Abstract
Purpose
To evaluate the relative effectiveness of letrozole compared with tamoxifen for patients with invasive ductal or lobular carcinoma.
Patients and Methods
Patients diagnosed with early-stage invasive ductal carcinoma (IDC) or classic invasive lobular carcinoma (ILC) who were randomly assigned onto the Breast International Group (BIG) 1-98 trial and who had centrally reviewed pathology data were included (N = 2,923). HER2-negative IDC and ILC were additionally classified as hormone receptor–positive with high (luminal B [LB] –like) or low (luminal A [LA] –like) proliferative activity by Ki-67 labeling index. Survival analyses were performed with weighted Cox models that used inverse probability of censoring weighted modeling.
Results
The median follow-up time was 8.1 years. In multivariable models for disease-free survival (DFS), significant interactions between treatment and histology (ILC or IDC; P = .006) and treatment and subgroup (LB like or LA like; P = .01) were observed. In the ILC subset, there was a 66% reduction in the hazard of a DFS event with letrozole for LB (hazard ratio [HR], 0.34; 95% CI, 0.21 to 0.55) and a 50% reduction for LA subtypes (HR, 0.50; 95% CI, 0.32 to 0.78). In the IDC subset, there was a significant 35% reduction in the hazard of a DFS event with letrozole for the LB subtype (HR, 0.65; 95% CI, 0.53 to 0.79), but no difference between treatments was noted for IDC and the LA subtype (HR, 0.95; 95% CI, 0.76 to 1.20).
Conclusion
The magnitude of benefit of adjuvant letrozole is greater for patients diagnosed with lobular carcinoma versus ductal carcinoma.
INTRODUCTION
Invasive lobular carcinoma (ILC) is the second-most common breast cancer subtype and accounts for approximately 10% of all breast cancers.1 ILC differs from invasive ductal carcinoma (IDC), the most common breast cancer subtype, with respect to epidemiology, clinicopathologic characteristics, and responsiveness to systemic therapies.1,2 Morphologically, ILC is commonly characterized as noncohesive infiltrating cells classified as hormone receptor–positive, with a low to intermediate histologic grade. In addition, morphologic variants of ILC (eg, pleomorphic, alveolar ILC) have been described to define ILC tumors that lack the characteristic diffuse, nonlinear growth pattern of classic ILC.3,4
Retrospective studies have consistently demonstrated that primary ILC is less responsive than IDC to chemotherapy.5–13 Limited information is available to compare the efficacy of hormonal therapy in ILC and IDC,14,15 and it is unclear whether the benefit of different therapies (ie, tamoxifen or aromatase inhibitors [AIs]) differs by histologic subtype (ie, ILC v IDC).
The Breast International Group (BIG) 1-98 study is a four-arm study comparing 5 years of monotherapy with tamoxifen, 5 years with letrozole, or the two treatments administered sequentially in postmenopausal women who have hormone receptor–positive early-stage breast cancer.16,17 In the present analysis, we investigated the magnitude of benefit of endocrine treatment (tamoxifen or letrozole) in patients diagnosed with classic ILC or IDC who were enrolled in the monotherapy arms of the study. It is important to note that the classification of breast cancer has evolved from a pure morphologic classification (ie, ILC, IDC) to include hormone receptors and human epidermal growth factor receptor 2 (HER2), and the classification now encompasses a group of heterogeneous, genomically defined breast cancer subtypes.18,19 BIG 1-98 enrolled participants diagnosed with hormone receptor–positive breast cancer and, as such, accounts for a population that could be genomically defined as having luminal breast cancer. Luminal breast cancers encompass at least two subgroups with distinct survival outcomes: luminal A (LA) is represented as estrogen receptor (ER) –positive tumors that have low proliferative activity; luminal B (LB) also is represented by ER-positive tumors, but have high proliferative activity and worse outcomes than LA types. In the present analysis, the differential effectiveness of tamoxifen versus letrozole in ILC versus IDC is performed while taking into the consideration the distribution of high- and low-proliferative ER subtypes in both subgroups.
PATIENTS AND METHODS
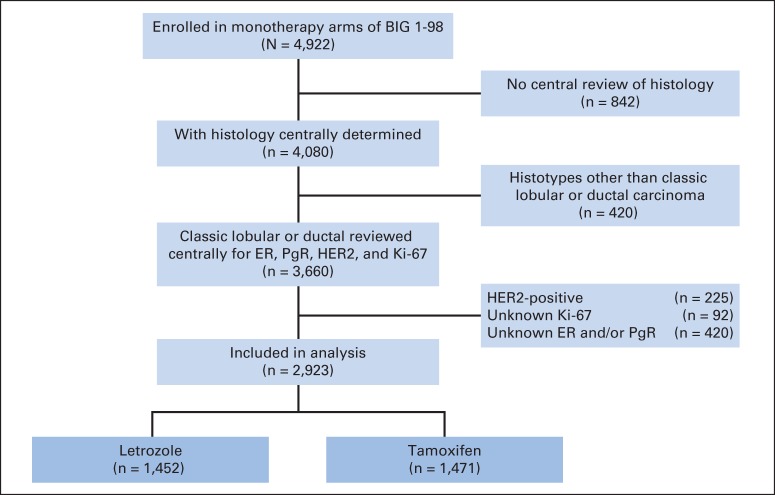
This analysis is limited to patients assigned to monotherapy with either tamoxifen or letrozole at a median of 8.1 years of follow-up time. There were 4,922 patients enrolled in the monotherapy arms of BIG 1-98. This analysis includes patients who had centrally reviewed histology data (n = 4,080) and whose tumors were classified as IDC or classic ILC (n = 2,923; Fig 1). HER2-positive tumors were not included in the present analysis. The BIG 1-98 patient population was postmenopausal women with early invasive breast cancer whose tumors were assessed by local pathologists as hormone receptor positive (ie, ER and/or progesterone receptor [PgR] positive). Between March 1998 and March 2000, patients were randomly assigned to receive adjuvant endocrine therapy in one of the monotherapy arms comprising either letrozole 2.5 mg/d or tamoxifen 20 mg/d for 5 years; from April 1999 to May 2003, patients were randomly assigned to all four arms, including the sequence of 2 years of tamoxifen followed by 3 years of letrozole, or 2 years of letrozole followed by 3 years of tamoxifen.
Fig 1.
CONSORT diagram showing the analytic cohort of 2,923 patients with tumors classified as ductal (n = 2,599) and lobular (n = 324) enrolled in the monotherapy arm of the BIG 1-98 clinical trial that compared 5 years of letrozole with 5 years of tamoxifen. ER, estrogen receptor; HER2, human epidermal growth factor receptor; PgR, progesterone receptor.
All participants provided written informed consent. Ethics committees and relevant health authorities approved the protocol.
Pathology Assessment
Central pathology evaluation of histologic subtype was performed at the University of Glasgow, United Kingdom. Data reported were the dominant and secondary histologic subtype, histologic grade, and peritumoral vascular invasion. This analysis is restricted to dominant IDC and dominant ILC (classic type) only. Data from patients who had dominant ductal and secondary ILC and vice versa (ie, mixed histology) were not included in the analysis.
Central pathology evaluation, including ER status, PgR status, HER2 status, and the Ki-67 labeling index (LI), was performed in the International Breast Cancer Study Group Central Pathology Laboratory, European Institute of Oncology, Milan, Italy. Expressions of ER, PgR, HER2, and the Ki-67 LI in the primary tumors were determined by immunohistochemistry (IHC).20–22 ER-negative and PgR-negative negative statuses were each defined as fewer than 1% immunoreactive cells, in accordance with recent guidelines.23 Whole tumor sections were incubated with the specific primary mouse monoclonal antibodies to ER (clone 1D5; 1:100 dilution) or PgR (clone 1A6; 1:800 dilution; DAKO, Glostrup, Denmark). HER2 status was assessed by IHC and fluorescence in situ hybridization.24 Tumors were considered HER2 positive if they were amplified by fluorescence in situ hybridization or if IHC was 3+ positive.24 The Ki-67 LI was assessed using the mouse monoclonal antibody MIB-1 (1:200 dilution; DAKO); the percentage of cells that showed definite nuclear immunoreactivity with MIB-1 among 2,000 invasive neoplastic cells in randomly selected high-power fields (×400) at the periphery of the tumor was recorded.
Subtype Definitions
Tumors were classified as LA-like or LB-like subtypes according to IHC surrogates. LA-like subtypes were ER and/or PgR positive and HER2 negative and had a Ki-67 LI less than 14%; LB-like subtypes were ER and/or PgR were positive and HER2 negative but had a Ki-67 LI ≥ 14%. The Ki-67 LI cutoff point of 14% was selected as the one most likely to represent LA and LB breast cancer molecular subtypes.18,25–27
End Points and Statistical Methods
The protocol-specified primary end point was disease-free survival (DFS), which was defined as the time from random assignment to the earliest time of an invasive locoregional recurrence, distant metastasis, invasive contralateral breast cancer, second primary malignancy, or death as a result of any cause. Overall survival (OS) was defined as the time from random assignment to death as a result of any cause. Estimates of 5-year and 8-year time-to-event percentages (for DFS and OS) were based on the Kaplan-Meier method and were compared across treatment groups with stratified log-rank tests. Analyses of outcomes employed both the intention-to-treat and inverse probability of censoring weighting (IPCW) approaches.
IPCW was used to account for selective cross over to letrozole of patients in the tamoxifen arm after the positive initial findings of BIG 1-98 were reported in 2005.28 The IPCW method17,29,30 weights the follow-up information provided by patients who remain on tamoxifen so that, in the analysis, their follow-up assessment accounts not only for themselves but also for patients with similar characteristics whose follow-up assessments were artificially censored at the time of selective cross over to letrozole. The weights used are those estimated on the basis of the full data set analysis, with a median of 8.1 years of follow-up time.
Cox models are stratified by prior chemotherapy use and the random assignment option (two-arm or four-arm assignment). Independent variables in the Cox models for interaction were ILC/IDC, treatment assignment, and their interaction. Multivariable models added patient age, tumor size, nodal status, tumor grade, local therapy received (mastectomy or less than mastectomy ± radiotherapy), and LA- or LB-like type to the covariates in the basic interaction models listed above. Statistical significance was defined as P ≤ .05; there were no adjustments for multiple comparisons.
RESULTS
A total of 2,923 patients who had early-stage breast cancer were analyzed in this study at a median follow-up time of 8.1 years, including 2,599 patients with IDC and 324 patients with ILC. Clinical and pathologic characteristics according to histologic subtypes are shown in Table 1. The percentages of LA and LB subtypes according to histology were 73.1% and 26.9%, respectively, for ILC, and 55.3% and 44.7%, respectively, for IDC. ILC tumors had larger tumor sizes than IDC tumors (ILC v IDC size ≥ 2 cm, 50% v 34.8%); nodal involvement was comparable between IDC and ILC. Rates of obesity, which may affect the risk of recurrence,31 were well balanced between women who had IDC and ILC. Treatment compliance, defined as completion of 5 years of treatment, was similar in the ILC and IDC subsets (67% and 71% of patients, respectively). Chemotherapy was administered before study initiation to 26.9% and 20.8% of patients with ILC and IDC, respectively. Additional information about clinical and pathologic characteristics according to histologic subtype and treatment is shown in Appendix Table A1 (online only).
Table 1.
Demographic, Disease, and Treatment Characteristics According to Histologic Subtype
| Characteristic | Histologic Subtype (N = 2,923) |
Fisher's Exact P | |||
|---|---|---|---|---|---|
| Ductal (n = 2,599) |
Lobular (n = 324) |
||||
| No. | % | No. | % | ||
| Chemotherapy strata | .01 | ||||
| No chemotherapy | 2,059 | 79.2 | 237 | 73.1 | |
| Received chemotherapy | 540 | 20.8 | 87 | 26.9 | |
| Random assignment strata | .001 | ||||
| 4-arm | 1,880 | 72.3 | 262 | 80.9 | |
| 2-arm | 719 | 27.7 | 62 | 19.1 | |
| Subtype | < .001 | ||||
| Lum A | 1,436 | 55.3 | 237 | 73.1 | |
| Lum B | 1,163 | 44.7 | 87 | 26.9 | |
| Age, years | .19 | ||||
| ≤ 55 | 584 | 22.5 | 86 | 26.5 | |
| 56-70 | 1,638 | 63.0 | 199 | 61.4 | |
| ≥ 71 | 377 | 14.5 | 39 | 12.0 | |
| Obese (BMI ≥ 30 kg/m2) | .93 | ||||
| No | 1,929 | 74.2 | 243 | 75.0 | |
| Yes | 561 | 21.6 | 67 | 20.7 | |
| Unknown | 109 | 4.2 | 14 | 4.3 | |
| Nodal status | .29 | ||||
| Nx/N0 | 1,473 | 56.7 | 178 | 54.9 | |
| N1-N3 | 709 | 27.3 | 83 | 25.6 | |
| ≥ N4 | 417 | 16.0 | 63 | 19.4 | |
| Tumor size, cm | < .001 | ||||
| < 2 | 1,685 | 64.8 | 158 | 48.8 | |
| ≥ 2 | 905 | 34.8 | 162 | 50.0 | |
| Missing | 9 | 0.3 | 4 | 1.2 | |
| Tumor grade (centrally assessed) | < .001 | ||||
| 1 | 553 | 21.3 | 13 | 4.0 | |
| 2 | 1,443 | 55.5 | 309 | 95.4 | |
| 3 | 589 | 22.7 | 2 | 0.6 | |
| Missing | 14 | 0.5 | — | — | |
| Peritumoral invasion (centrally assessed) | < .001 | ||||
| No | 2,304 | 88.6 | 317 | 97.8 | |
| Yes | 285 | 11.0 | 7 | 2.2 | |
| Missing | 10 | 0.4 | — | — | |
| Treatment assigned | .32 | ||||
| Letrozole | 1,300 | 50.0 | 152 | 46.9 | |
| Tamoxifen | 1,299 | 50.0 | 172 | 53.1 | |
| Local therapy | < .001 | ||||
| LTM/RT | 1,426 | 54.9 | 145 | 44.8 | |
| LTM/no RT | 85 | 3.3 | 4 | 1.2 | |
| Mastectomy/RT | 423 | 16.3 | 86 | 26.5 | |
| Mastectomy/no RT | 663 | 25.5 | 88 | 27.2 | |
| Other | 2 | 0.1 | 1 | 0.3 | |
Abbreviations: BMI, body mass index; LTM: less than mastectomy; Lum A, luminal A; Lum B, luminal B; RT, radiotherapy.
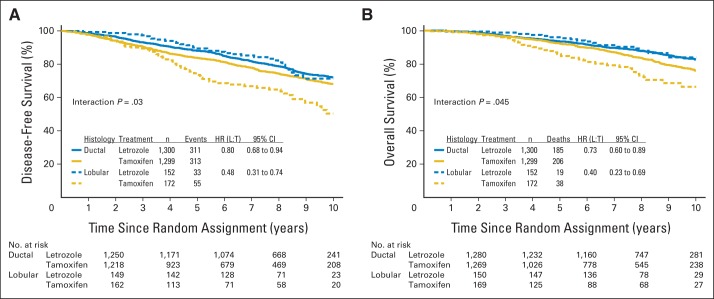
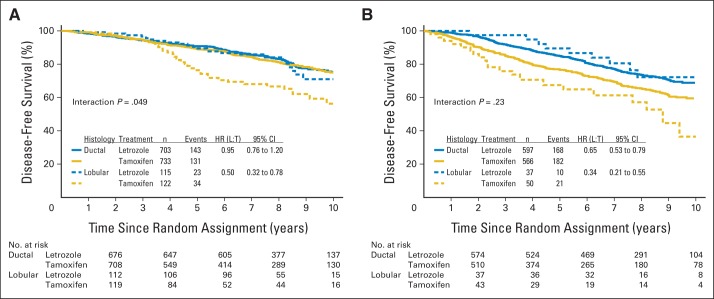
IPCW estimates for DFS and OS are shown in Figures 2A and 2B. The 8-year DFS estimate was 66% for tamoxifen compared with 82% for letrozole in the ILC subset (hazard ratio [HR], 0.48; 95% CI, 0.31 to 0.74) and was 75% for tamoxifen and 82% for letrozole in the IDC subset (HR, 0.80; 95% CI, 0.68 to 0.94). In this comparison, the effect of treatment on DFS depended on the histology of the tumor (interaction P = .03; Fig 2A). The 8-year OS estimate was 74% for tamoxifen compared with 89% for letrozole in the ILC subset (HR, 0.40; 95% CI, 0.23 to 0.69) and 84% for tamoxifen and 88% for letrozole in the IDC subset (HR, 0.73; 95% CI, 0.60 to 0.89). The effect of treatment on OS also depended on the histology of the tumor (interaction P = .045; Fig 2B). IPCW estimates for DFS according to breast cancer subtype in the LA-like and LB-like subsets are shown in Figure 3. ILC tumors classified as LA like had inferior outcomes when treated with tamoxifen, whereas ILC tumors classified as LB like and IDC tumors classified as either LA like or LB like had similar outcomes when treated with letrozole, as shown in the overlapping curves in Figure 3. Additional analyses were performed by using intention-to-treat analyses and showed similar estimates as observed with IPCW (Appendix Fig A1, online only).
Fig 2.
Inverse probability of censoring weighted Kaplan-Meier estimates of (A) disease-free survival and (B) overall survival according to histology (ductal, lobular) and treatment (letrozole, tamoxifen) among the 2,923 patients in the analytic cohort. HR, hazard ratio; L, letrozole; T, tamoxifen.
Fig 3.
Inverse probability of censoring weighted Kaplan-Meier estimates of disease-free survival according to treatment and histology within subgroups defined as (A) luminal A–like and (B) luminal B–like. HR, hazard ratio; L, letrozole; T, tamoxifen.
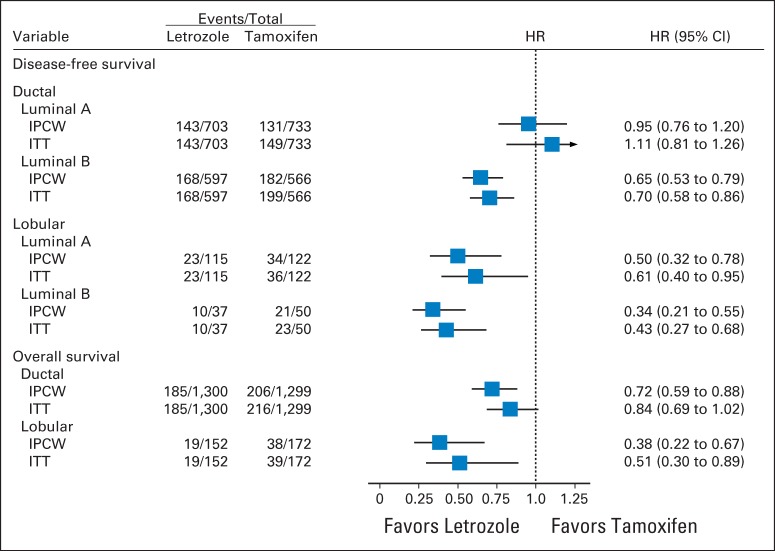
In multivariable models for DFS adjusted for classic clinicopathologic features, significant interactions between treatment and histology (ILC or IDC; interaction P = .006) and treatment and subtype (LB like or LA like; interaction P = .01) were observed (Fig 4). Women who had IDC and the LB-like subtype experienced a significant 35% reduction in the hazard of a DFS event with letrozole (HR, 0.65; 95% CI, 0.53 to 0.79); however, no difference between treatments was noted in women who had IDC and the LA-like subtype (HR, 0.95; 95% CI, 0.76 to 1.20). In the ILC subset, there was a 66% reduction in the hazard of a DFS event for LB-like subtypes (HR, 0.34; 95% CI, 0.21 to 0.55) and a 50% reduction in the hazard of a DFS event with letrozole for LA-like subtypes (HR, 0.50; 95% CI, 0.32 to 0.78).
Fig 4.
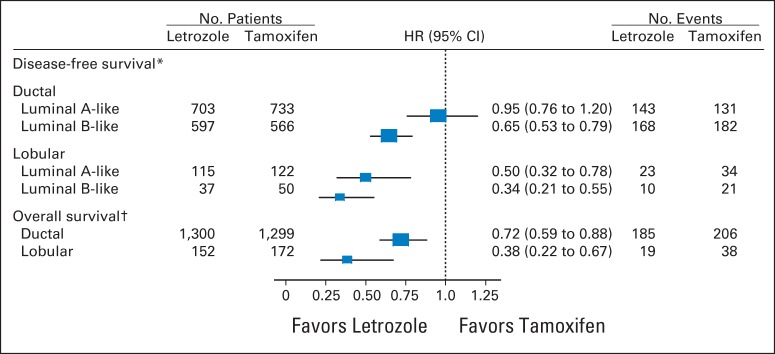
Disease-free survival and overall survival hazard ratio (HR) estimates and 95% CIs comparing the efficacy of letrozole versus tamoxifen for histologic subgroups. The results were based on multivariable models that included classic clinicopathologic variables as predictors: age, tumor size, nodal status, histologic grade, histology (ductal/lobular), local therapy, subtype (luminal A or B), and treatment. The box size is inversely proportional to the SE of the HR; the extended horizontal lines indicate the 95% CIs. (*) Treatment by histology (ductal/lobular), P = .006; treatment by subtype (LA/LB), P = .01. (†) Treatment by histology (ductal/lobular), P = .035.
In the multivariable analysis for OS, a significant interaction between treatment and histology (interaction P = .035) was observed, which suggests that the effect of letrozole compared with tamoxifen is significantly greater for ILC compared with IDC. Women with IDC experienced a statistically significant 28% reduction in the hazard of death with letrozole (HR, 0.72; 95% CI, 0.59 to 0.88). In women with ILC, there was a 62% reduction in the hazard of death with letrozole (HR, 0.38; 95% CI 0.22 to 0.67)
DISCUSSION
This study demonstrates that the magnitude of benefit of adjuvant letrozole varies by histologic subtype. Significant reductions in the risk of DFS events with letrozole monotherapy versus tamoxifen monotherapy were noted in women diagnosed with ILC regardless of whether the tumor was LA like or LB like. By contrast, in the IDC subset, there was a significant reduction in the risk of DFS events with letrozole for the LB-like subtype but no difference between letrozole and tamoxifen with the LA-like subtype.
A major strength of this study is that the population comes from a large, phase III clinical trial with a median patient follow-up time of more than 8 years.32 Central pathology review for histologic breast cancer subtypes allowed us to reliably identify patients diagnosed with classic ILC and investigate for the first time the effectiveness of adjuvant tamoxifen and letrozole in this subset. In addition, determination of biomarkers (ER, PgR, HER2, and Ki-67) was performed in a central laboratory with extensive breast cancer pathology experience.
The use of an IHC-based surrogate to divide the ER-positive tumors into low- and high-proliferative subsets (ie, LA like or LB like) is a limitation. Currently, the use of Ki-67 in clinical practice is limited by uncertain technical reproducibility and subjective interpretation.25,26 Moreover, the use of a reduced set of biomarkers is not expected to replace genomic classifiers. Despite the limitations, a good approximation of genomically defined breast cancer subtypes can be achieved by IHC-based surrogates when biomarkers are assessed with existing recommendations.26,33 In agreement with a retrospective analysis, in which genomic data from more than 180 ILC tumors classified the majority (75%) of ILC tumors as the LA type,4,34 the present analysis classified 73% of ILC tumors as LA like and 27% as LB like. In contrast, the IDC subset had a smaller representation (55%) of LA-like tumors and a higher representation (45%) of LB-like tumors.
Data from a retrospective analysis that included 2,000 women with breast cancers classified into subtypes according to the same IHC-based surrogate and observed for greater than 12 years, demonstrated a worse prognosis for LB-like tumors than LA-like tumors.35 It also showed a consistently higher hazard of early (< 5 years after diagnosis) and late (> 5 years) recurrence with LB-like tumors versus LA-like tumors. The present analysis takes into consideration the different distribution of LA-like and LB-like tumors in ILC and IDC subsets and allows for a more precise investigation of the benefit of AIs versus tamoxifen in both subsets.
There are other definitions of IHC-based surrogate classifications. One adds PgR expression to the existing definition of the LA-like subtype and requires that tumors have PgR ≥ 20%.36 Our results were consistent when the analysis was performed with this modified IHC-based surrogate (data not shown), and our conclusions were not dependent on one surrogate definition.
The retrospective nature of this analysis and the reduced number of ILC occurrences are limiting factors. It is unclear at this stage whether inferior outcomes for patients diagnosed with ILC and treated with tamoxifen would be confirmed in additional studies. In a randomized study comparing 2 years of tamoxifen with observation, the advantage of tamoxifen was more pronounced in the subset of patients with IDC than in those with ILC, but the number of patients with ILC was small (n = 43).14 In a retrospective subset analyses of patients enrolled on the Exemestane Adjuvant Multinational (TEAM) phase III study, similar outcomes were observed for patients with ILC and IDC who were treated with single-agent exemestane or tamoxifen followed by exemestane.37 The lack of a tamoxifen monotherapy arm and a shorter follow-up time are limiting factors to the interpretation of these results. More recently, results from the Austrian Breast and Colorectal Cancer Study Group (ABCSG) VIII trial showed improved OS for patients with ILC who were treated with tamoxifen followed by anastrozole versus those treated with tamoxifen monotherapy.38 Additional results from BIG 1-98 failed to demonstrate similar results for the comparison of tamoxifen followed by letrozole versus tamoxifen monotherapy.39 Comparisons of sequential arms (eg, tamoxifen followed by an AI) versus tamoxifen monotherapy should be interpreted with caution, and additional analysis with larger number of patients (ie, patients with ILC) is warranted before definitive conclusions are made.
The IPCW methodology, which attempts to remove a bias caused by treatment cross over, has strengths and limitations that have been noted in the published literature.30 Quantifying bias in IPCW estimates can be difficult but will be limited as long as data are available about factors that affect patient decisions to cross over and are prognostic for future outcomes. The BIG 1-98 study is well suited for the use of IPCW methods, because patients before recurrence were relatively healthy and because prognostic factors were recorded as part of study follow-up times and were available for estimation of the IPCW weights.
Preclinical and translational efforts have suggested the mechanisms of resistance to tamoxifen in the subset of ILC. This includes the expression of different forms of ERs, including estrogen-related receptor γ,40 persistence of ERβ expression,41 and fibroblast growth factor receptor signaling.42 In addition to the above-mentioned mechanisms, higher levels of ER and PgR expression in ILC than IDC could hypothetically contribute to a greater benefit of an AI in ILC, but data that link ER/PgR levels and AI efficacy are inconsistent across studies.22,43,44
In clinical practice, the choice of an AI versus tamoxifen usually includes the assessment of the baseline risk of disease recurrence as determined by tumor burden, tumor biology, comorbidities, toxicity profile of tamoxifen and the AI, and patient preference.32,45,46 Although the current results suggest the addition of histologic subtype (ie, ILC and IDC), in the choice of an AI versus tamoxifen, it is important to note that these findings are derived from a retrospective analysis and should be interpreted with caution.
Among patients diagnosed with IDC, the effect of letrozole seems confined to the LB cohort. This result is in agreement with previous analyses conducted with the BIG 1-98 data that show a greater magnitude of benefit for letrozole versus tamoxifen monotherapy among patients with high Ki-67.20 Despite our limited ability to classify tumors into LA- or LB-like subtypes in clinical practice, the results of the present study could be useful in special circumstances. As an example, switching from AI to tamoxifen among those experiencing severe adverse effects might represent a good option, specifically for patients diagnosed with IDC tumors that have features indicative of low-proliferative activity.
In conclusion, although the current data suggest a greater benefit of adjuvant letrozole than tamoxifen for patients diagnosed with ILC, subsequent validation in larger data sets is necessary before implementing a routine clinical recommendation of AI for patients diagnosed with ILC.
Acknowledgment
We thank the women, pathologists, physicians, nurses, and data managers who participated in the BIG 1-98 clinical trial.
Glossary Terms
- Aromatase inhibitor (AI):
Inhibitors used in treating breast cancer in postmenopausal women. Aromatase inhibitors inhibit the conversion of androgens to estrogens by the enzyme aromatase, thus depriving the tumor of estrogenic signals. Because of decreased production of estrogen, estrogen receptors, which are important in the progression of breast cancer, cannot be activated.
- Estrogen receptor (ER):
Ligand-activated nuclear proteins, belonging to the class of nuclear receptors, present in many breast cancer cells that are important in the progression of hormone-dependent cancers. After binding, the receptor-ligand complex activates gene transcription. There are two types of estrogen receptors (ERα and ERβ). ERα is one of the most important proteins controlling breast cancer function. ERβ is present in much lower levels in breast cancer, and its function is uncertain. ER status guides therapeutic decisions in breast cancer.
Appendix
BIG 1-98 Trial Participating Centers and Offices
Steering Committee: B. Thürlimann (Chair), S. Aebi, L. Blacher, H. Bonnefoi, A.S. Coates, T. Cufer, B. Ejlertsen, J.F. Forbes, R.D. Gelber, A. Giobbie-Hurder, A. Goldhirsch, A. Hiltbrunner, S.B. Holmberg, R. Maibach, A. Martoni, H. Bonnefoi, G. MacGrogan, H. T. Mouridsen, R. Paridaens, K.N. Price, M. Rabaglio, B.B. Rasmussen, M.M. Regan, A. Santoro, I.E. Smith, A. Wardley, G. Viale, H.A. Chaudri-Ross (Novartis).
International Breast Cancer Study Group Foundation Council (members from 1998 to 2012): S. Aebi, A.S. Coates, M. Colleoni, J.P. Collins, H. Cortés Funes, R.D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S.B. Holmberg, P. Karlsson, I Kössler, I. Láng, J. Lindtner, F Paganetti, M. de Stoppani, C.-M. Rudenstam, H.-J. Senn, R. Stahel, B. Thürlimann, A. Veronesi.
Coordinating Center (Bern, Switzerland): M. Castiglione (Chief Executive Officer 1998-2007), A. Hiltbrunner (Director), M. Rabaglio, G. Egli, H. Hawle, B. Cliffe, S. Ribeli-Hofmann, F. Munarini, R. Kammler, R. Studer, B. Ruepp, R. Maibach, N. Munarini.
Statistical Center (Dana-Farber Cancer Institute, Boston, MA): R. D. Gelber (Director), M.M. Regan (Group Statistician), K.N. Price (Director of Scientific Administration), A. Giobbie-Hurder (Trial Statistician), A. Keshaviah, H. Litman, B.F. Cole, Z. Sun, P.K. Gray, H. Huang, L.J. Somos, B. Timmers, L. Nickerson.
Data Management Center (Frontier Science and Technology Research Foundation, Amherst, NY): L. Blacher (Director of Data Management), T. Heckman Scolese (Coordinating Data Manager), M. Belisle, M. Caporale, J. Celano, L. Dalfonso, L. Dooley, S. Fischer, K. Galloway, J. Gould, R. Hinkle, M. Holody, G. Jones, R. Krall, S. Lippert, J. Meshulam, L. Mundy, A. Pavlov-Shapiro, K. Scott, M. Scott, S. Shepard, J. Swick, L. Uhteg, D. Weinbaum, C. Westby, T. Zielinski.
Central Pathology Review Office (University of Glasgow, Glasgow, United Kingdom): B.A. Gusterson, E. Mallon; (European Institute of Oncology, Division of Pathology, Milano, Italy): G. Viale, P. Dell'Orto, M. Mastropasqua, B. Del Curto.
Breast International Group: International Breast Cancer Study Group
Australia and New Zealand Breast Cancer Trials Group (ANZ BCTG): R.D. Snyder, J. Chirgwin, J.F. Forbes, A.S. Coates, F. Boyle, D. Lindsay, D. Preece, J. Cowell, D. Talbot, A. Whipp.
Australia.
The Cancer Council Victoria, Melbourne, Victoria: F. Abell, R. Basser, R. Bell, B. Brady, D. Blakey, P. Briggs, I. Burns, P. Campbell, M. Chao, J. Chirgwin, B. Chua, K. Clarke, J. Collins, R. De Boer, J.C. Din, R. Doig, A. Dowling, R. Drummond, N. Efe, S.T. Fan, M. Francis, P. Francis, V. Ganju, P. Gibbs, G. Goss, M. Green, P. Gregory, J. Griffiths, I. Haines, M. Henderson, R. Holmes, P. James, J. Kiffler, M. Lehman, M. Leyden, L. Lim, G. Lindeman, R. Lynch, B. Mann, J. McKendrick, S. McLachlan, R. McLennan, G. Mitchell, S. Mitra, C. Murphy, I. Parker, K. Phillips, I. Porter, G. Richardson, J. Scarlet, S. Sewak, J. Shapiro, R. Snyder, R. Stanley, C. Steer, D. Stoney, A. Strickland, G. Toner, C. Underhill, K. White, M. White, A. Wirth, S. Wong; W.P. Holman Clinic, Liverpool Hospital, Sydney, New South Wales: S. Della-Fiorentina, A. Goldrick, E. Hovey, E. Moylan, E. Segelov; Calvary Mater Newcastle, Newcastle, New South Wales: J.F. Forbes, F. Abell, S. Ackland, A. Bonaventura, S. Cox, J. Denham, R. Gourlay, D. Jackson, R. Sillar, J. Stewart; Princess Alexandra Hospital, Woollongabba, Queensland: E. Walpole, D. Thompson; Royal Brisbane and Women's Hospital, Brisbane, Queensland: M. Colosimo, R. Cheuk, L. Kenny, N. McCarthy, D. Wyld; Royal Hobart Hospital, Hobart, Tasmania: R. Young, R. Harrup, R. Kimber, R. Lowenthal; Toowoomba Hospital, Toowoomba, Queensland: E.A. Abdi, R. Brodribb, Z. Volobueva; Westmead Hospital, Sydney, New South Wales: P. Harnett, V. Ahern, H. Gurney, N. Wilcken.
New Zealand.
Auckland Hospital, Auckland: V.J. Harvey, B. Evans, W. Jones, M. McCrystal, D. Porter, P. Thompson, M. Vaughan; Christchurch Hospital, Christchurch: D. Gibbs, C. Atkinson, R. Burcombe, B. Fitzharris, B. Hickey, M. Jeffery, B. Robinson; Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez; Waikato Hospital, Hamilton: I.D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, I. Kennedy, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Brazil.
Hospital de Clinicas de Porto Alegre, Porto Alegre: C. Menke, J. Biazús, R. Cericatto, J. Cavalheiro, N. Xavier, A. Bittelbrunn, E. Rabin.
Chile.
Chilean Cooperative Group for Oncologic Research (GOCCHI): J. Gutiérrez (Chairman), R. Arriagada (Scientific Adviser), L. Bronfman (Principal Investigator), M. Zuñiga (Data Manager); Clinica Las Condes, Santiago: J. Gutiérrez, J.C. Acevedo, S. Torres, A. León, E. Salazar; Hospital DIPRECA, Las Condes, Santiago: L. Soto Diaz, R. Duval, N. Oddeshede, M.C. Venti; Hospital San Juan de Dios, Santiago: K. Peña, L. Puente, V. Maidana; Instituto de Radiomedicina (IRAM), Vitacura, Santiago: R. Baeza, R. Arriagada, P. Olfos, J. Solé, E. Vinés, C. Mariani.
Hungary.
National Institute of Oncology, Budapest: I. Láng, E. Hitre, E. Szabó, Z. Horváth, E. Ganofszky, E. Juhos.
Italy.
Centro di Riferimento Oncologico, Aviano: A. Veronesi, D. Crivellari, M.D. Magri, A. Buonadonna, F. Coran, E. Borsatti, E. Candiani, S. Massarut, M. Roncadin, M. Arcicasa, A. Carbone, T. Perin, A. Gloghini; Ospedali Riuniti di Bergamo, Bergamo: C. Tondini, R. Labianca, P. Poletti, A. Bettini; Ospedale degli Infermi, Biella: M. Clerico, M. Vincenti, A. Malossi, E. Seles, E. Perfetti, B. Sartorello; Spedali Civili, Brescia: E. Simoncini, G. Marini, P. Marpicati, R. Farfaglia, A.M. Bianchi, P. Grigolato, L. Lucini, P. Frata, A. Huscher, E. Micheletti, C. Fogazzi; U.O. Medicina Oncologica, Ospedale Capri, Ospedale Mirandola: F. Artioli, K. Cagossi, L. Scaltriti, E. Bandieri, L. Botticelli, G. Giovanardi; Ospedale di Cattolica Cervesi, Cattolica: A. Ravaioli, E. Pasquini, B. Rudnas; Ospedale Civile, Gorizia: L. Foghin; Ospedale A. Manzoni Lecco, Lecco: M. Visini, L. Zavallone, G. Ucci; Istituto Europeo di Oncologia, Milano: M. Colleoni, G. Viale, G. Renne, G. Pruneri, M. Mastropasqua, S. Dellapasqua, A. Balduzzi, M. Iorfida, G. Cancello, E. Montagna, A. Cardillo, G. Peruzzotti, R. Gisini, A. Luini, P. Veronesi, V. Galimberti, M. Intra, O. Gentilini, S. Zurrida, G. Curigliano, F. Nole, R. Orecchia, C. Leonardi, A. Goldhirsch; Ospedale Infermi, Rimini: A. Ravaioli, L. Gianni.
Peru.
Instituto de Enfermedades Neoplásicas, Lima: H. Gome.
Slovenia.
Institute of Oncology, Ljubljana: T. Cufer, B. Pajk, J. Cervek.
South Africa.
Groote Schuur Hospital and University of Cape Town, Cape Town: I.D. Werner, E. Murray, D. Govender, S. Dalvie, T. Erasmus, B. Robertson, B. Read, E. Nel, J. Toop, N. Nedeva, E. Panieri; Sandton Oncology Centre, Johannesburg: D. Vorobiof, M. Chasen, G. McMichael, C. Mohammed. Local funding provided by the Cancer Association of South Africa.
Sweden.
West Swedish Breast Cancer Study Group: S.B. Holmberg; Sahlgrenska U Hospital, Moelndal: S.B. Holmberg, J. Mattsson; Boras Hospital, Boras; Karlstads Hospital, Karlstads: H. Sellström; Kungalvs Hospital, Kungalvs: B. Lindberg.
Switzerland.
Swiss Group for Clinical Cancer Research (SAKK): A. Goldhirsch (to January 2004), R. Herrmann (from June 2004): Kantonsspital Aarau, Zentrum f. Onkologie, Aarau: A. Schönenberger, W. Mingrone, Ch. Honegger, E. Bärtschi, M. Neter, M. Rederer, G. Schär; University Hospital Basel, Basel: C. Rochlitz, R. Herrmann, D. Oertli, E. Wight, H. Moch; Institute of Oncology of Southern Switzerland: Ospedale San Giovanni, Bellinzona: J. Bernier, L. Bronz, F. Cavalli, E. Gallerani, A. Richetti, A. Franzetti; Ospedale Regionale di Lugano (Civico & Italiano), Lugano: M. Conti-Beltraminelli, M. Ghielmini, T. Gyr, S. Mauri, P.C. Saletti; Ospedale Regionale Beata Vergine, Mendrisio: A. Goldhirsch, O. Pagani, R. Graffeo, M. Locatelli, S. Longhi, P.C. Rey, M. Ruggeri; Ospedale Regionale La Carità, Locarno: E. Zucca, D. Wyss; Istituto Cantonale di Patologia, Locarno: L. Mazzucchelli, E. Pedrinis, T. Rusca; Inselspital, Bern: S. Aebi, M.F. Fey, M. Castiglione, M. Rabaglio; Kantonsspital Olten, Olten: S. Aebi, M.F. Fey, M. Zuber, G. Beck; Bürgerspital, Solothurn: S. Aebi, M.F. Fey, R. Schönenberger; Spital Thun-Simmental AG Thun: J.M. Lüthi, D. Rauch; Hôpital Cantonal Universitaire HCUG, Geneva: H. Bonnefoi; Rätisches Kantons- und Regionalspital, Chur: F. Egli, R. Steiner, P. Fehr; Centre Pluridisciplinaire d'Oncologie, Lausanne: L. Perey, P. de Grandi, W. Jeanneret, S. Leyvraz, J.-F. Delaloye; Kantonsspital St Gallen, St Gallen: B. Thürlimann, D. Köberle, F. Weisser, S., Mattmann, A. Müller, T. Cerny, B. Späti, M. Höfliger, G. Fürstenberger, B. Bolliger, C. Öhlschlegel, U. Lorenz, M. Bamert, J. Kehl-Blank, E. Vogel; Kantonales Spital Herisau, Herisau: B. Thürlimann, D. Hess, I. Senn, D. Köberle, A. Ehrsam, C. Nauer, C. Öhlschlegel, J. Kehl-Blank, E. Vogel; Stadtspital Triemli, Zürich: L. Widmer, M. Häfner; Universitätsspital Zürich, Zürich: B. C. Pestalozzi, M. Fehr, R. Caduff, Z. Varga, R. Trüb, D. Fink.
Swiss Private MDs.
Private Praxis, Zürich: B. A. Bättig; Sonnenhof-Klinik Engeried, Bern: K. Buser; Frauenklinik Limmattalspital, Schlieren: N. Bürki; Private Praxis, Birsfelden: A. Dieterle; Private Praxis, Biel: L. Hasler; Private Praxis, Baar: M. Mannhart-Harms; Brust-Zentrum, Zürich: C. Rageth; Private Praxis, Bern: J. Richner; Private Praxis, Bellinzona: V. Spataro; Private Praxis, Winterthur: M. Umbricht.
Danish Breast Cancer Cooperative Group
Danish Breast Cancer Cooperative Group (DBCG) Secretariate, Copenhagen: B. Ejlertsen; Rigshospitalet, Copenhagen: H.T. Mouridsen, B. Ejlertsen; Vejle Hospital, Vejle: E. Jakobsen; Odense University Hospital, Odense: S. Cold; Kas Herlev/Herlev University Hospital, Herlev: C. Kamby; Aalborg Sygehus Syd, Aalborg: M. Ewertz; Hilleroed Hospital, Hilleroed: P.M. Vestlev; Aarhus University Hospital, Aarhus: J. Andersen; Roskilde County Hospital, Roskilde: P. Grundtvig; Esbjerg Central Hospital, Esbjerg: E. Sandberg; Naestved Central Hospital, Naestved: P. Philip; Soenderborg Sygehus, Soenderborg: E.L. Madsen; Herning Central Hospital, Herning: K.A. Moeller; Viborg Sygehus, Viborg: V. Haahr; Landspitali University Hospital, Reykjavik, Iceland: J. Johansson.
French Federation of Cancer Centers Sarcoma Group
Institut Bergonié, Bordeaux: L. Mauriac, M. Debled, P. Campo, H. Bonnefoi; Centre Jean Perrin, Clermont-Ferrand: H. Auvray; Centre Georges François Leclerc, Dijon: C. De Gislain, F. Delille, M.-C. Porteret; Centre Oscar Lambret, Lille: V. Servent, M. Chapoutier; Centre Hospitalier Regional Universitaire-Limoges, Limoges: N. Tubiana-Mathieu, S. Lavau-Denes, P. Bosc; Centre Léon Bérard, Lyon: J.P. Guastalla, T. Bachelot, C. Arbault; C.H.G. André Boulloche, Montbéliard: V. Perrin, A. Monnier, Y. Hammoud; Clinique Francheville, Périgueux: L. Cany, C. Maguire; Hôpital de la Milétrie, Poitiers: A. Daban, M. Le Saux, C. Grandon; Centre Eugène Marquis, Rennes: P. Kerbrat, C. Catheline; Centre Henri Becquerel, Rouen: C. Veyret, E. Jugieau, V. Talon; Centre René Gauducheau, Saint-Herblain: A. Le Mevel, S. Maury; Centre Claudius Régaud, Toulouse: L. Gladieff, N. Lignon.
North Yorkshire Group
D. Dodwell; Harrogate District Hospital, Harrogate, North Yorkshire: D. Dodwell; Huddersfield Royal Infirmary, Huddersfield: J. Joffe; Castlehill Hospital, Hull: P. Drew; Airedale General Hospital, Keighley, W. Yorkshire: A. Nejim; Leeds General Infirmary, Leeds: D. Dodwell, K. Horgan; Weston Park Hospital, Sheffield: R.E. Coleman.
Independent Centers/Groups
Australia.
Flinders Medical Centre, Adelaide, South Australia: S. Birrell, M. Eaton, C. Hoffman; The Geelong Hospital, Geelong, Victoria: R. Bell, F. Abell, M. Francis, J. Kiffer, R. Lynch, R. McLennan, K. White; Western General Hospital, Melbourne, Victoria: M. Green, R. Basser, J. Collins, R. De Boer, J.C. Din, N. Efe, S.T. Fan, G. Lindeman, S. Wong; Calvary Mater Newcastle, Newcastle, New South Wales: J. Stewart, F. Abell, S. Ackland, A. Bonaventura; St George Hospital, Sydney, New South Wales: P. de Souza, M. Links.
Belgium.
Institut Jules Bordet, Brussels: J.M. Nogaret; University Hospitals Leuven, Leuven: M.R. Christiaens, P. Neven, R. Paridaens, A. Smeets, I. Vergote, C. Weltens, H. Wildiers; Les Cliniques St Joseph Association Sans But Lucratif, Liège: C. Focan; Clinique du Parc Léopold, Brussels: L. Marcelis; C.H. Etterbeek-Ixelles, Brussels: J.P. Kains.
Canada.
Cambridge Memorial Hospital, Cambridge: J. Gowing; CHUM-Campus Notre Dame, Montreal: L. Yelle; Hôpital Maisonneuve-Rosemont, Montreal: P. Dubé.
Chile.
Fundacion Lopez Perez, Santiago: C. Vogel; Hospital Carlos Van Buren, Valparaiso: M. León Prieto.
Czech Republic.
Institute of Oncology, Brno: K. Petrakova, M. Palacova, R. Demlova; Department of Clinical and Radiation Oncology, Ceske Budejovice: H. Siffnerova, J. Fischer, I. Bustova; Centre of Breast Diseases, Prague: H. Kankova, M. Pintova; Institute of Radiation Oncology, Prague: P. Vitek; University Hospital, Prague: J. Abrahamova, D. Kordikova; University Hospital Prague: L. Petruzelka, E. Sedlackova, H. Honova.
Germany.
Onkologische Gemeinschaftspraxis, Augsburg: B. Heinrich; Zentralklinikum/Frauenklinik, Augsburg: A. Wischnik; Universitätsklinikum Essen, Essen: C. Oberhoff, A.E. Schindler; Universitäts-Frauenklinik d. JLU Giessen, Giessen: K. Münstedt; Onkologische Gemeinschaftspraxis, Göttingen: D. Meyer; Martin-Luther-Universität Halle-Wittenberg, Halle: R. Grosse, H. Kölbl; Universitätskliniken des Saarlandes, Hamburg: W. Schmidt, D. Mink; Universitäts-Frauenklinik und Poliklinik Universitätskrankenhaus Eppendorf, Hamburg: F. Jänicke; Kliniken d. Med. Hochschule, Frauenklinik, Hannover: H.J. Lück; Krankenanstalt Mutterhaus der Borromäerinnen, Trier: W. Dornoff; Gynäkologische Abteilung des St Josefshospital, Wiesbaden: G. Hoffmann; Gynäkologische Abteilung d. Marienhospitals, Universität Witten-Herdecke, Witten: J. Hackmann, W. Bader.
Hungary.
SZOTE Onkoterápiás Klinika, Szeged: Z. Kahan; BM Központi Kórház, Budapest: G. Pajkos, K. Kristo; Almási Balogh Pál Kórház, Ózd: E. Kner.
Italy.
Policlinico S. Orsola-Malpighi, Bologna: A. Martoni, C. Zamagni, S. Giaquinta, E. Piana; Ospedale S. Croce, Fano: R. Mattioli, L. Imperatori; Azienda Ospedaliera San Filippo Neri, Rome: G. Gasparini, G. Sciarretta, A. Morabito; Az. Ospedaliera Treviglio-Caravaggio, Treviglio: S. Barni, M. Cazzaniga, M. Cabiddu; Policlinico Universitario (PUDG), Udine: F. Puglisi; Universitiy of Cagliari, Policlinico Universitario, Cagliari: G. Mantovani, E. Massa, G. Astara; Ospedale Civile Feltre, Feltre: R. Segati; Istituto Nazionali Ricerca Cancro, Genova: R. Rosso, L. Del Mastro, M. Venturini, C. Bighin; Istituto Nazionale dei Tumori, Milano: E. Bajetta, N. Zilembo, D. Paleari, G. Procopio; Azienda Ospedaliera S. Salvatore, Pesaro: G. Catalano, S. Luzi Fedeli; Azienda Ospedaliera Ospedale di Circolo e Fondazione Macchi Varese: G. Pinotti, G. Giardina, I. Vallini; Universitiy of Cagliari, Policlinico Universitario, Cagliari: B. Massidda, M.T. Ionta, M.C. Deidda; Ospedale Maggiore, Lodi: G. Nalli, G. Sita; Ospedale Civile dello Spirito Santo, Pescara: M. Lombardo, G. Pandoli, P. Di Stefano; Azienda Ospedaliera Santa Maria Nuova, Reggio Emilia: C. Boni, G. Bisagni, M.C. Banzi, P. Linarello; Azienda Ospedaliera Desenzano del Garda, Manerbio: G. Colosini, A. Spasiano, A. Caldonazzo; Ospedale Civile ASL 20, Tortona: M. G. Pacquola.
The Netherlands.
Catharina Ziekenhuis, Eindhoven: H.J.T. Rutten; St Anna Ziekenhuis, Geldrop: E.J.T. Luiten; Tweesteden Ziekenhuis, Tilburg: H.T.J. Roerdink; Maxima Medisch Centrum, Veldhoven: R.H.M. Roumen.
New Zealand.
Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez, K., Bayston, M. Pfieffer; Waikato Hospital, Hamilton: I. Kennedy, I.D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Poland.
Department of Oncology and Radiotherapy, Medical University of Gdansk, Gdansk: J. Jassem, M. Welnicka-Jaskiewicz, E. Senkus-Konefka, K. Matuszewska; Klinika Nowotworów Piersi i, Chirurgii Rekonstrukcyjnej-Warszawa, Warsaw: T. Pienkowski, E. Brewczynska, B. Bauer-Kosinska, R. Sienkiewicz-Kozlowska, A. Jagiello-Gruszfeld, K. Sudol; Oddzial Kliniczny Onkologiczny, Centralnego Szpitala Klinicznego Wojskowej, Akademii Medycznej-Warszawa, Warsaw: M. Górnasiowa.
Portugal.
Hospital de S. João, Porto: M. Damasceno; Instituto Português de Oncologia de Coimbra, Coimbra: J.Q. Albano; Hospital de Santa Maria, Lisbon: B. da Costa, L. Costa; Hospital Geral de Santo António, Porto: F. Marques.
Russia.
Moscow Municipal Hospital No. 62, Moscow: A.N. Makhson, N.V. Zabaznyi; N.N. Petrov Research Institute of Oncology, St Petersburg: V. Semiglazov, V. Ivanov.
South Africa.
Mamma Clinic, Tygerberg Hospital, Cape Town: J. Apffelstaedt; Southern Cross Hospital, Cape Town: D. Eedes; Pretoria Academic Hospital, Pretoria: C. Slabber; Pretoria East Hospital, Pretoria: M.A. Coccia-Portugal; Eastern Cape Oncology Centre, Port Elizabeth: K. Maart.
Spain.
Hospital Ruber Internacional, Madrid: J.E. Alés Martinez, P. Aramburo, R. Sánchez; Hospital Son Dureta, Palma del Mallorca: J. Rifa, J. Martin; Centro Oncológico Integral de Madrid (CONIM), Madrid: R. Pérez-Carrión, J.L. González Larriba, A. Cubillo; Hospital Universitario San Carlos, Madrid: M.M. Jiménez, A. Casado; Hospital Central de Asturias, Oviedo: J. Fra, J.M. Vieitez, E. Esteban, A.J. Lacave.
Switzerland.
Universitätsfrauenklinik, Basel: E. Wight, S. Bartens, R. Decio, U. Güth; Klinik am Park, Zürich: U. Breitenstein.
Turkey.
Ege University Medical School, Izmir: E. Ozdedeli.
United Kingdom.
The Royal Marsden Hospital, London, Royal Marsden National Health Service Trust, Surrey: I.E. Smith; University of Dundee, Dundee: A.M. Thompson; Christie Hospital National Health Service Trust, South Manchester University Hospital Trust, Manchester: A. Wardley; North Middlesex Hospital, London: F. Neave.
Table A1.
Baseline Demographic, Disease, and Treatment Characteristics by Histology and Treatment
| Characteristic | Treatment by Histologic Subtype |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ductal |
Lobular |
|||||||
| Letrozole (n = 1,300) |
Tamoxifen (n = 1,299) |
Letrozole (n = 152) |
Tamoxifen (n = 172) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Chemotherapy strata | ||||||||
| No chemotherapy | 1,040 | 80.0 | 1,019 | 78.4 | 111 | 73.0 | 126 | 73.3 |
| Received chemotherapy | 260 | 20.0 | 280 | 21.6 | 41 | 27.0 | 46 | 26.7 |
| Random assignment strata | ||||||||
| 4-arm | 941 | 72.4 | 939 | 72.3 | 123 | 80.9 | 139 | 80.8 |
| 2-arm | 359 | 27.6 | 360 | 27.7 | 29 | 19.1 | 33 | 19.2 |
| Subtype | ||||||||
| Lum A | 703 | 54.1 | 733 | 56.4 | 115 | 75.7 | 122 | 70.9 |
| Lum B | 597 | 45.9 | 566 | 43.6 | 37 | 24.3 | 50 | 29.1 |
| Age, years | ||||||||
| ≤ 55 | 288 | 22.2 | 296 | 22.8 | 41 | 27.0 | 45 | 26.2 |
| 56-70 | 818 | 62.9 | 820 | 63.1 | 90 | 59.2 | 109 | 63.4 |
| ≥ 71 | 194 | 14.9 | 183 | 14.1 | 21 | 13.8 | 18 | 10.5 |
| Obese (BMI ≥ 30 kg/m2) | ||||||||
| No | 974 | 74.9 | 955 | 73.5 | 109 | 71.7 | 134 | 77.9 |
| Yes | 275 | 21.2 | 286 | 22.0 | 34 | 22.4 | 33 | 19.2 |
| Unknown | 51 | 3.9 | 58 | 4.5 | 9 | 5.9 | 5 | 2.9 |
| Nodal status | ||||||||
| Nx/N0 | 740 | 56.9 | 733 | 56.4 | 83 | 54.6 | 95 | 55.2 |
| N1-N3 | 357 | 27.5 | 352 | 27.1 | 39 | 25.7 | 44 | 25.6 |
| ≥ N4 | 203 | 15.6 | 214 | 16.5 | 30 | 19.7 | 33 | 19.2 |
| Tumor size, cm | ||||||||
| < 2 | 855 | 65.8 | 830 | 63.9 | 73 | 48.0 | 85 | 49.4 |
| ≥ 2 | 441 | 33.9 | 464 | 35.7 | 78 | 51.3 | 84 | 48.8 |
| Missing | 4 | 0.3 | 5 | 0.4 | 1 | 0.7 | 3 | 1.7 |
| Tumor grade (centrally assessed) | ||||||||
| 1 | 268 | 20.6 | 285 | 21.9 | 8 | 5.3 | 5 | 2.9 |
| 2 | 729 | 56.1 | 714 | 55.0 | 144 | 94.7 | 165 | 95.9 |
| 3 | 296 | 22.8 | 293 | 22.6 | — | — | 2 | 1.2 |
| Missing | 7 | 0.5 | 7 | 0.5 | — | — | — | — |
| Peritumoral invasion (centrally assessed) | ||||||||
| No | 1,155 | 88.8 | 1,149 | 88.5 | 149 | 98.0 | 168 | 97.7 |
| Yes | 142 | 10.9 | 143 | 11.0 | 3 | 2.0 | 4 | 2.3 |
| Missing | 3 | 0.2 | 7 | 0.5 | — | — | — | — |
| Local therapy | ||||||||
| LTM/RT | 718 | 55.2 | 708 | 54.5 | 69 | 45.4 | 76 | 44.2 |
| LTM/no RT | 41 | 3.2 | 44 | 3.4 | 2 | 1.3 | 2 | 1.2 |
| Mastectomy/RT | 206 | 15.8 | 217 | 16.7 | 39 | 25.7 | 47 | 27.3 |
| Mastectomy/no RT | 334 | 25.7 | 329 | 25.3 | 41 | 27.0 | 47 | 27.3 |
| Other | 1 | 0.1 | 1 | 0.1 | 1 | 0.7 | — | — |
Abbreviations: BMI, body mass index; LTM: less than mastectomy; Lum A, luminal A; Lum B, luminal B; RT, radiotherapy.
Fig A1.
Inverse probability of censoring weighted (IPCW) and intention-to-treat (ITT) hazard ratio (HR) estimates of the relative effect of letrozole versus tamoxifen for disease-free survival and overall survival.
Footnotes
Written on behalf of the BIG 1-98 Collaborative Group.
Supported by Novartis (sponsorship of the BIG 1-98 trial) and coordinated by the International Breast Cancer Study Group (IBCSG). The IBCSG was supported by the Swedish Cancer Society, the Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, US National Institute of Health Grant No. CA-75362, Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2012, and at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00004205.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Otto Metzger Filho
Provision of study materials or patients: Giuseppe Viale, Beat Thürlimann, Marco Colleoni, Bent Ejlertsen, Marc Debled, Alan S. Coates, Aron Goldhirsch
Collection and assembly of data: Anita Giobbie-Hurder, Elizabeth Mallon, Barry Gusterson, Giuseppe Viale, Marco Colleoni, Bent Ejlertsen, Karen N. Price, Aron Goldhirsch
Data analysis and interpretation: Otto Metzger Filho, Anita Giobbie-Hurder, Eric P. Winer, Beat Thürlimann, Richard D. Gelber, Marc Debled, Karen N. Price, Meredith M. Regan, Alan S. Coates, Aron Goldhirsch
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Relative Effectiveness of Letrozole Compared With Tamoxifen for Patients With Lobular Carcinoma in the BIG 1-98 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Otto Metzger Filho
No relationship to disclose
Anita Giobbie-Hurder
No relationship to disclose
Elizabeth Mallon
Travel, Accommodations, Expenses: Genomic Health
Barry Gusterson
Stock or Other Ownership: Illumina
Giuseppe Viale
Honoraria: Roche
Consulting or Advisory Role: DAKO, Genentech
Speakers' Bureau: Novartis
Travel, Accommodations, Expenses: Roche
Eric P. Winer
No relationship to disclose
Beat Thürlimann
Stock or Other Ownership: Roche (I), Novartis (I)
Honoraria: Roche
Travel, Accommodations, Expenses: Pierre Fabre
Richard D. Gelber
Research Funding: AstraZeneca (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst)
Marco Colleoni
Honoraria: Novartis
Consulting or Advisory Role: Boehringer Ingelheim, Taiho Pharmaceutical, AbbVie, AstraZeneca, Pierre Fabre
Bent Ejlertsen
Research Funding: Amgen, Novartis, Roche
Travel, Accommodations, Expenses: Amgen, Roche
Marc Debled
No relationship to disclose
Karen N. Price
No relationship to disclose
Meredith M. Regan
Research Funding: Veridex (Inst), OncoGenex Pharmaceuticals (Inst), Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), Ferring Pharmaceuticals (Inst), Celgene (Inst)
Alan S. Coates
No relationship to disclose
Aron Goldhirsch
No relationship to disclose
REFERENCES
- 1.Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27:49–61. doi: 10.1053/j.semdp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 3.Iorfida M, Maiorano E, Orvieto E, et al. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res Treat. 2012;133:713–723. doi: 10.1007/s10549-012-2002-z. [DOI] [PubMed] [Google Scholar]
- 4.Metzger Filho O, Michiels S, Bertucci F, et al. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann Oncol. 2013;24:377–384. doi: 10.1093/annonc/mds280. [DOI] [PubMed] [Google Scholar]
- 5.Tubiana-Hulin M, Stevens D, Lasry S, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: A retrospective study on 860 patients from one institution. Ann Oncol. 2006;17:1228–1233. doi: 10.1093/annonc/mdl114. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu MC, Rouzier R, Llombart-Cussac A, et al. The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Cancer. 2004;40:342–351. doi: 10.1016/j.ejca.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Newman LA, Buzdar AU, Singletary SE, et al. A prospective trial of preoperative chemotherapy in resectable breast cancer: Predictors of breast-conservation therapy feasibility. Ann Surg Oncol. 2002;9:228–234. doi: 10.1007/BF02573059. [DOI] [PubMed] [Google Scholar]
- 8.Lips EH, Mukhtar RA, Yau C, et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res Treat. 2012;136:35–43. doi: 10.1007/s10549-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delpech Y, Coutant C, Hsu L, et al. Clinical benefit from neoadjuvant chemotherapy in estrogen receptor–positive invasive ductal and lobular carcinomas. Br J Cancer. 2013;108:285–291. doi: 10.1038/bjc.2012.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: Response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23:41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 11.Cocquyt VF, Blondeel PN, Depypere HT, et al. Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol. 2003;29:361–367. doi: 10.1053/ejso.2002.1404. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel C, Bartsch R, Hussian D, et al. Invasive ductal carcinoma and invasive lobular carcinoma of breast differ in response following neoadjuvant therapy with epidoxorubicin and docetaxel + G-CSF. Breast Cancer Res Treat. 2007;104:109–114. doi: 10.1007/s10549-006-9397-3. [DOI] [PubMed] [Google Scholar]
- 13.Bollet MA, Savignoni A, Pierga JY, et al. High rates of breast conservation for large ductal and lobular invasive carcinomas combining multimodality strategies. Br J Cancer. 2008;98:734–741. doi: 10.1038/sj.bjc.6604229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jirstrom K, Ryden L, Anagnostaki L, et al. Pathology parameters and adjuvant tamoxifen response in a randomized premenopausal breast cancer trial. J Clin Pathol. 2005;58:1135–1142. doi: 10.1136/jcp.2005.027185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakha EA, El-Sayed ME, Powe DG, et al. Invasive lobular carcinoma of the breast: Response to hormonal therapy and outcomes. Eur J Cancer. 2008;44:73–83. doi: 10.1016/j.ejca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Giobbie-Hurder A, Price KN, Gelber RD, et al. Design, conduct, and analyses of Breast International Group (BIG) 1-98: A randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clin Trials. 2009;6:272–287. doi: 10.1177/1740774509105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan MM, Price KN, Giobbie-Hurder A, et al. Interpreting Breast International Group (BIG) 1-98: A randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor–positive, early breast cancer. Breast Cancer Res. 2011;13:209. doi: 10.1186/bcr2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 20.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: Results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viale G, Regan MM, Mastropasqua MG, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008;100:207–212. doi: 10.1093/jnci/djm289. [DOI] [PubMed] [Google Scholar]
- 22.Ewertz M, Gray KP, Regan MM, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the Breast International Group 1-98 trial. J Clin Oncol. 2012;30:3967–3975. doi: 10.1200/JCO.2011.40.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen BB, Regan MM, Lykkesfeldt AE, et al. Adjuvant letrozole versus tamoxifen according to centrally assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: Supplementary results from the BIG 1-98 randomized trial. Lancet Oncol. 2008;9:23–28. doi: 10.1016/S1470-2045(07)70386-8. [DOI] [PubMed] [Google Scholar]
- 25.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of Patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: Dealing with the diversity of breast cancer—Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer, 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer, 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 29.Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–1124. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkelstein DM, Schoenfeld DA. Correcting for discretionary treatment crossover in an analysis of survival in the Breast International Group BIG 1-98 trial by using the inverse probability of censoring weighted method. J Clin Oncol. 2011;29:1093–1095. doi: 10.1200/JCO.2010.33.9374. [DOI] [PubMed] [Google Scholar]
- 31.Ligibel JA, Winer EP. Aromatase inhibition in obese women: How much is enough? J Clin Oncol. 2012;30:2940–2942. doi: 10.1200/JCO.2012.43.7244. [DOI] [PubMed] [Google Scholar]
- 32.Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor–positive breast cancer: The BIG 1-98 randomized clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12:1101–1108. doi: 10.1016/S1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metzger Filho O, Singhal SK, Michiels S, et al. Invasive lobular carcinoma: A luminal breast cancer histotype enriched for epithelial-to-mesenchymal transition features. Cancer Res. 2011;71:24s. (abstr P2-18-01) [Google Scholar]
- 35.Metzger Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: Results from International Breast Cancer Study Group trials VIII and IX. J Clin Oncol. 2013;31:3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor–positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Water W, Fontein DB, van Nes JG, et al. Influence of semi-quantitative estrogen receptor expression on adjuvant endocrine therapy efficacy in ductal and lobular breast cancer: A TEAM study analysis. Eur J Cancer. 2013;49:297–304. doi: 10.1016/j.ejca.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Knauer M, Gruber C, Dietze O, et al. Survival advantage of anastrozol compared to tamoxifen for lobular breast cancer in the ABCSG-8 study. Cancer Res. 2015;(suppl 9):75. abstr S2-06. [Google Scholar]
- 39.Metzger Filho O, Giobbie-Hurder A, Mallon EA, et al. Relative effectiveness of letrozole alone or in sequence with tamoxifen for patients diagnosed with invasive lobular carcinoma. J Clin Oncol. 2013;31:14s. doi: 10.1200/JCO.2015.60.8133. (abstr 529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riggins RB, Lan JP, Zhu Y, et al. ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68:8908–8917. doi: 10.1158/0008-5472.CAN-08-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang B, Omoto Y, Iwase H, et al. Differential expression of estrogen receptor alpha, beta1, and beta2 in lobular and ductal breast cancer. Proc Natl Acad Sci USA. 2014;111:1933–1938. doi: 10.1073/pnas.1323719111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikora MJ, Cooper KL, Bahreini A, et al. Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res. 2014;74:1463–1474. doi: 10.1158/0008-5472.CAN-13-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viale G, Regan MM, Dell'Orto P, et al. Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1-98 randomized trial. Ann Oncol. 2011;22:2201–2207. doi: 10.1093/annonc/mdq738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 45.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor–positive breast cancer: Status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 46.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]