Abstract
Medical research in regenerative medicine and cell-based therapy has brought encouraging perspectives for the use of stem cells in clinical trials. Multiple types of stem cells, from progenitors to pluripotent stem cells, have been investigated. Among these, dental pulp stem cells (DPSCs) are mesenchymal multipotent cells coming from the dental pulp, which is the soft tissue within teeth. They represent an interesting adult stem cell source because they are recovered in large amount in dental pulps with non-invasive techniques compared to other adult stem cell sources. DPSCs can be obtained from discarded teeth, especially wisdom teeth extracted for orthodontic reasons. To shift from promising preclinical results to therapeutic applications to human, DPSCs must be prepared in clinical grade lots and transformed into advanced therapy medicinal products (ATMP). As the production of patient-specific stem cells is costly and time-consuming, allogenic biobanking of clinical grade human leukocyte antigen (HLA)-typed DPSC lines provides efficient innovative therapeutic products. DPSC biobanks represent industrial and therapeutic innovations by using discarded biological tissues (dental pulps) as a source of mesenchymal stem cells to produce and store, in good manufacturing practice (GMP) conditions, DPSC therapeutic batches. In this review, we discuss about the challenges to transfer biological samples from a donor to HLA-typed DPSC therapeutic lots, following regulations, GMP guidelines and ethical principles. We also present some clinical applications, for which there is no efficient therapeutics so far, but that DPSCs-based ATMP could potentially treat.
Keywords: Adult stem cells, Multipotent stem cells, Cell-based therapy, Cell tissue bank
Core tip: To achieve clinical applications, stem cell-based therapy must shift from lab experimentation to clinical grade stem cells. We present here the development of advanced therapy medicinal products (ATMP) by the banking of dental pulp stem cells (DPSCs) for allogenic use. The dental pulp represents an efficient tool for industrial applications due to its accessibility after wisdom teeth extraction for orthodontic purpose. DPSC therapeutic batches can be produced in good manufacturing practice condition after human leukocyte antigen typing and stored in allogenic biobanks. We propose some clinical applications, for which there is no efficient therapeutics so far, but that DPSCs-based ATMP could potentially treat.
INTRODUCTION
Adult organisms contain postnatal somatic stem cells that are involved in symmetrical and asymmetrical cell divisions, allowing stem cell compartment maintenance and cell differentiation[1]. Thus, adult stem cells provide replacement and repair cells for normal turnover or injured tissues[2]. As these stem cells are able to renew particular tissues, they have motivated research on how to apply them in the clinic. Because of their self-renewal and ability to regenerate tissue, stem cells could provide long-lasting clinical benefits to recipients. Among these potentially beneficial cells, mesenchymal stromal cells are spindle-shaped, plastic-adherent cells isolated from bone marrow, adipose tissue, dental pulp and many other tissue sources[3,4]. They are also called mesenchymal stem cells (MSCs) in reference to their significant self-renewing properties and ability to form skeletal and connective tissue, and are suggested to be responsible for the normal turnover and maintenance of adult mesenchymal tissues[2,5]. MSCs are now the focus of intensive efforts in order to elucidate their nature and properties, and to develop cell-based therapies with real clinical applications[6]. Moreover, MSCs provide promising therapeutic benefits as they primarily mediate positive effects through paracrine mechanisms independent of cell differentiation[7]. Many preclinical and clinical trials have been completed and the major hurdles are now cell engraftment and survival, stem cell fate control, and donor-patient compatibility for allogenic applications. Several current efforts are directed at promoting the registration and banking of stem cell lines and providing associated data[8,9]. Banking MSCs, with shared materials and data, is an important step for the efficient progress of stem cell research and clinical translation. Emphasis on clinical applications is increasing, with an aim of establishing clinical grade, human leukocyte antigen (HLA)-matched banks for clinical translation[10].
DENTAL PULP STEM CELLS
Teeth are formed of two main parts, the crown and the root, that can be defined by anatomic criteria. They are linked by the periodontal ligament to the supporting alveolar bone, which is composed of both compact and trabecular bone. The dental crown consists of enamel, dentin, and dental pulp tissue. During tooth growth and development, ameloblasts form enamel and odontoblasts generate primary dentin. After tooth eruption, ameloblasts disappear from the surface of the enamel; consequently, enamel formation ceases to occur naturally in vivo. In contrast, odontoblasts, along the inner surface of the dentin inside the pulp chamber, continue to deposit dentin matrix to form secondary dentin throughout life. In addition to secondary dentin, odontoblasts can form tertiary (reparative) dentin in response to several stimuli, such as mechanical, chemical, and/or bacterial stimulation. Even when odontoblasts have been damaged, the reparative dentin can be formed in the dental pulp to protect against further disruption of the pulp tissue. This reparative dentinogenesis has been thought to be mediated by newly generated odontoblasts arising from dental pulp tissue. These findings led to the speculation that odontogenic progenitor cells or stem cells may exist in dental pulp tissue[11]. The first type of dental stem cell was subsequently isolated from the human pulp tissue and given the name dental pulp stem cells (DPSCs)[12]. Dental pulp is a soft connective tissue entrapped within the dental crown, and divided into four layers. The external layer is made up of odontoblasts producing dentin; the second layer is poor in cells and rich in collagen fibers; and the third layer contains progenitor cells and undifferentiated cells, some of which are considered stem cells. From this layer, undifferentiated cells migrate to various districts where they can differentiate under different stimuli and make new differentiated cells and tissues. The innermost layer is the core of the pulp and comprises the vascular area and nerves[13]. Dental pulp is an interesting source of adult stem cells because of the large amount of cells present and the non-invasiveness of the isolation methods compared to other adult tissue sources[13-15]. MSCs defined as dental stem cells can be obtained from human permanent and primary teeth, human wisdom teeth[12], human exfoliated deciduous teeth[16], apical papilla[17], the periodontal ligament[18,19] and the dental follicle[20,21].
Dental pulp tissue from human third molar, exfoliated deciduous or supernumerary teeth represent an easily accessible source for harvesting MSCs as these teeth are often discarded.
Stem cells that reside in dental pulp (DPSCs) have been described as a population of MSCs, as they match the definition given by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy[3]: DPSC are plastic-adherent when maintained in standard culture conditions; they express some specific surface molecules such as CD105, CD73, CD90 and lack expression of CD45, CD34, CD14, CD19 and HLA-DR surface molecules; and they have the ability to differentiate into osteoblasts, adipocytes and chondroblasts in vitro[11,12,18,22-24]. Moreover, DPSCs can differentiate into a large array of cells and tissues[25-28] and a comparison of their multipotency with Bone Marrow Stem Cells has demonstrated that proliferation, availability, and cell number of DPSCs were greater than for bone marrow MSCs[24,29].
In addition, DPSCs were also found to undergo myogenic and neurogenic differentiation capacities in vitro, expressing respective gene markers and exhibiting neuron-like cell morphologies. The plasticity and multipotential capability of DPSCs can be explained by the fact that dental pulp is made of both ectodermic and mesenchymal components, and contains neural crest-derived cells[13]. Concerning cell surface molecules, the persistence of negative results for CD45 demonstrates that these cells are not derived from a hematopoietic source, although they are of mesenchymal origin[25]. Like all MSCs, DPSCs are also heterogeneous and the various markers may be expressed differently by subpopulations of these stem cells[24]. A selected subpopulation of CD34+/CD45- DPSCs, which represented roughly 10% of dental pulp cells, has also been described. These cells displayed an increased capacity of self-expanding and differentiating in pre-osteoblasts, and were able to self-maintain and renew for long time[17]. Although MSCs were originally described as CD34 negative, it seems that this subpopulation of DPSCs expresses the CD34 cell surface antigen in the manner reserved for the most primitive stromal stem cells (other than hematopoietic) that was gradually lost after the differentiation of lineage committed progenitors[30].
DPSCS BIOBANKING
The term “biobank” describes various facilities that store biological samples, from small tissue collections to wide repositories featuring a variety of tissues and biological sample types[31,32].
Storage and collection of biological material might be accompanied by various medical and epidemiological data that are used for current research and for potential future works[33]. Biobanking has been defined as a structured resource for genetic and medical research and their therapeutic applications. It includes human biological material and extensive associated information[34,35].
Several types of biobanks can be distinguished, according to the purpose and target: case control, clinical trial, tissue, biomolecular resource center, stem cells[36]. Stem cell biobanks have received much attention as a new biologic resource for both research and clinical applications, leading to the development of stem cell banks around the world[37]. Stem cells of dental origin represent a promising source of new stem cells as, in western countries, 80% of teenagers and/or young adults have their wisdom teeth extracted. Furthermore, dental pulp is naturally protected within the pulp chamber, the inner cavity of the tooth, in a sterile environment. Pulp from one wisdom tooth generally contains between 200000 and 300000 DPSCs[38]. Studies have indicated that DPSC isolation was feasible for 5 d after tooth extraction[39]. Efficient results were obtained by cryopreserving second-passage DPSC cultures, but could also be achieved by isolating and cryopreserving entire pulp tissues, with digestion and culture performed post-thaw[40]. Such minimal processing may be of interest for the banking of samples for which there are no immediate plans for expansion and use. Furthermore, cell recovery could be achieved by mechanical disruption with a single-use device, in accordance with the GMP (European Good Manufacturing Practices) standard. Dental pulp and DPSC recovery is represented in Figure 1.
Figure 1.
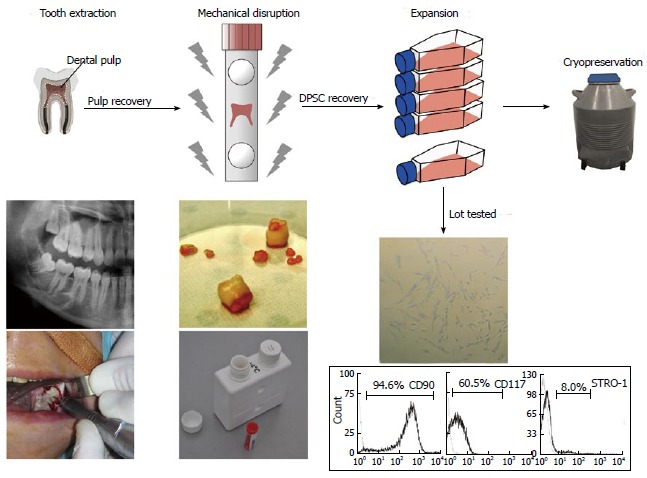
Dental Pulp tissue and dental pulp stem cell recovery. Wisdom teeth are extracted in aseptic conditions and transferred to the cell bank in a sterile transport tube. The teeth are then cracked opened and the pulps are mechanically disrupted in a tissue grinder/homogenizer. The cell suspensions obtained are screened for expression of stemness markers by flow cytometry, before storage in liquid nitrogen. DPSC: Dental pulp stem cell.
Immunologic considerations: Allogenic use and immunomodulation
Immune mechanisms confer immediate protection against foreign organisms (innate immunity) and specific immune responses to neutralize pathogens (adaptive immunity). The immune system recognizes tissue compatibility and can raise an effective immune response against pathogens or incompatible allogenic tissues. Tissue compatibility or incompatibility is determined from allelic similarities or disparities at genetic loci that encode the major histocompatibility complex (MHC) antigens, also called the HLA system. The HLA system encodes two major classes of highly polymorphic cell surface glycoproteins: HLA class I molecules are expressed on all nucleated cells and HLA class II molecules are expressed on antigen-presenting cells, thymic epithelial cells, and B lymphocytes. These immunological principles, which apply to organ or tissue transplantation, can be extended to transplantation of DPSCs or DPSC-derived tissue, especially for HLA class I molecules (HLA-A, HLA-B and HLA-C)[41]. Thus, a major clinical challenge to DPSC banking will be to overcome the immunological barriers to the transplantation of DPSC-derived tissues in order to prevent rejection[42,43]. Ensuring HLA compatibility is certainly the most interesting method of minimizing the risk of rejection. Embryonic stem cells have been shown to express very low levels of HLA class I proteins, with a moderate increase during differentiation[44]. Regarding MSCs, HLA expression remains unclear, but they have been extensively studied for their immunomodulatory properties[45-49]. Indeed, MSCs exert a profound inhibitory effect on T cell proliferation in vitro and in vivo, with similar effects on B cells, dendritic cells and natural killer cells[50]. Moreover, T-cell inhibition is not restricted by HLA type, and immunosuppressive effects are mediated through soluble factors and the generation of regulatory cells[47,51]. These findings suggest that MSCs can induce peripheral tolerance, enhancing their potential for therapeutic applications[45,48]. A higher immunosuppression of T-cell alloreactivity has also been demonstrated in DPSCs in comparison with bone marrow stem cells[52]. These properties distinguish DPSCs as one of the most accessible cell sources for cell-based therapy in regenerative medicine and inflammation-related diseases[46].
To overcome rejection and use DPSCs in transplantation medicine, the formation of a histocompatibility bank is an attractive option, where DPSCs are stored after HLA isotyping. Ideally, considering the large numbers of wisdom teeth extracted from genetically diverse populations, adequate levels of isotype matching with patients may be achieved. The establishment of an HLA-organized DPSC allogenic bank could be sufficient to provide stem cells for a large number of patients. The concept of haplobanking with HLA homozygous cell lines would also limit the number of HLA mismatches[53]. Several studies have been conducted to determine the number of donors needed to cover the population of a country. These findings are dependent on ethnic disparities of the population and the type of stem cells considered. The creation of a bank containing highly selected homozygous lines is an attractive approach to HLA matching. Selected homozygous lines can provide HLA matches for a wide percentage of populations. Estimates of the number of homozygous cell lines needed have mainly been established considering embryonic stem cells, for the main proteins HLA-A, HLA-B and HLA-DR. Data from cadaveric organ donors, cord blood bank or in vitro fertilization-derived embryos led to the estimate that approximately 150-190 human embryonic stem cell lines with various HLA genotypes, or a collection of 10-30 homozygous lines for the common HLA types, would be sufficient to provide HLA-matches for a wide part of the population in the United Kingdom[54], Japan[55,56], the United States[57] or China[58]. Because of the low incidence (1.5%) of HLA-homozygous individuals in the normal population[54], a systematic collection of discarded wisdom teeth would be of prime interest. The determination of the HLA types of 100 DPSC lines from teeth collected in Japan revealed 2 homozygous lines for all the 3 considered HLA loci. These 2 homozygous lines therefore have the potential to cover approximately 20% of the Japanese population with a perfect match[59].
Methods and good manufacturing practices
The production and marketing of stem cell-based therapy faces imperative steps, including product characterization, safety testing and clinical trials design. At both national and international levels, numerous standards and regulations must be followed in order to translate DPSCs into clinical products. There are variations in these international and national guidelines, and in the regulations that are applied to the collection and storage of human tissue, personal data and medical records[32]. The Food and Drug Administration, in the United States, and the European Medicines Agency (EMA), in Europe, are responsible for creating and enforcing these regulations. In Europe, stem cells for clinical therapies are classified under advanced therapy medicinal products (ATMP) unless they are minimally manipulated and intended for homologous use[60]. A Committee for advanced therapies (CAT) has even been created to evaluate cell production marketing by assessing the quality, safety and efficacy of ATMPs, in accordance with the regulatory framework. EMA regulation defines the current Good Manufacturing Practices (cGMP) guidelines to manufacture ATMPs[61]. Even though clinical grade production of DPSCs needs to be implemented, DPSCs can be isolated, stored, and eventually expanded by applying rational modifications to the commonly used methods[15,62], in order to continue complying with good manufacturing practices[63] from the donor (patient having his/her tooth extracted, in aseptic condition) to the storage tank. The critical step of enzymatic pulp tissue digestion can be replaced by mechanical disruption in single use devices, such as a tissue grinder/homogenizer. Fetal bovine serum usually required for in vitro expansion can be replaced by human serum supplements derived from peripheral blood serum, peripheral blood plasma, or platelet lysate[64]. Moreover, genetic stability has been demonstrated for DPSCs for up to 9 cell passages[65,66].
Legal and practical issues (consent, confidentiality, commercialization)
Translation of DPSC research into clinical applications relies on abundant in vitro and in vivo preclinical data. However, when it comes to potential therapeutic applications, some barriers can appear, due to restrictions specified in the consent document used for the collection of biological materials, questions about ownership of the collected DPSCs, and the confidentiality of the information associated with the cell lines[10]. The constitution of an allogenic DPSC bank contains procedures to ensure anonymity, although authorized parties can access some clinically relevant information.
The rights of donors and the interests of researchers are protected by incorporating relevant government legislation (ethical committee review) and procedures (e.g., anonymity and consent). It is crucial to explain the use and transfer of cells and data at the time of informed consent, especially highlighting features that distinguish collection for research from collection for a biobank[67]. The whole process, from the patient coming for tooth extraction to storage of DPSC lots, is presented in Figure 2.
Figure 2.
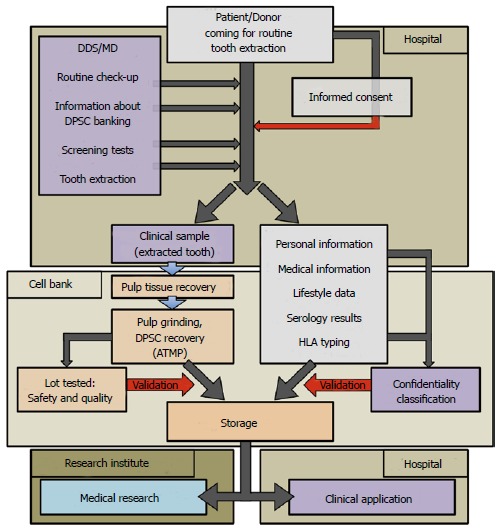
Process flowchart for current Good Manufacturing Practices manufacturing of dental pulp stem cell lots/therapeutic products. The chart is divided into 4 areas: Hospital for tooth recovery, Cell Bank, Research Institute and Hospital for clinical applications: (1) Hospital: direct contact between patients and authorized medical staff, such as Medical Doctor (MD) or Doctor of Dental Surgery (DDS). Clinical sample and donor’s personal data are recovered; (2) Cell Bank: current Good Manufacturing Practices manufacturing of Advanced Therapy Medicinal Products (ATMP) from the biological samples (dental pulps). All data concerning the donors are anonymous; (3) Research Institute: dental pulp stem cell (DPSC) lots are used for animal experiments to develop new therapeutics; and (4) Hospital: clinical grade DPSC lots are used for therapeutics. Red arrows represent critical parameters related to each step of processing (informed consent, quality, safety, confidentiality). HLA: Human leukocyte antigen.
Allogenic DPSC biobanking brings together a multitude of data on individuals, including health and lifestyle. Thus, the way the informed consent is obtained should reflect the personal information used for medical research, taking into account that the patient was originally coming for a routine tooth extraction. As informed consent is derived from the standard that every donor has the right to self-determination, the patient must be informed about the nature of biobanking, the procedures in which the tooth he has donated might be involved, and the expected outcome of the research[36,68]. The physical and intellectual property of biological samples collected must be clearly established and explained[69].
INNOVATIVE THERAPEUTICS
Upon discovery of stem cells in the dental pulp, DPSCs demonstrated their ability to regenerate a complex consisting of a mineralized matrix of odontoblasts and connective tissue containing blood vessels similar to that observed in normal human tooth[12]. Since then, the use range of potential medical applications based on DPSCs include the repair and regeneration of bone[13,70], the central nervous system[27,71,72], liver tissue[73], heart tissue[74], eyes[75], muscles[76,77], and salivary gland cells[78,79]. Overall, it holds great potential in the field of regenerative medicine and tissue engineering[13,23] alone or combined with various biomaterials[80-84]. Some have proposed that DPSCs may have greater potential than the current MSC gold standard, the bone marrow-derived MSC[29].
Allogenic banking of DPSCs could boost industrial and therapeutic innovations by providing tools for unsolved medical problems, including the production of advanced therapy medicinal products (ATMP). We present here some clinical applications, for which there is no efficient therapeutics so far, but that DPSCs-based ATMP could potentially treat.
Spinal cord injuries
Chronic medullary lesion, a result of spinal cord trauma, is characterized by neurologic deficiency without evolution. Six months after trauma, these lesions are considered chronic, with no chance of improvement. According to the Christopher Reeves foundation, these spinal cord lesions affect 1.2 million persons in the United States and 300000 persons in Europe. Numerous preclinical studies have been conducted to graft stem cells of various origins into injured spinal cords, such as neural stem cells[85], embryonic stem cells[86-88], with encouraging results[89]. DPSCs, due to their embryologic origin, express some markers of both mesenchymal and neuroectodermic origin[12,16]. Indeed, DPSCs originate from migrating cranial neural crest cells. During embryonic development, these neural crest cells differentiate into a wide variety of cell types, including neurons of the peripheral nervous system[27]. MSCs have been thought to be usable in the treatment of spinal cord injuries, and adult human DPSCs could provide an ideal source of stem cells for therapeutic applications in such neurological pathologies[90]. The efficiency of DPSCs in improving neural regeneration has been shown in vitro[72,73,91] and in vivo after spinal cord injury[92-94]. These preclinical data enhance the therapeutic potential of intrathecally administrated HLA-typed DPSC lots in treatment of nerve tissue injuries.
Sjögren’s syndrome
Sjögren’s syndrome is an autoimmune pathology affecting 0.2% to 3% of the general population[95]. It is a chronic inflammation of the salivary and lacrimal glands, characterized by lymphocytic infiltration of the exocrine glands with a polyclonal B cell activation[96]. Although the pathogenesis of primary Sjögren’s syndrome remains unclear, T cells and B cells have been shown to be involved. Pharmacological treatments have limited efficiency, with only the capacity to temporarily ameliorate symptoms, and with no modification of the overall course of the disease[97]. Given the lack of disease-modifying drugs, treatment options are now focused on biotherapies[98]. As detailed above, immunomodulatory properties of MSCs have been demonstrated in vitro and in vivo, suggesting a therapeutic potential for autoimmune disease treatments[47], especially through anti-inflammatory cytokines production and T regulatory cells promotion[99]. These immunomodulatory properties have also been demonstrated for DPSCs, identifying them as a cell source for cell-based therapy of immune and inflammation-related diseases[47]. Intravenous injection and local injection of DPSC lots into salivary glands represents a potential novel immunotherapeutic tool for autoimmune Sjögren’s syndrome.
Irradiated salivary glands
Cancers that originate from the aerodigestive epithelium, including carcinomas of the head and neck, are the leading causes of cancer-related mortality worldwide, accounting for about 2 million deaths and 500000 new cancers diagnosed annually[100,101]. Treatment involves chemotherapy, radiotherapy, and surgery. Radiation-induced salivary hypofunction is one of the major developments that affect survivors. Even though radiotherapy is focused on the cancerous area, radiations often affect salivary glands, causing severe hyposialia and oral dryness after orofacial cancer treatment; hyposalivation underlying xerostomia after radiotherapy is still a major problem in the treatment of head and neck cancer. To date, the only treatment for this oral dryness is the use of artificial saliva to supply salivary glands, a treatment with limited efficiency. Salivary stem cell (salisphere) transplantation has been shown to functionally restore salivary gland efficiency after radiation-induced impairment of salivary gland function and consequential xerostomia[79]. Furthermore, it was demonstrated that DPSCs used as a cell source for the treatment of salivary gland hypofunction could partially revert this hypofunction[80]. Thus, stem cell-based therapy has great potential in prevention or treatment of radiation-induced hyposalivation[102]. New therapeutic strategies are now being considered using stem cells injected intravenously or directly into salivary glands to allow salivary gland cell reactivation[103-106].
Acute periodontitis
Periodontal diseases are highly prevalent diseases that can affect up to 90% of the population worldwide. They have various forms, from gingivitis, the mildest form caused by dental plaque, to periodontitis, which induces the loss of connective tissue and bone support, and causes tooth loss in adults[107]. Acute periodontitis is an inflammatory disease of the periodontium triggered by the host’s immune response and resulting in the progressive loss of gingival tissue, periodontal ligament and supporting alveolar bone[108]. Actual therapeutics consist of the control of bacterial infection and the stabilization of tissue loss. Regenerative treatment using bone grafts, gingiva grafts, and growth factors offer interesting possibilities, but only in specific indications[109-111], and with unpredictable results[112]. In this context, topical application of stem cells in periodontal lesions appeared to be a promising strategy to regenerate periodontium[113]. In vitro studies demonstrated the ability of DPSCs to differentiate into osteoblast and cementoblast lineage, and to participate in periodontal ligament and cementum regeneration[114,115]. In vivo experiments enhanced the therapeutic potential of dental stem cell grafting to regenerate periodontal tissues[116-118]. Allogenic transplantation could enhance periodontal tissue repair and limit local inflammation through MSC immunomodulation[108,119].
Endodontic regeneration
Dental pulp, the soft connective tissue described above, is the tissue entrapped within the teeth, in which are recovered DPSCs. In case of dental decay, this tissue can be infected and become necrotic, because it is encased in a thick dentin wall, and consists of a microcirculatory system originating from a very small opening at the apex of the root. This anatomical configuration limits the development vascular supply during pulp regeneration. Endodontic treatment, when needed, includes the removal of vital and necrotic tissues from the root canal system, along with infected root dentine. It aims to prepare the canal space to facilitate disinfection by irrigants and medicaments. Prevention of reinfection is then achieved through the provision of a fluid-tight root canal filling and a coronal restoration[120]. The potential possibility of regeneration of pulp tissue by cell therapy is a promising approach for the future treatment of pulpitis or peri-apical disease assuring longevity of teeth and improved quality of life. It has been demonstrated that transplantation of DPSC was capable of inducing complete pulp regeneration in a root canal after pulpectomy[121]. Thus, the use of DPSC, combined with a supporting scaffold, could be used to treat and heal infected root canals, providing an interesting alternative to the actual inert fillings used in endodontics. Root canals anatomy limits the use of rigid scaffold systems in pulp regeneration: scaffolds for pulp regeneration should be injectable, with fibrous structures that ideally mimic the extracellular matrix of the pulp tissue and support stem cells growth.
Induced pluripotent stem generation from DPSC
Pluripotent stem cells can be induced from fibroblasts by retroviral introduction of Oct3/4, Sox2, c-Myc and Klf4[122]. These induced pluripotent stem (iPS) cells are similar to embryonic stem cells in morphology, proliferation and differentiation capacities[123]. They proliferate extensively and differentiate into virtually any desired cell type, providing an unlimited source of replacement cells for human therapy[124]. It has been shown that DPSCs could be also reprogrammed into iPS cells, with a higher efficiency rate than dermal fibroblasts. DPSCs-derived iPS cells were indistinguishable from human embryonic stem cells, highlighting the potential of DPSCs as an alternative source for generating iPS cells[125,126]. Many reprogrammed cell lines could easily be established from DPSCs obtained from young patients with a low risk of bacterial contamination and genetic modification, as extracted wisdom teeth are generally aseptically obtained from the mandible and are protected from ultraviolet and other damage by surrounding hard tissues. It was shown that iPS cells could be efficiently generated from DPSCs using the conventional 4 reprogramming factors (Oct3/4, Sox2, c-Myc and Klf4)[59,125,126], as well as using only 3 factors (Oct3/4, Sox2 and Klf4)[59], or even using only 2 non-oncogenic factors (Oct4 and Sox2)[30]. Interestingly, the efficiency rate of reprogramming was related to the donor’s age, with higher rate for younger patients with wisdom teeth still under maturation[59].
With respect to safety, it would be ideal not to use retrovirus vectors for transient expression of the reprogramming genes. DPSCs are assumed to offer high efficiency of iPS cell generation even with the use of non-integrating vectors such as Sendai viruses or modified mRNA. Clinical use of iPS in regenerative medicine is very promising. However, time-efficiency and financial considerations argue in favor of the use of allogenic rather than autologous iPS lines. Similarly to DPSCs, biobanking of iPS lines would be a reasonable strategy. In this setting, DPSC banking could be of great help to establish iPS cell banks with a sufficient repertoire of HLA types, since the establishment of clinical-grade iPS cell lines from individual patients would require much time and incur a high cost[59].
From DPSC to successful therapy: Limitations and issues
The notion of stem cells as postnatal units of organ or tissue regeneration allows imagining therapies such as tissue engineering. A stem cell that could be expanded and modified ex vivo, and transplanted in vivo encourages attempts to treat severe or lethal diseases. However, even when the use of stem cells could replace the lost cells, it does not guarantee that the regenerated cells could circumvent the cell death caused by the disease. And replacing lost cells, even with cells expressing several specific cell markers, is far from a successful therapy with fully functional cells. Indeed, clinically successful translation of stem cell science into medicine has been conducted following a simple framework in which organ-specific stem cells were used for organ-specific diseases[6]. The roles of DPSC, and MSCs in general, as niche cells, tissue organizers and skeletal or neural progenitors open opportunities and pose challenges. The molecular mechanisms by which stem cells become functional are still largely unknown. Its comprehension and identification may involve new methods that go far beyond the empirical injection of poorly characterized cultured cell strains[6]. Such methods may involve modeling of disease mechanisms, identification of cell-derived bioactive factors and their use as drugs (including factors mediating the interactions with endothelial and hematopoietic cells), definition of targeted specific disease mechanisms and organ-specific strategies to deliver DPSC to a site of interest[127].
CONCLUSION
DPSC-based therapy is now entering into a new stage of development, shifting from initial in vitro and in vivo studies to optimization of therapeutic products for clinical applications. As for other MSC, there are still many challenges concerning stem cell potency, age-related and disease-related tissue impairment, and production of clinical grade stem cells lots. The strategies presented in this review emphasize the potential of DPSC to be used for innovative clinical trials based on rational DPSC therapy, following GMP conditions.
Indeed, dental pulp is a remarkable site of stem cells, and the collection of stem cells from dental pulp is a non-invasive practice that can be performed after routine wisdom teeth extraction. DPSC can be recovered in GMP conditions and cryopreserved for long periods, after HLA typing. However, in the perspective of therapeutic use, optimization and better methods are still necessary for DPSC isolation, expansion and banking.
Although producing and storing patient-specific stem cells could resolve immunological problems, this procedure would be costly, laborious, and time-consuming. Allogenic DPSC banking containing clinical grade stem cell lines offers an alternative and provides stem cell lines from which it will be possible to choose a HLA match for the patient to be treated. There are variations in national and international regulations for the collection and storage of human tissue, but ethical principles related to biobanks always include safety, informed consent and confidentiality. The recovery of DPSC doesn’t involve any invasive procedure as they come from already extracted teeth. Thus, the main concerns are: (1) for the donor, clear explanations about the banking project and confidentiality of all personal and medical data; and (2) for the patient, safety of DPSC lots produced in accordance to guidelines. To date, despite promising preclinical data, clinical trials using DPSCs have not been widely reported. Allogenic biobanks represent a new strategy that aims to develop the clinical applications of the DPSC potential, involving both researchers and clinicians.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 14, 2014
First decision: January 8, 2015
Article in press: June 19, 2015
P- Reviewer: Jorge V, Politi LE S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Robey PG. Stem cells near the century mark. J Clin Invest. 2000;105:1489–1491. doi: 10.1172/JCI10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–774. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 5.Ma T. Mesenchymal stem cells: From bench to bedside. World J Stem Cells. 2010;2:13–17. doi: 10.4252/wjsc.v2.i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82–93. doi: 10.4252/wjsc.v6.i2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crook JM, Hei D, Stacey G. The International Stem Cell Banking Initiative (ISCBI): raising standards to bank on. In Vitro Cell Dev Biol Anim. 2010;46:169–172. doi: 10.1007/s11626-010-9301-7. [DOI] [PubMed] [Google Scholar]
- 9.Borstlap J, Luong MX, Rooke HM, Aran B, Damaschun A, Elstner A, Smith KP, Stein GS, Veiga A. International stem cell registries. In Vitro Cell Dev Biol Anim. 2010;46:242–246. doi: 10.1007/s11626-010-9295-1. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, Kimmelman J, Robert J, Sipp D, Sugarman J. Ethical and policy issues in the clinical translation of stem cells: report of a focus session at the ISSCR Tenth Annual Meeting. Cell Stem Cell. 2012;11:765–767. doi: 10.1016/j.stem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Sonoyama W, Yamaza T, Gronthos S, Shi S. Multipotent Stem Cells in Dental Pulp. In: Freshney RI, Stacey GN, Auerbach JM, editors. Culture of Human Stem Cells. Hoboken (NJ): John Wiley & Sons, Inc; 2007. pp. 187–206. [Google Scholar]
- 12.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d’Aquino R, De Rosa A, Laino G, Caruso F, Guida L, Rullo R, Checchi V, Laino L, Tirino V, Papaccio G. Human dental pulp stem cells: from biology to clinical applications. J Exp Zool B Mol Dev Evol. 2009;312B:408–415. doi: 10.1002/jez.b.21263. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 15.Tirino V, Paino F, De Rosa A, Papaccio G. Identification, isolation, characterization, and banking of human dental pulp stem cells. Methods Mol Biol. 2012;879:443–463. doi: 10.1007/978-1-61779-815-3_26. [DOI] [PubMed] [Google Scholar]
- 16.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 18.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 20.Huang AH, Chen YK, Lin LM, Shieh TY, Chan AW. Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med. 2008;37:571–574. doi: 10.1111/j.1600-0714.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 21.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–539. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 24.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 26.Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611–620. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- 27.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 28.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 29.Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, Gabriel Chu TM, Goebel WS. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo CH, Na HJ, Lee DS, Heo SC, An Y, Cha J, Choi C, Kim JH, Park JC, Cho YS. Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials. 2013;34:8149–8160. doi: 10.1016/j.biomaterials.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Riegman PH, Morente MM, Betsou F, de Blasio P, Geary P. Biobanking for better healthcare. Mol Oncol. 2008;2:213–222. doi: 10.1016/j.molonc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West CC, Murray IR, González ZN, Hindle P, Hay DC, Stewart KJ, Péault B. Ethical, legal and practical issues of establishing an adipose stem cell bank for research. J Plast Reconstr Aesthet Surg. 2014;67:745–751. doi: 10.1016/j.bjps.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Yuille M, Dixon K, Platt A, Pullum S, Lewis D, Hall A, Ollier W. The UK DNA banking network: a “fair access” biobank. Cell Tissue Bank. 2010;11:241–251. doi: 10.1007/s10561-009-9150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hewitt RE. Biobanking: the foundation of personalized medicine. Curr Opin Oncol. 2011;23:112–119. doi: 10.1097/CCO.0b013e32834161b8. [DOI] [PubMed] [Google Scholar]
- 35.Knoppers BM, Isasi R. Stem cell banking: between traceability and identifiability. Genome Med. 2010;2:73. doi: 10.1186/gm194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artene SA, Ciurea ME, Purcaru SO, Tache DE, Tataranu LG, Lupu M, Dricu A. Biobanking in a constantly developing medical world. ScientificWorldJournal. 2013;2013:343275. doi: 10.1155/2013/343275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322–324. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- 38.Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000;66:129–138. doi: 10.1007/pl00005833. [DOI] [PubMed] [Google Scholar]
- 39.Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14:149–156. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59:150–157. doi: 10.1016/j.cryobiol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auchincloss H, Bonventre JV. Transplanting cloned cells into therapeutic promise. Nat Biotechnol. 2002;20:665–666. doi: 10.1038/nbt0702-665. [DOI] [PubMed] [Google Scholar]
- 43.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 44.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montespan F, Deschaseaux F, Sensébé L, Carosella ED, Rouas-Freiss N. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. J Immunol Res. 2014;2014:230346. doi: 10.1155/2014/230346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Jiang CM, An S, Cheng Q, Huang YF, Wang YT, Gou YC, Xiao L, Yu WJ, Wang J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20:25–34. doi: 10.1111/odi.12086. [DOI] [PubMed] [Google Scholar]
- 47.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noël D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 48.Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14. doi: 10.1186/scrt55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 52.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–842. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann A, Preynat-Seauve O, Tiercy JM, Krause KH, Villard J. Haplotype-based banking of human pluripotent stem cells for transplantation: potential and limitations. Stem Cells Dev. 2012;21:2364–2373. doi: 10.1089/scd.2012.0088. [DOI] [PubMed] [Google Scholar]
- 54.Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 55.Nakajima F, Tokunaga K, Nakatsuji N. Human leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapy. Stem Cells. 2007;25:983–985. doi: 10.1634/stemcells.2006-0566. [DOI] [PubMed] [Google Scholar]
- 56.Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- 57.Faden RR, Dawson L, Bateman-House AS, Agnew DM, Bok H, Brock DW, Chakravarti A, Gao XJ, Greene M, Hansen JA, et al. Public stem cell banks: considerations of justice in stem cell research and therapy. Hastings Cent Rep. 2003;33:13–27. [PubMed] [Google Scholar]
- 58.Lin G, Xie Y, Ouyang Q, Qian X, Xie P, Zhou X, Xiong B, Tan Y, Li W, Deng L, et al. HLA-matching potential of an established human embryonic stem cell bank in China. Cell Stem Cell. 2009;5:461–465. doi: 10.1016/j.stem.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Tamaoki N, Takahashi K, Tanaka T, Ichisaka T, Aoki H, Takeda-Kawaguchi T, Iida K, Kunisada T, Shibata T, Yamanaka S, et al. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res. 2010;89:773–778. doi: 10.1177/0022034510366846. [DOI] [PubMed] [Google Scholar]
- 60.Thirumala S, Goebel WS, Woods EJ. Manufacturing and banking of mesenchymal stem cells. Expert Opin Biol Ther. 2013;13:673–691. doi: 10.1517/14712598.2013.763925. [DOI] [PubMed] [Google Scholar]
- 61.Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol. 2012;3:253. doi: 10.3389/fimmu.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Noce M, Paino F, Spina A, Naddeo P, Montella R, Desiderio V, De Rosa A, Papaccio G, Tirino V, Laino L. Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. J Dent. 2014;42:761–768. doi: 10.1016/j.jdent.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Tirino V, Paino F, d’Aquino R, Desiderio V, De Rosa A, Papaccio G. Methods for the identification, characterization and banking of human DPSCs: current strategies and perspectives. Stem Cell Rev. 2011;7:608–615. doi: 10.1007/s12015-011-9235-9. [DOI] [PubMed] [Google Scholar]
- 64.Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, Del Fante C, Novara F, de Silvestri A, Amendola G, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol. 2007;211:121–130. doi: 10.1002/jcp.20911. [DOI] [PubMed] [Google Scholar]
- 65.Govindasamy V, Ronald VS, Totey S, Din SB, Mustafa WM, Totey S, Zakaria Z, Bhonde RR. Micromanipulation of culture niche permits long-term expansion of dental pulp stem cells--an economic and commercial angle. In Vitro Cell Dev Biol Anim. 2010;46:764–773. doi: 10.1007/s11626-010-9332-0. [DOI] [PubMed] [Google Scholar]
- 66.Atari M, Gil-Recio C, Fabregat M, García-Fernández D, Barajas M, Carrasco MA, Jung HS, Alfaro FH, Casals N, Prosper F, et al. Dental pulp of the third molar: a new source of pluripotent-like stem cells. J Cell Sci. 2012;125:3343–3356. doi: 10.1242/jcs.096537. [DOI] [PubMed] [Google Scholar]
- 67.Cyranoski D. Stem-cell pioneer banks on future therapies. Nature. 2012;488:139. doi: 10.1038/488139a. [DOI] [PubMed] [Google Scholar]
- 68.Eriksson S, Helgesson G. Potential harms, anonymization, and the right to withdraw consent to biobank research. Eur J Hum Genet. 2005;13:1071–1076. doi: 10.1038/sj.ejhg.5201458. [DOI] [PubMed] [Google Scholar]
- 69.De Robbio A. Biobanks: Patents Or Open Science? 1st ed. Cambridge: Woodhead Publishing Limited; 2013. pp. 1–70. [Google Scholar]
- 70.Graziano A, d’Aquino R, Laino G, Proto A, Giuliano MT, Pirozzi G, De Rosa A, Di Napoli D, Papaccio G. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif. 2008;41:1–11. doi: 10.1111/j.1365-2184.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang AH, Snyder BR, Cheng PH, Chan AW. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells. 2008;26:2654–2663. doi: 10.1634/stemcells.2008-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Király M, Porcsalmy B, Pataki A, Kádár K, Jelitai M, Molnár B, Hermann P, Gera I, Grimm WD, Ganss B, et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55:323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 73.Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, et al. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 74.Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 75.Monteiro BG, Serafim RC, Melo GB, Silva MC, Lizier NF, Maranduba CM, Smith RL, Kerkis A, Cerruti H, Gomes JA, et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009;42:587–594. doi: 10.1111/j.1365-2184.2009.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 77.Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SA, Cabral RM, Maranduba CM, Gaiad TP, Morini AC, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J Transl Med. 2008;6:35. doi: 10.1186/1479-5876-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamura Y, Yamada H, Sakurai T, Ide F, Inoue H, Muramatsu T, Mishima K, Hamada Y, Saito I. Treatment of salivary gland hypofunction by transplantation with dental pulp cells. Arch Oral Biol. 2013;58:935–942. doi: 10.1016/j.archoralbio.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 80.Collart-Dutilleul PY, Secret E, Panayotov I, Deville de Périère D, Martín-Palma RJ, Torres-Costa V, Martin M, Gergely C, Durand JO, Cunin F, et al. Adhesion and proliferation of human mesenchymal stem cells from dental pulp on porous silicon scaffolds. ACS Appl Mater Interfaces. 2014;6:1719–1728. doi: 10.1021/am4046316. [DOI] [PubMed] [Google Scholar]
- 81.Haridas V, Sadanandan S, Collart-Dutilleul PY, Gronthos S, Voelcker NH. Lysine-appended polydiacetylene scaffolds for human mesenchymal stem cells. Biomacromolecules. 2014;15:582–590. doi: 10.1021/bm4015655. [DOI] [PubMed] [Google Scholar]
- 82.Panayotov IV, Collart-Dutilleul PY, Salehi H, Martin M, Végh A, Yachouh J, Vladimirov B, Sipos P, Szalontai B, Gergely C, et al. Sprayed cells and polyelectrolyte films for biomaterial functionalization: the influence of physical PLL-PGA film treatments on dental pulp cell behavior. Macromol Biosci. 2014;14:1771–1782. doi: 10.1002/mabi.201400256. [DOI] [PubMed] [Google Scholar]
- 83.Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, Shi S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101:3285–3294. doi: 10.1002/jbm.a.34546. [DOI] [PubMed] [Google Scholar]
- 84.Niu LN, Sun JQ, Li QH, Jiao K, Shen LJ, Wu D, Tay F, Chen JH. Intrafibrillar-silicified collagen scaffolds enhance the osteogenic capacity of human dental pulp stem cells. J Dent. 2014;42:839–849. doi: 10.1016/j.jdent.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 85.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumagai G, Okada Y, Yamane J, Nagoshi N, Kitamura K, Mukaino M, Tsuji O, Fujiyoshi K, Katoh H, Okada S, et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One. 2009;4:e7706. doi: 10.1371/journal.pone.0007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, Montenegro X, Gonzalez R, Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008;17:1277–1293. doi: 10.3727/096368908787648074. [DOI] [PubMed] [Google Scholar]
- 89.Sharp J, Keirstead HS. Therapeutic applications of oligodendrocyte precursors derived from human embryonic stem cells. Curr Opin Biotechnol. 2007;18:434–440. doi: 10.1016/j.copbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J Stem Cells. 2014;6:120–133. doi: 10.4252/wjsc.v6.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 92.Taghipour Z, Karbalaie K, Kiani A, Niapour A, Bahramian H, Nasr-Esfahani MH, Baharvand H. Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev. 2012;21:1794–1802. doi: 10.1089/scd.2011.0408. [DOI] [PubMed] [Google Scholar]
- 93.Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young F, Sloan A, Song B. Dental pulp stem cells and their potential roles in central nervous system regeneration and repair. J Neurosci Res. 2013;91:1383–1393. doi: 10.1002/jnr.23250. [DOI] [PubMed] [Google Scholar]
- 95.Dafni UG, Tzioufas AG, Staikos P, Skopouli FN, Moutsopoulos HM. Prevalence of Sjögren’s syndrome in a closed rural community. Ann Rheum Dis. 1997;56:521–525. doi: 10.1136/ard.56.9.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjögren‘s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren‘s Syndrome. Arthritis Rheum. 1999;42:1765–1772. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 97.Devauchelle-Pensec V, Pennec Y, Morvan J, Pers JO, Daridon C, Jousse-Joulin S, Roudaut A, Jamin C, Renaudineau Y, Roué IQ, et al. Improvement of Sjögren’s syndrome after two infusions of rituximab (anti-CD20) Arthritis Rheum. 2007;57:310–317. doi: 10.1002/art.22536. [DOI] [PubMed] [Google Scholar]
- 98.Tobón GJ, Saraux A, Pers JO, Youinou P. Emerging biotherapies for Sjögren’s syndrome. Expert Opin Emerg Drugs. 2010;15:269–282. doi: 10.1517/14728211003702392. [DOI] [PubMed] [Google Scholar]
- 99.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 101.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 102.Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis. 2011;17:143–153. doi: 10.1111/j.1601-0825.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 103.Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, Coppes RP. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol. 2013;108:458–463. doi: 10.1016/j.radonc.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 104.Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim HJ, Song SU, Kim YM. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. 2013;49:136–143. doi: 10.1016/j.oraloncology.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Lim JY, Ra JC, Shin IS, Jang YH, An HY, Choi JS, Kim WC, Kim YM. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS One. 2013;8:e71167. doi: 10.1371/journal.pone.0071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sumita Y, Liu Y, Khalili S, Maria OM, Xia D, Key S, Cotrim AP, Mezey E, Tran SD. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol. 2011;43:80–87. doi: 10.1016/j.biocel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 108.Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 2012;59:203–227. doi: 10.1111/j.1600-0757.2012.00443.x. [DOI] [PubMed] [Google Scholar]
- 109.Gonçalves PF, Gurgel BC, Pimentel SP, Sallum EA, Sallum AW, Casati MZ, Nociti FH. Effect of two different approaches for root decontamination on new cementum formation following guided tissue regeneration: a histomorphometric study in dogs. J Periodontal Res. 2006;41:535–540. doi: 10.1111/j.1600-0765.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 110.Needleman IG, Worthington HV, Giedrys-Leeper E, Tucker RJ. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006;(2):CD001724. doi: 10.1002/14651858.CD001724.pub2. [DOI] [PubMed] [Google Scholar]
- 111.Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647–662. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: A clinical case report. Int J Periodontics Restorative Dent. 2006;26:363–369. [PubMed] [Google Scholar]
- 113.Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75:1281–1287. doi: 10.1902/jop.2004.75.9.1281. [DOI] [PubMed] [Google Scholar]
- 114.Ikeda E, Hirose M, Kotobuki N, Shimaoka H, Tadokoro M, Maeda M, Hayashi Y, Kirita T, Ohgushi H. Osteogenic differentiation of human dental papilla mesenchymal cells. Biochem Biophys Res Commun. 2006;342:1257–1262. doi: 10.1016/j.bbrc.2006.02.101. [DOI] [PubMed] [Google Scholar]
- 115.Flores MG, Hasegawa M, Yamato M, Takagi R, Okano T, Ishikawa I. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J Periodontal Res. 2008;43:364–371. doi: 10.1111/j.1600-0765.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 116.Nakahara T, Nakamura T, Kobayashi E, Kuremoto K, Matsuno T, Tabata Y, Eto K, Shimizu Y. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004;10:537–544. doi: 10.1089/107632704323061898. [DOI] [PubMed] [Google Scholar]
- 117.Lin NH, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Putative stem cells in regenerating human periodontium. J Periodontal Res. 2008;43:514–523. doi: 10.1111/j.1600-0765.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 118.Khorsand A, Eslaminejad MB, Arabsolghar M, Paknejad M, Ghaedi B, Rokn AR, Moslemi N, Nazarian H, Jahangir S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implantol. 2013;39:433–443. doi: 10.1563/AAID-JOI-D-12-00027. [DOI] [PubMed] [Google Scholar]
- 119.Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Collart Dutilleul PY, Fonseca CG, Zimányi L, Romieu O, Pozos-Guillén AJ, Semetey V, Cuisinier F, Pérez E, Levallois B. Root canal hydrophobization by dentinal silanization: improvement of silicon-based endodontic treatment tightness. J Biomed Mater Res B Appl Biomater. 2013;101:721–728. doi: 10.1002/jbm.b.32874. [DOI] [PubMed] [Google Scholar]
- 121.Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 123.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 124.Ramirez JM, Bai Q, Dijon-Grinand M, Assou S, Gerbal-Chaloin S, Hamamah S, De Vos J. Human pluripotent stem cells: from biology to cell therapy. World J Stem Cells. 2010;2:24–33. doi: 10.4252/wjsc.v2.i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19:469–480. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beltrão-Braga PC, Pignatari GC, Maiorka PC, Oliveira NA, Lizier NF, Wenceslau CV, Miglino MA, Muotri AR, Kerkis I. Feeder-free derivation of induced pluripotent stem cells from human immature dental pulp stem cells. Cell Transplant. 2011;20:1707–1719. doi: 10.3727/096368911X566235. [DOI] [PubMed] [Google Scholar]
- 127.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]


