Abstract
Purpose
Previous studies report that early palliative care is associated with clinical benefits, but there is limited evidence on economic impact. This article addresses the research question: Does timing of palliative care have an impact on its effect on cost?
Patients and Methods
Using a prospective, observational design, clinical and cost data were collected for adult patients with an advanced cancer diagnosis admitted to five US hospitals from 2007 to 2011. The sample for economic evaluation was 969 patients; 256 were seen by a palliative care consultation team, and 713 received usual care only. Subsamples were created according to time to consult after admission. Propensity score weights were calculated, matching the treatment and comparison arms specific to each subsample on observed confounders. Generalized linear models with a γ distribution and a log link were applied to estimate the mean treatment effect on cost within subsamples.
Results
Earlier consultation is associated with a larger effect on total direct cost. Intervention within 6 days is estimated to reduce costs by −$1,312 (95% CI, −$2,568 to −$56; P = .04) compared with no intervention and intervention within 2 days by −$2,280 (95% CI, −$3,438 to −$1,122; P < .001); these reductions are equivalent to a 14% and a 24% reduction, respectively, in cost of hospital stay.
Conclusion
Earlier palliative care consultation during hospital admission is associated with lower cost of hospital stay for patients admitted with an advanced cancer diagnosis. These findings are consistent with a growing body of research on quality and survival suggesting that early palliative care should be more widely implemented.
INTRODUCTION
Palliative care has been recommended by the American Society of Clinical Oncology as standard care and is increasingly available to patients with serious illness earlier in their care trajectory with observable benefits.1,2 For example, concurrent palliative care from point of diagnosis and use of trigger checklists on hospital admission to identify and then serve patients with palliative care needs have demonstrated beneficial impact on quality and survival.3–6 Palliative care programs have also been shown to reduce the cost of care, but there is little evidence on whether earlier treatment has economic benefits.7,8 This article addresses a recognized limitation in the evidence base for organization of care to patients with serious illness with the following research question: Does time to consult after admission impact palliative care consultation team (PCCT) effect on cost of hospital care?
PATIENTS AND METHODS
Study Design and Setting
We conducted a multisite, prospective cohort study to examine the effect of PCCTs on patient and family outcomes (symptom control, satisfaction with care), processes of care (prescribing, transition management, advance planning), and utilization (hospital costs, length of stay [LOS]) for patients with advanced cancer admitted to five hospitals from 2007 to 2011 (the Palliative Care for Cancer study9).
Because a randomized controlled trial of palliative care was not deemed to be feasible or ethical,10–15 a prospective observational study was conducted instead. To reduce selection bias, we used propensity scores to balance observed confounders across the treatment and comparison groups.
All five participating hospitals (Mount Sinai Medical Center, New York, NY; Virginia Commonwealth University-Massey Cancer Center, Richmond, VA; University of Pittsburgh Medical Center, Pittsburgh, PA; Mount Carmel Health System, Columbus, OH; and Froedtert Hospital of the Medical College of Wisconsin, Milwaukee, WI) are high-volume tertiary-care medical and cancer centers with established PCCTs using existing practice guidelines for pain and symptom management and communication. We received permission to recruit patients from more than 95% of primary physicians at all hospitals. All participants signed written informed consent, and the study was approved by the institutional review board of each participating facility.
Intervention
The intervention was a palliative care consultation with a specialist-led interdisciplinary team that assists in the treatment of seriously ill patients through identification and treatment of pain and other symptoms, clarifying treatment options, establishing goals of care and advance plans, and helping patients and family members select treatments that match their goals. Consultation was initiated at the request of the attending physician. Teams across all sites were trained in a standardized protocol approach to consultation including a standard rounding form, symptom assessment tool, and team membership. Adherence to protocols was monitored. Repeat training was given to all new personnel joining any PCCT.
Usual care comprised each individual hospital's and service's approach to routine assessment of pain and other symptoms, function, nutrition, sleep, and emotional concerns. Symptoms identified at admission and response to treatment were monitored daily. Chaplaincy and psychiatric support were available at all sites. These services were also received by and available to PCCT patients at all sites.
Participants
Patients were considered for enrollment if they were admitted to a participating hospital, were age 18 years or older, and had a primary diagnosis of metastatic solid tumor, CNS malignancy, locally advanced head, neck, or pancreatic cancers, metastatic melanoma, or transplant-ineligible lymphoma or multiple myeloma. Patients were excluded if their attending physician did not give permission to recruit their patients or if they did not speak English, had a diagnosis of dementia, were unresponsive or nonverbal, were admitted for routine chemotherapy, died or were discharged within 48 hours of admission, or had previously received a palliative care consultation. For inclusion in the study, patients had to be enrolled within 48 hours of admission.
Variables
Outcome of interest.
Our primary outcome of interest was total direct cost of hospital care. Direct costs are those directly attributable to the provision of medications, tests, procedures, supplies, or services including the room and board costs associated with nurse staffing (eg, intensive care unit [ICU] v medical-surgical).16 We excluded indirect costs, which represent the overhead costs of operating a hospital (eg, operations, information technology, maintenance), because these are not impacted by treatment.17 The cost measure did not include physician services billed separately from the hospital bill. Direct cost data were extracted from hospital accounting databases and therefore reflect the actual cost (US dollars) to the hospital of providing each medication, test, procedure, or service to each patient. The main outcome measure was incremental treatment effect on total direct cost (ie, the estimated mean effect on cost of moving a patient from comparison to treatment group, holding all other values constant).
Primary independent variable.
The primary independent variable was a binary treatment variable: Did patients receive a consultation from the specialist inpatient palliative care team during hospital admission? Patients who were seen by a PCCT, as defined earlier, were placed into the treatment group; those who did not were placed into the usual care only group.
In the first instance, we defined treatment as a consultation at any time during admission, per previous studies in this field.8 We also explored the following four definitions of treatment that incorporated timing of the first PCCT consult: within 20 days of admission (97.5% of all PCCT patients), within 10 days of admission (95%), within 6 days of admission (90%), or within 2 days of admission (75%). Patients who received PCCT after the cutoff for each definition (97.5th, 95th, 90th, and 75th, respectively) were dropped from the respective analysis.
Our reasons for choosing this approach over the alternatives are as follows. First, stratifying the treatment arm by specific treatment days creates smaller subsamples; covariate balance between these subsamples and the control arm is generally weaker. Second, we are interested less in the point estimate treatment effect for a consultation on a specific day than in the general association between treatment timing and effect on cost; by creating overlapping subsamples, we leverage a greater proportion of the data to examine this association and avoid overspecification of treatment. Third, using dose-response interaction terms with a propensity score–matched sample assumes balance of interaction terms between treatment and control arms, an assumption that may not be valid.
Other predictors.
Treatment and comparison groups were balanced on 33 baseline covariates covering demographic, socioeconomic, health system, and clinical factors. Specifically, we used binary variables for age (three strata), sex, race (three strata), insurance status (three strata), education level (three strata), advance directive status, lymphoma diagnosis, specific activities of daily living (Activities of Daily Living-618), visiting home services before admission, and levels of pain (three strata) and fatigue (two strata). We used continuous variables for comorbidities (Elixhauser index19), mean physical and psychological Edmonton System Assessment Scale scores,20 and the Condensed Memorial Symptom Assessment Scale21). See Table 1 for details. All regressions were performed against these 33 variables (random effects) plus fixed effects for each hospital.
Table 1.
Weighted Matched Sample (N = 969) by All 33 Covariates Included in Propensity Score
| Variable | % of Patients |
Absolute Standardized Difference (%) | |
|---|---|---|---|
| UC (n = 713) | PC (n = 256) | ||
| Age, years | |||
| 55 to 75 | 51.6 | 53.5 | 3.8 |
| > 75 | 13.0 | 10.9 | 6.4 |
| Sex: female | 55.0 | 53.5 | 3.0 |
| Race | |||
| White | 61.2 | 61.7 | 1.1 |
| Black | 33.5 | 33.2 | 0.6 |
| Living will: yes | 40.2 | 40.2 | 0.1 |
| Proxy: yes | 45.9 | 44.9 | 1.9 |
| Insurance | |||
| Medicare only | 19.5 | 19.5 | 0.0 |
| Medicaid (and Medicare) | 25.8 | 25.4 | 0.9 |
| Education | |||
| High schoola | 55.0 | 55.5 | 1.0 |
| Collegea | 37.0 | 36.3 | 1.3 |
| Visiting nurse services: yesb | 13.3 | 12.9 | 1.4 |
| Home health aide, total hoursb,c | 0.25 | 0.25 | 0.0 |
| Primary diagnosis: lymphoma/myeloma | 6.1 | 5.9 | 0.6 |
| Complication(s): yesd | 2.7 | 2.3 | 1.8 |
| Comorbidities: Elixhauser index (mean) | 3.94 | 3.96 | 1.1 |
| Activities of daily living | |||
| Needs partial assistance bathing | 37.9 | 37.5 | 1.0 |
| Needs partial assistance transferring from chair | 35.3 | 36.3 | 2.3 |
| Needs complete assistance with ≥ one activity | 14.6 | 14.8 | 0.9 |
| ESAS score | |||
| ESAS Physical: at admission (mean) | 1.98 | 1.97 | 1.1 |
| ESAS Psychological: at admission (mean) | 1.57 | 1.61 | 2.8 |
| ESAS Physical: at consult/reference day (mean)e | 1.82 | 1.82 | 0.7 |
| ESAS Psychological: at consult/reference day (mean)e | 1.43 | 1.37 | 4.5 |
| CMSAS score | |||
| Numberf: at admission (mean) | 9.08 | 9.02 | 2.0 |
| Numberf: at consult/reference day (mean)e | 7.87 | 7.83 | 1.3 |
| Severityf: at admission (mean) | 16.1 | 16.0 | 1.3 |
| Severityf: at consult/reference day (mean)e | 12.6 | 12.7 | 1.5 |
| Morphine: equivalent dose, mgc,g | 3.1 | 3.3 | 4.8 |
| Pain | |||
| Somewhath | 10.5 | 11.3 | 2.5 |
| Quite a bith | 28.5 | 27.7 | 1.9 |
| Very muchh | 32.7 | 34.0 | 3.1 |
| Fatigue | |||
| A little, somewhat, or quite a bith | 37.2 | 36.7 | 1.1 |
| Very muchh | 29.7 | 30.5 | 1.7 |
NOTE. Reference case for binary variables: no. Reference cases for: age = < 55 years; race = neither white nor black; insurance = neither Medicare nor Medicaid; education = elementary school. Reference cases for pain and fatigue: none.
Abbreviations: CMSAS, Condensed Memorial Symptom Assessment Scale; ESAS, Edmonton System Assessment Scale; PC, palliative care; UC, usual care.
Highest level attained.
In 2 weeks before hospitalization.
Raw data square root transformed.
Presence of a major/minor complication before consultation/reference day during the hospitalization.
For PC patients, the reference day was the day of consult; for UC patients, the reference day was the day they had symptom severity most similar to that of PC patients.
Number indicates number of physical symptoms on the CMSAS. Severity indicates number of physical symptoms multiplied by the mean severity of physical symptoms on the CMSAS.
In week before hospitalization.
Taken at consult/reference day with none as the reference category.
Data Sources
Clinical data come from medical record review completed by trained project staff and patient baseline interviews and daily symptom inventories. Cost data were extracted from hospital databases and adjusted for regional variation in health care costs using the Medicare Wage Index.22 All costs were standardized to US dollars in 2011, the final year of data collection, using the Consumer Price Index.23
Bias
Patient clinical characteristics are likely correlated with receipt of palliative care and with hospital costs. To minimize confounding as a result of selection bias, propensity score kernel weights were calculated to balance observed confounders between treated and comparison patients.24 Balance across groups was evaluated with standardized differences both before and after weighting the sample. Propensity scores were stratified by subsample; where treatment was redefined, separate weights were calculated for each subsample25 (Data Supplement).
Statistical Methods
We estimated generalized linear models (GLMs) with a γ distribution and a log link and calculated the average incremental effect of PCCT on total direct hospital costs. Incremental effects were calculated as the average of finite differences across the sample and using bootstrapped SEs (1,000 replications).26 All analyses were performed using Stata (version 12; StataCorp, College Station, TX).27
Secondary Analyses
We repeated the primary analysis with different outcomes of interest, including LOS and total direct costs for specific utilization categories (room and board, ICU, pharmacy, laboratory, and imaging). Taken together, these analyses allow us to understand the mechanism of any treatment effect on cost.
Confirmatory Analyses
We performed sensitivity analyses on our primary results, rerunning analyses with late-consult outliers moved to the control group (rather than being excluded), long-stay outliers removed from both groups, patients who died during hospitalization included alongside those discharged alive, and an unweighted GLM (γ, log) and a weighted ordinary least squares specification to check sensitivity to model specification.
RESULTS
Participants
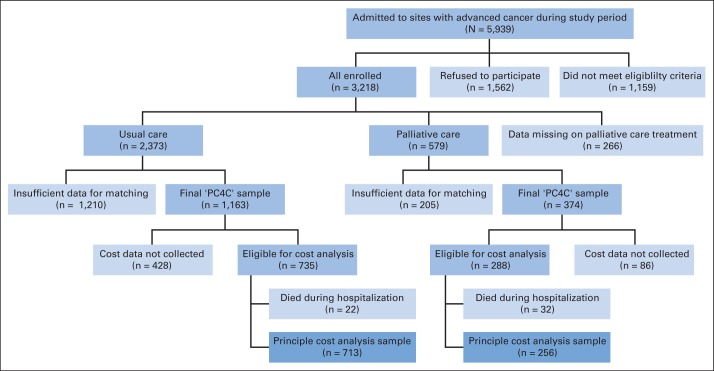
A total of 5,939 patients were admitted to study sites with an advanced cancer diagnosis during the study period. Of these, 1,562 (26%) refused to participate and 1,159 (20%) did not meet the eligibility criteria, meaning that 3,218 patients were enrolled onto the study. There were 266 patients with missing treatment data and a further 1,415 patients for whom insufficient data were collected at baseline (to match for the propensity score) and/or on relevant outcomes (to include in analysis); high levels of attrition and missing data are a familiar challenge in studying populations with serious illness.10,28
Therefore, after data cleaning and matching, a subsample of 1,537 patients was identified for whom there were adequate data for matching and analysis (palliative care, n = 374; usual care, n = 1,163). Palliative care patients were more likely than usual care patients to be in the final analytic sample (P < .001). One site (site 4) collected clinical, satisfaction, and process data for all other parts of the Palliative Care for Cancer project but did not collect cost data. Therefore, site 4 patients (n = 513) and one additional patient for whom cost data were missing were excluded from economic evaluation papers. Patients from four sites with cost data who died during hospitalization (n = 54; 51 patients died during hospitalization, survival data are missing for three patients) were excluded because they do not constitute a sufficient sample to support a propensity score and pooling patients irrespective of discharge status may lead to a heterogeneity problem.29–32 Thus, the final subsample for economic evaluation was 969 patients (palliative care, n = 256; usual care, n = 713); this group had no missing data on any propensity score variable or cost data, and no patients were lost to matching (Fig 1).
Fig 1.
Participants in the Palliative Care for Cancer (PC4C) study.
Descriptive Data
The variables in the treatment and comparison arms, weighted according to 33 covariates in the propensity score, are listed in Table 1. There is a demonstrable balance, with 32 standardized differences no greater than 5% and all within the 10% rule of thumb for acceptable difference.33 Corresponding tables for subsamples where later consults are excluded show similar balance (Data Supplement).
Utilization Data
Direct cost of hospital stay, LOS, and time to consult after admission are listed in Table 2. Palliative care patients had slightly higher costs and LOS on average, accounted for by long right-hand tails. Time to consult is clustered within 2 days of admission; 77% of PC patients saw a PCCT by the end of their second day in hospital; the protocol specified consultation within 48 hours when possible.
Table 2.
Unweighted Utilization Data: Total Cost, Length of Stay, and Time to Consult
| Measure | Total Direct Cost ($) |
Length of Stay (days) |
Time to Consult (days) |
||
|---|---|---|---|---|---|
| UC (n = 713) | PC (n = 256) | UC (n = 713) | PC (n = 256) | PC (n = 256) | |
| Mean | 9,550 | 11,150 | 8.0 | 9.0 | 2.3 |
| Median | 7,379 | 7,400 | 6 | 7 | 1 |
| 25th-75th percentile | 4,943-11,791 | 4,805-12,282 | 5-9 | 5-10 | 0-2 |
| Standard deviation | 7,558 | 13,130 | 5.0 | 7.5 | 4.8 |
Abbreviations: PC, palliative care; UC, usual care.
Main Results
Total direct cost treatment effect estimates taking into account time to consult are listed in Table 3. The results demonstrate a clear pattern—earlier treatment is associated with larger cost-saving effect. As late-consult patients are removed from the sample, the estimated treatment effect grows. PCCT interventions within at least 6 days of admission had an estimated mean treatment effect of −$1,312 (95% CI, −$2,568 to −$56; P = .04) compared with no intervention, and intervention within 2 days had an estimated mean treatment effect of −$2,280 (95% CI, −$3,741 to −$819; P = .002). The magnitude of cost saving implied is 14% of total direct hospital costs for a consult within 6 days and 24% for a consult within 2 days.
Table 3.
Estimated Treatment Effect on Total Cost, by Time to Consult
| Treatment: Time of Consultation After Hospital Admission (percentile) | No. of Patients |
Estimated Treatment Effect ($) (95% CI) | P | Implied Saving (%)* | ||
|---|---|---|---|---|---|---|
| UC | PC | All | ||||
| Any time (100th) | 713 | 256 | 969 | 153 (−1,266 to 1,572) | .83 | −2 |
| Within 20 days (97.5th) | 713 | 249 | 962 | −706 (−2,007 to 596) | .29 | 7 |
| Within 10 days (95th) | 713 | 244 | 957 | −927 (−2,283 to 429) | .18 | 10 |
| Within 6 days (90th) | 713 | 231 | 944 | −1,312 (−2,568 to −56) | .04 | 14 |
| Within 2 days (75th) | 713 | 197 | 910 | −2,280 (−3,438 to −1,122) | < .01 | 24 |
Abbreviations: PC, palliative care; UC, usual care.
Implied saving in total cost of hospital stay from receiving treatment compared with receiving UC only.
Secondary Analyses
We reran our primary analysis with different outcomes of interest to examine the mechanism for the treatment effect on cost observable in Table 3. The results with LOS as an outcome of interest are listed in Table 4. The pattern is consistent with Table 3 and demonstrates a small, statistically insignificant positive treatment effect when late consult outliers are included and negative coefficients of increasing magnitude once they are excluded, although in this case, the association between treatment and outcome reduction is statistically significant only when the first consult occurred within 2 days.
Table 4.
Estimated Treatment Effect on Length of Stay, by Time to Consult
| Treatment: Time of Consultation After Hospital Admission (percentile) | No. of Patients |
Length of Stay |
||||
|---|---|---|---|---|---|---|
| UC | PC | All | Estimated Treatment Effect (days) (95% CI) | P | Implied Reduction (%) | |
| Any time (100th) | 713 | 256 | 969 | 0.6 (−0.3 to 1.5) | .18 | −8 |
| Within 20 days (97.5th) | 713 | 249 | 962 | −0.1 (−0.9 to 0.7) | .81 | 1 |
| Within 10 days (95th) | 713 | 244 | 957 | −0.3 (−1.0 to 0.4) | .41 | 4 |
| Within 6 days (90th) | 713 | 231 | 944 | −0.5 (−1.3 to 0.2) | .14 | 6 |
| Within 2 days (75th) | 713 | 197 | 910 | −1.0 (−1.7 to −0.2) | < .01 | 13 |
Abbreviations: PC, palliative care; UC, usual care.
The results by utilization category are listed in in Table 5. Laboratory costs are significantly reduced irrespective of treatment timing, and the magnitude of this effect is greater for earlier palliative care treatment. For other categories, statistically significant results are observable for pharmacy and ICU when the first consult was performed within 2 days of admission. Moreover, for all categories, coefficients and P values decrease with time to consult (ie, earlier consult seems to reduce systematically all major cost categories compared with later consult).
Table 5.
Estimated Treatment Effect on Total Cost for Specific Utilization Categories, by Time to Consult
| Treatment: Time of Consultation After Hospital Admission (percentile) | Room and Board |
ICU* |
Pharmacy |
Laboratory |
Imaging |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC Effect ($) (95% CI) | P | PC Effect ($) (95% CI) | P | PC Effect ($) (95% CI) | P | PC Effect ($) (95% CI) | P | PC Effect ($) (95% CI) | P | |
| Any time (100th) | 536 (141 to 932) | < .01 | −255 (−1,495 to 984) | .69 | 180 (−259 to 619) | .42 | −149 (−228 to −71) | < .01 | −3 (−146 to 140) | .96 |
| Within 20 days (97.5th) | 351 (−23 to 726) | .07 | −696 (−1,757 to 364) | .20 | 56 (−400 to 513) | .81 | −185 (−260 to −110) | < .01 | −33 (−174 to 109) | .65 |
| Within 10 days (95th) | 123 (−226 to 471) | .49 | −624 (−1,784 to 535) | .29 | −162 (−542 to 218) | .40 | −203 (−272 to −135) | < .01 | −53 (−193 to 87) | .46 |
| Within 6 days (90th) | 42 (−286 to 370) | .80 | −842 (−1,878 to 193) | .11 | −253 (−1,313 to 806) | .64 | −228 (−303 to −154) | < .01 | −73 (−206 to 61) | .28 |
| Within 2 days (75th) | −144 (−1,113 to 825) | .77 | −1,162 (−2,133 to −191) | .02 | −332 (−651 to −12) | .04 | −284 (−525 to −45) | .02 | −136 (−283 to 12) | .07 |
Abbreviations: ICU, intensive care unit; PC, palliative care;
For all categories except ICU, the primary analysis was repeated with each cost category as the outcome of interest. This was not possible with ICU cost data because only 11% (n = 111) of the sample had nonzero ICU costs. Therefore, the ICU results were generated instead with an unweighted, unmatched regression for patients with nonzero ICU costs. Sensitivity analyses of the primary analysis show that unweighted regressions typically estimate a smaller cost-saving effect than weighted regressions because the weights compensate for a higher illness burden among PC patients (Data Supplement). A comparison of PC and usual care patients with nonzero ICU costs exhibits a similar difference. All else being equal, therefore, the ICU treatment effect estimates are likely underestimates of the cost-saving effect, but it is not possible to verify with a weighted analysis.
Confirmatory Analyses
We performed a series of additional analyses to confirm the robustness of the primary analysis. In all cases, the results did not change substantively. For details, see Data Supplement.
DISCUSSION
Our primary analysis demonstrates that earlier palliative care during hospital admission is associated with significantly larger cost savings. Care within 6 days is estimated to reduce direct costs by 14% compared with no intervention, and care within 2 days is estimated to reduce costs by 24% compared with no intervention.
Secondary analysis shows that the cost savings are attributable to a combination of reduced LOS and reduced intensity of hospital stay. PCCT interventions reduce laboratory costs, irrespective of timing and LOS, and ICU and pharmacy costs if consult occurred within 2 days of admission. The magnitude of effect on ICU costs is notably large, suggesting that in hospitals with limited palliative care services, savings could be maximized by prioritizing critically ill patients. The effect on room and board is the least substantial, reflecting that a proportion of these costs is incurred at the start of the hospitalization and thus not amenable to treatment.
There are a number of limitations to our study. Although measures have been taken to minimize observed confounding as a result of selection bias, there is likely unobserved heterogeneity between treated and comparison patients, including reasons contributing to time to consult after admission. It was not possible to identify an instrumental variable for this sample.34 As a sensitivity analysis, we ran GLM and ordinary least squares regressions on the unweighted sample, and results were substantively similar (Data Supplement).
The data were collected at hospitals with well-established palliative care programs in the United States. It is not clear how generalizable the results are to new programs, to patients with diagnoses other than cancer, or to programs in other health systems. In addition, we experienced significant attrition from recruitment to final sample, excluding approximately 50% of enrolled patients for incomplete data. The most common reason for incomplete data among those who consented to participate was that the patient became too ill to participate. These data can only be generalized to hospitalized patients with cancer whose illness severity made them a candidate for palliative care but who were still able to participate in follow-up interviews.
These results are consistent with a growing body of research on quality, survival, and cost suggesting that early palliative care should be more widely implemented.2–6 Our analysis is able to control for a large number of observed covariates that may be confounders for palliative care consultation, including presence of advance directives, as well as multiple physical and psychological function measures.
Our methods diverge from those in previous cost analyses of PCCTs. Prior studies have variously controlled for LOS by trimming the sample of short- and long-stay patients,30–32,35 including LOS as a covariate,35 or matching for LOS in propensity scoring.35,36 These strategies are intended to control for unobserved heterogeneity in observational studies but may have a significant unintended effect on results.37,38 Propensity score methods are designed to estimate a counterfactual given baseline characteristics only,14 whereas LOS is likely connected to both treatment and outcome, and adjusting the sample by a variable that may lie on the causal pathway between treatment and outcome can obscure treatment effect and bias estimates.39
Indeed, we found that treatment effect estimates with our data were sensitive to use of LOS strategies and chose not to control for LOS in primary analysis. The difference between cost-saving treatment effect estimates in prior studies8 and our finding that there is no association between costs and palliative care at any time is a result of the absence of LOS controls. If we had controlled for LOS, then a statistically significant cost-saving effect for palliative care at any time is observable with our data. The fact that the any-time-treatment effect estimate is so sensitive (trimming the sample by late-consult or high-LOS outliers markedly affects results) shows that it is a poor, unreliable estimation of the relationship between treatment and cost.
In summary, our primary analysis results show that timely palliative care consultation reduces hospital costs and the earlier the consult, the greater are the savings. These findings are robust (minimizing endogeneity concerns and robust to multiple sensitivity analyses) and extend the PCCT literature on costs (revealing the clear and important association between timing and treatment effect).
These results indicate a large potential cost saving from increased early palliative care consultation on hospital admission for patients with advanced cancer. In our study, less than a quarter of all patients with an advanced cancer diagnosis admitted to hospitals with well-established palliative care teams saw a PCCT within 2 days of administration.
Timely palliative care after hospital admission is associated with cost savings, and earlier treatment delivers a larger cost-saving effect. These results are consistent with a growing body of research on quality and survival, suggesting that early palliative care should be more widely implemented. As the evidence base on palliative care programs continues to grow, researchers should consider incorporating timing into definitions of treatment and measurement of outcomes.
Acknowledgment
Presented, in part, at the Annual Assembly of the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association, Philadelphia, PA, February 25-28, 2015. We thank Robert Arnold, Phil Santa Emma, Mary Beth Happ, Tim Smith, and David Weissman for their contributions to the Palliative Care for Cancer project.
See accompanying editorial on page 2723
Support information appears at the end of this article.
The study sponsors had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. All authors are independent of the study sponsors. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Support
Supported by the National Cancer Institute (NCI) and the National Institute of Nursing Research in the United States. P.M. is sponsored by a health economics fellowship from the Health Research Board (Ireland) and NCI. M.M.G. is supported by a Veterans Affairs Health Services Research and Development career development award (CDA 11-201/CDP 12-255). T.J.S. is supported by NCI Grant No. P30 CA 006973 to Sidney Kimmel Comprehensive Cancer Center. R.S.M. was the recipient of a Midcareer Investigator Award in Patient-Oriented Research (5K24AG022345) during the course of this work. This work was supported by the National Institute on Aging, Claude D. Pepper Older Americans Independence Center at the Icahn School of Medicine at Mount Sinai (Grant No. 5P30AG028741), and the National Palliative Care Research Center. A.S.K. time was funded by the National Institute on Aging (Grant No. 1K23AG040774-01A1).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Diane E. Meier, R. Sean Morrison
Financial support: Diane E. Meier, R. Sean Morrison
Administrative support: Thomas J. Smith, R. Sean Morrison
Provision of study materials or patients: Diane E. Meier, Thomas J. Smith, R. Sean Morrison
Collection and assembly of data: Melissa M. Garrido, J. Brian Cassel, Diane E. Meier, Thomas J. Smith, R. Sean Morrison
Data analysis and interpretation: Peter May, Melissa M. Garrido, J. Brian Cassel, Amy S. Kelley, Diane E. Meier, Charles Normand, Lee Stefanis, R. Sean Morrison
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Peter May
No relationship to disclose
Melissa M. Garrido
Employment: Spectra Laboratories (I)
J. Brian Cassel
No relationship to disclose
Amy S. Kelley
No relationship to disclose
Diane E. Meier
No relationship to disclose
Charles Normand
Speakers' Bureau: Novartis, Pfizer
Thomas J. Smith
Stock or Other Ownership: United Healthcare
Lee Stefanis
Stock or Other Ownership: Sangamo BioSciences
R. Sean Morrison
No relationship to disclose
REFERENCES
- 1.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 2.Parikh RB, Kirch RA, Smith TJ, et al. Early specialty palliative care: Translating data in oncology into practice. N Engl J Med. 2013;369:2347–2351. doi: 10.1056/NEJMsb1305469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 5.Adelson K, Paris J, Smith CB, et al. Standardized criteria for required palliative care consultation on the solid tumor oncology service. J Clin Oncol. 2013;(suppl 31):31. doi: 10.1200/JOP.2016.016808. abstr 37. [DOI] [PubMed] [Google Scholar]
- 6.Bakitas M, Tosteson T, Li Z, et al. The ENABLE III randomized controlled trial of concurrent palliative oncology care. J Clin Oncol. 2014;(suppl 5s):32. doi: 10.1200/JCO.2014.58.6362. abstr 9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith S, Brick A, O'Hara S, et al. Evidence on the cost and cost-effectiveness of palliative care: A literature review. Palliat Med. 2014;28:130–150. doi: 10.1177/0269216313493466. [DOI] [PubMed] [Google Scholar]
- 8.May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: Review of current evidence and directions for future research. J Palliat Med. 2014;17:1054–1063. doi: 10.1089/jpm.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health. Bethesda, MD: US Department of Health & Human Services; 2006. Project information: Palliative care for hospitalized cancer patients. http://projectreporter.nih.gov/project_info_description.cfm?projectnumber=5R01CA116227-04. [Google Scholar]
- 10.Ewing G, Rogers M, Barclay S, et al. Recruiting patients into a primary care based study of palliative care: Why is it so difficult? Palliat Med. 2004;18:452–459. doi: 10.1191/0269216304pm905oa. [DOI] [PubMed] [Google Scholar]
- 11.Penrod J, Morrison RS, Meier DE. Studying the effectiveness of palliative care. JAMA. 2008;300:1022–1023. doi: 10.1001/jama.300.9.1022-b. [DOI] [PubMed] [Google Scholar]
- 12.Marcus S, Gibbons R. Estimating the efficacy of receiving treatment in randomized clinical trials with noncompliance. Health Serv Outcomes Res Methodol. 2001;2:247–258. [Google Scholar]
- 13.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. The design versus the analysis of observational studies for causal effects: Parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 15.Aldridge Carlson MD. Research methods priorities in geriatric palliative medicine. J Palliat Med. 2013;16:838–842. doi: 10.1089/jpm.2013.9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taheri PA, Butz D, Griffes LC, et al. Physician impact on the total cost of care. Ann Surg. 2000;231:432–435. doi: 10.1097/00000658-200003000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 18.Katz S, Ford A, Moskowitz R, et al. The index of ADL: A standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 21.Chang VT, Hwang SS, Kasimis B, et al. Shorter symptom assessment instruments: The Condensed Memorial Symptom Assessment Scale (CMSAS) Cancer Invest. 2004;22:526–536. doi: 10.1081/cnv-200026487. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services. FY Wage Index (Table 2). US Department of Health and Human Services; 2011. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files-Items/CMS1234173.html.
- 23.Bureau of Labor Statistics. CPI Database (All Urban Consumers) US Department of Labor. 2014. http://www.bls.gov/cpi/data.htm.
- 24.Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–1720. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green KM, Stuart EA. Examining moderation analyses in propensity score methods: Application to depression and substance use. J Consult Clin Psychol. 2014;82:773–783. doi: 10.1037/a0036515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abadie A, Imbens GW. Notes and comments on the failure of the bootstrap for matching estimators. Econometrica. 2008;76:1537–1557. [Google Scholar]
- 27.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 28.Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med. 2006;20:745–754. doi: 10.1177/0269216306073112. [DOI] [PubMed] [Google Scholar]
- 29.Cassel JB, Kerr K, Pantilat S, et al. Palliative care consultation and hospital length of stay. J Palliat Med. 2010;13:761–767. doi: 10.1089/jpm.2009.0379. [DOI] [PubMed] [Google Scholar]
- 30.Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168:1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 31.Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood) 2011;30:454–463. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy IM, Robinson C, Huq S, et al. Cost savings from palliative care teams and guidance for a financially viable palliative care program. Health Serv Res. 2015;50:217–236. doi: 10.1111/1475-6773.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penrod JD, Goldstein NE, Deb P. When and how to use instrumental variables in palliative care research. J Palliat Med. 2009;12:471–474. doi: 10.1089/jpm.2009.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitford K, Shah ND, Moriarty J, et al. Impact of a palliative care consult service. Am J Hosp Palliat Care. 2014;31:175–182. doi: 10.1177/1049909113482746. [DOI] [PubMed] [Google Scholar]
- 36.Starks H, Wang S, Farber S, et al. Cost savings vary by length of stay for inpatients receiving palliative care consultation services. J Palliat Med. 2013;16:1215–1220. doi: 10.1089/jpm.2013.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marazzi A, Paccaud F, Ruffieux C, et al. Fitting the distributions of length of stay by parametric models. Med Care. 1998;36:915–927. doi: 10.1097/00005650-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Garrido MM. Propensity scores and palliative care. J Palliat Med. 2014;17:261. doi: 10.1089/jpm.2013.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrido MM. Propensity scores: A practical method for assessing treatment effects in pain and symptom management research. J Pain Symptom Manage. 2014;48:711–718. doi: 10.1016/j.jpainsymman.2014.05.014. [DOI] [PubMed] [Google Scholar]