nm; 1 Isolation-MY: goethite 100%/5.3 nm; 1 Isolation-MF goethite 100%/6.2 nm. So, besides goethite, the phase of lepidocrocite was detected only in the sample 1 Isolation-MP. This might result from the type of cultivation (bioreactor and cultivation in the flasks without shaking). Phase and size analyses of the AM cultivated sample 2DE show lepidocrocite 59.67%/29.9 nm, magnetite 21.56%/23.9 nm, goethite 18.77%/12.0 nm.
Remarkably, only iron-containing phases were disclosed in the studied biogenic samples. Bear in mind that goethite (α-FeOOH), lepidocrocite (γ-FeOOH), hematite (α-Fe2O3), and maghemite (γ-Fe2O3) constitute the main natural occurrences with magnetite (Fe3O4) for ferric oxides and oxyhydroxides [3]. The iron ions are in different oxidation states and coordination conforming to the corresponding iron oxide phase.
The structure of hematite, α-Fe2O3, is isostructural with corundum, α-Al2O3, and has rhombohedral symmetry, but it was not found in the studied samples, implying that it was not formed by the bacteria either in nature or under artificial conditions. Magnetite and maghemite are cubic and crystallize with an inverse spinel-like crystal structure. However, while magnetite is a mixed valence (Fe2+, Fe3+ ions) compound because it contains Fe2+ in tetrahedral coordination, and both tetrahedrally and octahedrally coordinated Fe3+, in maghemite all the iron cations are in a trivalent state and the charge neutrality of the unit cell is guaranteed by the presence of cation vacancies. Goethite, α-FeO(OH) and lepidocrocite, γ-FeO(OH), are orthorhombic but the corresponding crystal structure differs substantially by symmetry.
The quantitative analyses of X-ray diffraction data and comparison with the ICDD database unambiguously reveal that the magnetite phase is non-stoichiometric and can be represented as Fe3-xO4, reflecting creation of cation vacancies x in octahedral sites under an excess of oxygen.
It is interesting to notice that the occurrence of spinel γ-Fe2O3 (maghemite) with octahedrally and tetrahedrally coordinated Fe3+ was not established while the identified two polymorphs of the iron hydroxides γ-FeOOH (lepidocrocite) and α-FeOOH (goethite) contain iron ions in a trivalent state and octahedral coordination. Also, one could draw the conclusion that in contrast to the cultivation in Adler’s medium, the use of the Isolation medium is more efficient for the conversion of lepidocrocite (γ-FeOOH) to the thermally more stable polymorph, goethite (α-FeOOH).
Conclusions
Biogenic nanostructured materials were obtained after cultivation of the Leptothrix sp. in Adler’s and Isolation media. A high enrichment level of iron was found by the INAA technique in cultivated isolates as compared to the reference sample (natural material). The enrichment rate varied between 3.8 times for the Adler medium and 7.4 times for the Isolation medium. Three types of iron oxide compounds were found after cultivation in Adler’s medium: lepidocrocite (γ-FeOOH), non-stoichiometric magnetite (Fe3-xO4) and goethite (α-FeOOH). The cultivation in the Isolation medium yielded products identified as a single phase – goethite. The XRD and SEM and TEM investigations showed that the biogenic oxides formed are nanosized and could be used in appropriate nano- and biotechnologies.
Acknowledgments
This work was supported by the Bulgarian National Science Fund under project DID02/38/2009. The partial financial support through the HAS (RISSPO) – BAS (INRNE) bilateral agreement for collaboration 2013/2015 and the EU-FP7 NMI3 project is gratefully acknowledged.
References
- 1.Emerson D, Fleming EJ, McBeth JM. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu. Rev. Microbiol. 2010;64:561–583. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- 2.Schüler D, Frankel RB. Bacterial magnetosomes: microbiology, biomineralization and biotechnological applications. Appl. Microbiol. Biotechnol. 1999;52:464–473. doi: 10.1007/s002530051547. [DOI] [PubMed] [Google Scholar]
- 3.Cornell RM, Schwertmann U. Iron Oxides. 2. New York: John Wiley & Sons; 2003. p. 484. [Google Scholar]
- 4.Sawayama M. Isolation of a Leptothrix strain, OUMS1, from ocherous deposits in groundwater. Curr. Microbiol. 2011;63:173–180. doi: 10.1007/s00284-011-9957-6. [DOI] [PubMed] [Google Scholar]
- 5.Budapest Neutron Centre, http://www.bnc.hu
- 6.Bergey’s Manual of Determinative Bacteriology, 8th edition, Edited by R. E. Buchanan and N. E. Gibbons, p.1246. Williams & Wilkins Company, Baltimore (1974)
- 7.Handbook of Nuclear Chemistry, Vértes, A., Nagy, S., Klencsár, Z., Lovas, R.G., Rösch, F. (eds.) 2nd ed., in vol. 6, p. 3049. Springer Science & Business Media, ISBN 978-1-4419-0719-6. (2011)
- 8.Ellis D. Microbiology of the Iron-Depositing Bacteria. Palm Springs, CA: Wexford College Press; 2003. [Google Scholar]
- 9.Eaton AD, Clesceri LS, Rice W, Greenberg AW. Standard Methods for the Examination of Water and Wastewater. 21. Washington, D.C.: APHA; 2005. [Google Scholar]
- 10.Simonits A, De Corte F, Hoste J. Zirconium as a multi-isotopic flux ratio monitor and a single comparator in reactor-neutron activation analysis. J. Radioanal. Nucl. Chem. 1976;31:467–486. doi: 10.1007/BF02518511. [DOI] [Google Scholar]