Abstract
Although experimental data regarding cross-protection of horse West Nile virus (WNV) vaccines against lineage 2 infections exist, the cross-protective efficacy of these vaccines under field conditions has not been demonstrated. This study was conducted to evaluate the capability of an inactivated lineage 1 vaccine (Equip WNV) to protect against natural infections from the Nea Santa-Greece-2010 lineage 2 strain. In total, 185 WNV-seronegative horses in Thessaloniki, Greece, were selected during 2 consecutive years (2011 and 2012); 140 were immunized, and 45 were used as controls. Horses were examined for signs compatible with WNV infection. Neutralizing antibody titers against the Greek strain and the PaAn001/France lineage 1 strain were determined in immunized horses. WNV circulation was detected during both years in the study area. It was estimated that 37% and 27% of the horses were infected during 2011 and 2012, respectively. Three control animals developed clinical signs, and the WNV diagnosis was confirmed. Signs related to WNV infection were not observed in the vaccinated animals. The nonvaccinated animals had a 7.58% ± 1.82% higher chance of exhibiting signs than immunized animals (P < 0.05). Neutralizing antibodies raised against both strains in all immunized horses were detectable 1 month after the initial vaccination course. The cross-protective capacity of the lowest titer (1:40) was evident in 19 animals which were subsequently infected and did not exhibit signs. Neutralizing antibodies were detectable until the annual booster, when strong anamnestic responses were observed (geometrical mean titer ratio [GMTR] for lineage 1 of 30.2; GMTR for lineage 2 of 27.5). The results indicate that Equip WNV is capable of inducing cross-protection against natural infections from a virulent lineage 2 WNV strain in horses.
INTRODUCTION
West Nile virus (WNV) is a single-stranded RNA virus within the Japanese encephalitis virus serocomplex, which belongs to the genus Flavivirus (family Flaviviridae) (1). WNV is maintained in nature by enzootic transmission cycles between certain bird species and ornithophilic mosquitoes (2). Mosquitoes mainly belonging to the genus Culex can also act as bridge vectors, transmitting the virus to other animal species, including incidental hosts (3–6). Humans and horses are regarded as incidental (dead-end) hosts, as the virus titer developed in their blood is generally too low to infect mosquitoes (7). Nevertheless, WNV infection in susceptible hosts may eventually cause neurological disease (8). Regarding horses, the reported clinical signs may vary, and these include fever, paraparesis or tetraparesis, and ataxia, recumbency, and behavioral changes, while in many clinically affected horses muscle fasciculation and tremors are also present. It is expected that deaths will occur in a small percentage of the affected animals (9–13).
Phylogenetic analyses of WNV strains isolated worldwide have resulted in the identification of 8 genetic lineages of the virus so far (14). Until 2004, only viral strains belonging to lineages 1 and 3 had been found in Europe. The majority of the strains isolated from European outbreaks belong to lineage 1 (15, 16). Lineage 2 includes strains from sub-Saharan Africa and Madagascar, and these have so far been considered low virulence (17). Such strains belonging to lineage 2 were isolated in Hungary (2004), in Austria (2008), and in Italy (2008) (16, 18). However, a virulent lineage 2 strain (Nea Santa-Greece-2010) was found to be responsible for the occurrence of 4 consecutive epidemic periods (2010-2013) in Greece, with neuroinvasive disease (West Nile neuroinvasive disease [WNND]) cases in humans and horses during all these years (19, 20). An amino acid substitution (H249P) in the nonstructural protein 3 (NS3), absent from other closely related European strains, is suspected to be associated with the high virulence and neuroinvasiveness of the Greek strain (19). Enzootic transmission of the virus was detected once again in Central Macedonia, the epicenter of the 2010 epidemic, during June 2014, using backyard chickens (21).
Experimental vaccinations in birds have been applied outside Europe (although bird vaccines against WNV are not commercially available) to a limited extent, especially in endangered bird species (e.g., in California condors) to protect them from fatal WNV infection or in bird reservoir hosts (e.g., American crows and robins), with the aim of reducing WNV viremia in them and preventing subsequent transmission of the virus to competent vectors (22–26). With regard to dead-end hosts, for humans only passive immunization (intravenous immunoglobulin or hyperimmune gammaglobulin administration) has been used to a limited extent for treatment of patients with WNND (27). No human vaccines against WNV are commercially available at this time, and, as a result, active immunization of humans is not possible (28). In contrast, several inactivated and recombinant WNV vaccines for horses have been produced, evaluated, and licensed in the United States. Specifically, two inactivated vaccines have been licensed and are being used at this time in the United States: West Nile-Innovator (Fort Dodge, IA, USA) and Vetera WNV (Boehringer Ingelheim Vetmedica, MO, USA). A recombinant vaccine with a canarypox virus vector (Recombitek Equine West Nile virus; Merial, GA, USA) is also marketed (19, 26, 29). It has been shown that all of these immunological agents induce the production of WNV-specific neutralizing antibodies (NAbs) (30), and they have proven to be very effective in protecting horses from meningoencephalitis in North America (31). Additionally, a DNA vaccine (West Nile-Innovator DNA; Fort Dodge, IA, USA) was approved by the USDA in 2005. Finally, a chimeric vaccine (PreveNile; Intervet, KS, USA) containing a strain of yellow fever virus (YFV-17D) was approved for marketing in 2006 by the USDA and was later remarketed as a killed vaccine, under the name EquiNile (19, 26, 32). All of these immunological agents have been developed using lineage 1 WNV strains.
Along with the emergence of virulent lineage 2 WNV strains in Europe, two of the aforementioned vaccines, West Nile-Innovator (under the name Equip WNV [Zoetis]) and Recombitek Equine West Nile virus (under the name Proteq West Nile [Merial]), were authorized in 2011 and are being commercialized in European countries (33, 34). Concomitantly, questions arose as to whether these commercially available WNV vaccines for horses are effective in protection against virulent strains belonging to lineage 2, since both of them contain lineage 1 antigens and their protection had not been extensively evaluated for other lineages. Previous experimental studies indicated that both of these vaccines can lead to the development of cross-protective immunity. Specifically, the recombinant vaccine ALVAC-WNV (Merial) is capable of immunity induction in horses challenged with the goshawk-Hungary/04 lineage 2 strain (35). Another study, which was conducted in mice immunized with the inactivated vaccine Duvaxyn/Equip WNV, showed that the vaccine provided complete protection against challenge with the SPU93/01 lineage 2 strain (36). A more recent study was conducted in horses, showing that immunization with the Equip WNV vaccine resulted in reductions in the number of viremic animals and in the duration and severity of clinical signs of disease and mortality, following experimental infection with the virulent Nea Santa-Greece-2010 lineage 2 WNV strain (37). As a result, Equip WNV was recently authorized also for lineage 2 strains, although the duration of immunity has not been established for these strains (33). Nevertheless, results regarding the evaluation of the cross-protection of these vaccines in field conditions are lacking.
It has been demonstrated that, under experimental conditions, the effects of needle WNV inoculation in chickens might differ significantly from those of mosquito-borne natural infections (38). It has also been demonstrated that experimental WNV challenge in horses via needle inoculation or mosquito feeding was not able to induce significant clinical signs (30). In addition, under experimental conditions, cell culture-adapted and -passaged viruses are used as challenge strains. All of these cultivation procedures might have consequent effects on the virulence of the viral strains. Therefore, field evaluation of viral vaccines is of utmost importance in order to truly estimate the degree of cross-protection among different strains. In the present study, we evaluated the capacity of the inactivated Equip WNV vaccine to offer cross-protective immunity in horses against natural infections from the highly virulent Nea Santa-Greece-2010 lineage 2 WNV strain in field conditions.
MATERIALS AND METHODS
Animals.
In total, 185 mixed-breed horses, aged 5 to 18 years old, were included in this 2-year study, which took place during the 2011 and 2012 epidemic periods in Greece. The horses were from 6 horse-riding clubs in Central Macedonia, the epicenter of the 2010 Greek epidemic. None of the horses had been previously exposed to WNV, as indicated by serological testing with competitive enzyme-linked immunosorbent assays (cELISAs) and serum neutralization tests (SNTs) as described below. Specifically, serological testing was conducted twice: (i) 1 week prior to the initiation of the immunizations in both years and (ii) at the time the first dose of the primary vaccination was administered for both years. The health status of each horse was determined prior to its inclusion in the study. Immunizations, blood samplings, and clinical examinations of the animals were performed by experienced veterinarians. A mixture composed of oats, muesli, and hay/alfalfa hay was administered to the horses, and water was available ad libitum. Trained technicians were responsible for animal husbandry procedures.
Vaccine and immunization plan.
The commercially available ready-to-use vaccine Equip WNV (Zoetis, Louvain-la-Neuve, Belgium) was used in this 2-year study. This vaccine contains the inactivated lineage 1 WNV strain New York 1999/VM-2 (isolated from the brain of an infected horse during the 1999 epidemic period in New York, NY, USA) formulated in MetaStim oil emulsion adjuvant, consisting of squalene, poloxamer 401 (Pluronic L121), and polysorbate 80 (33). Vaccine lots 387BYC01L and 387BYA08A were used in 2011 and 2012, respectively. Each dose was administered via a single intramuscular injection in the neck of the animals.
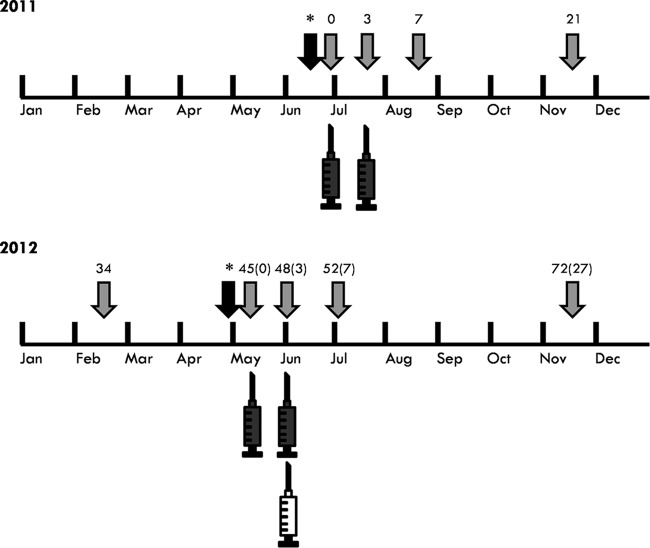
During June to July 2011, an initial double primary vaccination (two doses administered 3 weeks apart) of 85 horses was performed (Fig. 1), while 33 horses were used to form the control group (Table 1). During May to June 2012, 79 of the aforementioned vaccinated animals received an annual booster immunization dose of the vaccine. Six of the original 85 horses were excluded during the second year for various reasons, e.g., they were moved out of the study area or were euthanized due to causes not related to WNV infection. In addition, in May to June 2012, another 55 horses which were seronegative to WNV received a double immunization with the vaccine (Fig. 1). During this period, 21 of the 2011 control animals which were determined to be seronegative were kept and, along with 12 additional seronegative horses, were used as naive controls for the 2012 epidemic period (Table 1). The total number of control horses (n = 45) was intentionally limited to approximately 33% of the total number of horses used in the study, for humane reasons. In each participating horse-riding club, the vaccinated and control animals were comingled and managed similarly.
FIG 1.
Timeline of the immunizations and blood serum samplings performed in horses for the evaluation of the cross-protective immunity offered by the inactivated vaccine. The black syringes indicate the double primary vaccinations; the white syringe indicates the annual booster vaccination. Arrows depict the time points of blood serum samplings. Weeks in which these samplings were conducted are displayed above the arrows. The two black arrows marked with asterisks depict samplings performed 1 week prior to the initiation of the primary vaccinations of 2011 and 2012, respectively, in order to detect and select WNV-seronegative horses.
TABLE 1.
Numbers of immunized and control horses which were included in the efficacy study during 2011 and 2012a
| Parameterb | 2011c |
2012 |
|||
|---|---|---|---|---|---|
| IH (WNV seronegative, primo-vaccinated) | CH (WNV seronegative) | IH (WNV seronegative, primo-vaccinated) | IH (primo-vaccinated in 2011 but not infected, received annual booster vaccine dose in 2012) | CH (WNV seronegative of 2011 but not infected + WNV seronegative, selected in 2012) | |
| No. of horses per group | 85 | 33 | 55 | 52 | 33 (21 + 12) |
| No. (%) of WNV naturally infected horses determined by cELISA and/or SNT | 32/85 (38) | 12/33 (36) | 14/55 (26) | 15/52 (29)d | 9/33 (27) |
| No. (%) of WNV naturally infected horses with clinical signs, confirmed by MAC-ELISA | 0/32 (0) | 1/12 (8) | 0/14 (0) | 0/15 (0) | 2/9 (22) |
| Total no. (%) of infected horses per year | 44/118 (37) | 38/140 (27) | |||
Numbers and infection rates of horses per year and numbers of horses which exhibited neurological signs due to WNV infection are also included in the table.
cELISA, competitive enzyme-linked immunosorbent assay; SNT, serum neutralization test; MAC-ELISA, IgM antibody capture enzyme-linked immunosorbent assay.
IH, immunized horses; CH, control horses.
Indirect determination.
Clinical examination and blood samplings.
Physical and special neurological examinations were performed on each of the participating horses at least 1 week prior to the initiation of immunizations and until the end of the respective epidemic period. Monitoring was performed regularly (every 5 to 6 days) for signs compatible with WNV infection (e.g., anxiety, muscle fasciculation, head tremor, lip twitching, teeth grinding, ataxia, paresis, head shaking, etc.), along with any other abnormal conditions. Besides the evaluation for the presence of clinical signs due to WNV infection, horses were also evaluated for local and systemic adverse reactions due to the vaccination. Clinical evaluations were done independent of knowledge of the immunization status.
Blood samples were collected from all horses in 10-ml plain vacuum tubes at specific time points. For the animals participating from 2011, these time points were week 0 (W0), W3, W7, W21, W34, W48, W52, W66, and W72 (Fig. 1). For the animals that participated in the study only during 2012, the respective time points for blood collection were W45 (0), W48 (3), W52 (7), and W72 (27) (Fig. 1). The numbers in parentheses indicate the exact week numbers in which samples were obtained from the horses that participated only in 2012, beginning from the week that these animals received the first dose of the vaccine (0). Numbers outside parentheses indicate the corresponding week numbers from the beginning of the study (2011). For example, W45 (0) indicates week 0 for the horses participating in 2012 (conduction of the first immunization). Concomitantly, this is also week 45, counting from the day in which the first vaccine dose was administered during 2011 (W0).
Additionally, for the confirmation of the diagnosis of WNV infection in horses with neurological signs, blood samples were drawn shortly after the clinical signs were noticed. Blood samples were allowed to clot, and the tubes were centrifuged (3,000 × g, 10 min, 4°C). Sera were transferred to clear 2-ml microcentrifuge tubes and stored at −80°C until they were assayed.
Serological and virological testing.
Serum samples obtained from all control horses after the end of both 2011 and 2012 epidemic periods [W21 and W72 (27) of the study, respectively] were tested for WNV-specific antibodies (indication of seroconversion), using a commercially available cELISA kit (ID Screen West Nile competition; ID.vet, Montpellier, France). This analysis was performed in order to confirm that the virus was circulating in the participating horse-riding clubs and to estimate the percentage of animals which were exposed to the virus during each epidemic period.
In order to confirm that the vaccine induced the development of cross-protective immunity, sera obtained 1 month after the completion of the double primary vaccination course from all vaccinated horses of both years [W7 and W52 (7), respectively] were tested for the presence of NAbs specifically directed against the Nea Santa-Greece-2010 lineage 2 strain, following an existing SNT protocol (39) with slight modifications. Briefly, after heat inactivation at 56°C for 30 min, sera were 2-fold serially diluted (1:5 to 1:2,560, in duplicate in 96-well cell culture plates) in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen-Gibco, Groningen, The Netherlands), and 50 μl of DMEM containing 100 50% tissue culture infective doses (TCID50) of the Nea Santa-Greece-2010 strain was added. Controls, reference sera, and back titration of the antigen were also included. After incubation of the plates at 37°C for 1.5 h, 2 × 104 Vero cells in 100 μl of DMEM with 2% penicillin (100 IU/ml) and streptomycin (100 μg/ml), 2% sodium pyruvate, and 10% fetal bovine serum (Invitrogen-Gibco) were added to every well. Plates were incubated at 37°C for 5 days, and wells were examined under an inverted light microscope for evidence of viral cytopathic effects. The NAb titer of each serum sample was calculated as the highest serum dilution in which protection of the cell monolayer was observed. Sera were considered positive if cells were protected at a dilution of ≥1:10.
Moreover, sera obtained from all primo-vaccinated horses (naive horses which received the initial two-dose vaccination course) after the end of each epidemic period [November, W21 and W72 (27) of the study] were also tested with the aforementioned SNT protocol, and NAb titers were compared to the respective titers developed 1 month after the double primary vaccination [W7 and W52 (7), respectively] to detect the occurrence of anamnestic humoral immune responses, indicative of natural infections.
In order to evaluate the levels and the duration of the NAbs produced, 23 primo-vaccinated animals of the first year (2011) which were not exposed to the virus, as indicated by the results of the aforementioned analysis (∼40% of the total number of vaccinated animals which were determined not to have been exposed to WNV during that year), were tested by samples obtained from the day of the first immunization (W0) until 1 month after the annual booster (W52). SNTs were used to determine the NAb titers against two WNV strains: the Nea Santa-Greece-2010 lineage 2 strain and the PaAn001/France lineage 1 strain (kindly provided by Sylvie Lecollinet, UMR 1161 Virology, INRA-ANSES-ENVA, France).
For the confirmation of the diagnosis in control horses with clinical signs, the collected serum samples were tested for the presence of WNV-specific IgM antibodies, using a commercially available IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) (IgM WNV Ab test; IDEXX-Institut Pourquier, Montpellier, France), following the manufacturer's instructions. Furthermore, RNA was extracted from the serum samples obtained from the horses with neurological signs using the NucleoSpin RNA virus kit (Macherey-Nagel, Düren, Germany). Extracts were examined using a WNV-specific, one-tube real-time reverse transcription (RT)-PCR, using the primer pair WNPolUp (5′-TTTTGGGAGATGGTGGATGARGA-3′) and WNPolDo2 (5′-CCACATGAACCAWATGGCTCTGC-3′) at a final concentration of 0.6 μM each, and the TaqMan probe WNPolProb2 (5′-FAM-TCTCTCTCTTTCCCATCATGTTGT-ZNA5-BHQ1-3′ at a final concentration of 0.2 μM), targeting a 144-bp part of the nonstructural protein 5 (NS5) genomic region of WNV. The limit of detection was previously determined to be 1 TCID50/ml (40). Amplification reactions were run in a total volume of 25 μl using 5 μl of RNA extract and 20 μl of reaction buffer of a commercial RT-PCR kit (OneStep RT-PCR kit; Qiagen, Hilden, Germany). The thermal cycling conditions were as follows: 50°C for 30 min, followed by 95°C for 15 min and 50 cycles in 2 steps, (i) 95°C for 30 s (denaturation) and (ii) 60°C for 40 s (annealing and extension). The fluorescence levels were measured at the end of each cycle. The assay was performed using the CFX96 Touch Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). Data were analyzed using CFX software (v.3.0; Bio-Rad Laboratories).
Statistical analysis.
The effect of immunization on the presence of clinical signs was assessed with an odds ratio and a mixed-model analysis.
The odds ratio analysis considered nonvaccinated animals the control and vaccinated animals the intervention (case) group. The odds ratio was calculated as follows (41):
| (1) |
where OR is the odds ratio, a is the number of vaccinated horses with clinical signs, b is the number of vaccinated horses without clinical signs, c is the number of nonvaccinated horses with clinical signs, and d is the number of nonvaccinated horses without clinical signs.
The significance of the odds ratio was assessed by the confidence interval, which was calculated as follows:
| (2) |
where 95% CI is the 95% confidence interval, ln is the natural logarithm, SE is the standard error, and OR is the odds ratio as in equation 1. The standard error was calculated as follows:
| (3) |
where SE is as in equation 2 and a, b, c, and d are as in equation 1. In order to accommodate possible values equal to zero in the calculation of the odds ratio or its standard error, 0.5 may be added to all cells (42, 43). An odds ratio significantly smaller than unity suggests that the intervention (i.e., immunization) is better than the control.
The mixed-model analysis was based on the following model:
| (4) |
where Y is the presence or absence of clinical signs for the lth animal, μ is the overall mean, CY is the fixed effect of calendar year i (i = 2011-2012), RC is the fixed effect of riding club j (j = 1 to 6), VS is the vaccination status k (k = 0 for nonvaccinated and k = 1 for vaccinated animals), A is the random animal effect reflecting the individual response of each horse, and e is the random residual.
Model 4 fitted a logit function to account for the binary nature of the trait (presence or absence of clinical signs). The outcomes of this model served as confirmation of the odds ratio analysis with the additional benefit of the quantification of the vaccination effect on the presence of clinical signs, adjusted for all other factors included in model 4. The mixed-model analysis was conducted with ASReml software (44).
The two analyses, odds ratio and mixed model, were run once considering all animals and then a second time including only the infected horses.
NAb titers were used to calculate the geometrical mean titer (GMT) for each sampling time point and against each viral strain. A comparison of the GMTs against the two viral lineage antigens was performed at all sampling time points, using a paired two-tailed Student's t test. A P value of <0.05 was considered to be statistically significant. In order to estimate the strength of the anamnestic immune responses (e.g., due to the booster) between two sampling points (w, z) and against the same lineage (x), the geometrical mean titer ratio (GMTR) was calculated as follows: GMTRx = GMTz/GMTw.
Investigation of the immunological similarity between the vaccine and the circulating viral strains.
In an effort to interpret the immunological cross-reactivity between the NAbs produced against the vaccine strain (New York 1999/VM-2; GenBank accession no. AF260967) and the lineage 2 strain circulating in the study area (Nea Santa-Greece-2010; GenBank accession no. HQ537483), we compared the identities of the envelope (E) protein peptide sequences of the two strains. Furthermore, the respective peptide sequence of a lineage 2 strain isolated in South Africa (SA93/01; GenBank accession no. EF429198) was included in these comparisons. Multiple alignments of E protein sequences were conducted using MEGA v.6.06 software (45), and amino acid substitutions were visualized using BioEdit v.7.2.5 software (46).
Animal ethics.
Animal studies were performed in accordance with the International Guiding Principles for Biomedical Research Involving Animals, as issued by the Council for International Organizations of Medical Sciences. All horse owners gave their consent for the immunizations, blood sampling, and serological testing prior to the commencement of the study. This study was performed in compliance with national guidelines and European Union regulations as well as with those of the local ethics committees of the School of Veterinary Medicine, Aristotle University of Thessaloniki.
RESULTS
WNV circulation in the study area.
The presence and circulation of the virus in the study area were confirmed for both 2011 and 2012 by studies conducted in captive sentinel chickens and mosquitoes, as already described (40, 47, 48). Specifically, chickens were placed in cages in close proximity to the participating horse-riding clubs and exposed to mosquitoes throughout both epidemic periods, followed by serological and virological testing. Mosquitoes which were collected throughout May to October of both years were also tested. Molecular characterization of the circulating viral strain during both 2011 and 2012 in chickens and mosquitoes confirmed that the virulent Nea Santa-Greece-2010 strain was the only strain detected in Central Macedonia (40, 47, 48).
WNV natural infections in control and immunized horses.
Serological testing of control horses during November of each year [W21 and W72 (27)] indicated that WNV circulated in all the participating horse-riding clubs, during both the 2011 and 2012 epidemic seasons. Specifically, 12 out of 33 control animals of the 2011 period (36%) and 9 out of 33 control animals of the 2012 period (27%) seroconverted to WNV as evidenced by cELISA (Table 1).
Comparative evaluation of the NAb titers of sera obtained from all primo-vaccinated animals 1 month after the double vaccination and after the end of each epidemic period (November) indicated that anamnestic humoral responses (WNV infections) were evident in 32 of 85 primo-vaccinated horses of the 2011 period (38% of the immunized horses) and in 14 of 55 primo-vaccinated horses of the 2012 period (26% of the horses which received a primary immunization during the later year). Specifically, the GMT increased from 1:67 to 1:1,083 (GMTR of 16.2, ∼log2 4-fold increase). Natural infections, during 2012, of the horses that were exposed for a second consecutive year were not determined directly by SNTs (due to the booster) but were calculated indirectly based on the respective percentages of the seroconverted control horses, as well as on the percentages of primo-vaccinated horses in which anamnestic humoral responses due to infections were detected for the 2 years. As a result, it is estimated that of the 52 horses which remained uninfected during 2011 and received an annual booster in 2012, 15 animals were subsequently infected with WNV during the second epidemic season (Table 1).
Combinatory analysis of all these results obtained from serological testing applied in control and vaccinated horses indicates that in total 44 out of 118 horses (37%) were infected during 2011. The respective infection rate for 2012 was estimated to be 27% (Table 1). Infection rates for the horse-riding clubs ranged between 18 and 60% for 2011 and between 18 and 47% for 2012.
Vaccine safety.
Regarding the adverse reactions of the applied vaccine, only 1 out of 140 immunized animals (0.7%) developed a local reaction on the site of the injection. This was a mild swelling which developed after the second injection of the first year, and it was observed again in the same animal after the annual booster. During the aforementioned occurrences, resolution of the lesion was observed within a few days, without any interventions and without other effects on the health of the animal.
Neutralizing antibody responses in immunized horses.
One month after the initial double vaccination, NAbs against the Greek lineage 2 strain were induced in all vaccinated animals with a GMT of 1:102 (titer range, 1:40 to 1:320). Briefly, in 67 out of the 140 vaccinated animals (47.9%; 95% confidence interval [CI], 39 to 56%), an intermediate neutralizing activity was observed (titer range, 1:40 to 1:80). Twenty-six of these animals developed a NAb titer of 1:40, and 41 animals presented with a titer of 1:80. Higher neutralizing responses were observed in sera from the remaining 73 vaccinated animals (52.1%; 95% CI, 44 to 61%). Nineteen out of the 46 primo-vaccinated horses of 2011 and 2012 (41%) which were subsequently infected (32 and 14, respectively), as determined by SNTs (Table 1), had an NAb titer of 1:40 against the Nea Santa-Greece-2010 strain 1 month after the primary immunization course.
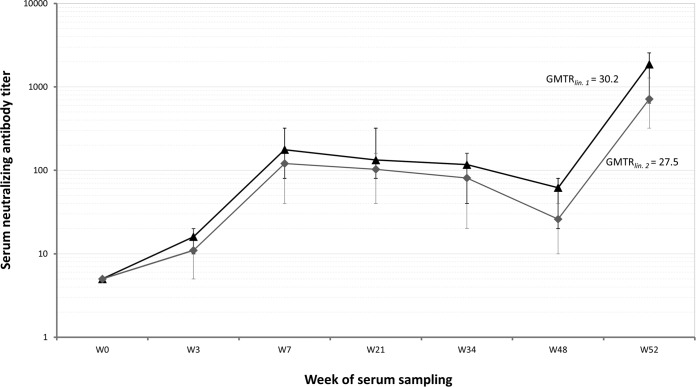
Moreover, use of SNTs in 23 of the 53 primo-vaccinated horses in 2011, which were revealed to not be naturally infected, indicated that GMTs against the lineage 1 strain were higher than the respective titers against the lineage 2 strain at all sampling points. NAbs against the two viral lineage antigens were consistently detected in the sera of these animals at all sampling points and until the annual booster immunization (Fig. 2). However, paired t test analysis revealed no significant differences in the GMTs against the two WNV strains in the sera of the 23 vaccinated horses at all sampling points (P > 0.28). Specifically, analysis of the NAb titers raised against the two viral lineage antigens 1 month after the completion of the double primary vaccination course (i.e., at W7) indicated that the GMT for lineage 1 was 1:175 (titer range, 1:80 to 1:320), while the respective value for lineage 2 was 1:112 (titer range, 1:40 to 1:160). Individual NAb titers indicated that in 9 out of 23 animals (39.1; 95% CI, 20 to 61%), NAb titers were the same against both antigens. In 13 out of 23 animals (56.5; 95% CI, 35 to 76%), titers against the lineage 1 antigen were higher than the respective titer raised against the lineage 2 antigen by one serial dilution, while in one serum sample (4.4; 95% CI, 0.1 to 24%), a titer difference of 2 dilutions between the two viral lineage antigens was observed. Further comparisons of NAb titers throughout the year indicate that those for lineage 1 were consistently higher than the respective values determined for lineage 2.
FIG 2.
Neutralizing antibody (NAb) geometrical mean titers (GMTs) of 23 immunized horses which were not infected against the Nea Santa-Greece-2010 lineage 2 strain (gray curve, ◆) as well as the PaAn001/France lineage 1 strain (black curve, ▲). SNTs were performed in sera collected from W0 (the time of the first dose of the double primary vaccination in 2011) and until W52 (i.e., 1 month after the annual booster immunization). Paired t test analysis revealed no significant differences in the NAb GMTs against the two antigens at any sampling point (P > 0.28). Error bars encompass the range of the individual NAb titers against each antigen and for every sampling time point. The geometrical mean titer ratio (GMTR) calculated for the annual booster against each strain is also presented.
One month after the annual booster, a strong titer increase against both strains was observed (Fig. 2). The NAb titers achieved were well above their initial peak (1 month after the initial double primary vaccination). Specifically, ≥log2 4-fold titer increases were observed in all cases for the NAb titers against both PaAn001/France lineage 1 (GMTRlin.1 of 30.2) and Nea Santa-Greece-2010 lineage 2 (GMTRlin.2 of 27.5) strains. A similar degree of immunoreactivity (titer increase of ≥4 2-fold serial dilutions) was also observed in the vaccinated animals which were naturally infected. In all these anamnestic immune responses, NAb titers were determined to be ≥1:320. The GMT for the lineage 1 antigen 1 month after the annual booster (i.e., at W52) was determined to be 1:1,894 (titer range, 1:640 to ≥1:2,560), whereas the respective value for the lineage 2 antigen was 1:722 (titer range, 1:320 to 1:1,280). In terms of NAb titer differences between the two viral lineage antigens, it was indicated that in 15 out of 23 animals (65.2; 95% CI, 43 to 83%) NAb titers against the lineage 1 antigen were higher than the respective titers raised against the lineage 2 antigen by one serial dilution. In 7 out of 23 animals (30.4; 95% CI, 14 to 53%) titers differed by two serial dilutions, and in one serum sample (4.4; 95% CI, 0.1 to 24%), a titer difference of 3 dilutions was observed.
Clinical signs, confirmation of the diagnosis, and cross-protective efficacy of the vaccine.
None of the 140 vaccinated horses (0%) showed any clinical signs related to WNV infection during either epidemic period. In contrast, 3 out of 45 control animals (7%) showed clinical signs due to WNV infection. The odds ratio, considering all animals, was 0.0432 (95% CI, 0.0022 to 0.8534). The fact that the CI did not cross 1 implies a statistically significant (P < 0.05) difference between the vaccinated and nonvaccinated horses with regard to the presence of clinical signs. The same analysis based on the infected animals only (61 immunized and 21 controls) returned an odds ratio of 0.0423 (95% CI, 0.0021 to 0.08562), implying that immunization was also beneficial for the animals that were naturally infected.
More specifically, during the 2011 epidemic period, clinical signs were detected in 1 out of the 12 seroconverted control animals (August 13). During the 2012 epidemic period, 2 out of the 9 seroconverted horses of the control group showed clinical signs (August 25 and September 8, respectively). These signs included fever, weakness of the hind limbs, ataxia, muscle twitching, and tremors in all 3 affected animals. The diagnosis was confirmed, as WNV-specific IgM antibodies were detected in all of the animals by MAC-ELISA. WNV RNA was not detected (no threshold cycle [CT] values obtained). Consequently, it was not possible to detect the virus in the blood sera obtained, since the samples were drawn after the initiation of the clinical signs and probably past the viremia stage. The 3 horses received supportive treatment (dexamethasone, vitamin B complex supplements, and phenylbutazone) that led to the resolution of clinical signs within a few days.
The effect of vaccination on preventing clinical signs was confirmed and quantified with mixed-model analysis. The vaccination status had a significant effect on clinical signs, with nonimmunized animals being associated with a 7.58% ± 1.82% (P < 0.05) higher chance of exhibiting signs than immunized animals. This value was derived from the analysis of all animals with mixed-model 4. The value reflects the effect of immunization on the presence of clinical signs and describes the difference between the marginal means of vaccinated and nonvaccinated animals, adjusted for all other effects in model 4. The corresponding value from the analysis of infected animals only was 14.22% ± 1.43% (P < 0.05), suggesting that the vaccination effect was even stronger for naturally infected horses.
E protein amino acid sequence comparisons.
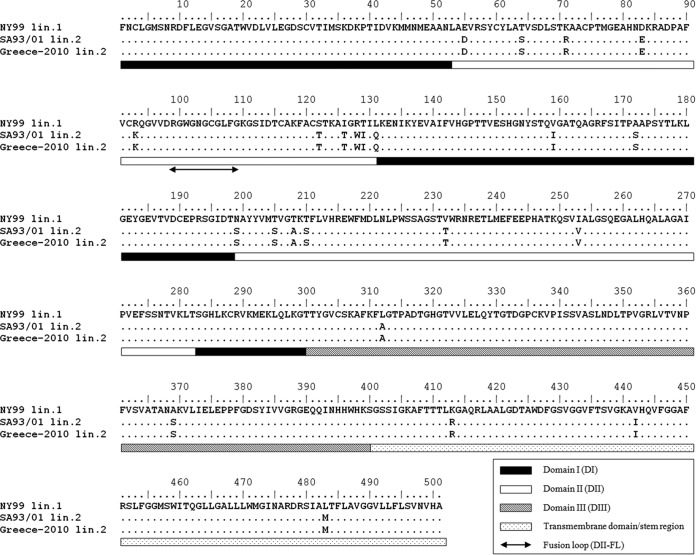
For the interpretation of the reactivity between the NAbs raised against the vaccine strain and virus strains belonging to lineage 2, the immunological similarity of E protein peptide sequences was investigated. No differences were observed between the peptide sequences of the Greek and African lineage 2 strains. A comparison of the sequences of the lineage 1 vaccine strain with those of the lineage 2 strains indicated 23 amino acid substitutions. Specifically, 3 of these substitutions were observed in structural domain I (DI) (L131Q, V159I, and A172S), 15 substitutions were identified in DII (E55D, T64S, K71R, D83E, R93K, S122T, I126T, R128W, T129I, N199S, T205S, T208A, T210S, V232T, and I253V), 2 substitutions were present in DIII (L312A and A369S), and 3 more substitutions were found in the transmembrane domain/stem region (K413R, V442I, and L483M) (Fig. 3).
FIG 3.
Alignment of the E protein amino acid sequences from the vaccine lineage 1 strain New York 1999/VM-2 (GenBank accession no. AF260967), the circulating lineage 2 strain Nea Santa-Greece-2010 (GenBank accession no. HQ537483), and the South African lineage 2 strain SA93/01 (GenBank accession no. EF429198) of West Nile virus. Dots indicate amino acid identities. The domains are indicated by bars, as explained in the legend. The investigation of the immunological similarity among the three peptides revealed no differences between the two lineage 2 strains. The comparison of the lineage 1 (vaccine) and the lineage 2 peptide sequences of the E protein revealed 23 amino acid substitutions.
DISCUSSION
Two doses of the vaccine, administered 3 weeks apart in immunologically naive horses, resulted in the development of adequate cross-protective immunity against the manifestation of neurological signs due to natural infections from the Nea Santa-Greece-2010 lineage 2 strain during the following epidemic period, as indicated by the lack of occurrence of clinical signs in any of the immunized animals. Immunization using the aforementioned vaccine may not prevent horses from being infected by lineage 2 strains but can reduce the number of viremic horses, the viremia duration and titer in the infected animals, the duration and severity of clinical signs, and the mortality, as has already been described (34, 37). In our case, although the detection of severe cases in horses was effective due to the experience of the veterinarians involved, it is possible that mild clinical occurrences might not have been noticed. However, since no supportive treatment was required, the impact of these cases was insignificant. Adverse reactions due to the vaccine were minimal. Our findings confirm that although the majority of infections in horses were subclinical, a high percentage (14%) of the seroconverted nonvaccinated horses exhibited neurological signs. This is in agreement with a similar percentage (19%) of neurological manifestations to infections reported for this virus strain during the 2010 epidemic in Greece (49). Interestingly, a slightly lower morbidity rate (10%) within infected horses has been reported for lineage 1 WNV strains (50–52).
Despite the use of adjuvants, long-term immunity is not a feature of inactivated vaccines. Although the duration of immunity for lineage 1 strains has been determined (12 months after the primary vaccination course) (33), relevant information for lineage 2 strains is lacking. Previous studies with Equip WNV have indicated that immunized horses maintained NAb titers of ≥1:100 against lineage 1 strains for 5 to 7 months, as determined by plaque reduction neutralization tests (PRNT) (53). In another study, it was shown that neutralizing responses were maintained for 6 months after vaccination of immunologically naive horses (54). In a more recent study, it has been demonstrated that NAbs may be detected in samples obtained 1 year after the primary vaccination course in naive horses, although a decline in neutralizing titers was observed (55). In our case, NAbs against the lineage 2 strain developed in all vaccinated animals (titer range, 1:40 to 1:320, GMT of 1:102) 1 month after the initial double vaccination. Although a titer decline was observed through time, as evidenced by testing of vaccinated animals that were not infected (GMT of <1:100 at week 34), NAbs were detectable until the annual booster. The lowest neutralizing response of 1:40 against the lineage 2 strain was observed in 41% of the primo-vaccinated horses of both years (n = 19) which were subsequently infected. The fact that these 19 horses did not exhibit clinical signs due to WNV infection indicates that NAb titers as low as 1:40 1 month after the primary immunization course can be protective against natural infections from the Nea Santa-Greece-2010 lineage 2 strain. It is hypothesized that humoral immunity against lineage 2 strains lasts at least until W21, based solely on the GMTs against the lineage 2 strain, which were >1:100 for these sampling time points, although individual titers of >1:100 were detected until W34. GMTs against the PaAn001/France lineage 1 strain were higher than the respective titers against the lineage 2 strain, which is in agreement with other studies (36, 55). In our case, comparison of the GMTs against the two lineages revealed no significant differences. The applied immunization scheme resulted in development of adequate B-cell memory, as indicated by the strong responses observed after annual boosters and natural infections. The results regarding these responses are supported by a previous study in which significant NAb titer increases (log2 5-fold) against both lineages is described, and the titers achieved were well above their peaks observed after the initial vaccination (55). It has been previously demonstrated that horses immunized with this vaccine also developed antigen-specific cellular responses (CD4+ and CD8+ alpha interferon [IFN-α] expression, cellular proliferation, and interleukin-4 [IL-4] expression in CD4+ peripheral blood mononuclear cells [PBMCs]) (54), indicating that, besides humoral immunity, the vaccine induces T-cell responses, which might also contribute to the cross-protection of the naturally infected horses.
WNV E protein is a major determinant of tropism and is the primary target of NAbs. Neutralizing epitopes have been identified, mainly on DIII of E protein and, specifically, on residues 306, 307, 330, and 332 (56–58). Additional neutralizing epitopes have been identified on several residues of DI and DII, although the observed neutralizing activity for these regions is weaker (59). In our case, no changes were observed at residues S306, K307, T330, and T332, which serve as major DIII neutralizing epitopes (56–58). However, escape from neutralization has been associated with the L312A substitution, which was present in the Nea Santa-Greece-2010 strain, as it is in several WNV strains (60). No changes were observed in residues W101, G106, and L107, antigenic sites of the fusion loop located within DII (DII-FL) (residues 98 to 109), which act as targets of cross-NAbs among different species of the genus Flavivirus (61–63). Despite the L312A substitution, the findings of the present study ultimately suggest that under field conditions, adequate cross-neutralization is capable of providing a high degree of protection.
Different WNV lineages, characterized by varying levels of virulence and neuroinvasiveness, cocirculate in Europe (63), and knowledge regarding cross-protection is a prerequisite. However, since outbreaks in horses were limited and unpredictable, immunizations have been performed extensively (26), regardless of the degree of cross-protection between circulating and vaccine strains. For the purpose of in-field evaluations of arbovirus vaccines, identification of the circulating strain is a necessity. In our case, it was not possible to detect the virus in the affected horses. This was anticipated, given that in horses WNV detection is hampered by the short viremia duration which precedes the onset of clinical signs (7, 49, 51). Therefore, WNV surveillance data from birds and mosquitoes, indicating that the only strain circulating during both years was the Nea Santa-Greece-2010 strain, were utilized (46–48). Mixed-model analysis seems to be a more accurate approach for in-field vaccine evaluations, as many factors are involved and should be taken into consideration. It was also possible to quantify the favorable effect of the immunization on the presence of clinical signs. Immunizations using inactivated lineage 1 vaccines can effectively protect horses from the development of neurological signs due to natural infections of virulent lineage 2 WNV strains. Since the pathogenesis and antiviral immune responses against WNV in horses and humans are similar, our results might be of value in the future for the possible evaluation of a candidate human vaccine.
ACKNOWLEDGMENTS
We acknowledge Eleni Pavlidou (Zoetis Inc., Athens, Greece) for her support. We also acknowledge all of the horse owners who gave their consent for the immunizations, blood samplings, and laboratory testing, as well as all horse-riding club owners and the animal husbandry technical staff who allowed access to their premises and aided us with our study. We thank Panagiota Tyrnenopoulou (Equine Unit, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki) for her contribution with immunizations and blood samplings, Sylvie Lecollinet (UMR 1161 Virology, INRA-ANSES-ENVA, France) for providing the PaAn001/France lineage 1 WNV strain, and Orestis Papadopoulos (Laboratory of Microbiology and Infectious Diseases, School of Veterinary Medicine, Aristotle University of Thessaloniki) for valuable discussions.
This work was supported by Zoetis Inc. Serafeim C. Chaintoutis was supported by the Alexander S. Onassis Public Benefit Foundation.
REFERENCES
- 1.Zeller HG, Schuffenecker I. 2004. West Nile virus: an overview of its spread in Europe and the Mediterranean Basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis 23:147–156. doi: 10.1007/s10096-003-1085-1. [DOI] [PubMed] [Google Scholar]
- 2.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis 11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappole JH, Hubálek Z. 2003. Migratory birds and West Nile virus. J Appl Microbiol 94(Suppl):47S–58S. [DOI] [PubMed] [Google Scholar]
- 4.Linke S, Niedrig M, Kaiser A, Ellerbrok H, Müller K, Müller T, Conraths FJ, Mühle RU, Schmidt D, Köppen U, Bairlein F, Berthold P, Pauli G. 2007. Serologic evidence of West Nile virus infections in wild birds captured in Germany. Am J Trop Med Hyg 77:358–364. [PubMed] [Google Scholar]
- 5.Kramer LD, Styer LM, Ebel GD. 2008. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol 53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 6.Engler O, Savini G, Papa A, Figuerola J, Groschup MH, Kampen H, Medlock J, Vaux A, Wilson AJ, Werner D, Jöst H, Goffredo M, Capelli G, Federici V, Tonolla M, Patocchi N, Flacio E, Portmann J, Rossi-Pedruzzi A, Mourelatos S, Ruiz S, Vázquez A, Calzolari M, Bonilauri P, Dottori M, Schaffner F, Mathis A, Johnson N. 2013. European surveillance for West Nile virus in mosquito populations. Int J Environ Res Public Health 10:4869–4895. doi: 10.3390/ijerph10104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauphin G, Zientara S, Zeller H, Murgue B. 2004. West Nile: worldwide current situation in animals and humans. Comp Immunol Microbiol Infect Dis 27:343–355. doi: 10.1016/j.cimid.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. 2005. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis 11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantile C, di Guardo G, Eleni C, Arispici M. 2000. Clinical and neuropathological features of West Nile virus equine encephalomyelitis in Italy. Equine Vet J 32:31–35. doi: 10.2746/042516400777612080. [DOI] [PubMed] [Google Scholar]
- 10.Steinman A, Banet C, Sutton GA, Yadin H, Hadar S, Brill A. 2002. Clinical signs of West Nile virus encephalomyelitis in horses during the outbreak in Israel in 2000. Vet Rec 151:47–49. doi: 10.1136/vr.151.2.47. [DOI] [PubMed] [Google Scholar]
- 11.Weese JS, Baird JD, DeLay J, Kenney DG, Staempfli HR, Viel L, Parent J, Smith-Maxie L, Poma R. 2003. West Nile virus encephalomyelitis in horses in Ontario: 28 cases. Can Vet J 44:469–473. [PMC free article] [PubMed] [Google Scholar]
- 12.Venter M, Human S, Zaayman D, Gerdes GH, Williams J, Steyl J, Leman PA, Paweska JT, Setzkorn H, Rous G, Murray S, Parker R, Donnellan C, Swanepoel R. 2009. Lineage 2 West Nile virus as cause of fatal neurologic disease in horses, South Africa. Emerg Infect Dis 15:877–884. doi: 10.3201/eid1506.081515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutasi O, Bakonyi T, Lecollinet S, Biksi I, Ferenczi E, Bahuon C, Sardi S, Zientara S, Szenci O. 2011. Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. J Vet Intern Med 25:586–591. doi: 10.1111/j.1939-1676.2011.0715.x. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez A, Sánchez-Seco MP, Ruiz S, Molero F, Hernández L, Moreno J, Magallanes A, Tejedor CG, Tenorio A. 2010. Putative new lineage of West Nile virus, Spain. Emerg Infect Dis 16:549–552. doi: 10.3201/eid1603.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, Nowotny N. 2006. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis 12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calistri P, Giovannini A, Hubalek Z, Ionescu A, Monaco F, Savini G, Lelli R. 2010. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virol J 4:29–37. doi: 10.2174/1874357901004020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murgue B, Zeller H, Deubel V. 2002. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr Top Microbiol Immunol 267:195–221. [DOI] [PubMed] [Google Scholar]
- 18.Savini G, Capelli G, Monaco F, Polci A, Russo F, Di Gennaro A, Marini V, Teodori L, Montarsi F, Pinoni C, Pisciella M, Terregino C, Marangon S, Capua I, Lelli R. 2012. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Vet Microbiol 158:267–273. doi: 10.1016/j.vetmic.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Marka A, Diamantidis A, Papa A, Valiakos G, Chaintoutis SC, Doukas D, Tserkezou P, Giannakopoulos A, Papaspyropoulos K, Patsoula E, Badieritakis E, Baka A, Tseroni M, Pervanidou D, Papadopoulos NT, Koliopoulos G, Tontis D, Dovas CI, Billinis C, Tsakris A, Kremastinou J, Hadjichristodoulou C. 2013. West Nile Virus state of the art report of MALWEST Project. Int J Environ Res Public Health 10:6534–6610. doi: 10.3390/ijerph10126534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellenic Center for Disease Control and Prevention. 2013. Report on West Nile virus epidemic. http://goo.gl/lsJaeC. [Google Scholar]
- 21.Chaintoutis SC, Gewehr S, Danis K, Kalaitzopoulou S, Antalis V, Papanastassopoulou M, Mourelatos S, Panagiotopoulos T, Hadjichristodoulou C, Dovas CI. 2014. Surveillance and early warning of West Nile virus lineage 2 using domestic pigeons and backyard chickens. In Proceedings of the 19th Congress of the European Society for Vector Ecology, Thessaloniki, Greece. [Google Scholar]
- 22.Turell MJ, Bunning M, Ludwig GV, Ortman B, Chang J, Speaker T, Spielman A, McLean R, Komar N, Gates R, McNamara T, Creekmore T, Farley L, Mitchell CJ. 2003. DNA vaccine for West Nile virus infection in fish crows (Corvus ossifragus). Emerg Infect Dis 9:1077–1081. doi: 10.3201/eid0909.030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunning ML, Fox PE, Bowen RA, Komar N, Chang G-JJ, Speaker TJ, Stephens MR, Nemeth N, Panella NA, Langevin SA, Gordy P, Teehee M, Bright PR, Turell MJ. 2007. DNA vaccination of the American crow (Corvus brachyrhynchos) provides partial protection against lethal challenge with West Nile virus. Avian Dis 51:573–577. doi: 10.1637/0005-2086(2007)51[573:DVOTAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Chang G-JJ, Davis BS, Stringfield C, Lutz C. 2007. Prospective immunization of the endangered California condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine 25:2325–2330. doi: 10.1016/j.vaccine.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Kilpatrick AM, Dupuis AP, Chang G-JJ, Kramer LD. 2010. DNA vaccination of American robins (Turdus migratorius) against West Nile virus. Vector Borne Zoonotic Dis 10:377–380. doi: 10.1089/vbz.2009.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck C, Jimenez-Clavero MA, Leblond A, Durand B, Nowotny N, Leparc-Goffart I, Zientara S, Jourdain E, Lecollinet S. 2013. Flaviviruses in Europe: complex circulation patterns and their consequences for the diagnosis and control of West Nile Disease. Int J Environ Res Public Health 10:6049–6083. doi: 10.3390/ijerph10116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal A-G, Petersen LR. 2003. Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis 188:1–4. doi: 10.1086/376871. [DOI] [PubMed] [Google Scholar]
- 28.Brandler S, Tangy F. 2013. Vaccines in development against West Nile virus. Viruses 5:2384–2409. doi: 10.3390/v5102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng T, Hathaway D, Jennings N, Champ D, Chiang YW, Chu HJ. 2003. Equine vaccine for West Nile virus. Dev Biol (Basel) 114:221–227. [PubMed] [Google Scholar]
- 30.Seino KK, Long MT, Gibbs EP, Bowen RA, Beachboard SE, Humphrey PP, Dixon MA, Bourgeois MA. 2007. Comparative efficacies of three commercially available vaccines against West Nile virus (WNV) in a short-duration challenge trial involving an equine WNV encephalitis model. Clin Vaccine Immunol 14:1465–1471. doi: 10.1128/CVI.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epp T, Waldner C, Townsend HGG. 2007. A case-control study of factors associated with development of clinical disease due to West Nile virus, Saskatchewan 2003. Equine Vet J 39:498–503. doi: 10.2746/042516407X248476. [DOI] [PubMed] [Google Scholar]
- 32.Intervet Schering-Plough Animal Health. EquiNile with Havlogen: material safety data sheet no. SP002579. http://www.merck-animal-health-usa.com/products/equi-nile_with_havlogen/overview.aspx. [Google Scholar]
- 33.European Medicines Agency. 2015. EPAR product information: Equip WNV. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/veterinary/000137/WC500063683.pdf. [Google Scholar]
- 34.European Medicines Agency. 2015. EPAR summary for the public: Proteq West Nile. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/veterinary/002005/WC500110366.pdf. [Google Scholar]
- 35.Minke JM, Siger L, Cupillard L, Powers B, Bakonyi T, Boyum S, Nowotny N, Bowen R. 2011. Protection provided by a recombinant ALVAC-WNV vaccine expressing the prM/E genes of a lineage 1 strain of WNV against a virulent challenge with a lineage 2 strain. Vaccine 29:4608–4612. doi: 10.1016/j.vaccine.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 36.Venter M, van Vuren PJ, Mentoor J, Paweska J, Williams J. 2013. Inactivated West Nile virus (WNV) vaccine, Duvaxyn WNV, protects against a highly neuroinvasive lineage 2 WNV strain in mice. Vaccine 31:3856–3862. doi: 10.1016/j.vaccine.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 37.Bowen RA, Bosco-Lauth A, Syvrud K, Thomas A, Meinert TR, Ludlow DR, Cook C, Salt J, Ons E. 2014. Protection of horses from West Nile virus lineage 2 challenge following immunization with a whole, inactivated WNV lineage 1 vaccine. Vaccine 32:5455–5459. doi: 10.1016/j.vaccine.2014.07.093. [DOI] [PubMed] [Google Scholar]
- 38.Styer LM, Bernard KA, Kramer LD. 2006. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am J Trop Med Hyg 75:337–345. [PubMed] [Google Scholar]
- 39.Chaintoutis SC, Dovas CI, Papanastassopoulou M, Gewehr S, Danis K, Beck C, Lecollinet S, Antalis V, Kalaitzopoulou S, Panagiotopoulos T, Mourelatos S, Zientara S, Papadopoulos O. 2014. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp Immunol Microbiol Infect Dis 37:131–141. doi: 10.1016/j.cimid.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Chaskopoulou A, Dovas CI, Chaintoutis SC, Kashefi J, Koehler P, Papanastassopoulou M. 2013. Detection and early warning of West Nile virus circulation in Central Macedonia, Greece, using sentinel chickens and mosquitoes. Vector Borne Zoonotic Dis 13:723–732. doi: 10.1089/vbz.2012.1176. [DOI] [PubMed] [Google Scholar]
- 41.Altman DG. 2006. Practical statistics for medical research, 2nd ed Chapman & Hall/CRC tests in statistical science. Chapman & Hall/CRC, London, United Kingdom. [Google Scholar]
- 42.Pagano M, Gauvreau K. 2000. Principles of biostatistics, 2nd ed Brooks/Cole, Belmont, CA. [Google Scholar]
- 43.Deeks JJ, Higgins JPT. 2010. Statistical algorithms in Review Manager 5. http://ims.cochrane.org/revman/documentation/Statistical-methods-in-RevMan-5.pdf. [Google Scholar]
- 44.Gilmour AR, Gogel BJ, Cullis BR, Thompson R. 2009. ASReml user guide release 3.0. VSN International Ltd., Hemel Hempstead, United Kingdom. [Google Scholar]
- 45.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98. [Google Scholar]
- 47.Chaskopoulou A, Dovas CI, Chaintoutis SC, Bouzalas I, Ara G, Papanastassopoulou M. 2011. Evidence of enzootic circulation of West Nile virus (Nea Santa-Greece-2010, lineage 2), Greece, May to July 2011. Euro Surveill 16(31):pii=19933 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19933. [PubMed] [Google Scholar]
- 48.Chaintoutis SC, Chaskopoulou A, Chassalevris T, Koehler PG, Papanastassopoulou M, Dovas CI. 2013. West Nile virus lineage 2 strain in Greece, 2012. Emerg Infect Dis 19:827–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouzalas IG, Diakakis N, Chaintoutis SC, Brellou GD, Papanastassopoulou M, Danis K, Vlemmas I, Seuberlich T, Dovas CI. 7 February 2015. Emergence of equine West Nile encephalitis in Central Macedonia, Greece, 2010. Transbound Emerg Dis doi: 10.1111/tbed.12334. [DOI] [PubMed] [Google Scholar]
- 50.Long MT, Porter MB, Hernandez J, Giguere S, Fontaine GL, Jodoin EA, Gillis KD. 2003. Preliminary data regarding the subclinical exposure rate of horses to West Nile virus during the 2001 Florida enzootic, p 397–398. In Proceedings of the 49th Annual Meeting of the American Association of Equine Practitioners, New Orleans, LA. [Google Scholar]
- 51.Castillo-Olivares J, Wood J. 2004. West Nile virus infection of horses. Vet Res 35:467–483. doi: 10.1051/vetres:2004022. [DOI] [PubMed] [Google Scholar]
- 52.Gardner IA, Wong SJ, Ferraro GL, Balasuriya UB, Hullinger PJ, Wilson D, Shi PY, MacLachlan NJ. 2007. Incidence and effects of West Nile virus infection in vaccinated and unvaccinated horses in California. Vet Res 38:109–116. doi: 10.1051/vetres:2006045. [DOI] [PubMed] [Google Scholar]
- 53.Davidson AJ, Traub-Dargatz JL, Rodeheaver RM, Ostlund EN, Pedersen DD, Moorhead RG, Stricklin JB, Dewell RD, Roach SD, Long RE, Albers SJ, Callan RJ, Salman MD. 2005. Immunologic responses to West Nile virus in vaccinated and clinically affected horses. J Am Vet Med Assoc 226:240–245. doi: 10.2460/javma.2005.226.240. [DOI] [PubMed] [Google Scholar]
- 54.Davis EG, Zhang Y, Tuttle J, Hankins K, Wilkerson M. 2008. Investigation of antigen specific lymphocyte responses in healthy horses vaccinated with an inactivated West Nile virus vaccine. Vet Immunol Immunopathol 126:293–301. doi: 10.1016/j.vetimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Pearce MC, Venter M, Schouwstra T, Van Eeden C, Jansen van Vuren P, Paweska J, Liu B, du Plessis A. 2013. Serum neutralising antibody response of seronegative horses against lineage 1 and lineage 2 West Nile virus following vaccination with an inactivated lineage 1 West Nile virus vaccine. J South Afr Vet Assoc 84. doi: 10.4102/jsava.v84i1.1052. [DOI] [Google Scholar]
- 56.Beasley DWC, Barrett ADT. 2002. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol 76:13091–13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sánchez MD, Pierson TC, McAllister D, Hanna SL, Puffer BA, Valentine LE, Murtadha MM, Hoxie JA, Doms RW. 2005. Characterization of neutralizing antibodies to West Nile virus. Virology 336:70–82. doi: 10.1016/j.virol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 59.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. 2006. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J Virol 80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Barrett ADT, Beasley DWC. 2005. Differential expression of domain III neutralizing epitopes on the envelope proteins of West Nile virus strains. Virology 335:99–105. doi: 10.1016/j.virol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Crill WD, Chang GJ. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol 78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiasny K, Kiermayr S, Holzmann H, Heinz FX. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol 80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donadieu E, Bahuon C, Lowenski S, Zientara S, Coulpier M, Lecollinet S. 2013. Differential virulence and pathogenesis of West Nile viruses. Viruses 5:2856–2880. doi: 10.3390/v5112856. [DOI] [PMC free article] [PubMed] [Google Scholar]