Abstract
The objectives of the present study were to assess the mucosal, cellular, and humoral immune responses induced by two different infectious bronchitis virus (IBV) vaccination regimes and their efficacy against challenge by a variant IBV Q1. One-day-old broiler chicks were vaccinated with live H120 alone (group I) or in combination with CR88 (group II). The two groups were again vaccinated with CR88 at 14 days of age (doa). One group was kept as the control (group III). A significant increase in lachrymal IgA levels was observed at 4 doa and then peaked at 14 doa in the vaccinated groups. The IgA levels in group II were significantly higher than those in group I from 14 doa. Using immunohistochemistry to examine changes in the number of CD4+ and CD8+ cells in the trachea, it was found that overall patterns of CD8+ cells were dominant compared to those of CD4+ cells in the two vaccinated groups. CD8+ cells were significantly higher in group II than those in group I at 21 and 28 doa. All groups were challenged oculonasally with a virulent Q1 strain at 28 doa, and their protection was assessed. The two vaccinated groups gave excellent ciliary protection against Q1, although group II's histopathology lesion scores and viral RNA loads in the trachea and kidney showed greater levels of protection than those in group I. These results suggest that greater protection is achieved from the combined vaccination of H120 and CR88 of 1-day-old chicks, followed by CR88 at 14 doa.
INTRODUCTION
The prevention of infectious bronchitis (IB) in chickens is achieved through the use of live and inactivated vaccines, which provide protection against virulent field IB viruses (IBVs) in the event of an exposure. Despite these preventative measures, outbreaks of IB frequently occur in many poultry producing countries (1–3). This is probably due to the emergence of new variants of infectious bronchitis virus (1–5). For the successful protection of chickens against infection, it is essential to identify the prevalent genotypes in the region, determine the cross-protective potential of available vaccines, and optimize strategic vaccination programs.
IB was first described in the United States during the 1930s and was identified in the United Kingdom in 1948. Thereafter, many IBV variants were isolated from Europe, significantly a variant called 793B that emerged in the 1990s (6). Later, IBV QX was first identified in China (7) before spreading to Europe (8). Another IBV genotype, Q1, genetically and serologically distinct from the classical IBVs, was also reported in China (9), the Middle East (10), and Europe (11). To contain this strain, an effective vaccination program is needed. However, very little is known about the cross protection induced by the commercially available vaccines or vaccination regimes against this variant Q1.
An effective and long-lasting protection against IBV infection requires the activation of effector, memory cell-mediated, and humoral immune responses (HIRs) against the virus (12). A number of studies have reported the systemic and local humoral immune response to IBV vaccination (12–14). In chickens experimentally challenged with IBV, the development of a cell-mediated immune response (CMI) has been correlated with effective virus clearance, reduction of clinical signs, and resolution of lesions (15, 16). The presence of CD8+ cytotoxic T lymphocytes (CTL) represents a good correlation for decreasing infection and corresponds with a reduction in clinical signs, as CTL activity is major histocompatibility complex restricted, and these T cells mediate cytolysis (17). It has additionally been shown that the transfer of CTLs obtained from the spleens of IBV-infected chickens was protective to naive chicks against a subsequent IBV challenge (15, 18). During the course of experimental viral infection, Kotani et al. showed that the clearance of the IBV from the tracheal mucosa occurred at an early phase of the infection and that CTLs at the tracheal mucosa were proposed to be involved in this clearance (19). To date, there is no information available on the tracheal mucosal leukocytes after vaccination with live IBV vaccines. Nevertheless, Okino et al. quantified the relative expression of the CTL genes in tracheal samples from vaccinated and further challenged birds (20). The upregulation of these genes in the tracheal mucosa of the full-dose-vaccinated birds was significantly increased at 24 h postinfection (hpi), demonstrating the development of a memory CMI (20). However, these researchers did not directly measure the activity of CMI, such as the cytotoxic mechanism of CTLs.
Despite all of these reports, the kinetics of and the relationship between local and systemic HIR and CMI induced by different IBV vaccination regimes need to be better understood for protection against emerging IBV strains. Thus, the objective of our study was to measure the local and systemic HIR and CMI induced by two different IBV vaccination regimes administered to commercial broiler chicks and to estimate the protection achieved against a recently isolated virulent Q1 strain.
MATERIALS AND METHODS
Birds.
We obtained 120 broiler chicks, aged 1 day, from a commercial hatchery. Birds were allowed ad libitum access to feed and drinking water. All procedures were undertaken according to the United Kingdom legislation on the use of animals for experiments as permitted under the project license PPL 40/3723, which was approved by the University of Liverpool ethical review committee.
Challenge virus.
The virulent Q1 isolate used in this study was kindly provided by Merial Animal Health. PCR confirmed that the allantoic fluid from eggs used to propagate the virus was free of Newcastle disease, avian influenza, infectious bursal disease, infectious laryngotracheitis, and avian metapneumoviruses. Q1 IBV was also free of bacterial or fungal contaminants. The virus was titrated in chicken tracheal organ culture (TOC) as described before and expressed in 50% (median) ciliostatic doses (CD50) per milliliter (21).
Vaccine preparation.
As recommended by the manufacturer (Merial Animal Health Limited, United Kingdom), the vaccines were prepared by thoroughly mixing one vial of live IBV H120 (Bioral H 120) vaccine with 100 ml of sterile water (SW). For combined vaccinations, one vial each of the Bioral H 120 and the live IBV CR88 (Gallivac IB88) vaccine were mixed together in 100 ml of SW. Immediately after preparation, the vaccines and SW were kept in a cold box (at 0°C). Each chick received a total of 100 μl of the appropriate vaccine ocularly (50 μl) and nasally (50 μl) or SW. To quantify the virus, titration of live IBV vaccine for H120 and CR88 was performed by using 9- to 11-day-old specific pathogen free (SPF) embryonated chicken eggs (ECE) inoculated via the allantoic cavity. The ECE were examined for IBV specific lesions (curling and dwarfing) of the embryos up to 5 days postinoculation. Viral titers were calculated according to Reed and Muench (22) and expressed as the 50% egg infective dose (EID50/ml). The titers of the vaccine viruses used were 3.5 log10 EID50/chick and 4.25 log10 EID50/chick for the H120 strain and CR88 strain, respectively.
Experimental design.
We divided 120 broiler chicks, aged 1 day, into three groups (n = 40 chicks/group) (Table 1). Chicks in group I were inoculated oculonasally with 100 μl of live H120 vaccine alone. In group II, chicks were inoculated oculonasally with 100 μl of live H120 and CR88 vaccines simultaneously. Chicks in the two groups (I and II) were again inoculated with a live CR88 vaccine at 14 days of age (doa). Group III received only 100 μl of SW oculonasally and was kept as a control. Samples (5 birds/group) of serum, tears, and heparinized blood were collected at 0, 4, 7, 14, 21, and 28 doa before sacrificing the birds. The tears and serum samples were stored at −20°C, and blood samples were processed immediately for peripheral blood mononuclear lymphocyte isolation. Five chickens from each group per interval were humanely euthanized for the collection of approximately 1 cm of the upper trachea in OCT to be snap-frozen in liquid nitrogen for immunohistochemistry (IHC). The rest of the trachea was used for tracheal washes. At 28 doa, 10 birds from each group were challenged via ocular-nasal route with the Q1 (104.0 CD50/bird) and observed daily for clinical signs. After 5 days postchallenge (dpc), all 10 birds from each group were necropsied and tracheal samples were collected; portions were placed in the RNAlater (Qiagen, Crawley, United Kingdom) and stored at −70°C until processing for examination of viral RNA load. The remaining portions were examined by histopathology and ciliostasis tests. The kidneys from all groups were also taken for histopathology and viral RNA load examination.
TABLE 1.
Study design showing groups, vaccine, and vaccination regimesa
| IBV vaccine (dosage/chick in 100 μl) | Group I |
Group II |
Group III |
|||
|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | |
| H120 (3.5 log10 EID50) | √ | √ | ||||
| CR88 (4.25 log10 EID50) | √ | √ | √ | |||
| Sterile water | √ | √ | ||||
At 28 days of age, 10 chicks from each group were challenged with a virulent IBV Q1.
Sample collection for antibody detection.
The potential of the vaccines to induce antibody production was assessed individually by using samples of serum, tears, and tracheal washes. Tears were collected using sodium chloride as described before (23) and immediately centrifuged at 3,000 × g for 3 min before the supernatant was stored at −70°C until used. To collect the tracheal washes, the trachea was clamped with two artery forceps at each end and washed with 1 ml phosphate-buffered saline (PBS) using a syringe with a 19-gauge needle (24). The collected samples were centrifuged at 3,000 × g for 3 min, and the supernatant was stored at −70°C until further use.
ELISAs.
To detect IBV antibodies, serum samples were tested with a commercial IBV enzyme-linked immunosorbent assay (ELISA) kit (FlockChek; IDEXX Laboratories, Inc., Westbrook, ME, USA), and immunoglobulin A (IgA) in tears and tracheal washes was assayed using a commercial IgA chicken ELISA kit (Abcam, Cambridge, United Kingdom). The two assays were carried out according to the respective manufacturer's instructions.
Hemagglutination inhibition test.
For the hemagglutination inhibition (HI) test, M41 and 793B hemagglutinin (HA) antigens were obtained from GD Animal Health Service (Deventer, Netherlands). The Q1 HA antigen was prepared in our laboratory as described earlier (25). The HI test was conducted according to standard procedures (OIE) using 4 HA units of antigen per well. The HI titers were read as the reciprocal of the highest dilution showing complete inhibition, and the HI geometric mean titers were expressed as reciprocal log2.
Cellular immune responses. (i) Analysis of T lymphocyte subset (CD4+/CD8+) ratio in peripheral blood.
To determine the percentage of T-lymphocyte subpopulations, blood was collected from the cephalic vein in heparin tubes (Sigma-Aldrich Co., St. Louis, MO, USA) at final concentrations of 20 μg/ml of blood and further diluted (1:1) with RPMI 1640 medium (Sigma-Aldrich Co.). The prepared blood samples (1 ml each) were then layered onto 0.5 ml of Histopaque −1.077 gradient (Sigma-Aldrich Co.) and centrifuged in 1.5-ml Eppendorf vials at 8,000 × g for 90 s. After centrifugation, the buffy coat formed of mononuclear cells was gently collected, washed twice with an RPMI 1640 medium, and adjusted to 1 × 107 cells/ml. The cells were resuspended in 0.5% bovine serum albumin (BSA) (Sigma-Aldrich Co.) in PBS (blocking solution) and incubated at room temperature for 15 min. The sample (100 μl) was incubated with antibodies against surface domains of CD4 (mouse anti-chicken CD4-fluorescein isothiocyanate [FITC] clone CT-4; 0.5 mg/ml; SouthernBiotech, Birmingham, AL, USA) and CD8 (mouse anti-chicken CD8a-FITC clone CT-8; 0.5 mg/ml; SouthernBiotech) receptors of T lymphocytes (antibody final concentrations as 0.2 μl/100 μl of sample) for 30 min in the dark. The stained cells were detected by flow cytometry (BD Accuri C6; BD Bioscience, San Jose, CA, USA) to count the T lymphocytes. The unstained cell sample was used as a negative control to adjust the threshold.
(ii) Immunohistochemical detection of CD4+, CD8+, and IgA-bearing B cells in tracheal sections.
The OCT-embedded tracheal samples were cut into 5-μm sections, fixed in ice-cold acetone for 10 min, air dried at room temperature, and stored at −80°C until staining. Just prior to staining, slides were removed from −80°C storage and air dried at room temperature for 10 min. After endogenous peroxidase inhibition using 0.03% hydrogen peroxide in PBS for 20 min, the endogenous biotin or biotin-binding proteins in tissue sections were blocked with blocking serum using Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). Following blocking, tissue sections were stained overnight at 4°C in the dark to detect CD4+, CD8+, and IgA+ cells by using mouse monoclonal antibodies to chicken CD4 (clone CT-4; 0.5 mg/ml) and CD8a (clone CT-8; 0.5 mg/ml) at 1:1,000 and to chicken IgA (clone A-1; 0.5 mg/ml) at 1:2,000. All monoclonal antibodies were procured from SouthernBiotech (Birmingham, AL, USA). The staining procedure was performed as described earlier (26). For each sample, the average number of positive cells/×400 microscopic field was calculated for each cell type (26).
Ciliary protection.
At 5 dpc, trachea samples were evaluated according to standard procedure for ciliary movement, and the ciliary protection for each group was calculated (27).
Histopathological evaluation.
At 5 dpc, kidneys and tracheas from humanely euthanized birds were collected and fixed in 10% formalin. The tissues were embedded in paraffin wax (50 to 60°C) and sections were cut to 7 μm thickness. Tissue sections were stained by hematoxylin and eosin (H&E) for microscopic evaluation, and scores were attributed according to histopathological severity and determined by recommendations described previously (28, 29).
Real time RT-qPCR.
Total RNA extractions from the tracheas and kidneys collected from the challenged birds were performed immediately using the RNeasy minikit (Qiagen, Crawley, United Kingdom) according to the manufacturer's instructions. Quantification of the viral RNA was done by quantitative real-time reverse transcription-PCR (RT-qPCR) using IBV 3′untranslated region (UTR) gene-specific primers and probes as described previously (30). The RT-qPCR was performed according to the manufacturer's instructions using the OneStep RT-PCR kit (Qiagen) and 40 ng of total RNA per reaction. Amplification plots were recorded and analyzed, and the threshold cycle (CT) was determined with Rotor-Gene Q thermocycler software (Qiagen). The CT values were converted to log-relative equivalent units (REU) of viral RNA, done through generation of a standard curve of five 10-fold dilutions of extracted RNA from infective allantoic fluid of 106 EID50 of M41 as described earlier (31).
Statistical analysis.
The comparisons of the means of anti-IBV antibody levels, CD4+/CD8+ ratio in peripheral blood, immunohistochemical detection of CD4+, CD8+, and IgA-bearing B cells in tracheal sections were performed using one-way analysis of variance (ANOVA), followed by the post hoc least significant difference (LSD) multiple-comparison test using GraphPad Prism version 6.00 software. The Kruskal-Wallis test followed by Dunn's test was used for statistical analysis of the nonparametric RT-qPCR and histopathological evaluation data. Differences were considered significant at a P of <0.05.
RESULTS
Systemic humoral immune response. (i) ELISA.
On the day of vaccination, the mean of the maternally derived anti-IBV antibody titer was 1,750 ± 203. Subsequently, the antibody levels in all three groups declined to below the cutoff point (396) by 14 doa. After the booster vaccination with CR88 at 14 doa, significant increases in the antibody titers until 28 doa were observed in groups I and II, as shown in Fig. 1. At these time points, the levels of antibodies were not significantly different between the vaccinated groups (P < 0.05). After 14 doa, though, the antibody titers in group III were always less than the cutoff value of 396 in this assay.
FIG 1.
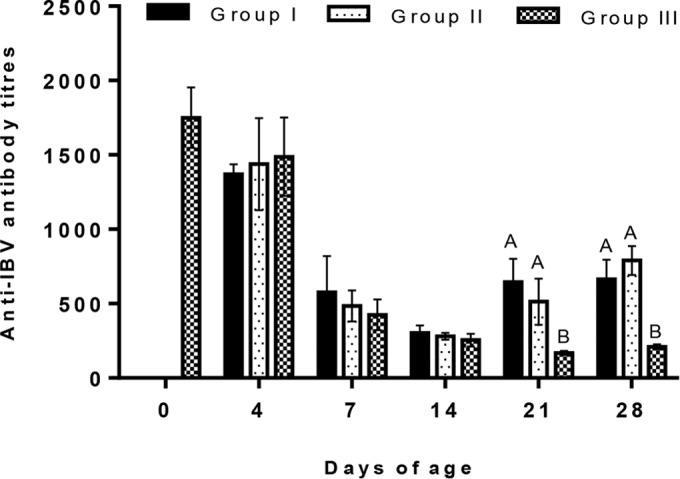
Anti-infectious bronchitis virus (IBV) antibody titers of the different groups vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. One group (group III) was kept as a control. Significantly (P < 0.05) different values are shown with different letters, and all other values were not significantly (P < 0.05) different between the groups at those sampling points.
(ii) HI test.
The level of serotype specific antibodies against homologous and heterologous antigens was evaluated by an HI test (Table 2). The HI antibody responses against all the antigens used showed no significant difference (P < 0.05) between the groups from 0 to 7 doa. However, a lower antibody response was obtained in all groups when the antigen used in the HI test was heterologous (Q1) to the viruses used in the vaccination. At 14 doa, the mean HI antibody titer to M41 was significantly higher (P < 0.05) in group II than those in group I and III. Thereafter, at 28 doa, the levels of antibodies to M41 in groups I and II were very similar and significantly higher than those in group III (P < 0.05). At 21 doa, group II showed a significant increase of HI antibody response against CR88 following revaccination with a homologous antigen. A similar increase was observed in group I on the same sample day. Thereafter, at 28 doa, the HI antibody titer to the CR88 antigen was overall significantly higher in group II (log2 8.2) than that in group I (log2 4.4) and that in group III (log2 1.8). At 21 doa, the titers to Q1 in groups I and II were higher than those in group III (P < 0.05). At 28 doa, the mean HI titer to Q1 was significantly higher (P < 0.05) in group II than that in group I, with a mean difference of 1.2 log2.
TABLE 2.
Geometric mean anti-IBV HI antibody titer (log2) in serum of chickens vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1a
| HI antigen | Group | Anti-IBV HI antibody titer (log2) (geometric mean ± standard error) at days of ageb |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 14 | 21 | 28 | ||
| M41 | I | 9.2 ± 0.374A | 8.2 ± 0.970A | 7.4 ± 0.400A | 5 ± 0.000B | 5 ± 0.548A | 4 ± 0.447A |
| II | 9.2 ± 0.374A | 8.8 ± 0.374A | 7.4 ± 0.400A | 6.4 ± 0.510A | 5.4 ± 0.400A | 4.4 ± 0.400A | |
| III | 9.2 ± 0.374A | 7.2 ± 0.374A | 6.4 ± 0.748A | 4.8 ± 0.200B | 4 ± 0.632A | 2 ± 0.632B | |
| CR88 | I | 8.4 ± 0.400A | 7.8 ± 0.374A | 6.8 ± 0.490A | 5 ± 0.316A | 6.8 ± 0.583A | 4.4 ± 0.678B |
| II | 8.4 ± 0.400A | 8.4 ± 0.245A | 6.8 ± 0.374A | 5.8 ± 0.200A | 7 ± 0.548A | 8.2 ± 0.583A | |
| III | 8.4 ± 0.400A | 7.6 ± 0.400A | 7.4 ± 0.245A | 4.6 ± 0.600A | 3.4 ± 0.245B | 1.8 ± 0.490C | |
| Q1 | I | 7 ± 0.316A | 3 ± 0.316A | 2.4 ± 0.245A | 2.2 ± 0.200B | 4.4 ± 0.510A | 4.4 ± 0.510B |
| II | 7 ± 0.316A | 3 ± 0.678A | 3.4 ± 0.400A | 3.8 ± 0.374A | 5 ± 0.548A | 5.6 ± 0.245A | |
| III | 7 ± 0.316A | 3.4 ± 0.316A | 2.2 ± 0.200A | 2 ± 0.000B | 2 ± 0.000B | 2 ± 0.000C | |
Both groups were again vaccinated with CR88 at 14 days of age. One group (group III) was kept as a control.
Significant differences between the groups (n = 5 per group) for each homologous as well as heterologous antigen for each interval are represented by different letters (P < 0.05).
(iii) Mucosal humoral immune responses.
In groups I and II, the levels of IgA in tears increased significantly (P < 0.05) compared to that in the control group III from 4 doa, continuing to rise until and initially peaking on 14 doa. In the vaccinated groups, IgA values fell after the second vaccination at day 14 and then increased slightly again to 28 doa (the day of challenge). The IgA level in group II was significantly higher (P < 0.05) than that in group I from 14 doa until 28 doa, the end of the observation period (Fig. 2a). The levels of IgA in tracheal washes in the vaccinated groups were detected from 4 doa, peaking at 7 doa before declining until 28 doa. No significant (P < 0.05) difference in the levels of IgA in tracheal washes induced by the two vaccine groups was observed at any time point (Fig. 2b). IgA levels in the tears and tracheal washes of the two vaccinated groups were significantly higher than the levels from the unvaccinated control group.
FIG 2.
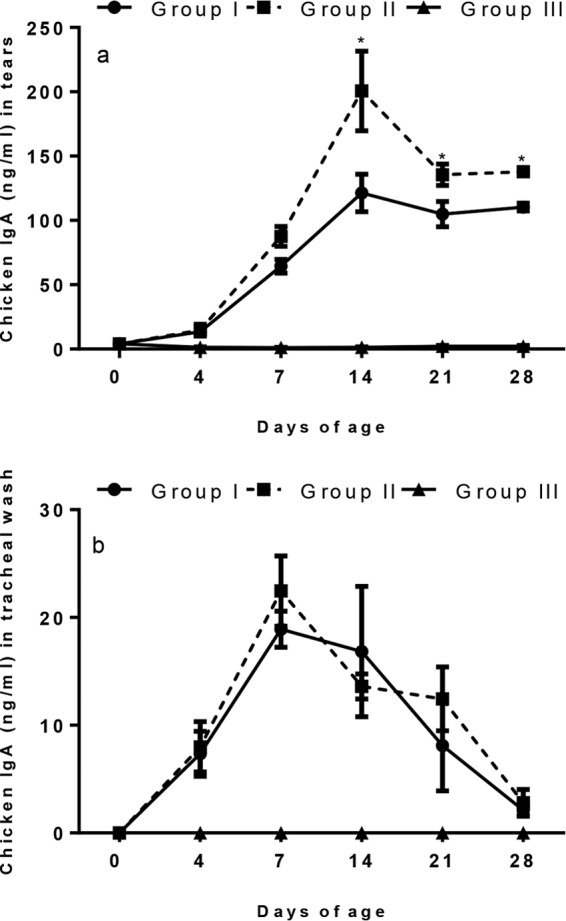
Detection of IgA production using ELISA in tears (a) and tracheal wash (b) of chickens (n = 5 per group) vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. One group (group III) was kept as a control. The IgA antibody levels in tears and tracheal wash from control chickens (group III) remained below the detectable level. Asterisks indicate that values between the two vaccine groups were significantly different (P < 0.05) at those time points. Error bars indicate standard error of the mean.
Systemic cell-mediated immune response. (i) CD4+/CD8+ ratio in peripheral blood.
Flow cytometry results showed that at 7 doa the CD4+/CD8+ ratios were slightly higher in the two vaccinated groups than in the nonvaccinated group, although there was no significant difference (P < 0.05) between the CD4+/CD8+ ratios of the vaccinated and nonvaccinated groups observed up to 14 doa (Fig. 3). After the booster vaccination with the CR88 at 14 doa, the ratio of CD4+/CD8+ at 21 doa showed a slight increase in the vaccinated groups, as it was significantly higher (P < 0.05) in group I than in groups II and III. At 28 doa, the ratio was significantly higher (P < 0.05) in group II than in groups I and III.
FIG 3.
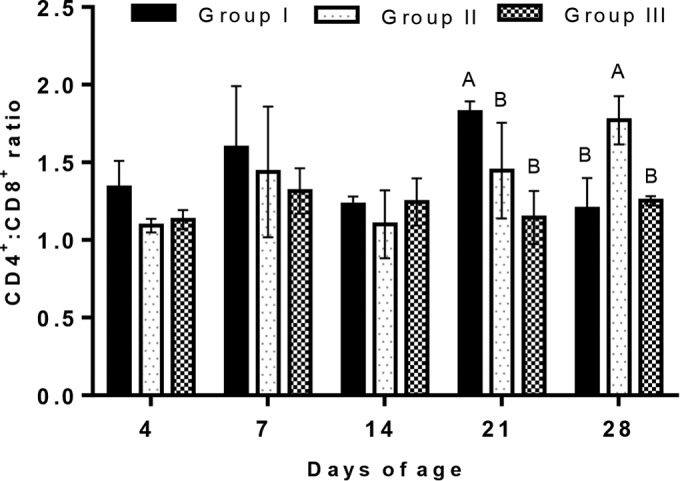
The ratio of CD4+/CD8+ cells analyzed by flow cytometry in the peripheral blood of chickens vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. One group (group III) was kept as a control. Depicted are the mean values (n = 5 per group) and one standard error. Significantly (P < 0.05) different values are shown with different letters, and all other values were not significantly (P < 0.05) different between the groups at those time points.
(ii) Mucosal cell-mediated immune responses in the trachea.
The kinetics of CD4+, CD8+, and IgA-bearing B lymphocytes in the trachea were studied by IHC (Fig. 4). The number of CD4+ lymphocytes in the trachea increased significantly (P < 0.05) from 4 doa in the vaccinated groups compared to that in the control (Fig. 5a). The number of CD4+ cells reached its peak at 4 doa in group I and at 7 doa in group II before gradually decreasing until 14 doa. After the second immunization, CD4+ cells strongly increased in number by 21 doa compared to those of the nonvaccinated controls before declining again. The difference between vaccinated groups I and II was not statistically significant (P < 0.05). The CD8+ cell subpopulation in groups I and II started to increase significantly (P < 0.05) at 4 doa, reaching a peak at 7 doa and then declining (Fig. 5b). After revaccination with CR88 at 14 doa, the two vaccinated groups showed a strong increase in the number of CD8+ cells. The number of CD8+ cells was significantly higher in group II than that in group I at 21 and 28 doa (P < 0.05). Overall, the dynamics of the CD8+ cell subpopulations in the two vaccinated groups were more dominant than those of the CD4+ cells. At 7 doa, the IgA-bearing B cells increased in vaccinated groups I and II, peaking at 14 doa and showing significant difference compared with those of the unvaccinated group (P < 0.05). The number of IgA-bearing B cells was significantly higher in group II than that in group I at 21 doa, whereas no significant (P < 0.05) difference was observed between the two vaccinated groups at 28 doa (Fig. 5c).
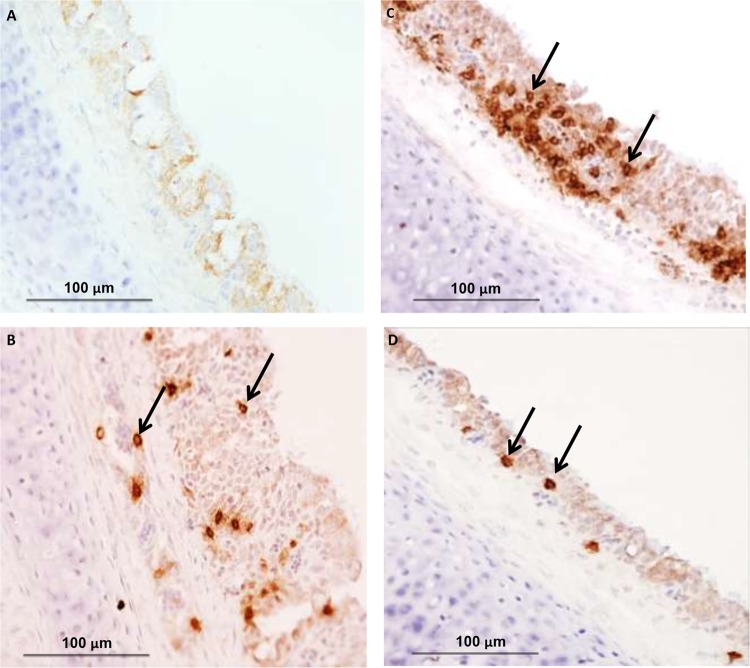
FIG 4.
Immunohistochemical detection of CD4+ cells in group II at 28 days of age (B), CD8+ cells in group II at 28 days of age (C), and IgA-bearing B cells in group II at 28 days of age (D) in tracheas of chickens vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. (A) One group (group III) was kept as a control. Magnification, ×400. Arrows indicate positive cells.
FIG 5.
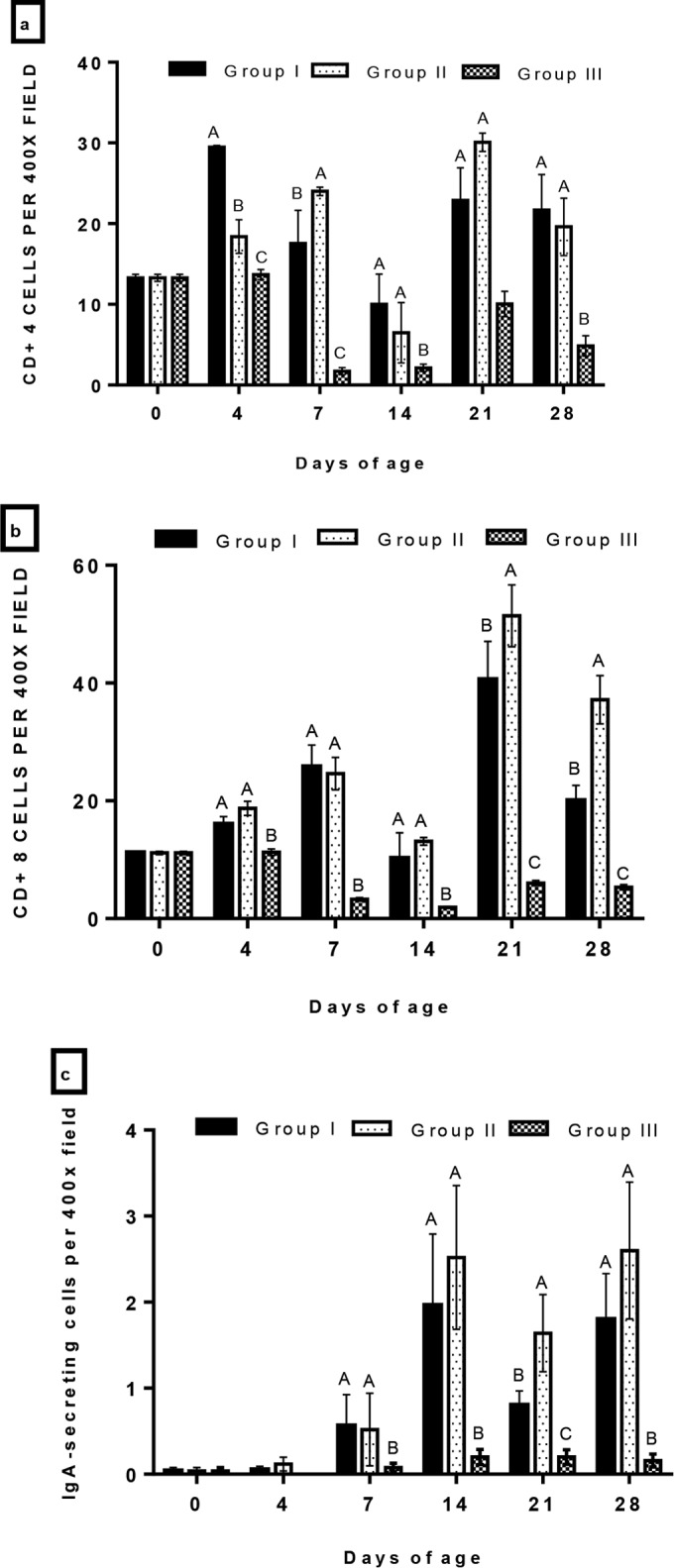
Summary of CD4+ cells (a), CD8+ cells (b), and IgA-secreting cells (c) determined by immunohistochemical staining in the tracheas of chickens vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. One group (group III) was kept as a control. Depicted are the mean values (n = 5 per group) and one standard error. Significantly (P < 0.05) different values are shown with different letters, and all other values were not significantly (P < 0.05) different between the groups at those time points.
Protection.
After challenge, no clinical signs were observed in either vaccinated group. In the unvaccinated group, respiratory signs such as coughing, sneezing, head shaking, tracheal rales, and nasal discharge were observed until 5 dpc. The highest percentage of ciliary protection (97%) was observed in group II, followed by group I (89.75%). The unvaccinated challenged group (group III) showed little protection (12%) compared to the vaccinated challenged groups.
Viral RNA loads, in all tracheal samples collected, were significantly higher (P < 0.05) (4.416 log REU RNA) in the unvaccinated challenged group (III) than those in the vaccinated groups (I and II) as measured by real-time RT-qPCR at 5 dpc. Vaccinated groups I and II showed mean log REUs of viral RNA of 1.016 and 0.555, respectively, with no significant difference between these groups (Fig. 6). Overall viral RNA in the kidney samples of all the groups was low compared to that in tracheal samples. The viral RNA load in kidneys in group III was significantly higher (P < 0.05) than that in group II, whereas group I showed no significant difference (P < 0.05) in log REU of viral RNA with either group II or group III.
FIG 6.
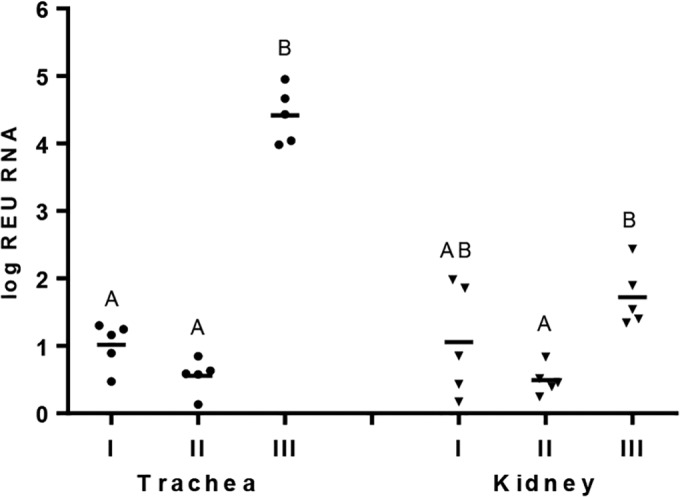
Quantification of infectious bronchitis virus (IBV), expressed as log REU of RNA, in trachea and kidney measured by real-time RT-PCR after 5 dpc from chickens experimentally challenged at 28 days of age with Q1 strain of IBV (n = 10 per group). The chickens were previously vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. One group (group III) was kept as a control and received sterile water. Significant differences between the groups were detected by Kruskal-Wallis test followed by Dunn's mean test and are indicated with different letters (P < 0.05).
Histopathological lesions in the tracheas and kidneys were induced by challenge virus in all the groups at 5 dpc. Marked histopathological changes occurred in group III (nonvaccinated group) with mean scores of 10.2 and showed significant differences with group II (P < 0.05) but not with group I (Fig. 7). The mean lesion scores for kidneys in group III were significantly higher (P < 0.05) than those in group II, whereas group I showed no significant difference (P < 0.05) in mean lesion scores with either group II or group III. However, overall mean lesion scores in kidneys were low compared to mean tracheal lesion scores.
FIG 7.
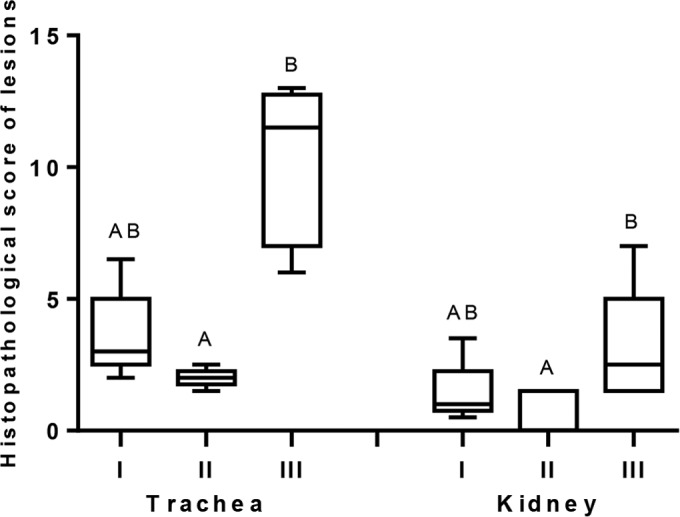
Means of histopathological scores of lesions in trachea and kidney samples after 5 dpc from chickens experimentally challenged at 28 days of age with the Q1 strain of infectious bronchitis virus (n = 10 per group). The chickens were previously vaccinated with live H120 alone (group I) or in combination with CR88 (group II) at day 1. The two groups were again vaccinated with CR88 at 14 days of age. One group (group III) kept as a control was inoculated with sterile water. Significant differences between the groups were detected by Kruskal-Wallis test followed by Dunn's mean test and are indicated with different letters (P < 0.05).
DISCUSSION
The vaccination regime chosen in this study for group I is based on research demonstrating that improved protection was seen when two vaccines used were of different serotypes (27, 32). These researchers emphasized that the vaccination program used in their experiments may not protect the respiratory tract against challenge with every new IBV serotype to emerge. It is also evident that despite the use of Mass-type vaccine at day 0, followed by 793B-type vaccine at 14 doa (the same as in group 1 in this study), a significant number of new IBVs are still emerging under field conditions, e.g., QX, IS/885/00, IS/1494/06, and most recently Q1. Therefore, in order to optimize the use of currently available vaccines, to achieve better immunity, and to assess protection against the newly emerged Q1 strain, the vaccination regime for group II was also included in this work.
At 1 day of age, chicks had high ELISA anti-IBV antibody titers in all groups, which declined and dropped to below the cutoff point by 14 doa. In the groups that received the vaccine at 1 day of age, these low antibody levels may result from the partial neutralization of the vaccine virus in the target tissues by the maternal antibodies present in the broilers at that age, with a consequently low replication of the vaccine virus and poor stimulation of the humoral response (16, 33, 34). Later, after the second vaccination at 14 doa, an increase in the antibody titers was observed until 28 doa (day of challenge) in groups I and II, with no significant difference in antibody levels between these vaccinated groups (P < 0.05). HI antibody levels declined by 14 doa against the homologous and heterologous virus antigens, showing similar patterns to declining ELISA titers. Interestingly, by 28 doa, there was no significant difference between vaccinated groups I and II in terms of the levels of antibodies to M41, whereas the HI titers to 793B and Q1 were significantly higher in group II than those in group I (P < 0.05). The role of antibodies in the control of IBV infection remains controversial, as workers have shown that circulating antibody titers did not correlate with protection from IBV infection (16, 35, 36). However, other studies demonstrated the importance of humoral immunity in disease recovery and virus clearance (37, 38). In our study, as expected, the higher HI titers were obtained using antigen homologous to vaccine strains. However, the chicks also appeared to be protected against heterologous challenge. This may be due to the presence of local immunity of the upper respiratory tract induced by vaccination, which reduces the replication of challenge virus.
The role of IgA antibodies is important for mucosal immunity to IBV, and the presence of these antibodies in tears following IBV antigen inoculation has been reported previously (37, 39). In this study, gradual increases in IgA levels were observed in tears for the two vaccinated groups during the first 2 weeks after vaccination. These results are in agreement with previous research reporting similar kinetics of lachrymal fluid IgA production to H120 vaccination (20, 40). In addition, after the second vaccination, lachrymal IgA levels decreased in the two vaccinated groups, though the levels in group II remained significantly higher (P < 0.05) than those in group I. This observation may indicate a decrease of lachrymal IgA levels after the second vaccination and is most likely due to partial neutralization of the anti-IBV IgA. A sharp decrease of IgA-IBV in vaccinated chicks was also observed after challenge (41). In addition, no significant rise in specific lachrymal IgA of vaccinated chickens was detected after subsequent challenge with the Ark-IBV isolate, explaining the probable role of neutralizing antibodies in the lachrymal fluid at the time of challenge (42).
IBV-specific IgA can also be found in tracheal washes after an infection with the IBV strain M41 (38, 39). In this study, the patterns of IgA in tracheal washes in vaccinated groups I and II were closely parallel, reaching a peak at 7 doa and, thereafter, declining until 28 doa, suggesting a short duration of the local humoral immunity in the trachea. Although there have been conflicting reports on the relative concentrations of IgA in the avian respiratory tract (43–45), our results are consistent with those of Hawkes et al., which showed IgA antibodies in tracheal washes only at day 7 after vaccination (46). Interestingly, in the two vaccinated groups, the second vaccination did not cause any rise in tracheal IgA level. Similar findings have also been reported, revealing that the revaccination with homologous IBV (M41 or H strains) (44) and secondary M41 IBV exposure (38) did not induce the secondary secretory antibody response in tracheobronchial washings.
Consistent with the notion that CMI is protective against IBV (18, 47), we next sought to study the level of systemic and local cellular immune responses. CD4+ cells may directly produce antiviral cytokines, which increase B cell activity and promote the proliferation, maturation, and functional activity of CD8+ CTLs, thus playing a critical role in controlling IBV infection (48, 49). The ratio of CD4+/CD8+ cells has been widely shown to be indicative of the general immune system status (50, 51). In this study, the CD4+/CD8+ ratio showed no significant (P < 0.05) variation among the groups until 14 doa. Nevertheless, the ratio at 28 doa was found to be significantly higher (P < 0.05) in group II than those in groups I and III, indicating that the second vaccination at 14 doa in group II probably enhanced the cellular immunity by promoting the differentiation and proliferation of CD4+ cells in peripheral blood. There are no specific data regarding the effects of different IBV vaccinations on the CD4+/CD8+ ratio in peripheral blood with which to compare the present findings; however, Yohannes et al. reported a significantly (P < 0.05) higher CD4+/CD8+ ratio in IBV-infected chicks than in the controls (52). In addition, the high CD4+/CD8+ ratio has been associated with increased humoral incompetence in chickens, and a low CD4+/CD8+ ratio and a reduced response against sheep red blood cells were reported earlier (53). In this study, significantly (P < 0.05) higher HI titers at 28 doa to 793B and Q1 in group II than those in group I may be attributed to the high CD4+/CD8+ ratio in group II at that time point. However, the significance of this in relation to protection remains to be determined.
The results of IHC in tracheal tissue showed that the number of CD4+ lymphocytes started increasing from 4 doa in the two vaccinated groups compared to that of the control. At 28 doa, no significant difference was reported between the vaccinated groups. The CD8+ cell subpopulation in the two vaccinated groups started to increase significantly (P < 0.05) at 4 doa, reaching peak at 7 doa, and then declining in number until 14 doa, suggesting that infiltration and recruitment of these cells occur in the first 2 weeks of initial IBV vaccination. Similar to the findings of the present study, in the trachea, CD8+ cell recruitment in response to infection was at a maximum by 7 days postinfection (dpi), and CD4+ cells were not recruited until 5 dpi. This was reported by Raj and Jones (54). This work also showed an overall higher infiltration of CD8+ cells than of CD4+ cells in the two vaccinated groups. This observation is consistent with a previous study (54), where CD8+ cells were also found to predominate compared to CD4+ cells in the trachea after IBV infection. Moreover, the current study also documents the significantly higher numbers of CD8+ cells in vaccinated chicks of group II than of group I at 21 and 28 doa. The IgA-bearing B cells in the vaccinated groups reached their peak at 14 doa; however, the number of these cells was significantly higher in group II than in group I at 21 and 28 doa (P < 0.05). This pattern of recruitment of B cells later than either class of T cells is in accordance with earlier studies (55, 56) that contended that local immunity against IBV is mediated mainly by T cells.
In this study, following the Q1 challenge, ciliary protection was higher in group II, vaccinated with mixed H120 and CR88 vaccines at 1 day old, than that in group I, vaccinated at 1 day old with H120 alone. Furthermore, the results of RT-qPCR showed that the viral RNA load at 5 dpc in the trachea was higher in group I than that in group II, although the difference was not statistically significant (P < 0.05). In agreement with this, the scores of histopathology in the trachea showed that the damage caused by the Q1 was higher in group I than that in group II and showed no significant difference in mean lesion scores with those of either group II or group III. On the basis of these tracheal histopathological assessments, chickens in group II were better protected than those in group I, and this better protection may be attributed to various factors, including those discussed below.
Although the anti-IBV ELISA antibody titer results indicated that there was no significant difference between the two vaccinated groups at the day of challenge, group II showed higher ciliary protection than that of group I. This observation is consistent with previous studies that have shown that circulating antibody levels were of minor importance in the protection of the respiratory mucosa against IBV challenge (14, 43).
From our results, it appears that such overall higher protection may be due to significantly higher levels of CD8+ cells in the tracheal tissues in group II than those in group I at the day of challenge. Previous studies have shown that CD8+ cells are important contributors to viral clearance in respiratory virus infections, utilizing contact-dependent effector functions, gamma interferon (IFN-γ), and tumor necrosis factor alpha (57). Therefore, we may speculate that group II's higher CD8+ cell reaction than that of group I may have contributed to the faster viral clearance after challenge with Q1, explaining the differences in tracheal protection between the vaccinated groups. This possible explanation agrees with other studies that emphasized the involvement of local CD8+ cells in the infection of chickens with respiratory pathogens, such as Newcastle disease virus (58) and Mycoplasma gallisepticum (59). Additionally, group II's higher levels of IgA in lachrymal fluid than those of group I reduced the tracheal histopathological damage, which also corroborates the hypothesis that the traditional role of IgA is to prevent pathogen entry at mucosal surfaces and neutralize virus in infected epithelial cells (60). IBV-specific IgA antibodies in lachrymal fluid were correlated with resistance to IBV reinfection (37, 39, 41). Our results are in agreement with a recent study by Okino et al. in which the authors concluded that IBV IgA antibodies in lachrymal secretions and the expression of granzyme-A and CD8 genes in tracheal tissues after H120 vaccination provide a reliable approach to monitor immune protection status in the trachea, as shown by examination for ciliostasis, histopathology, and viral replication (20). For our study, we aimed to stain for a variety of cell-surface markers and thereby identify the T cell populations infiltrating the trachea. This provides further information about the role of cell-mediated immunity in protection given by different live IBV vaccination regimes against a novel IBV Q1 challenge.
The results of RT-qPCR and scores of histopathology in the kidneys showed that the damage caused by Q1 was higher in group I than that in group II and showed no significant difference in mean lesion scores with those of either group II or group III. Specific cytotoxic T lymphocytes have been shown to be important for the systemic clearance of nephropathogenic IBV and reduction of kidney lesions (15). A plausible explanation is that a higher CD8+ cell response in the tracheal tissues (the portal of entry of challenge virus) in group II than in group I may have prevented the challenge virus from becoming viremic, thus failing to reach the kidneys. This provided an efficient prevention of kidney infection as measured by viral RNA load and histopathological lesion scores in renal tissue.
In conclusion, chicks vaccinated with H120 and CR88 at 1 day old followed by CR88 at 14 doa showed significantly higher CD8+ responses in the trachea and higher lachrymal IgA levels than those vaccinated with H120 alone. In terms of ciliary protection against Q1, though the two vaccinated groups were protected, the combined vaccination of H120 and CR88 of 1 day old chicks, followed by CR88 at 14 doa, showed higher ciliary protection and lower RNA load in the trachea and kidneys, wherein histopathological lesions are reduced. This study highlighted the potential modulation of chick immune response with the use of currently available live vaccines so that better protection against variant IBVs can be afforded.
ACKNOWLEDGMENTS
Rajesh Chhabra is a Commonwealth Scholar, funded by the United Kingdom government.
This document is provided for scientific purposes only. Any reference to a brand or trademark herein is for information purposes only and is not intended for a commercial purpose or to dilute the rights of the respective owner(s) of the brand(s) or trademark(s). Gallivac is a registered trademark of Merial. All other trademarks are the property of their respective owners.
REFERENCES
- 1.Liu S, Kong X. 2004. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol 33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough RE, Randall CJ, Dagless M, Alexander DJ, Cox WJ, Pearson D. 1992. A ‘new’ strain of infectious bronchitis virus infecting domestic fowl in Great Britain. Vet Rec 130:493–494. doi: 10.1136/vr.130.22.493. [DOI] [PubMed] [Google Scholar]
- 3.Gelb J Jr, Wolff JB, Moran CA. 1991. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis 35:82–87. doi: 10.2307/1591298. [DOI] [PubMed] [Google Scholar]
- 4.Pohuang T, Chansiripornchai N, Tawatsin A, Sasipreeyajan J. 2009. Detection and molecular characterization of infectious bronchitis virus isolated from recent outbreaks in broiler flocks in Thailand. J Vet Sci 10:219–223. doi: 10.4142/jvs.2009.10.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia W, Karaca K, Parrish CR, Naqi SA. 1995. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch Virol 140:259–271. doi: 10.1007/BF01309861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons D, Ellis MM, Cavanagh D, Cook JK. 1992. Characterisation of an infectious bronchitis virus isolated from vaccinated broiler breeder flocks. Vet Rec 131:408–411. doi: 10.1136/vr.131.18.408. [DOI] [PubMed] [Google Scholar]
- 7.Wang YD, Wang YL, Zhang ZC, Fan GC, Jiang YH, Liu XE, Ding J, Wang SS. 1998. Isolation and identification of glandular stomach type IBV (QX IBV) in chickens. Chinese J Anim Quarantine 15:1–3. [Google Scholar]
- 8.Worthington KJ, Currie RJ, Jones RC. 2008. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol 37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Jiang Y, Low S, Wang Z, Nam SJ, Liu W, Kwangac J. 2001. Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens. Avian Dis 45:416–424. doi: 10.2307/1592981. [DOI] [PubMed] [Google Scholar]
- 10.Ababneh M, Dalab AE, Alsaad S, Al-Zghoul M. 2012. Presence of infectious bronchitis virus strain CK/CH/LDL/97I in the Middle East. ISRN Vet Sci 2012:201721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toffan A, Terregino C, Mazzacan E, Castaldello I, Capua I, Bonci M. 2011. Detection of Chinese Q1 strain of infectious bronchitis virus in Europe. Vet Rec 169:212–213. doi: 10.1136/vr.d5285. [DOI] [PubMed] [Google Scholar]
- 12.Cavanagh D. 2007. Coronavirus avian infectious bronchitis virus. Vet Res 38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 13.Mockett AP, Darbyshire JH. 1981. Comparative studies with an enzyme-linked immunosorbent assay (ELISA) for antibodies to avian infectious bronchitis virus. Avian Pathol 10:1–10. doi: 10.1080/03079458108418453. [DOI] [PubMed] [Google Scholar]
- 14.Ignjatovic J, Galli L. 1994. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch Virol 138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collisson EW, Pei J, Dzielawa J, Seo SH. 2000. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev Comp Immunol 24:187–200. doi: 10.1016/S0145-305X(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 16.Raggi LG, Lee GG. 1965. Lack of correlation between infectivity, serologic response and challenge results in immunization with an avian infectious bronchitis vaccine. J Immunol 94:538–543. [PubMed] [Google Scholar]
- 17.Pei J, Sekellick MJ, Marcus PI, Choi IS, Collisson EW. 2001. Chicken interferon type I inhibits infectious bronchitis virus replication and associated respiratory illness. J Interferon Cytokine Res 21:1071–1077. doi: 10.1089/107999001317205204. [DOI] [PubMed] [Google Scholar]
- 18.Seo SH, Pei J, Briles WE, Dzielawa J, Collisson EW. 2000. Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection. Virology 269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotani T, Wada S, Tsukamoto Y, Kuwamura M, Yamate J, Sakuma S. 2000. Kinetics of lymphocytic subsets in chicken tracheal lesions infected with infectious bronchitis virus. J Vet Med Sci 62:397–401. doi: 10.1292/jvms.62.397. [DOI] [PubMed] [Google Scholar]
- 20.Okino CH, Alessi AC, Montassier MDFS, Rosa AJDM, Wang X, Montassier HJ. 2013. Humoral and cell-mediated immune responses to different doses of attenuated vaccine against avian infectious bronchitis virus. Viral Immunol 26:259–267. doi: 10.1089/vim.2013.0015. [DOI] [PubMed] [Google Scholar]
- 21.Cook JK, Darbyshire JH, Peters RW. 1976. Growth kinetic studies of avian infectious bronchitis virus in tracheal organ cultures. Res Vet Sci 20:348–349. [PubMed] [Google Scholar]
- 22.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am J Hygiene 27:493–497. [Google Scholar]
- 23.Ganapathy K, Cargill PW, Jones RC. 2005. A comparison of methods of inducing lachrymation and tear collection in chickens for detection of virus-specific immuoglobulins after infection with infectious bronchitis virus. Avian Pathol 34:248–251. doi: 10.1080/03079450500112344. [DOI] [PubMed] [Google Scholar]
- 24.Raj GD, Jones RC. 1996. Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus. Vet Immunol Immunopathol 53:147–161. doi: 10.1016/0165-2427(95)05545-2. [DOI] [PubMed] [Google Scholar]
- 25.King DJ, Hopkins SR. 1983. Evaluation of the hemagglutination-inhibition test for measuring the response of chickens to avian infectious bronchitis virus vaccination. Avian Dis 27:100–112. doi: 10.2307/1590376. [DOI] [PubMed] [Google Scholar]
- 26.Rautenschlein S, Aung YH, Haase C. 2011. Local and systemic immune responses following infection of broiler-type chickens with avian metapneumovirus subtypes A and B. Vet Immunol Immunopathol 140:10–22. doi: 10.1016/j.vetimm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Cook JKA, Orbell SJ, Woods MA, Huggins MB. 1999. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol 28:477–485. doi: 10.1080/03079459994506. [DOI] [PubMed] [Google Scholar]
- 28.Andrade LF, Villegas P, Fletcher OJ, Laudencia R. 1982. Evaluation of ciliary movement in tracheal rings to assess immunity against infectious bronchitis virus. Avian Dis 26:805–815. doi: 10.2307/1589867. [DOI] [PubMed] [Google Scholar]
- 29.Chen BY, Hosi S, Nunoya T, Itakura C. 1996. Histopathology and immunohistochemistry of renal lesions due to infectious bronchitis virus in chicks. Avian Pathol 25:269–283. doi: 10.1080/03079459608419141. [DOI] [PubMed] [Google Scholar]
- 30.Jones RM, Ellis RJ, Cox WJ, Errington J, Fuller C, Irvine RM, Wakeley PR. 2011. Development and validation of RT-PCR tests for the detection and S1 genotyping of infectious bronchitis virus and other closely related gammacoronaviruses within clinical samples. Transbound Emerg Dis 58:411–420. doi: 10.1111/j.1865-1682.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Londt BZ, Brookes SM, Kelly MD, Nash BJ, Brown IH. 2013. Failure to infect pigs co-housed with ducks or chickens infected experimentally with A/turkey/Turkey/1/2005 (H5N1) highly pathogenic avian influenza virus. Vet Microbiol 162:944–948. doi: 10.1016/j.vetmic.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Terregino C, Toffan A, Beato MS, De Nardi R, Vascellari M, Meini A, Ortali G, Mancin M, Capua I. 2008. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol 37:487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- 33.Davelaar FG, Kouwenhoven B. 1977. Influence of maternal antibodies on vaccination of chicks of different ages against infectious bronchitis. Avian Pathol 6:41–50. doi: 10.1080/03079457708418211. [DOI] [PubMed] [Google Scholar]
- 34.Mondal SP, Naqi SA. 2001. Maternal antibody to infectious bronchitis virus: its role in protection against infection and development of active immunity to vaccine. Vet Immunol Immunopathol 79:31–40. doi: 10.1016/S0165-2427(01)00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelb J Jr, Nix WA, Gellman SD. 1998. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis 42:364–374. doi: 10.2307/1592487. [DOI] [PubMed] [Google Scholar]
- 36.Gough RE, Alexander DJ. 1979. Comparison of duration of immunity in chickens infected with a live infectious bronchitis vaccine by three different routes. Res Vet Sci 26:329–332. [PubMed] [Google Scholar]
- 37.Toro H, Fernandez I. 1994. Avian infectious bronchitis: specific lachrymal IgA level and resistance against challenge. Zentralbl Veterinarmed B 41:467–472. [DOI] [PubMed] [Google Scholar]
- 38.Thompson G, Mohammed H, Bauman B, Naqi S. 1997. Systemic and local antibody responses to infectious bronchitis virus in chickens inoculated with infectious bursal disease virus and control chickens. Avian Dis 41:519–527. doi: 10.2307/1592140. [DOI] [PubMed] [Google Scholar]
- 39.Cook KA, Otsuki K, Martins NR, Ellis MM, Huggins MB. 1992. The secretory antibody response of inbred lines of chicken to avian infectious bronchitis virus infection. Avian Pathol 21:681–692. doi: 10.1080/03079459208418890. [DOI] [PubMed] [Google Scholar]
- 40.Toro H, Espinoza C, Ponce V, Rojas V, Morales MA, Kaleta EF. 1997. Infectious bronchitis: effect of viral doses and routes on specific lacrimal and serum antibody responses in chickens. Avian Dis 41:379–387. doi: 10.2307/1592193. [DOI] [PubMed] [Google Scholar]
- 41.Davelaar FG, Noordzij A, Vanderdonk JA. 1982. A study on the synthesis and secretion of immunoglobulins by the Jarderian gland of the fowl after eyedrop vaccination against infectious bronchitis at 1-day-old. Avian Pathol 11:63–79. doi: 10.1080/03079458208436082. [DOI] [PubMed] [Google Scholar]
- 42.Joiner KS, Hoerr FJ, Ewald SJ, van Santen VL, Wright JC, van Ginkel FW, Toro H. 2007. Pathogenesis of infectious bronchitis virus in vaccinated chickens of two different major histocompatibility B complex genotypes. Avian Dis 51:758–763. doi: 10.1637/0005-2086(2007)51[758:POIBVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Holmes HC. 1973. Neutralizing antibody in nasal secretions of chickens following administration of avian infectious bronchitis virus. Arch Gesamte Virusforsch 43:235–241. doi: 10.1007/BF01250418. [DOI] [PubMed] [Google Scholar]
- 44.Gillette KG. 1981. Local antibody response in avian infectious bronchitis: virus-neutralizing antibody in tracheobronchial secretions. Avian Dis 25:431–443. doi: 10.2307/1589935. [DOI] [PubMed] [Google Scholar]
- 45.Chhabra PC, Goel MC. 1980. Normal profile of immunoglobulins in sera and tracheal washings of chickens. Res Vet Sci 29:148–152. [PubMed] [Google Scholar]
- 46.Hawkes RA, Darbyshire JH, Peters RW, Mockett AP, Cavanagh D. 1983. Presence of viral antigens and antibody in the trachea of chickens infected with avian infectious bronchitis virus. Avian Pathol 12:331–340. doi: 10.1080/03079458308436175. [DOI] [PubMed] [Google Scholar]
- 47.Chubb R, Huynh V, Bradley R. 1988. The induction and control of delayed type hypersensitivity reactions induced in chickens by infectious bronchitis virus. Avian Pathol 17:371–383. doi: 10.1080/03079458808436455. [DOI] [PubMed] [Google Scholar]
- 48.Seo SH, Collisson EW. 1998. Cytotoxic T lymphocyte responses to infectious bronchitis virus infection. Adv Exp Med Biol 440:455–460. [DOI] [PubMed] [Google Scholar]
- 49.Raj GD, Jones RC. 1997. Cross-reactive cellular immune responses in chickens vaccinated with live infectious bronchitis virus vaccine. Avian Pathol 26:641–649. doi: 10.1080/03079459708419240. [DOI] [PubMed] [Google Scholar]
- 50.Chen HY, Cui P, Cui BA, Li HP, Jiao XQ, Zheng LL, Cheng G, Chao AJ. 2011. Immune responses of chickens inoculated with a recombinant fowlpox vaccine coexpressing glycoprotein B of infectious laryngotracheitis virus and chicken IL-18. FEMS Immunol Med Microbiol 63:289–295. doi: 10.1111/j.1574-695X.2011.00850.x. [DOI] [PubMed] [Google Scholar]
- 51.Dalgaard TS, Norup LR, Pedersen AR, Handberg KJ, Jorgensen PH, Juul-Madsen HR. 2010. Flow cytometric assessment of chicken T cell-mediated immune responses after Newcastle disease virus vaccination and challenge. Vaccine 28:4506–4514. doi: 10.1016/j.vaccine.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 52.Yohannes T, Sharma AK, Singh SD, Goswami TK. 2012. Immunopathological effects of experimental T-2 mycotoxocosis in broiler chicken co-infected with infectious bronchitis virus (IBV). Vet Immunol Immunopathol 146:245–253. doi: 10.1016/j.vetimm.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Dunnington EA, Larsen CT, Gross W, Siegel PB. 1992. Antibody responses to combinations of antigens in white Leghorn chickens of different background genomes and major histocmpatibility complex genotypes. Poultry Sci 71:1801–1806. doi: 10.3382/ps.0711801. [DOI] [PubMed] [Google Scholar]
- 54.Raj GD, Jones RC. 1996. Immunopathogenesis of infection in SPF chicks and commercial broiler chickens of a variant infectious bronchitis virus of economic importance. Avian Pathol 25:481–501. doi: 10.1080/03079459608419157. [DOI] [PubMed] [Google Scholar]
- 55.Janse EM, van Roozelaar D, Koch G. 1994. Leukocyte subpopulations in kidney and trachea of chickens infected with infectious bronchitis virus. Avian Pathol 23:513–523. doi: 10.1080/03079459408419021. [DOI] [PubMed] [Google Scholar]
- 56.Dhinakar Raj G, Jones RC. 1996. Protectotypic differentiation of avian infectious bronchitis viruses using an in vitro challenge model. Vet Microbiol 53:239–252. doi: 10.1016/S0378-1135(96)01258-8. [DOI] [PubMed] [Google Scholar]
- 57.Bruder D, Srikiatkhachorn A, Enelow RI. 2006. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol 19:147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 58.Russell PH, Dwivedi PN, Davison TF. 1997. The effects of cyclosporin A and cyclophosphamide on the populations of B and T cells and virus in the Harderian gland of chickens vaccinated with the Hitchner B1 strain of Newcastle disease virus. Vet Immunol Immunopathol 60:171–185. doi: 10.1016/S0165-2427(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 59.Javed MA, Frasca S Jr, Rood D, Cecchini K, Gladd M, Geary SJ, Silbart LK. 2005. Correlates of immune protection in chickens vaccinated with Mycoplasma gallisepticum strain GT5 following challenge with pathogenic M. gallisepticum strain R(low). Infect Immun 73:5410–5419. doi: 10.1128/IAI.73.9.5410-5419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamm ME. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]