Abstract
The distinction between causes of acute infections is a major clinical challenge. Current biomarkers, however, are not sufficiently accurate. Human neutrophil lipocalin (HNL) concentrations in serum or whole blood activated by formyl-methionine-leucine-phenylalanine (fMLP) were shown to distinguish acute infections of bacterial or viral cause with high accuracy. The aim was therefore to compare the clinical performance of HNL with currently used biomarkers. Seven hundred twenty-five subjects (144 healthy controls and 581 patients with signs and symptoms of acute infections) were included in the study. C-reactive protein (CRP), the expression of CD64 on neutrophils, procalcitonin (PCT), and blood neutrophil counts were measured by established techniques, and HNL concentrations were measured in whole-blood samples after activation with fMLP. All tested biomarkers were elevated in bacterial as opposed to viral infections (P < 0.001). CRP, PCT, and CD64 expression in neutrophils was elevated in viral infections compared to healthy controls (P < 0.001). In the distinction between healthy controls and patients with bacterial infections, the areas under the receiver operating characteristic (ROC) curves were >0.85 for all biomarkers, whereas for the distinction between bacterial and viral infections, only HNL concentration in fMLP-activated whole blood showed an area under the ROC curve (AUROC) of >0.90 and superior clinical performance. The clinical performance of HNL in fMLP-activated whole blood was superior to current biomarkers and similar to previous results of HNL in serum. The procedure can be adopted for point-of-care testing with response times of <15 min.
INTRODUCTION
Human neutrophil lipocalin (HNL) is also called lipocalin 2 or neutrophil gelatinase-associated lipocalin (NGAL) (1). HNL is stored in the secondary granules of neutrophil granulocytes, but the production of the protein may also be induced in epithelial cells and monocytes/macrophages under certain conditions (2–4). Sensitive immunoassays were developed to measure HNL/NGAL in various bodily fluids, and the concentrations in blood were shown to be raised in patients with bacterial infections and in urine in patients with acute kidney injury (5–8). Indeed, serum concentrations of HNL were shown to discriminate between acute bacterial and viral infections with high accuracy and with sensitivities and specificities of >90% (7). The discriminatory power of HNL concentrations in serum was seen in additional studies on children and adults, indicating that HNL measurements in serum might be a clinically useful biomarker for the distinction between bacterial or viral causes of acute infection (9, 10). However, the high discriminatory power of HNL concentration was seen only with measurements in serum and not with EDTA-plasma. This suggested that the neutrophils in the test tube ex vivo continued to release their HNL during blood coagulation.
To be useful in the emergency department or a doctor's office, the total assay time from blood to result of any biomarker should be short, i.e., <15 to 20 min, which is the philosophy behind the development of point-of-care (POC) assays (11, 12). Such requirements are not possible using serum measurements of HNL. In a recent report (13), we showed that the activation of purified neutrophils for a few minutes by the well-established neutrophil activator tripeptide formyl-methionine-leucine-phenylalanine (fMLP) might circumvent this problem, since the release of HNL from neutrophils obtained from both healthy and infected patients very closely correlated to the concentrations of HNL in serum. Moreover, we showed that the clinical performance of the activation of neutrophils in whole blood was superior to the measurement of HNL concentrations in plasma, with sensitivities and specificities in the discrimination between bacterial and viral causes of acute infections of >90%. In this study, we examined the diagnostic performance of HNL concentrations in whole blood after activation with fMLP in comparison to that with established and novel biomarkers, such as blood neutrophil counts, C-reactive protein (CRP), the expression on neutrophils of the Fc receptor CD64, and procalcitonin.
MATERIALS AND METHODS
Patients.
The total study cohort included 725 participants. Of the patients with signs and symptoms of acute infection, 253 were males (mean ± standard deviation [SD] age, 52.7 ± 20.0 years), and 328 were females (mean ± SD age, 46.4 ± 19.3 years). The 144 healthy controls had an average ± SD age of 43.6 ± 12.8 years and consisted of 57 males (mean ± SD age, 41.3 ± 12.7 years) and 87 females (mean ± SD age, 45.0 ± 12.8 years).
The number of patients with a confirmed etiology of their acute infection was 288 (49.6% of all patients). Of these patients, 185 had a bacterial infection, 54 had a viral infection, 26 had a mycoplasmal infection, and 23 had a bacterial infection as a secondary infection to influenza. The distribution of the confirmed infections is shown in Table 1. The number of patients remaining with an uncertain diagnosis was 293 (50.4%).
TABLE 1.
Patients with signs and symptoms of acute infections and confirmed etiology
| Type of infection | No. of patients with confirmed etiology | Age (mean ± SD) (% male) |
|---|---|---|
| Viral (influenza A, RSV, dengue, etc.) | 54 | 49.7 ± 20.1 (51) |
| Bacterial pneumonia | 45 | 63.9 ± 15.2 (51) |
| Mycoplasma pneumonia | 27 | 40.4 ± 15.2 (48) |
| Streptococcal tonsillitis | 39 | 34.8 ± 11.4 (34) |
| Urinary tract | 41 | 57.2 ± 19.8 (49) |
| Bacterial gastrointestinal | 26 | 42.9 ± 22.0 (42) |
| Erysipelas | 16 | 55.8 ± 13.9 (56) |
| Sepsis, endocarditis | 17 | 61.6 ± 19.6 (63) |
| Bacterial secondary to influenza virus | 23 | 56.5 ± 18.0 (50) |
| Total infected | 288 | 50.5 ± 20.1 (47) |
| Healthy controls | 145 | 43.5 ± 12.8 (40) |
The study was approved by the Uppsala Regional Ethics Committee.
Methods.
The inclusion criteria for patients in the study were fever of >38°C and signs and symptoms of an acute infection. An exclusion criterion was known chronic viral infection, such as human immunodeficiency virus infection or hepatitis. In addition, children age <18 years and patients who could not give informed consent were excluded from this study. The patients were admitted to the infectious disease department at the University Hospital in Uppsala (n = 449) or to a primary care unit in Uppsala (n = 132). A blood sample was drawn before the start of antibiotic treatment.
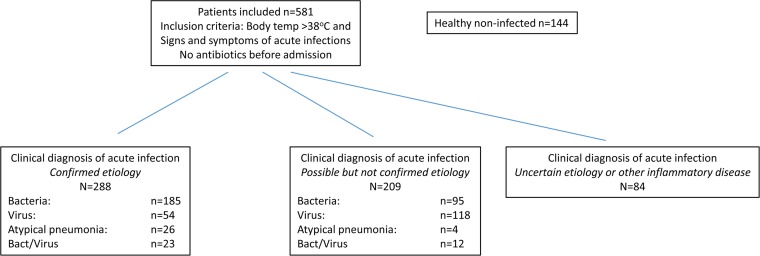
The patients were classified into three groups, as shown in Fig. 1. In the group with confirmed etiology of acute infection, clinical findings and assessment were documented, including white blood cell counts and CRP levels, and verified with objective tests used in the routine diagnostics. In the pneumonia group, the diagnosis was verified with a positive chest X-ray and supported by positive culture or PCR test from the lower respiratory tract samples. The diagnosis of respiratory tract infection with viruses, for example, influenza A/B and atypical pneumonia, such as mycoplasma pneumonia, was supported by PCR testing of samples from the respiratory tract. Viral infections, such as dengue fever, Epstein-Barr virus, or cytomegalovirus infection, were supported by IgG/IgM serology results. The diagnosis of bacterial infections was supported by cultures from blood, urine, stool, wound abscesses, and the respiratory tract, when appropriate. Tonsillitis was diagnosed by a rapid test for group A Streptococcus and supported by positive culture. Endocarditis diagnosis was made from positive blood cultures and findings on echocardiography.
FIG 1.
Description of patient inclusion numbers and clinical adjudication diagnosis. Bact, bacterial.
In the group with possible etiology, only a clinical assessment of findings, including white blood cell counts and CRP levels, without any confirmed etiology by objective tests was performed. The objective tests were either not taken or had given a negative result.
In the third group with unclear infection, the diagnosis could not be determined or was no infection but with a tumor or polymyalgia rheumatica.
Biomarkers of infections.
CRP levels and white blood cell (WBC) counts with differentials were analyzed at the routine department of clinical chemistry at Uppsala University Hospital.
HNL was measured in heparinized blood (B-HNL) after preactivation with the synthetic tripeptide formyl-methionine-leucine-phenylalanine (fMLP) (BioXtra; Sigma-Aldrich, St. Louis, MO, USA). HNL in whole blood was assayed by a prototype POC assay on the Meritas platform (Fiomi Diagnostics, Uppsala, Sweden), as described recently (13). The coefficient of variation (CV) profile showed an imprecision rate of the POC assay of <10% being >75 μg/liter. The POC assay correlated well with the ELISA results (r = 0.9594; fit POC assay = 1.04 × ELISA + 2.4).
Procalcitonin was measured in EDTA-plasma by means of the Kryptor instrument and according to the instructions of the manufacturer (Thermo Fisher Scientific, Hennigsdorf, Germany).
The expression on blood neutrophils of the Fc receptor CD64 was measured by flow cytometry, as previously described (14).
Statistics.
The data are expressed as the means ± SD or geometric means with 95% confidence interval (CI) wherever appropriate. Comparisons of two groups, either dependent or independent, were performed by Student's paired or unpaired t test and comparisons of >2 groups by one-way analysis of variance (ANOVA). Pearson's linear correlation was applied. P values of <0.05 were considered significant. In order to estimate the clinical performance of the biomarker assays, receiver operating characteristic (ROC) analyses were performed and comparisons of the areas under the curve were analyzed by c-statistics. After logarithmic transformation of the biomarker results, logistic regression analysis was performed to test for the diagnostic performances of more than one biomarker. For the calculations, the statistics programs MedCalc Statistical Software version 14.8.1 (MedCalc Software, Ostend, Belgium) and Statistica 64 version 12 (StatSoft, Tulsa, OK, USA) were used.
RESULTS
Heparinized whole-blood and EDTA-plasma samples were collected from 581 patients with fever of >38°C and symptoms of infection and from 144 apparently noninfected healthy subjects. Without knowledge of the investigated biomarker (HNL, PCT, and CD64 expression on blood neutrophils) results, the infected patients were classified as having a bacterial or viral cause of their disease. The primary study group included patients with confirmed etiology of their infections but excluded mycoplasmal infections and patients with mixed infections. The study group consisted of 383 subjects (144 healthy noninfected controls, 185 with bacterial infections, and 54 with viral infections) (Table 1).
Diagnostic performances of B-HNL, CD64 expression on neutrophils, and procalcitonin.
In Fig. S1a to d in the supplemental material, we show the distribution of the four additional biomarkers in the healthy control group compared to the results in those with bacterial or viral infections. Data on B-HNL were recently described (13). The results are summarized in Table 2 and show elevated levels in both viral and bacterial infections compared to those in the controls for all biomarkers, except blood neutrophil counts, which were unaltered in the virally infected group.
TABLE 2.
Concentrations and expression of studied biomarkers
| Group | Geometric mean concn (95% CI) for: |
||||
|---|---|---|---|---|---|
| B-HNL (μg/liter) | CRP (mg/liter) | Blood neutrophil count (109/liter) | CD64-PMN (MFI)a | Procalcitonin (μg/liter) | |
| Healthy | 98 (90–107) | 1.06 (0.90–1.26) | 3.59 (3.38–3.81) | 0.70 (0.65–0.75) | 0.042 (0.038–0.047) |
| Bacterial infection | 337 (300–379)b | 81.0 (68.8–95.4)b | 8.21 (7.60–8.88)b | 3.13 (2.78–3.52)b | 0.262 (0.205–0.335)b |
| Viral infection | 117 (101–136)c | 20.7 (14.7–29.0)b | 3.82 (3.23–4.53) | 1.59 (1.26–2.01)b | 0.117 (0.093–0.149)b |
PMN, polymorphonuclear leukocyte; MFI, mean fluorescence intensity.
P < 0.001 versus healthy controls.
P < 0.05 versus healthy controls.
In Fig. 2, we show the distribution of CD64 expression, procalcitonin, and B-HNL concentrations separated by verified clinical diagnosis. Compared to healthy subjects, all three biomarkers were elevated in all diagnoses with a bacterial cause. Compared to the levels with viral infections, CD64 expression on neutrophils was significantly higher in all diagnoses (P < 0.01) except erysipelas and sepsis. With respect to PCT, the results for bacterial pneumonia, bacterial urinary tract infections (UTI), and sepsis were significantly higher (P < 0.01 for bacterial pneumonia and P < 0.001 for UTI and sepsis) than the results in the viral group, whereas the results for the other bacterial infections and for mycoplasma pneumonia were nonsignificantly different. B-HNL concentrations were significantly elevated for bacterial pneumonia, tonsillitis, UTI, erysipelas, and sepsis, compared to the levels with viral infections (P < 0.001 for all comparisons), and for mycoplasma pneumonia (P < 0.001) and bacterial gastrointestinal infections (P < 0.01).
FIG 2.
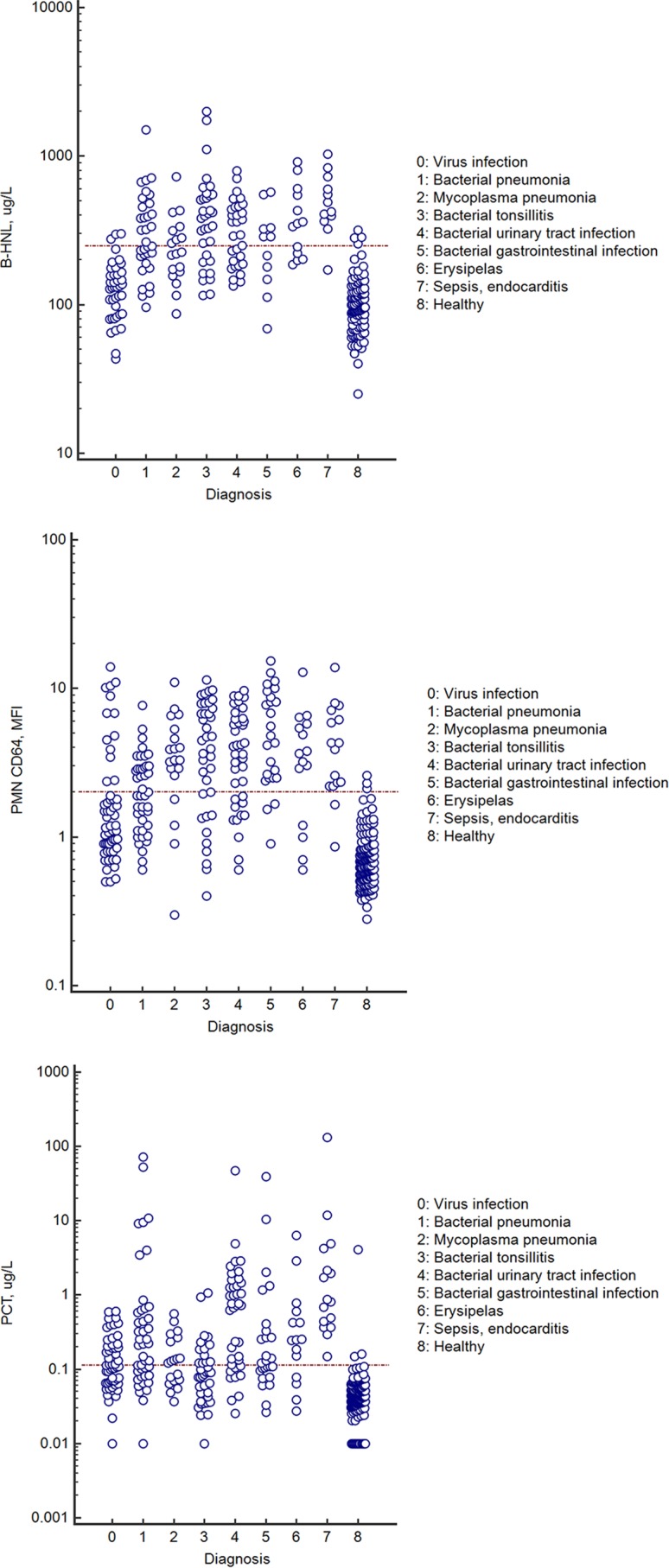
Top, B-HNL concentrations in the different diagnostic groups, as indicated by the numbers. Middle, expression of CD64 on PMN in the different diagnostic groups. Bottom, concentrations of procalcitonin in the different diagnostic groups. For all panels, only results with verified etiologies are presented. The horizontal line indicates the 97.5th percentile of the healthy control group.
Figure 3 shows the comparisons of the clinical performance of the three studied biomarkers. The areas under the ROC curves (AUROCs) are given in Table 3. In the distinction between healthy and bacterial infections, two biomarkers showed AUROCs of >90%, and these were B-HNL and the expression of CD64 on blood neutrophils. The AUROC of B-HNL was significantly higher than the AUROC of procalcitonin (P = 0.02), and the AUROC of the expression of CD64 on neutrophils was higher than that for PCT (P = 0.03).
FIG 3.
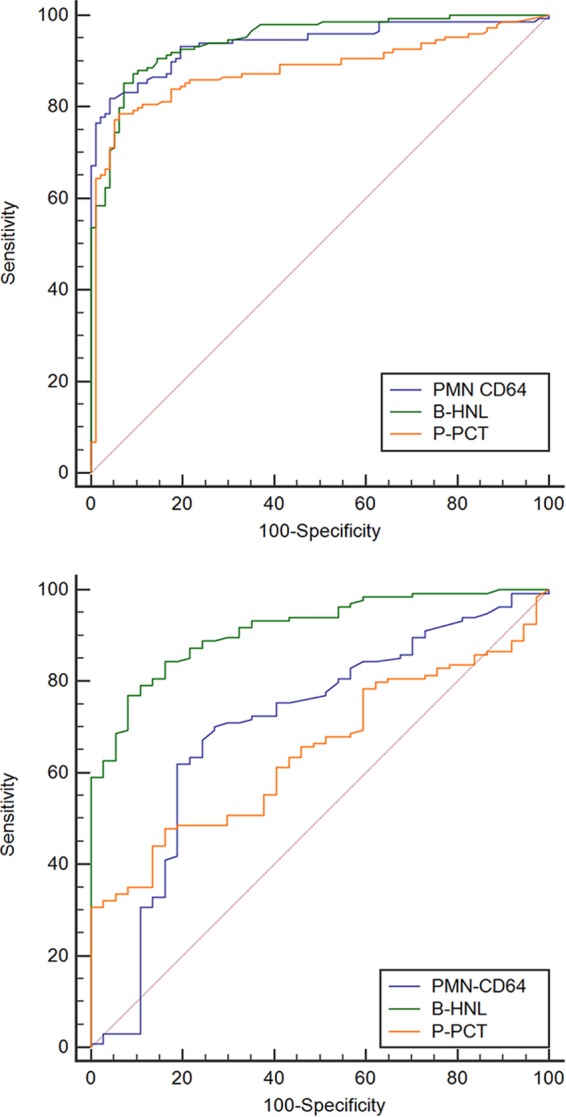
Receiver operating characteristic curves of B-HNL, expression of CD64 on PMN, and plasma PCT (P-PCT) in the discrimination between healthy noninfected subjects and patients with bacterial infections (top), and between patients with bacterial and viral infections (bottom). The areas under ROC curve results are summarized in Table 3.
TABLE 3.
Areas under the ROC curves of the studied biomarkers
| Biomarker by group comparison | AUROC (95% CI) |
|---|---|
| Healthy vs bacteria | |
| B-HNL | 0.94 (0.91–0.97) |
| CD64 expression on PMN | 0.94 (0.91–0.97) |
| Procalcitonin | 0.88 (0.83–0.92)a |
| Bacteria vs virus | |
| B-HNL | 0.91 (0.86–0.95) |
| CD64 expression on PMN | 0.71 (0.63–0.78)b |
| Procalcitonin | 0.64 (0.57–0.72)b |
P < 0.05, compared to B-HNL.
P < 0.001, compared to B-HNL.
In the distinction between bacterial and viral infections, only the AUROC of B-HNL was >90%. This was significantly different from CD64 expression on neutrophils and procalcitonin (P < 0.001 for both comparisons).
In Table 4, the AUROCs are shown of the three biomarkers in the distinction between the results with viral infections and the various diagnoses of bacterial infections or mycoplasma pneumonia. The clinical performance of B-HNL is superior to the other two biomarkers in bacterial pneumonia, streptococcal tonsillitis, and erysipelas but similar in sepsis and gastrointestinal infections.
TABLE 4.
Diagnostic distinction between viral infections and various diagnoses of bacterial and mycoplasma infections
| Diagnosis | AUROC (95% CI) for: |
||
|---|---|---|---|
| B-HNL | CD64 | PCT | |
| Bacterial pneumonia | 0.868 (0.771–0.935) | 0.641 (0.523–0.748)a | 0.647 (0.529–0.753)a |
| Mycoplasma pneumonia | 0.789 (0.674–0.878) | 0.776 (0.659–0.867) | 0.551 (0.426–0.671)b |
| Streptococcal tonsillitis | 0.905 (0.815–0.960) | 0.765 (0.653–0.855)c | 0.537 (0.418–0.653)a |
| Urinary tract infection | 0.899 (0.805–0.957) | 0.707 (0.588–0.808)b | 0.808 (0.698–0.891) |
| Bacterial gastrointestinal infection | 0.773 (0.635–0.887) | 0.832 (0.703–0.921) | 0.698 (0.555–0.817) |
| Erysipelas | 0.951 (0.856–0.991) | 0.683 (0.544–0.802)b | 0.676 (0.534–0.991)b |
| Sepsis, endocarditis | 0.980 (0.899–0.999) | 0.824 (0.697–0.914) | 0.938 (0.838–0.986) |
P < 0.001, compared to the AUROC of B-HNL.
P < 0.01, compared to the AUROC of B-HNL.
P < 0.05, compared to the AUROC of B-HNL.
Diagnostic performance of CRP, blood neutrophil counts, and combinations of biomarkers.
In the supplemental material, we show the ROC curve analysis of CRP levels and blood neutrophil counts in the distinction between bacterial and viral infections. These two biomarkers were justified for use in this comparison, because the diagnoses of the patients included in the analysis had been supported by other objective tests. As can be seen in Fig. S3 in the supplemental material, CRP (P = 0.009) and blood neutrophil counts (P = 0.03) had a lower area under the ROC curve than that of B-HNL.
The diagnostic performances of combinations of biomarkers were tested by logistic regression analysis. All three biomarkers, in addition to age and sex, were included in the analysis. The biomarkers were log transformed before the analysis. In the distinction between healthy noninfected subjects and those with bacterial infections, the expression of CD64 on neutrophils and PCT added to the performance of B-HNL, with an increase in the AUROC from 0.95 (95% CI, 0.91 to 0.97) to 0.98 (95% CI, 0.96 to 1.00). Odds ratios are given in Table 5 and show that in the discrimination between healthy noninfected subjects and patients with bacterial infections, high odds ratios were achieved for B-HNL (odds ratio [OR], 42.0) and the expression of CD64 on neutrophils (OR, 28.4), and the two biomarkers independently contributed to the discrimination. Also, PCT added independently to the diagnostic performance (OR, 2.3). With the inclusion of CRP concentrations and neutrophil counts in the model, the AUROC increased further to 0.996 (95% CI, 0.976 to 1.0) but only with CRP concentration as an independent variable (see Table S1 in the supplemental material). In the distinction between bacterial and viral infections, none of the other biomarkers added to the diagnostic performance of B-HNL. The odds ratio for B-HNL was 37.4 (95% CI, 10.7 to 130).
TABLE 5.
Results of the logistic regression analysis of the studied biomarkersa
| Biomarker by group comparison | Odds ratio (95% CI) | P value |
|---|---|---|
| Healthy vs bacterial infectionsb | ||
| B-HNL | 42.0 (8.7–203) | <0.001 |
| CD64-PMN | 28.4 (6.8–118) | <0.001 |
| PCT | 2.3 (1.3–4.4) | 0.005 |
| Bacterial vs viral infectionsc | ||
| B-HNL | 37.4 (10.7–130) | <0.001 |
Included in the model were the following variables (only independent variables are shown in the table): age, sex, CD64-PMN, B-HNL, P-HNL, and PCT. Continuous variables, except age, were included in the model after logarithmic transformation.
AUROC, 0.98 (95% CI, 0.96 to 1.00).
AUROC, 0.91 (95% CI, 0.86 to 0.95).
DISCUSSION
Symptoms of acute infections are probably the most common reason for seeking health care worldwide. In a preliminary survey of a large primary health care center in Sweden, 49% of all patients who visited the center did so because of symptoms of acute infection. The judgment of the doctor of whether to treat the infection with antibiotics is commonly based on clinical symptoms and in some cases is supported by laboratory tests, such as white blood cell counts, CRP concentrations, and rapid tests for bacterial or viral agents (15). The sensitivities and specificities of such tests, however, often preclude an accurate distinction between various causes of infection, with the consequent prescription of antibiotics to be used if needed. The unnecessary prescription of antibiotics or sales of antibiotics without any prescription over the counter add to the rapid development of antibiotic resistance, which is seen as a serious threat to mankind (16, 17). Thus, the development of diagnostic tools that are accurate and easily available is highly desirable. In previous publications, we showed that the neutrophil secretory protein HNL may represent a prominent step in this direction, since serum concentrations of HNL allowed an accurate distinction between bacterial or viral cause of acute infections, with negative and positive predictive values of >90%, which were superior to results obtained using white blood cell counts and CRP (7, 9, 10). However, in order to make HNL easily available and attractive to a primary care physician or to an emergency doctor, the results of HNL concentrations in blood should be reported within 15 to 20 min, with similar performance as serum measurement of HNL in the laboratory. In this study, we investigated the possible use of whole-blood samples for this purpose and showed that whole-blood measurement after activation with the neutrophil activator fMLP (13) is a superior assay for the identification of patients with bacterial infections in comparison to currently used assays, such as CRP, CD64 expression on neutrophils, procalcitonin, and blood neutrophil counts. It is also important that normal concentrations of HNL with high likelihood ruled out bacteria as the causative agents of acute infections.
Our previous finding that plasma measurements of HNL were less diagnostic in the distinction between bacterial and viral infections than the previously reported findings with serum indicates a unique quality of serum. This quality is due to the additional release in the extracellular environment of HNL during coagulation and this is an active process which is time and temperature dependent and most likely reflects the inherent state of activity of neutrophils in the blood. Our attempts in the recent study to try to mimic coagulation activation of HNL release by the activation of the blood neutrophils with fMLP showed a high concordance between this release and serum HNL concentrations measured in the same individual. The recent demonstration that HNL/NGAL may be induced in monocytes/macrophages (4), however, should be considered, although our previous investigations did not show any immunostaining of HNL in human blood monocytes (our unpublished data). Thus, the release from blood monocytes may contribute somewhat to the levels of HNL found after fMLP activation of whole blood. Our results prompted us to compare this diagnostic modality with those currently available, since HNL in fMLP-activated whole blood may be suitable for point-of-care applications.
In the comparisons of clinical performance, we estimated the diagnostic performance of the distinction between bacterial infection and healthy controls in addition to the distinction between bacterial and viral infections. In these comparisons, we included only those patients for whom the infectious etiology was objectively supported. This approach obviously reduced the numbers of patients in the two cohorts by 60% but was taken to minimize the numbers of false classifications, since it is well known that it is difficult to make a true distinction between different causes of infections, bacterial or viral, based on clinical judgment only (15).
One limitation of the comparisons of HNL with other biomarkers is that both CRP concentrations and WBC counts were used in the clinical judgment of the diagnosis. A bias toward these two biomarkers precluded an accurate evaluation of their diagnostic performance. However, with this caution in mind, CRP seemed to be a powerful tool for ruling out patients with bacterial infections, since the overlap in plasma concentrations between healthy noninfected subjects and those having a bacterial infection was minor. In viral infections, the considerable overlap with bacterial infections, however, indicated that CRP is less useful in this distinction, which corroborates current knowledge of the diagnostic power of CRP levels (18–23). In our earlier studies, serum measurements of HNL were clearly superior to those of CRP and also showed differences between CRP and serum HNL in kinetics after the start of antibiotic treatment, since serum concentrations of HNL normalized within 2 to 3 days, in contrast to CRP, which stayed elevated several days after the infection had subsided. Thus, HNL concentration seems to more closely reflect the ongoing infection than CRP concentration. Another limitation of CRP as a diagnostic means in acute infections is the low specificity of CRP, since this biomarker is found to be raised in most other inflammatory diseases. HNL, on the other hand, was found to be nonelevated in patients with active rheumatoid arthritis (24).
The diagnostic performance of procalcitonin was unexpectedly poor in this study, in particular in the discrimination between acute bacterial and viral infections. The inferior performance compared to that with most other biomarkers was explained by a 3-fold rise in concentrations in viral infections and a considerable overlap with the plasma levels of patients with bacterial infections. The highest concentrations of procalcitonin were found in patients with sepsis and urinary tract infections, such as pyelonephritis, which is in line with current understanding (21–23, 25–32). However, in lower respiratory tract infections, PCT levels were only occasionally found to be elevated. Thus, the clinical usefulness of procalcitonin may be in patients with very severe bacterial infections, such as sepsis, but not in the diagnosis of less severe infections and in the distinction against viral infections.
The expression of the Fc receptor CD64 on neutrophils is very low in healthy subjects, whereas the expression is considerably increased on the surface of neutrophils obtained from patients with bacterial infections. We and others showed previously that CD64 expression discriminated well between septic and nonseptic patients in both children and adults (14, 33–39). An additional study showed a concordance between the expression of CD64 on neutrophils and serum HNL, which indicated to us that these two biomarkers might reflect similar processes, i.e., the inherently increased activity of neutrophils in patients with bacterial infections (14, 34). The kinetics of these two biomarkers, however, are different, with a slow onset of CD64 expression compared to that of serum HNL and a slower normalization. Our findings in this study confirm the earlier findings of increased expression in bacterial infections, whereas the increase also in patients with viral infections seems to preclude CD64 expression as an acceptable diagnostic means to distinguish viral from bacterial infections. However, to what extent this diagnostic inferiority in this study relates to the above-mentioned notion of differences in kinetics cannot be concluded. One interesting notion in this regard is the large differences seen in mycoplasma pneumonia with highly elevated expression of CD64 on neutrophils compared to healthy persons in contrast to minor elevations of B-HNL and PCT levels.
We conclude from this study that neutrophil release of HNL in whole blood induced by the neutrophil activator fMLP is increased in blood samples obtained from patients with bacterial infections as opposed to blood samples from healthy controls and patients with viral infections. The increased propensity of neutrophils in this regard mimics the propensity of neutrophils to release HNL at coagulation. Similar to recent studies on serum HNL, the clinical performance of fMLP activation of whole blood showed high positive and negative predictive values in the distinction between acute bacterial and viral infections and was superior to current biomarkers, such as procalcitonin levels and CD64 expression on neutrophils. Building point-of-care applications based on this principle with a response time of <15 min should be the next step in the development of diagnostic tools as aids in the reduction of antibiotic abuse.
ACKNOWLEDGMENTS
We thank all research nurses and laboratory staff who contributed to the collection of clinical information and blood samples from patients and healthy controls and for the expert technical assistance.
This work was funded by a BIO-X grant from Uppsala BIO, Diagnostics Development, and Governmental Funding of Clinical Research within the National Health Services of Sweden (ALF) at Uppsala University Hospital.
The authors are part of research groups at Uppsala University belonging to the Centre of Excellence, Inflammation at Uppsala University Hospital (Akademiska sjukhuset), Uppsala, Sweden.
Per Venge and Shengyuan Xu own patent rights to measure HNL in bodily fluids in inflammatory disease (U.S. patent 6136526 A). Per Venge is a shareholder of Diagnostics Development.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00347-15.
REFERENCES
- 1.Xu S, Venge P. 2000. Lipocalins as biochemical markers of disease. Biochim Biophys Acta 1482:298–307. doi: 10.1016/S0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 2.Cai L, Rubin J, Han W, Venge P, Xu S. 2010. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol 5:2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. 1996. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zughaier SM, Tangpricha V, Leong T, Stecenko AA, McCarty NA. 2013. Peripheral monocytes derived from patients with cystic fibrosis and healthy donors secrete NGAL in response to Pseudomonas aeruginosa infection. J Invest Med 61:1018–1025. [DOI] [PubMed] [Google Scholar]
- 5.Mårtensson J, Xu S, Bell M, Martling CR, Venge P. 2012. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin Chim Acta 413:1661–1667. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Xu SY, Petersson CG, Carlson M, Venge P. 1994. The development of an assay for human neutrophil lipocalin (HNL)–to be used as a specific marker of neutrophil activity in vivo and vitro. J Immunol Methods 171:245–252. doi: 10.1016/0022-1759(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 7.Xu SY, Pauksen K, Venge P. 1995. Serum measurements of human neutrophil lipocalin (HNL) discriminate between acute bacterial and viral infections. Scand J Clin Lab Invest 55:125–131. doi: 10.3109/00365519509089604. [DOI] [PubMed] [Google Scholar]
- 8.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. 2005. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 9.Björkqvist M, Källman J, Fjaertoft G, Xu S, Venge P, Schollin J. 2004. Human neutrophil lipocalin: normal levels and use as a marker for invasive infection in the newborn. Acta Paediatr 93:534–539. [PubMed] [Google Scholar]
- 10.Fjaertoft G, Foucard T, Xu S, Venge P. 2005. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: a study of the kinetics. Acta Paediatr 94:661–666. doi: 10.1080/08035250510031610. [DOI] [PubMed] [Google Scholar]
- 11.Pfäfflin A, Schleicher E. 2009. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem 393:1473–1480. doi: 10.1007/s00216-008-2561-3. [DOI] [PubMed] [Google Scholar]
- 12.Bingisser R, Cairns C, Christ M, Hausfater P, Lindahl B, Mair J, Panteghini M, Price C, Venge P. 2012. Cardiac troponin: a critical review of the case for point-of-care testing in the ED. Am J Emerg Med 30:1639–1649. doi: 10.1016/j.ajem.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Venge P, Håkansson LD, Garwicz D, Peterson C, Xu S, Pauksen K. Human neutrophil lipocalin in fMLP-activated whole blood as a diagnostic means to distinguish between acute bacterial and viral infections. J Immunol Methods, in press. [DOI] [PubMed] [Google Scholar]
- 14.Fjaertoft G, Håkansson LD, Pauksens K, Sisask G, Venge P. 2007. Neutrophil CD64 (FcgammaRI) expression is a specific marker of bacterial infection: a study on the kinetics and the impact of major surgery. Scand J Infect Dis 39:525–535. doi: 10.1080/00365540601113693. [DOI] [PubMed] [Google Scholar]
- 15.Hopstaken RM, Muris JW, Knottnerus JA, Kester AD, Rinkens PE, Dinant GJ. 2003. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract 53:358–364. [PMC free article] [PubMed] [Google Scholar]
- 16.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. 2013. Antibiotic resistance–the need for global solutions. Lancet Infect Dis 13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 17.Nathan C, Cars O. 2014. Antibiotic resistance–problems, progress, and prospects. N Engl J Med 371:1761–1763. doi: 10.1056/NEJMp1408040. [DOI] [PubMed] [Google Scholar]
- 18.Andreeva E, Melbye H. 2014. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: an open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam Pract 15:80. doi: 10.1186/1471-2296-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calviño O, Llor C, Gómez F, González E, Sarvisé C, Hernández S. 2014. Association between C-reactive protein rapid test and group A streptococcus infection in acute pharyngitis. J Am Board Fam Med 27:424–426. doi: 10.3122/jabfm.2014.03.130315. [DOI] [PubMed] [Google Scholar]
- 20.Dupuy AM, Philippart F, Pean Y, Lasocki S, Charles PE, Chalumeau M, Claessens YE, Quenot JP, Guen CG, Ruiz S, Luyt CE, Roche N, Stahl JP, Bedos JP, Pugin J, Gauzit R, Misset B, Brun-Buisson C, Maurice Rapin Institute Biomarkers Group . 2013. Role of biomarkers in the management of antibiotic therapy: an expert panel review. I. Currently available biomarkers for clinical use in acute infections. Ann Intensive Care 3:22. doi: 10.1186/2110-5820-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hausfater P. 2014. Biomarkers and infection in the emergency unit. Med Mal Infect 44:139–145. doi: 10.1016/j.medmal.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Ip M, Rainer TH, Lee N, Chan C, Chau SS, Leung W, Leung MF, Tam TK, Antonio GE, Lui G, Lau TK, Hui DS, Fuchs D, Renneberg R, Chan PK. 2007. Value of serum procalcitonin, neopterin, and C-reactive protein in differentiating bacterial from viral etiologies in patients presenting with lower respiratory tract infections. Diagn Microbiol Infect Dis 59:131–136. doi: 10.1016/j.diagmicrobio.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Toikka P, Irjala K, Juvén T, Virkki R, Mertsola J, Leinonen M, Ruuskanen O. 2000. Serum procalcitonin, C-reactive protein and interleukin-6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J 19:598–602. doi: 10.1097/00006454-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Torsteinsdottir I, Håkansson L, Hällgren R, Gudbjörnsson B, Arvidson NG, Venge P. 1999. Serum lysozyme: a potential marker of monocyte/macrophage activity in rheumatoid arthritis. Rheumatology (Oxford) 38:1249–1254. doi: 10.1093/rheumatology/38.12.1249. [DOI] [PubMed] [Google Scholar]
- 25.Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, Taneja S, Ray S. 2014. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care 218:e7–e12. doi: 10.1016/j.jcrc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Friend KE, Burgess JN, Britt RC, Collins JN, Weireter LN, Novosel TJ, Britt LD. 2014. Procalcitonin elevation suggests a septic source. Am Surg 80:906–909. [PubMed] [Google Scholar]
- 27.Gu WJ, Liu JC. 2014. Procalcitonin-guided therapy in severe sepsis and septic shock. Crit Care 18:427. doi: 10.1186/cc13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meisner M. 2014. Update on procalcitonin measurements. Ann Lab Med 34:263–273. doi: 10.3343/alm.2014.34.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller B, Becker KL. 2001. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Med Wkly 131:595–602. [DOI] [PubMed] [Google Scholar]
- 30.Schlattmann P, Brunkhorst FM. 2014. Procalcitonin as a diagnostic marker for sepsis. Lancet Infect Dis 14:189. doi: 10.1016/S1473-3099(13)70325-6. [DOI] [PubMed] [Google Scholar]
- 31.Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, Walker A, Bailey MJ, Johnson B, Millis D, Ding G, Peake S, Wong H, Thomas J, Smith K, Forbes L, Hardie M, Micallef S, Fraser JF, ProGUARD Study Investigators, ANZICS Clinical Trials Group . 2014. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med 190:1102–1110. doi: 10.1164/rccm.201408-1483OC. [DOI] [PubMed] [Google Scholar]
- 32.Franz AR, Kron M, Pohlandt F, Steinbach G. 1999. Comparison of procalcitonin with interleukin 8, C-reactive protein and differential white blood cell count for the early diagnosis of bacterial infections in newborn infants. Pediatr Infect Dis J 18:666–671. doi: 10.1097/00006454-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Fjaertoft G, Håkansson L, Ewald U, Foucard T, Venge P. 1999. Neutrophils from term and preterm newborn infants express the high affinity Fcgamma-receptor I (CD64) during bacterial infections. Pediatr Res 45:871–876. doi: 10.1203/00006450-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Fjaertoft G, Pauksen K, Håkansson L, Xu S, Venge P. 2005. Cell surface expression of FcgammaRI (CD64) on neutrophils and monocytes in patients with influenza A, with and without complications. Scand J Infect Dis 37:882–889. doi: 10.1080/00365540500348929. [DOI] [PubMed] [Google Scholar]
- 35.Fjaertoft G, Håkansson L, Foucard T, Ewald U, Venge P. 2005. CD64 (Fcgamma receptor I) cell surface expression on maturing neutrophils from preterm and term newborn infants. Acta Paediatr 94:295–302. doi: 10.1111/j.1651-2227.2005.tb03072.x. [DOI] [PubMed] [Google Scholar]
- 36.Allen E, Bakke AC, Purtzer MZ, Deodhar A. 2002. Neutrophil CD64 expression: distinguishing acute inflammatory autoimmune disease from systemic infections. Ann Rheum Dis 61:522–525. doi: 10.1136/ard.61.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis BH. 1996. Quantitative neutrophil CD64 expression: promising diagnostic indicator of infection or systemic acute inflammatory response. Clin Immunol Newsl 16:123–130. [Google Scholar]
- 38.Farias MG, de Lucena NP, Dal Bó S, de Castro SM. 2014. Neutrophil CD64 expression as an important diagnostic marker of infection and sepsis in hospital patients. J Immunol Methods 414:65–68. doi: 10.1016/j.jim.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Ng PC, Li G, Chui KM, Chu WC, Li K, Wong RP, Chik KW, Wong E, Fok TF. 2004. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res 56:796–803. doi: 10.1203/01.PDR.0000142586.47798.5E. [DOI] [PubMed] [Google Scholar]