Abstract
The continued discovery and development of adjuvants for vaccine formulation are important to safely increase potency and/or reduce the antigen doses of existing vaccines and tailor the adaptive immune response to newly developed vaccines. Adjuplex is a novel adjuvant platform based on a purified lecithin and carbomer homopolymer. Here, we analyzed the adjuvant activity of Adjuplex in mice for the soluble hemagglutinin (HA) glycoprotein of influenza A virus. The titration of Adjuplex revealed an optimal dose of 1% for immunogenicity, eliciting high titers of HA-specific IgG but inducing no significant weight loss. At this dose, Adjuplex completely protected mice from an otherwise lethal influenza virus challenge and was at least as effective as the adjuvants monophosphoryl lipid A (MPL) and alum in preventing disease. Adjuplex elicited balanced Th1-/Th2-type immune responses with accompanying cytokines and triggered antigen-specific CD8+ T-cell proliferation. The use of the peritoneal inflammation model revealed that Adjuplex recruited dendritic cells (DCs), monocytes, and neutrophils in the context of innate cytokine and chemokine secretion. Adjuplex neither triggered classical maturation of DCs nor activated a pathogen recognition receptor (PRR)-expressing NF-κB reporter cell line, suggesting a mechanism of action different from that reported for classical pathogen-associated molecular pattern (PAMP)-activated innate immunity. Taken together, these data reveal Adjuplex to be a potent and well-tolerated adjuvant with application for subunit vaccines.
INTRODUCTION
Vaccines based upon recombinant, purified, or inactivated microorganism-derived antigens generally have suboptimal immunogenicity in the absence of an adjuvant. The field of vaccine adjuvant discovery has gained rapid momentum following the discovery of innate immune pathogen recognition receptors (PRRs), including Toll-like, nucleotide-binding and oligomerization domain (NOD)-like, and retinoic acid-inducible gene (RIG)-like receptors (TLRs, NLRs, and RLRs, respectively) that activate and condition innate and adaptive immunity (1). Despite this, the absolute requirement to demonstrate adjuvant safety along with other considerations has limited the licensing of vaccines containing novel adjuvants. New vaccines, particularly those aimed at driving robust adaptive immune responses in situations in which these are limited, such as influenza virus vaccination in the aged or HIV-1 antibody-based vaccines, may require more potent and/or tailored adjuvants. Adjuvant discovery therefore remains an essential area of vaccine research (1).
Adjuplex is a biodegradable matrix of carbomer homopolymer (also known as Carbopol) and submicron-sized liposomes (nanoliposomes) derived from purified soy lecithin. Carbomers, a species of cross-linked polyacrylic acids with long and broad use in biomedicine (2), have been evaluated as experimental adjuvants in veterinary vaccines against swine parvovirus (3), circovirus type 2 (4), Staphylococcus aureus in sheep (5), and equine influenza virus (6). These reports demonstrate that biodegradable carbomers, such as Carbopol, are not harmful to mammals and stimulate a more robust immune response than that with antigen alone. Indeed, carbomers are a component of a licensed veterinary vaccine in pigs (Suvaxyn; Wyeth). Despite its veterinary use, there is little information available in the published literature relating to the type and magnitude of innate or adaptive immune response induced by carbomers compared to those of other well-characterized adjuvants. Lecithin is a biocompatible naturally occurring surfactant derived from the lipid matrix of biological membranes (7). The term lecithin is loosely applied to various fractions of lipids commonly sourced from plant seeds, such as soybeans, or egg yolk. Natural lecithin contains a complex mixture of phosphatides and triglycerides, fatty acids, and carbohydrates, whereas refined deoiled lecithin exclusively contains phosphatides or simply phosphatidylcholine, the major component of the phosphatide fraction (7). Due to lecithin's properties as an emulsifier, stabilizer, antioxidant, and dispersing agent and its propensity to form multilamellar vesicles (liposomes), lecithin is utilized throughout the pharmaceutical industry, notably for drug and vaccine delivery (7–9). Lecithin is a principal component of the widely used commercial veterinary adjuvant Amphigen (10).
Our own recent work highlighted the potent adjuvant activity of carbomers, either used alone with subunit antigens (11) or coformulated with the proprietary oil-in-water formulation MF59 (12, 13), and suggested that they compare favorably with other adjuvants in terms of tolerability and potency. The coformulation of carbomer and lecithin that comprises Adjuplex has also been demonstrated to be a potent yet well-tolerated adjuvant suitable for use with a variety of antigens and immunization regimens. In a range of animal species from mice to nonhuman primates, Adjuplex induced antibodies to various HIV-1 (14, 15) and malaria (16) antigens and small-molecule addictive-drug analogs (cocaine and nicotine) (17–22). Notably, in a nonhuman primate study of HIV-1 envelope glycoprotein trimer antigen comparing DNA prime/protein boost regimens, in which all animals received protein antigen adjuvanted with Adjuplex, neutralizing antibody breadth and titers exceeded the levels achieved by previous vaccine regimens in primates (15).
Despite these studies, little is known of the immunological profile of Adjuplex or its ability to elicit protective antibodies against a common human pathogen. For these reasons, we assessed the adjuvant activity of Adjuplex in the context of soluble influenza virus HA antigen, the target of neutralizing antibodies and a potential component of a human vaccine against this virus. We found that Adjuplex is well tolerated in mice and elicits a strong and balanced adaptive immune response driving potent antibody production that is protective against influenza virus challenge. Based on these results, we propose that further exploration and development of this adjuvant are warranted.
MATERIALS AND METHODS
Antigens and adjuvants.
Influenza virus hemagglutinin (HA) bromelain released and purified from the H1N1 PR8 isolate (23) was obtained from J. Skehel, National Institute for Medical Research (NIMR), United Kingdom. The following adjuvants were used: Adjuplex was supplied by Advanced BioAdjuvants LLC as a 100% solution used at the doses shown, aluminum salts (alum) (alhydrogel; Brenntag; stock 2%) was used at 100 μg per dose, and monophosphoryl lipid A (MPL) (MPLA-SM VacciGrade; InvivoGen, Inc.) was used at 10 μg per dose. All adjuvants were mixed with HA diluted in endotoxin-free phosphate-buffered saline (PBS) at room temperature approximately 30 min prior to administration.
Animals, immunizations, and viral challenge.
C57BL/6 mice were purchased from Harlan, Inc. and were immunized in groups, as described in the figure legends, between 6 and 8 weeks of age. Antigen-adjuvant formulations were prepared under sterile conditions in endotoxin-free PBS in a total volume of 100 μl and were administered subcutaneously in prime or prime/boost regimens, as described in Results. Blood samples were taken via tail bleed at the time points described in Results. In some experiments, the mice were sacrificed, and spleens were taken, disaggregated, and cultured in vitro, as described below. For the protection experiments, each animal was intranasally challenged with 16 hemagglutinating units (HAU) (corresponding to 1.17 × 105 PFU) of mouse-adapted PR8 H1N1 on day 15 after immunization. The animals were monitored for weight loss and euthanized when a humane endpoint was reached, defined based on clinical scoring or weight loss. All animal experiments were performed under the appropriate national licenses in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986 and were authorized by the United Kingdom Home Office and the Oxford local institutional ethics review board.
ELISA.
Serum samples were allowed to clot for 30 min at room temperature (RT), cleared by centrifugation, and stored at −20°C until analysis. Antigen-coated and blocked enzyme-linked immunosorbent assay (ELISA) plates were incubated with serial dilutions of the samples. Bound antibodies were detected with the appropriate secondary reagents (anti-mouse IgG-horseradish peroxidase [HRP] [STAR120P; Serotec] and anti-mouse IgG1-HRP and IgG2c-HRP [product no. GTX77297; Genetex]), and the ELISA was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Thermo Fisher Scientific), using previously described methods (24).
Hemagglutination inhibition assay.
The serum samples were pretreated for 2 h at 4°C with 0.5% turkey red blood cells (TRBC) (bioTRADING Benelux B.V., The Netherlands) in PBS. TRBC were removed, and the serum samples were incubated overnight at 37°C in 10% CO2 with a 1:25 dilution of receptor-destroying enzyme (Sigma-Aldrich). The serum samples were serially 2-fold diluted from 1:8 in duplicate and were incubated for 1 h at RT with 8 HAU influenza virus A/PuertoRico/8/1934 in 50 μl. After incubation, 1% TRBC were added and incubated for 1 h at RT. The hemagglutination inhibition (HI) titer was determined as the reciprocal of the final dilution without agglutination and reported as log2-tranformed values.
T-cell assays.
For the assay of HA-specific responses, splenocytes were cultured in the presence or absence of 20 μg/ml HA for 72 h and [3H]thymidine incorporation was measured overnight using a scintillation counter (Beckman Coulter). Alternatively, proliferation was determined using a bromodeoxyuridine (BrdU) labeling kit (eBioscience, CA, USA) and fluorescent staining for CD3 and CD4 or CD8 (BD Biosciences, NJ, USA), acquired on a CyAn ADP analyzer (Beckman Coulter, USA) and analyzed using the FlowJo software (Tree Star, Ashland, OR, USA). Supernatants were cleared by centrifugation and analyzed using a multiplex cytokine assay.
Cell recruitment assays.
Adjuplex in endotoxin-free PBS or PBS alone was administered (100 μl) intraperitoneally. Each mouse was euthanized 24 h later, and small-volume (2 ml) and large-volume (5 ml) peritoneal lavages were sequentially performed using ice-cold PBS-EDTA. Supernatants from the small-volume lavages were used in cytokine/chemokine analyses, and cells from both lavages were combined for flow cytometric analysis.
Flow cytometry and antibodies.
The following antibodies were used for flow cytometry: CD11b (M1/70), CD19 (ID3), CD3 (145-2C11), Ly6C (AL-21), Ly6G (1A8), F4/80 (CI:A3-1), CD11c (HL3), and major histocompatibility complex class II (MHC-II) (2G9). Peritoneal leukocytes were stained for flow cytometry, and absolute numbers of monocytes (CD11b+ Ly6C2+ Ly6G− F4/80int), macrophages (CD11b+ F4/80hi Ly6G− Ly6C−), neutrophils (CD11b+ Ly6Ghi Ly6C+ F4/80−), and dendritic cells (CD11b−/int CD11chi F4/80−/lo MHC-IIhi) were determined.
Multiplex cytokine assays.
Supernatants were separated from either peritoneal lavage fluid or cultured cells via centrifugation and stored at −80°C until use. Cytokine concentrations from undiluted cell culture supernatants or peritoneal lavage fluid were determined using the Bio-Plex cytokine array (Bio-Rad Laboratories). The panel tested was tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12) p40 and p70, IL-1β, IL-4, IL-6, RANTES, and granulocyte-macrophage colony-stimulating factor (GM-CSF).
DC maturation and reporter cell line assays.
Immature bone marrow-derived dendritic cells (BMDCs) were derived as described in reference 24. Cells were pulsed with Adjuplex at the concentrations shown in that study, washed, blocked in BD mouse Fc block (BD Biosciences), stained for surface markers with anti-mouse MHC-II (BioLegend), CD80 (Serotec), CD86 (BD Biosciences), CD11c (APC), CD40 (BioLegend), and OX40L (BioLegend), and subsequently acquired and analyzed using a FACSCalibur flow cytometer (BD Biosciences) and the FlowJo software (Tree Star). Thp1-Blue cells (InvivoGen) expressing TLR1/2, TLR2, TLR2/6, TLR4, TLR5, TLR8, NOD1, and NOD2 were stimulated with Adjuplex or PRR ligands (InvivoGen) for 24 h, and the supernatants were tested for secreted embryonic alkaline phosphatase (SEAP) expressed following NF-κB or AP1 activation, using QUANTI-Blue substrate (InvivoGen).
Statistical analysis.
The antibody titer data were log10-transformed and then tested for normality (Kolmogorov-Smirnov test). If the data showed a normal distribution within each comparison group, a one-way analysis of variance (ANOVA) was used to assess for statistical significance, defined as a P value of <0.05. If the data were not normally distributed, they were analyzed using a Kruskal-Wallis test with the same significance limit. Direct comparisons between individual groups were tested for significance using the appropriate posttests.
RESULTS
Dose optimization of Adjuplex.
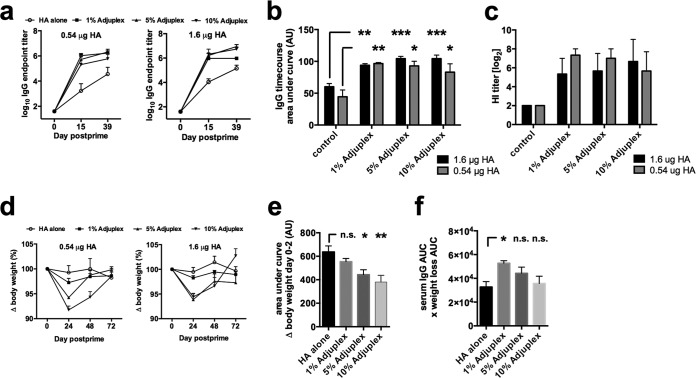
To define the optimal combination of tolerability and potency, Adjuplex was titrated from stock over three doses (1, 5, and 10%) using two doses of antigen (0.54 and 1.6 μg of HA). The HA used was soluble bromelain-cleaved HA purified from whole influenza A virus lysates (23). Each mouse was immunized subcutaneously on days 0 and 14, and antigen-specific IgG endpoint titers were determined by ELISA. The IgG titers rose more rapidly for HA adjuvanted in Adjuplex than for HA alone and reached maximal values of ∼106 for adjuvanted HA, which were higher than those for nonadjuvanted HA (Fig. 1a). Area under the concentration-time curve (AUC) analysis revealed no significant differences in overall IgG titers between the 1, 5, and 10% Adjuplex groups and no obvious difference between the two antigen doses (Fig. 1b). A similar pattern of results was obtained using a hemagglutination inhibition (HI) assay (Fig. 1c). We therefore chose the 0.54-μg antigen dose for further study. Weight loss as an indication of adjuvant tolerability was measured >72 h after immunization. The two highest does of Adjuplex showed clear transient decreases in weight compared to those with the lowest dose and HA alone (Fig. 1d). Quantification of these data by AUC analysis for both doses of antigen pooled confirmed no significant difference between antigen alone and antigen with 1% Adjuplex, whereas both 5 and 10% Adjuplex triggered significant weight loss (Fig. 1e). No signs of local toxicity or intolerance, assessed by swelling or scratching caused by irritation, were noted at any adjuvant dose (results not shown). When the immunogenicity-to-toxicity (determined by weight loss) ratio was calculated, 1% Adjuplex was significantly better than HA alone and showed a trend toward a more beneficial outcome than that with 5 or 10% Adjuplex (Fig. 1f). We therefore chose 1% Adjuplex for further study.
FIG 1.
Optimization of Adjuplex and antigen dose. (a) Mice (C57BL/6, 3 per group) were immunized on days 0 and 14 with HA in Adjuplex, and blood was taken on days −1, 14, and 28. (b) Total antigen-specific IgG production quantified by AUC analysis. (c) Hemagglutination inhibition (HI) titers were measured and expressed as log2 values. (d) Weight measured over 48 h and plotted as the percent change from starting weight (100%). (e) Weight loss quantified by AUC. (f) The immunogenicity-to-toxicity ratio was determined by multiplying weight loss by the IgG titer. Data represent the mean ± standard error of the mean (SEM). *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
Comparative adjuvanticity of Adjuplex, alum, and MPL.
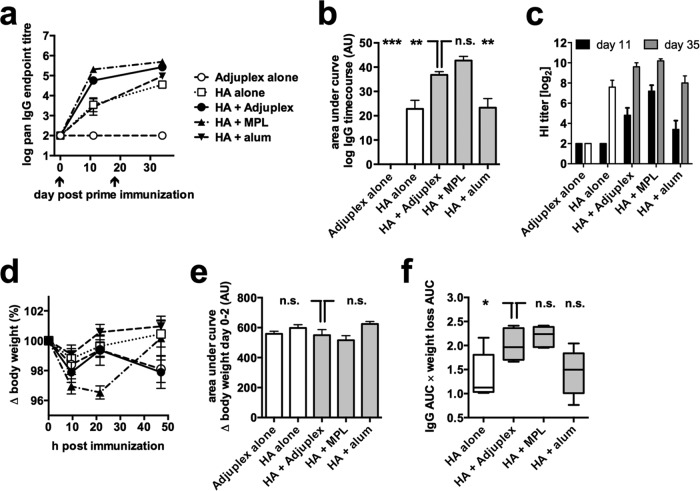
We evaluated the comparative adjuvanticity and safety of Adjuplex with two well-characterized adjuvants: alum, the most widely used licensed vaccine adjuvant, and monophosphoryl lipid A (MPL), a TLR-4 ligand-based licensed adjuvant used in hepatitis B virus and human papillomavirus (HPV) vaccines (1). Alum and MPL were used at doses similar to those previously published as being safe and immunogenic in mice (25–27). Each mouse was immunized on days 0 and 18 of the study, and total antigen-specific IgG responses were assayed postprime at day 11 and postboost at day 34. Adjuplex and MPL induced faster kinetics of IgG production than that with HA alone and HA in alum (Fig. 2a). When quantified by AUC analysis, Adjuplex induced responses that were significantly higher than those with HA alone or in alum but indistinguishable from those with MPL (Fig. 2b). HI titers were determined postprime and postboost and reflected the ELISA binding values (Fig. 2c). Weight loss was measured over 48 h from prime, and quantification revealed no significant differences between any of the groups (Fig. 2d and e). When the immunogenicity/weight loss index was calculated, Adjuplex and MPL showed a strong trend toward an increased index compared to alum (Fig. 2f). Thus, Adjuplex is as well tolerated and potent in IgG induction as MPL.
FIG 2.
Comparison of relative activity of Adjuplex and other commonly used adjuvants. (a) Mice (C57BL/6, 5 per group) were immunized on days 0 and 18 with HA alone, Adjuplex alone, or HA in Adjuplex, alum, or MPL; blood was taken on days −1, 10, and 36, and HA-specific IgG titers were determined. (b) HA-specific IgG titers quantified by AUC analysis. (c) Hemagglutination inhibition (HI) titers measured and expressed as log2 values. (d) Weight measured over 48 h and plotted as the percent change from starting weight (100%). (e) Weight loss quantified by AUC analysis. (f) Box (interquartile range) and whisker (complete range) plots of toxicity relative to adjuvanticity for each condition, quantified by determining the ratio of weight loss to IgG titer. (b to e) Data represent the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
Antigen-specific B- and T-cell responses.
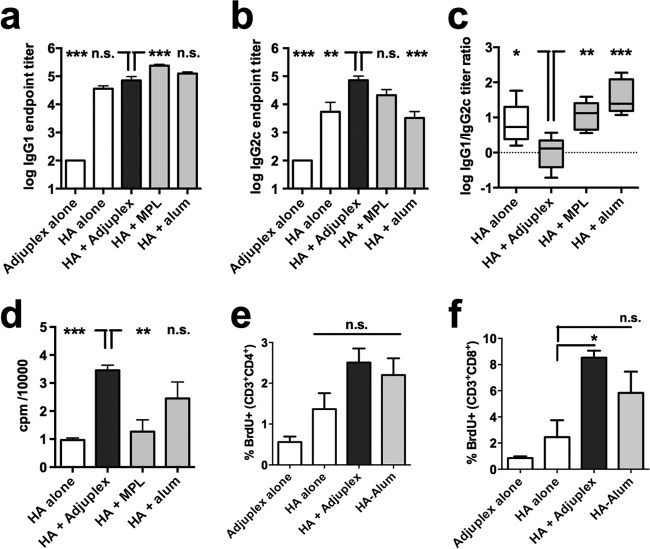
To investigate T-cell responses, we first carried out an IgG isotype analysis, as this informs the type of T-helper cell bias imposed upon the B cells producing antibody. HA formulated in Adjuplex gave significantly higher IgG1 titers than HA alone, equivalent titers to HA in alum, and significantly lower titers than HA in MPL (Fig. 3a). IgG2c titers were highest in the Adjuplex and MPL groups and significantly higher than those with HA alone or HA in alum (Fig. 3b). The IgG1-to-IgG2c ratio confirmed a significantly greater Th2-type balance for HA alone or adjuvanted with alum or MPL than for Adjuplex, which gave a balanced IgG1-to-IgG2c response (Fig. 3c). In vitro HA restimulation of splenocytes followed by [3H]thymidine incorporation showed a strong proliferative response for HA in Adjuplex that was significantly greater than that obtained with HA alone or HA in MPL (Fig. 3d). To define whether the antigen-specific proliferative response triggered by Adjuplex was shared by both CD4+ and CD8+ T cells, mice were primed and boosted and splenocytes restimulated in vitro as before, and CD4+ and CD8+ T-cell subsets were analyzed for activation by BrdU labeling. Figure 3e shows that CD4+ T cells were equivalently activated by exposure to HA in Adjuplex or alum, but these responses were not significantly greater than those elicited by antigen alone. In contrast, Adjuplex elicited a robust CD8+ T-cell response that was significantly greater than that achieved with HA alone, whereas the response to HA in alum was not significantly greater than that with antigen alone (Fig. 3f). To further characterize antigen-specific T-cell responses, we analyzed cytokines released from the restimulated splenocyte culture. Adjuplex-adjuvanted HA stimulated release of several Th1-associated cytokines (TNF-α, gamma interferon [IFN-γ], IL-12 p70, GM-CSF, and IL-2) to levels significantly higher than those for HA alone or MPLA-adjuvanted HA, and levels of IL-12 p70 and GM-CSF were higher than those for HA with alum (Fig. 4). In contrast, IL-17 levels were not significantly different between groups. The Th2-associated cytokine IL-4 was significantly greater in the Adjuplex group than in all other groups, and Adjuplex elicited greater IL-5 and IL-10 release than HA alone or in MPL (Fig. 4). When these data are taken together, and consistent with the literature, alum is a Th2-biasing adjuvant (28, 29). In contrast, MPL induces relatively modest levels of Th1- and Th2-type cytokines, with IFN-γ being the only cytokine that is highly induced compared to HA alone, and Adjuplex elicits a robust and balanced Th1/Th2 response, as previously described for Carbopol (11).
FIG 3.
B- and T-cell activity induced by immunization. (a to d) Mice (C57BL/6, 5 per group) were immunized for the experiment with results shown in Fig. 2, and HA-specific IgG1 (a) and IgG2c (b) endpoint titers were determined. (c) Box (interquartile range) and whisker (complete range) plots of the ratio of IgG1 to IgG2c titer. (d) Splenocytes were harvested on day 38 postprime, and HA-specific responses were assayed by [3H]thymidine incorporation. (e and f) Splenocytes from mice immunized with Adjuplex alone, HA alone, or in Adjuplex or alum were HA pulsed in vitro, fixed and labeled for incorporated nuclear BrdU and surface CD3 and either CD4 or CD8, and analyzed by flow cytometry. (a, b, and d to f) Data represent the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
FIG 4.
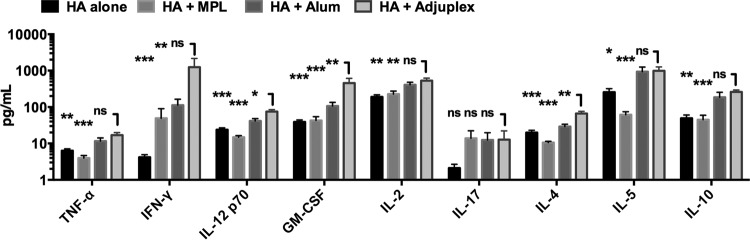
Cytokines induced by immunization. Supernatants from HA-restimulated splenocytes from mice (C57BL/6, 5 per group) immunized for the experiment with results shown in Fig. 2 were analyzed by multiplex array for cytokines. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
Protection from influenza virus challenge.
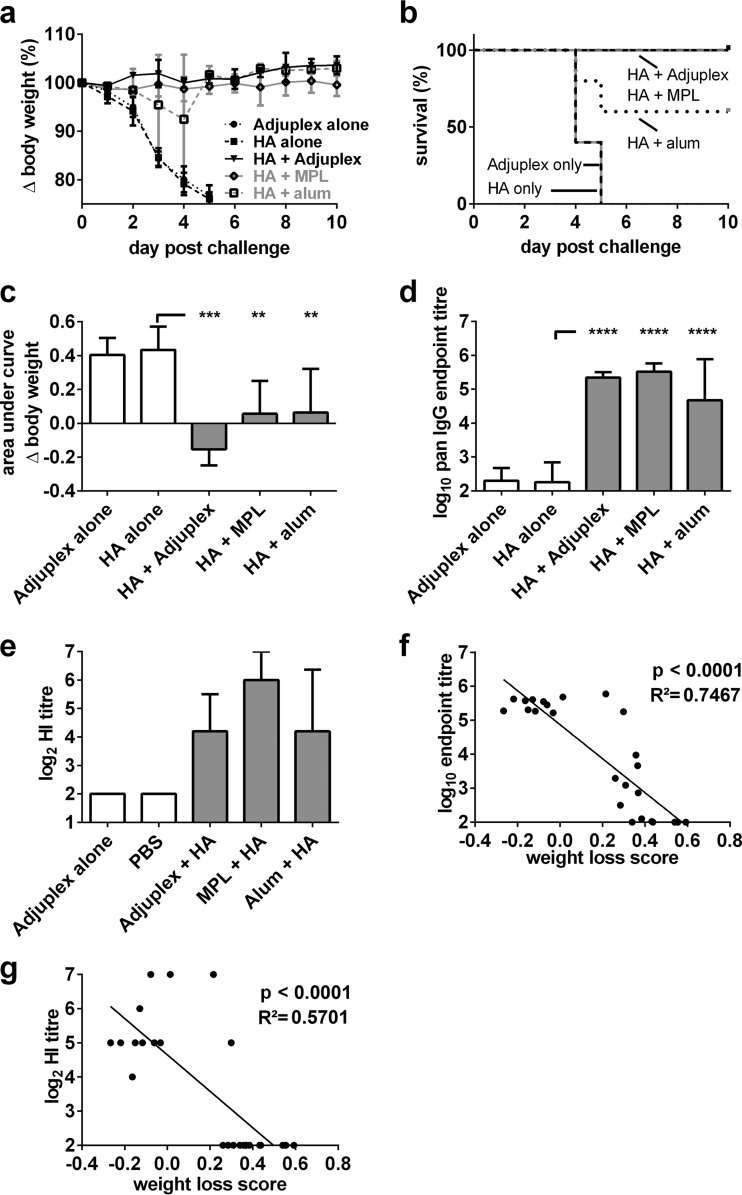
To assess whether adjuvanting HA might lead to increased protection from an otherwise lethal intranasal inoculation of mouse-adapted influenza virus, mice were subcutaneously inoculated with a single 0.54-μg dose of HA alone, Adjuplex alone, or the same dose of HA formulated in MPL or alum. Mice were challenged 15 days later with 16 HAU, corresponding to 1.17 × 105 PFU, of PR8 H1N1 influenza A virus, and weight loss was measured over the subsequent 10 days. As shown in Fig. 5a, mice administered Adjuplex or HA alone lost weight dramatically over the first 5 days, and all reached the humane endpoint by day 5. In contrast, 3/5 alum-adjuvanted mice and all MPL- and Adjuplex-adjuvanted mice survived, with minimal or no weight loss (Fig. 5b). AUC analysis of the weight loss data revealed that all three adjuvants protected mice, with a trend toward Adjuplex eliciting the greatest protection (Fig. 5c). Measurement of HA-specific IgG elicited in each group at challenge showed robust responses in all adjuvanted groups (Fig. 5d), and the pattern of HI titers was similar to that of the ELISA binding antibodies (Fig. 5e). The titers of antigen-specific IgG and HI both correlated strongly with protection from disease (Fig. 5f and g).
FIG 5.
Protection from influenza virus challenge. (a) Mice (C57BL/6, 5 per group) were immunized on day 0 with Adjuplex or 0.5 μg of HA alone or HA formulated in Adjuplex, MPL, or alum, and mice were challenged on day 15 after immunization with 16 HAU, corresponding to 1.17 × 105 PFU, of H1N1 PR8 influenza A virus. Weight loss was measured over 10 days and is expressed as the percent change from the starting weight, which was set at 100%. (b) Kaplan-Meier survival curves for each group: mice losing ≥25% of their weight were humanely sacrificed. (c) Weight change over the period of measurement was calculated using AUC analysis. (d) Blood was taken on the day of challenge, and HA-specific serum IgG endpoint titers were determined. (e) Hemagglutination inhibition (HI) titers were determined and are expressed as log2 values. (f) Linear regression analysis of correlation between weight loss and antigen-specific IgG titer. (g) Linear regression analysis of correlation between weight loss and HI titer. (a and c to e) Data are expressed as the mean ± SEM. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
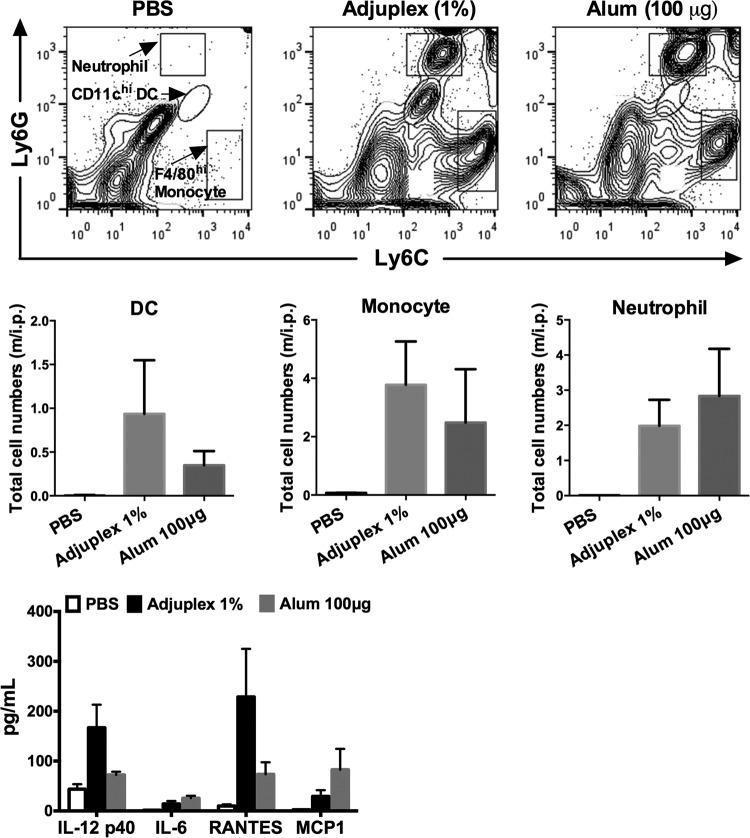
Immune cell recruitment and cytokine production after intraperitoneal administration.
The administration of adjuvants into the peritoneal cavity, while not representing a route relevant to human vaccination, allows a facile analysis of local immune cell infiltration and exit and cytokine production. This route has been used for analyses of alum-elicited immune cell recruitment and cytokine responses (29, 30) and so is relevant to the current study. Mice were administered 100 μg of alum or 5% Adjuplex in 200 μl, and 24 h later, peritoneal lavage fluid was obtained to capture innate immune cell infiltration and local cytokine release. Cells were labeled with a panel of antibodies, and individual populations were gated and quantified (Fig. 6, top panels). The data summarized in Fig. 6 (middle panels) show that alum and Adjuplex recruited equivalent numbers of monocytes, whereas Adjuplex had a trend toward decreased neutrophil and increased dendritic cell (DC) counts compared to those with alum. Cytokine responses were analyzed at the 24-h time point, and the positive results are shown in Fig. 6 (bottom panel). Adjuplex induced greater IL-12 p40 and RANTES responses than alum, whereas alum induced greater IL-6 and monocyte chemoattractant protein 1 (MCP-1) responses. In sum, these data imply that Adjuplex induces an early burst of cytokines and chemokines and attracts inflammatory monocytes and DCs to the site of adjuvant administration. The presence of DCs and monocytes is of particular interest, as DCs are professional antigen-presenting cells that would be expected to trap antigen and migrate with it to the draining lymph nodes for presentation to T cells (30), and monocytes may differentiate into antigen-presenting dendritic cells (31, 32) in the inflammatory cytokine milieu induced by Adjuplex.
FIG 6.
Immune cell recruitment and local cytokine production induced by Adjuplex. Intraperitoneal administration of PBS, Adjuplex, or alum into 2 or 3 mice was followed 24 h later by peritoneal lavage and analysis of cell subsets by flow cytometry for DCs, monocytes, and neutrophils (top panels), and the results are summarized for each subset (middle panels). m/i.p., million cells/intraperitoneal lavage. (Bottom panel) Innate immune cytokines and chemokines in the peritoneal exudate were analyzed by multiplex bead array. Data in the middle and bottom panels are expressed as the mean ± SEM.
Adjuplex does not trigger classical DC maturation.
Adjuvants that bind PRRs trigger NF-κB activation pathways, leading to the maturation of immature DCs and upregulation of costimulatory surface molecules that allow efficient antigen-specific priming of T cells. We therefore tested whether Adjuplex might represent a PRR ligand by pulsing DCs with two concentrations of Adjuplex and reading out surface HLA and costimulatory marker expression. The highest dose (5%) of Adjuplex generated a high fluorescent background in the BMDCs and so could not be used for the analysis. In contrast with lipopolysaccharide (LPS), which upregulated MHC-II, CD80, CD86, CD40, and OX40L expression, there was no significant increase in any surface markers in the presence of Adjuplex (Fig. 7a). To confirm that Adjuplex was unable to directly trigger PRRs, we pulsed an NF-κB myeloid reporter cell line, Thp1-Blue, expressing many functional PRRs, including TLR1/2, TLR2, TLR2/6, TLR4, TLR5, TLR8, NOD1, and NOD2, with a range of Adjuplex concentrations and read out NF-κB activation. Whereas the known ligands LPS (TLR4), lipoteichoic acid from S. aureus (LTA-SA) (TLR2), FSL-1 (TLR2/6), and muramyl dipeptide (MDP) (NOD2) all activated NF-κB, none of the 3 concentrations of Adjuplex induced any significant activation (Fig. 7b). Taken together with the lack of canonical DC maturation, we therefore conclude that the major adjuvant activity of Adjuplex is unlikely to depend upon direct DC activation via PRRs.
FIG 7.
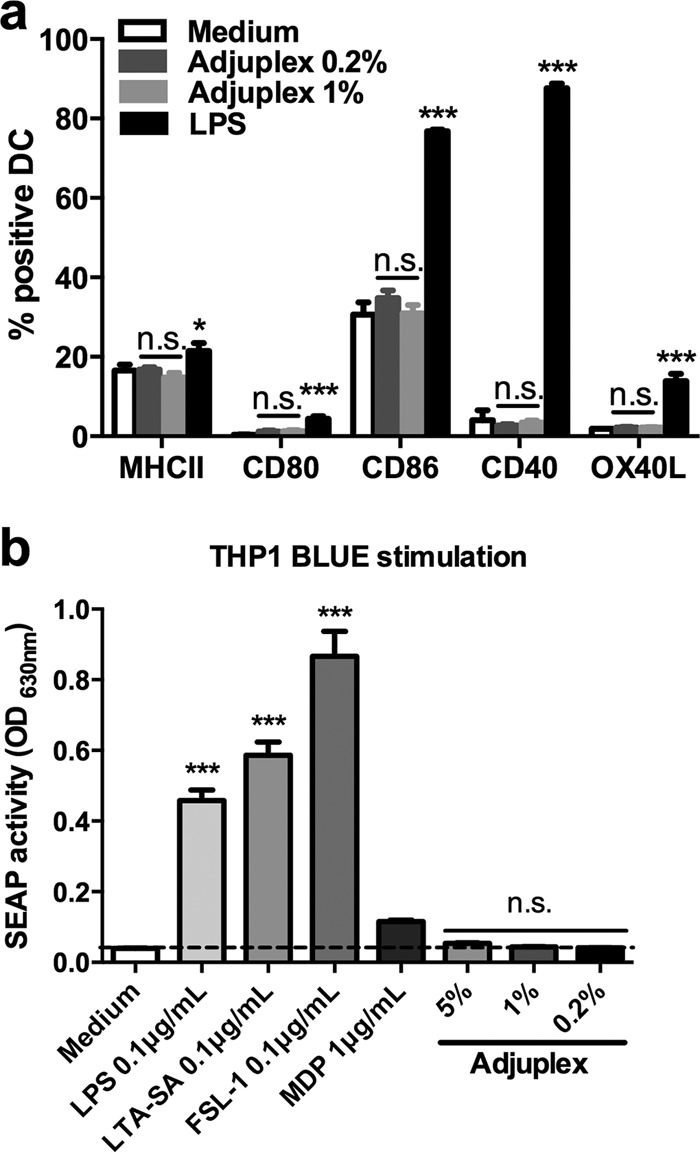
Lack of innate immune cell and receptor triggering by Adjuplex. (a) Bone marrow-derived dendritic cells (BMDCs) were incubated with the concentrations of Adjuplex or the PRR ligands indicated, and surface marker expression was analyzed by flow cytometry and expressed as the percentage of positively stained cells compared to isotype controls. (b) Thp1-Blue reporter cells were pulsed with the compounds shown for 24 h, and the supernatants were assayed for secreted embryonic alkaline phosphatase. Data represent the mean ± SEM. *, P < 0.05; ***, P < 0.001; n.s., not significant; OD630nm, optical density at 630 nm.
DISCUSSION
Adjuplex compared favorably in terms of tolerability and potency with both licensed adjuvants tested, alum and MPL, inducing antigen-specific IgG titers of equal (MPL) or greater (alum) magnitude without imposing detectable weight loss. In line with this, Adjuplex was at least as effective as the other adjuvants in protecting mice from an otherwise lethal influenza virus challenge, and it was highly significantly superior to antigen alone. In contrast with currently used alum-based adjuvanted vaccine formulations, carbomer-based formulations are nontoxic in vitro and exhibit excellent biocompatibility and biodegradability (33, 34).
Adjuplex imposed a more balanced Th response than that of the other adjuvants, as defined by the IgG1-to-IgG2a ratio, the increased levels of Th1 cytokines, including IL-12 and GM-CSF, and the antigen-induced proliferation of CD8+ T cells. This is unsurprising with respect to alum, which has well-characterized Th2-biasing properties (35). The TLR4 agonist MPL has been demonstrated to act primarily by triggering TIR-domain-containing adapter-inducing beta-interferon (TRIF)-mediated signaling (36) and thus induces, in contrast with LPS, a nonproinflammatory innate immune response, which may be suboptimal for the effective induction of memory CD8+ T-cell activity (37). In addition to the potent induction of Th1-related cytokines, Adjuplex induced robust Th2-type cytokines, most notable of which was IL-4, a cytokine favoring B-cell activation and differentiation into plasma cells.
Adjuplex is a coformulation of carbomer and lecithin. It seems likely that the major immunoactivating activity comes from the carbomer component, while the lecithin, configured as nanoliposomes, may function primarily to enhance the bioavailability of the antigen and facilitate delivery to antigen-presenting cells. Because of their biocompatibility, biodegradability, and smaller size than conventional liposomes, nanoliposomes are thought to enhance the performance of bioactive agents by improving their solubility, bioavailability, and stability (38). Moreover, the type of adaptive immunoactivating properties of Adjuplex we report here are coordinated with the balanced Th response and the robust elicitation of cytokines we observed previously for Carbopol (11). The lecithin nanoliposomes may carry intrinsic immunostimulating activity (39) but may impart additional favorable properties to the Carbopol formulation, such as eliciting enhanced antibody titers and avidity, as observed for the coformulations of carbomer and the oil-in-water emulsion MF59 (12, 13). Indeed, in oral immunization studies in mice, a formulation of bovine serum albumin (BSA) with lecithin had no adjuvant effect on the induction of anti-BSA antibodies, and carbomers were only moderately effective, whereas the combination of lecithin with carbomers increased IgG antibody titers 5-fold over those with carbomers alone (40).
Unlike MPL, which has a clearly defined mode of action by triggering TLR4 on antigen-presenting cells, leading to their maturation and enhanced priming of T cells, the mechanism of action of Adjuplex remains to be defined. We have clearly demonstrated that Adjuplex does not trigger canonical DC activation and that therefore it must act by an alternative mechanism. Since we assume that at least part of the adjuvant activity of Adjuplex is derived from the Carbopol component, further study of Adjuplex and the mode of action of Adjuplex and carbomers is warranted.
ACKNOWLEDGMENTS
We thank Eirikur Saeland for assistance with the hemagglutination assays.
This work was supported by Advanced BioAdjuvants LLC. Q.J.S. is a Jenner Vaccine Institute Investigator and James Martin Vaccine Research Institute Senior Fellow. We declare funding from Advanced BioAdjuvants LLC.
REFERENCES
- 1.Reed SG, Orr MT, Fox CB. 2013. Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux P. 1984. Water-soluble synthetic polymers in immunology and biomedicine. Asian Pac J Allergy Immunol 2:301–310. [PubMed] [Google Scholar]
- 3.Gualandi GL, Losio NM, Muratori G, Foni E. 1988. The ability by different preparations of porcine parvovirus to enhance humoral immunity in swine and guinea pigs. Microbiologica 11:363–369. [PubMed] [Google Scholar]
- 4.Hoogland MJ, Opriessnig T, Halbur PG. 2006. Effects of adjuvants on porcine circovirus type 2-associated lesions. J Swine Health Prod 14:133–139. [Google Scholar]
- 5.Tollersrud T, Nørstebø PE, Engvik JP, Andersen SR, Reitan LJ, Lund A. 2002. Antibody responses in sheep vaccinated against Staphylococcus aureus mastitis: a comparison of two experimental vaccines containing different adjuvants. Vet Res Commun 26:587–600. doi: 10.1023/A:1020960402112. [DOI] [PubMed] [Google Scholar]
- 6.Mumford JA, Wilson H, Hannant D, Jessett DM. 1994. Antigenicity and immunogenicity of equine influenza vaccines containing a carbomer adjuvant. Epidemiol Infect 112:421–437. doi: 10.1017/S0950268800057848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shchipunov Y. 2006. Lecithin, p 3299–3318. In Somasundaran P. (ed), Encyclopedia of surface and colloidal science, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 8.Murdan S. 2005. Organogels in drug delivery. Expert Opin Drug Deliv 2:489–505. doi: 10.1517/17425247.2.3.489. [DOI] [PubMed] [Google Scholar]
- 9.Allen TM, Cullis PR. 2013. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 10.Kullenberg FW. January 1992. Adjuvant for dose treatment with antigens. US patent 5,084,269.
- 11.Krashias G, Simon AK, Wegmann F, Kok WL, Ho LP, Stevens D, Skehel J, Heeney JL, Moghaddam AE, Sattentau QJ. 2010. Potent adaptive immune responses induced against HIV-1 gp140 and influenza virus HA by a polyanionic carbomer. Vaccine 28:2482–2489. doi: 10.1016/j.vaccine.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 12.Lai RP, Seaman MS, Tonks P, Wegmann F, Seilly DJ, Frost SD, LaBranche CC, Montefiori DC, Dey AK, Srivastava IK, Sattentau Q, Barnett SW, Heeney JL. 2012. Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund's adjuvants. PLoS One 7:e35083. doi: 10.1371/journal.pone.0035083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey AK, Burke B, Sun Y, Hartog K, Heeney JL, Montefiori D, Srivastava IK, Barnett SW. 2012. Use of a polyanionic carbomer, Carbopol971P, in combination with MF59, improves antibody responses to HIV-1 envelope glycoprotein. Vaccine 30:2749–2759. doi: 10.1016/j.vaccine.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koff WC, Phogat S. January 2012. Method of inducing high-titer neutralizing antibody responses in a host by administering immune complexes comprising anti-HIV-1 Env antibodies and the HIV-1 Env. US patent 8,105,600.
- 15.Chakrabarti BK, Feng Y, Sharma SK, McKee K, Karlsson Hedestam GB, Labranche CC, Montefiori DC, Mascola JR, Wyatt RT. 2013. Robust neutralizing antibodies elicited by HIV-1 JRFL envelope glycoprotein trimers in nonhuman primates. J Virol 87:13239–13251. doi: 10.1128/JVI.01247-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta PK, Mukherjee P, Dhawan S, Pandey AK, Mazumdar S, Gaur D, Jain SK, Chauhan VS. 2014. Production and preclinical evaluation of Plasmodium falciparum MSP-119 and MSP-311 chimeric protein, PfMSP-Fu24. Clin Vaccine Immunol 21:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wee S, Hicks MJ, De BP, Rosenberg JB, Moreno AY, Kaminsky SM, Janda KD, Crystal RG, Koob GF. 2012. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology 37:1083–1091. doi: 10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koob G, Hicks MJ, Wee S, Rosenberg JB, De BP, Kaminsky SM, Moreno A, Janda KD, Crystal RG. 2011. Anti-cocaine vaccine based on coupling a cocaine analog to a disrupted adenovirus. CNS Neurol Disord Drug Targets 10:899–904. doi: 10.2174/187152711799219334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg JB, Hicks MJ, De BP, Pagovich O, Frenk E, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, Worgall S, Tignor N, Mezey JG, Crystal RG. 2012. AAVrh.10-mediated expression of an anti-cocaine antibody mediates persistent passive immunization that suppresses cocaine-induced behavior. Hum Gene Ther 23:451–459. doi: 10.1089/hum.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De BP, Pagovich OE, Hicks MJ, Rosenberg JB, Moreno AY, Janda KD, Koob GF, Worgall S, Kaminsky SM, Sondhi D, Crystal RG. 2013. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Hum Gene Ther 24:58–66. doi: 10.1089/hum.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maoz A, Hicks MJ, Vallabhjosula S, Synan M, Kothari PJ, Dyke JP, Ballon DJ, Kaminsky SM, De BP, Rosenberg JB, Martinez D, Koob GF, Janda KD, Crystal RG. 2013. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology 38:2170–2178. doi: 10.1038/npp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks MJ, Kaminsky SM, De BP, Rosenberg JB, Evans SM, Foltin RW, Andrenyak DM, Moody DE, Koob GF, Janda KD, Ricart Arbona RJ, Lepherd M, Crystal RG. 2014. Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anticocaine vaccine. Hum Gene Ther Clin Dev 25:40–49. doi: 10.1089/humc.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruigrok RW, Aitken A, Calder LJ, Martin SR, Skehel JJ, Wharton SA, Weis W, Wiley DC. 1988. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J Gen Virol 69(Pt 11):2785–2795. [DOI] [PubMed] [Google Scholar]
- 24.Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho LP, Lambe T, Puthia M, Svanborg C, Scherer EM, Krashias G, Williams A, Blattman JN, Greenberg PD, Flavell RA, Moghaddam AE, Sheppard NC, Sattentau QJ. 2012. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol 30:883–888. doi: 10.1038/nbt.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakur A, Kaur H, Kaur S. 2014. Evaluation of the immunoprophylactic potential of a killed vaccine candidate in combination with different adjuvants against murine visceral leishmaniasis. Parasitol Int 64:70–78. [DOI] [PubMed] [Google Scholar]
- 26.Pouliot K, Buglione-Corbett R, Marty-Roix R, Montminy-Paquette S, West K, Wang S, Lu S, Lien E. 2014. Contribution of TLR4 and MyD88 for adjuvant monophosphoryl lipid A (MPLA) activity in a DNA prime-protein boost HIV-1 vaccine. Vaccine 32:5049–5056. doi: 10.1016/j.vaccine.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tregoning JS, Buffa V, Oszmiana A, Klein K, Walters AA, Shattock RJ. 2013. A “prime-pull” vaccine strategy has a modest effect on local and systemic antibody responses to HIV gp140 in mice. PLoS One 8:e80559. doi: 10.1371/journal.pone.0080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HogenEsch H. 2002. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 20(Suppl 3):S34–S39. [DOI] [PubMed] [Google Scholar]
- 29.Korsholm KS, Petersen RV, Agger EM, Andersen P. 2010. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology 129:75–86. doi: 10.1111/j.1365-2567.2009.03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. 2008. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 32.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. 2008. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 33.Sahoo S, Chakraborti CK, Mishra SC. 2011. Qualitative analysis of controlled release ciprofloxacin/Carbopol 934 mucoadhesive suspension. J Adv Pharm Technol Res 2:195–204. doi: 10.4103/2231-4040.85541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang C, Yin L, Yin C, Pei Y. 2007. Swelling behaviour and biocompatibility of Carbopol-containing superporous hydrogel composites. J Appl Polym Sci 104:2785–2791. doi: 10.1002/app.25930. [DOI] [Google Scholar]
- 35.Oleszycka E, Lavelle EC. 2014. Immunomodulatory properties of the vaccine adjuvant alum. Curr Opin Immunol 28C:1–5. [DOI] [PubMed] [Google Scholar]
- 36.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 37.Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, Kaech SM. 2014. TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T cell differentiation. J Immunol 192:4221–4232. doi: 10.4049/jimmunol.1302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mozafari MR. 2010. Nanoliposomes: preparation and analysis. Methods Mol Biol 605:29–50. doi: 10.1007/978-1-60327-360-2_2. [DOI] [PubMed] [Google Scholar]
- 39.Sloat BR, Sandoval MA, Hau AM, He Y, Cui Z. 2010. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J Control Release 141:93–100. doi: 10.1016/j.jconrel.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerber JD. August 2013. Methods for preparing and delivering adjuvant compositions. US patent 8,501,221 B2.