ABSTRACT
The macrophage response to planktonic Staphylococcus aureus involves the induction of proinflammatory microbicidal activity. However, S. aureus biofilms can interfere with these responses in part by polarizing macrophages toward an anti-inflammatory profibrotic phenotype. Here we demonstrate that conditioned medium from mature S. aureus biofilms inhibited macrophage phagocytosis and induced cytotoxicity, suggesting the involvement of a secreted factor(s). Iterative testing found the active factor(s) to be proteinaceous and partially agr-dependent. Quantitative mass spectrometry identified alpha-toxin (Hla) and leukocidin AB (LukAB) as critical molecules secreted by S. aureus biofilms that inhibit murine macrophage phagocytosis and promote cytotoxicity. A role for Hla and LukAB was confirmed by using hla and lukAB mutants, and synergy between the two toxins was demonstrated with a lukAB hla double mutant and verified by complementation. Independent confirmation of the effects of Hla and LukAB on macrophage dysfunction was demonstrated by using an isogenic strain in which Hla was constitutively expressed, an Hla antibody to block toxin activity, and purified LukAB peptide. The importance of Hla and LukAB during S. aureus biofilm formation in vivo was assessed by using a murine orthopedic implant biofilm infection model in which the lukAB hla double mutant displayed significantly lower bacterial burdens and more macrophage infiltrates than each single mutant. Collectively, these findings reveal a critical synergistic role for Hla and LukAB in promoting macrophage dysfunction and facilitating S. aureus biofilm development in vivo.
IMPORTANCE
Staphylococcus aureus has a propensity to form multicellular communities known as biofilms. While growing in a biofilm, S. aureus displays increased tolerance to nutrient deprivation, antibiotic insult, and even host immune challenge. Previous studies have shown that S. aureus biofilms thwart host immunity in part by preventing macrophage phagocytosis. It remained unclear whether this was influenced solely by the considerable size of biofilms or whether molecules were also actively secreted to circumvent macrophage-mediated phagocytosis. This is the first report to demonstrate that S. aureus biofilms inhibit macrophage phagocytosis and induce macrophage death through the combined action of leukocidin AB and alpha-toxin. Loss of leukocidin AB and alpha-toxin expression resulted in enhanced S. aureus biofilm clearance in a mouse model of orthopedic implant infection, suggesting that these toxins could be targeted therapeutically to facilitate biofilm clearance in humans.
INTRODUCTION
Highly opportunistic pathogens possess attributes that facilitate persistent infections in part by shielding themselves from immune-mediated attack (1–3). Staphylococcus aureus is one such example, and in addition to its well-known arsenal of virulence determinants, biofilm formation represents another means to circumvent immune-mediated clearance in the host (4, 5). Biofilms are heterogeneous bacterial communities encased in a complex matrix composed of extracellular DNA, proteins, and polysaccharides (6–9). S. aureus has a propensity to form biofilms on medical devices, such as prostheses and indwelling catheters, and the organism remains a major cause of health care- and community-associated infections (10–12).
Many S. aureus virulence factors target innate immune pathways that are elicited during acute planktonic infection, such as phagocytosis and proinflammatory transcription factor activation (4, 13–15). Phagocytosis leads to the killing of extracellular pathogens, as well as proinflammatory cytokine and chemokine production, which collectively orchestrate local and systemic inflammatory responses and initiate adaptive immunity (16–18). Recent studies have demonstrated that biofilms formed by various bacterial species interfere with classical host antibacterial effector mechanisms (19–24). With regard to S. aureus, work in our laboratory and others has shown that biofilms polarize macrophages toward an anti-inflammatory phenotype by dampening proinflammatory responses and limiting macrophage invasion in vivo (4, 15, 25–27). This response is considered detrimental to biofilm clearance, since polarized macrophages possess poor microbicidal activity and instead promote fibrosis (4). Similar findings of macrophage dysfunction have been reported in response to S. epidermidis biofilms (28–30), suggesting the existence of a conserved effort to thwart efficient biofilm recognition and clearance by the host. However, the molecules responsible for the ability of S. aureus biofilms to attenuate macrophage proinflammatory responses remain ill defined.
The objective of this study was to identify S. aureus biofilm-derived products that induce macrophage dysfunction and facilitate biofilm persistence. Quantitative mass spectrometry identified alpha-toxin (Hla) and the bicomponent leukotoxin leukocidin AB (LukAB, also known as LukGH) as potential candidates responsible for inhibition of macrophage phagocytosis and promotion of cytotoxicity, which was confirmed by using hla and lukAB mutants. A synergistic effect was demonstrated with a lukAB hla double mutant that also revealed decreased biofilm formation in vivo in a murine model of orthopedic implant biofilm infection. The reduction in macrophage phagocytosis, concomitant with enhanced cell death, likely facilitates the ability of S. aureus to avoid destructive host responses when organized as a biofilm.
RESULTS
S. aureus biofilms secrete a proteinaceous factor(s) that inhibits macrophage phagocytosis.
Our previous studies demonstrated that macrophages are unable to phagocytose S. aureus biofilms (4, 15); however, the mechanism responsible for this phenomenon remained to be identified. While it is known that the physical size of a biofilm is one factor that impedes phagocytosis (4), we investigated the possibility that a secreted factor(s) is also involved. In order to assess the effect of biofilm-conditioned medium on macrophage phagocytosis, we utilized fluorescent microspheres instead of live bacteria, since live S. aureus actively secretes factors during planktonic growth that would have been impossible to differentiate from biofilm-derived molecules. Using this approach, we were able to readily distinguish differences in phagocytosis and viability of murine macrophages exposed to fresh medium (Fig. 1A), S. aureus biofilm-conditioned medium (Fig. 1B), and S. aureus planktonic culture-conditioned medium (Fig. 1C) by confocal microscopy. Of note, similar effects of S. aureus biofilm-conditioned medium on macrophage phagocytosis were obtained with fluorescent microspheres and intact S. aureus in pilot studies (see Fig. S1 in the supplemental material), supporting the validity of this approach. Macrophage phagocytic activity was significantly reduced after treatment with conditioned medium from intact biofilms of the methicillin-resistant S. aureus (MRSA) clinical isolate USA300 LAC (31–34) (Fig. 1B and D), revealing a role for an extracellular factor(s). To determine whether this effect relied on an intact biofilm structure, fresh medium was added to mature biofilms that were disrupted by trituration, whereupon conditioned medium was harvested 24 h later. Treatment of macrophages with supernatants collected from disrupted biofilms had less of an impact on phagocytosis (Fig. 1D), suggesting that the putative extracellular factor(s) is enriched in intact biofilms, perhaps via a quorum-sensing system that is disturbed upon destruction of the biofilm architecture. Similarly, conditioned medium from planktonic organisms was less effective at blocking macrophage phagocytosis (Fig. 1C and D), even when cultures were grown to a high cell density (i.e., late stationary phase; data not shown), demonstrating the enrichment of this secreted factor(s) in intact biofilms. Importantly, these differences did not result from alterations in bacterial density or secreted protein levels, since titers and extracellular protein concentrations of intact biofilms, disrupted biofilms, and planktonic cultures were similar (see Fig. S2 in the supplemental material; data not shown).
FIG 1 .
S. aureus biofilms secrete a proteinaceous factor(s) that inhibits macrophage phagocytosis. (A to C) Representative two-dimensional confocal images (×63) of bone marrow-derived macrophage phagocytosis of fluorescent microspheres (yellow-white) and cell death with PI stain (red-purple) after exposure to fresh medium (A), S. aureus biofilm-conditioned medium (B), or S. aureus planktonic culture-conditioned medium (C). (D and E) Bone marrow-derived macrophages were exposed to fresh medium or conditioned medium collected from an intact biofilm, a mature biofilm that was mechanically disrupted, or a similar number of planktonic S. aureus cells. After a 3-h treatment period, macrophage phagocytosis of fluorescent microspheres (D) and cell viability (E) were quantitated by confocal microscopy. (F and G) Conditioned medium from biofilms treated with either PAS (10 µg/ml) or lysostaphin (50 µg/ml) was added to macrophages to assess the relative importance of active biofilm secretion versus passive release of products via autolysis, respectively, on macrophage phagocytosis (F) and cell death (G). (H and I) Biofilm-conditioned supernatants were treated with proteinase K (10 µg/ml) prior to macrophage exposure to assess the chemical nature of the inhibitory molecule(s). Significant differences are denoted by asterisks (***, P < 0.001; one-way ANOVA, followed by Bonferroni’s multiple-comparison test). Results are representative of at least two independent experiments.
Whereas little information is currently available regarding the S. aureus biofilm secretome, the importance of autolysis to biofilm formation has been well established (6, 35–39). To determine whether the putative biofilm extracellular factor(s) was actively secreted or a byproduct of cell lysis, mature biofilms were treated for 24 h with polyanethole sodium sulfanate (PAS) to inhibit lysis (40) or disrupted by trituration and treated with lysostaphin to artificially induce lysis. Only conditioned medium from PAS-treated biofilms maintained inhibitory activity (Fig. 1F), suggesting that S. aureus biofilms actively secrete a molecule(s) that impedes macrophage phagocytosis. To elucidate the chemical nature of the secreted inhibitory factor(s), conditioned medium from intact biofilms was treated with proteinase K prior to macrophage addition. Proteinase K completely ablated the inhibitory effect of S. aureus biofilm-conditioned medium on macrophage phagocytosis, implicating the action of a protein(s) in this phenomenon (Fig. 1H).
In addition to impaired phagocytosis, our prior report demonstrated that S. aureus biofilms also induced macrophage cytotoxicity (4). This could result from frustrated phagocytosis based on the inability of macrophages to physically engulf the bulky biofilm structure combined with the action of secreted toxins, such as Hla or leukocidins with known cytotoxic activity (13, 18, 41). Exposure of murine macrophages to conditioned medium from intact S. aureus biofilms induced significant cell death, whereas minimal cytotoxicity was observed following treatment with medium from either disrupted biofilms or planktonic S. aureus (Fig. 1A to C and E). Similar to the approach employed for phagocytosis, biofilms were treated with lysostaphin or PAS, where only lysostaphin prevented the cytotoxic effects of biofilm-conditioned medium (Fig. 1G), again revealing the action of an actively secreted protein based on its proteinase K-sensitive nature (Fig. 1I).
S. aureus biofilm-induced macrophage dysfunction is partially agr-dependent.
Our findings that disrupted biofilms were not as effective at blocking macrophage phagocytosis or inducing cytotoxicity suggested that quorum-sensing systems enriched during biofilm formation may regulate the putative inhibitory molecule(s). The expression of numerous virulence factors in S. aureus, including secreted proteases, leukocidins, and Hla, is either directly or indirectly influenced by two-component regulatory systems, such as the agr quorum-sensing system (42). agr modulates virulence factor expression and is an important regulatory switch between planktonic and biofilm lifestyles in S. aureus (43–47). Conditioned medium from a Δagr mutant biofilm induced minimal macrophage cell death (Fig. 2B), whereas the phagocytic block was significantly attenuated but only partially influenced by agr (Fig. 2). Since the macrophage-inhibitory phenotypes upon exposure to S. aureus biofilms were partially agr-dependent, the Δagr mutant strain was utilized for subsequent proteomic comparisons with wild-type (WT) biofilms in an attempt to identify secreted proteins enriched during biofilm growth that are capable of inducing macrophage dysfunction.
FIG 2 .
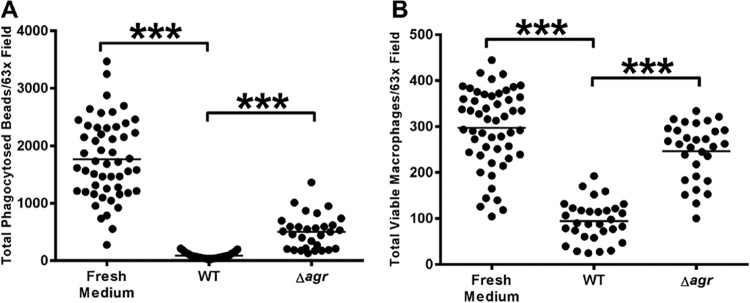
S. aureus biofilm-induced macrophage dysfunction is partially agr-dependent. Bone marrow-derived macrophages were exposed to fresh or conditioned medium from S. aureus WT or isogenic Δagr mutant biofilms for 2 h, whereupon phagocytosis of fluorescent microspheres (A) and total viable macrophages (B) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least three independent experiments.
SWATH-MS as a tool to identify S. aureus biofilm factors that induce macrophage dysfunction.
We next employed a proteomic approach to identify candidate molecules that might be responsible for biofilm-mediated macrophage dysfunction. Our proteomic strategy utilized the Δagr mutant strain as a comparator with WT biofilm, since the macrophage-inhibitory phenotypes were partially agr-dependent (Fig. 2). A second comparison between biofilm and planktonic conditions was done because the macrophage-inhibitory factors were enriched during biofilm growth (Fig. 1). To identify differentially expressed proteins in conditioned medium from WT and agr mutant biofilms and planktonic cultures, trichloroacetic acid (TCA)-precipitated proteins were analyzed by quantitative sequential windowed data-independent acquisition of the total high-resolution mass spectra (SWATH-MS) (48). As expected, conditioned medium from WT and Δagr mutant biofilms displayed vastly different proteomic profiles, with 68 (44%) of 153 proteins significantly enriched in WT biofilms, 23% of which were either secreted proteases or known virulence factors, such as Hla (Fig. 3A and B; see Table S1 in the supplemental material). In contrast, cell wall and structural proteins were more abundant in Δagr mutant biofilm-conditioned medium than in WT biofilm-conditioned medium (Fig. 3A and B). Similarly, proteomic comparisons showed that conditioned medium from WT biofilm and WT planktonic cultures differed significantly, with 108 (36%) of 301 proteins enriched in WT biofilm, including several secreted virulence factors such as toxins and proteases (see Table S2 in the supplemental material). A functional proteomic network constructed with overlapping hits from both comparisons (WT biofilm versus Δagr mutant biofilm and WT biofilm versus WT planktonic culture) identified 17 proteins, including two serine proteases and two leukocidin components, as candidates to account for the inhibitory effects of biofilm-conditioned medium on macrophage function (Fig. 3C). Additionally, Hla was significantly more enriched in WT than in Δagr mutant biofilms (see Table S1), which represented another toxin of interest for its potential role in the regulation of macrophage dysfunction. Importantly, both Hla and LukAB were significantly more enriched in biofilm-conditioned medium than in planktonic culture-conditioned medium (Fig. 4), suggesting that these two proteins may be responsible for biofilm-induced macrophage dysfunction. LukAB is a bicomponent leukotoxin also involved in S. aureus-mediated killing of host phagocytes (49–51). Therefore, Hla and LukAB were the focus of subsequent mechanistic studies, as they likely influence macrophage dysfunction and death in this setting.
FIG 3 .
SWATH-MS identifies potential biofilm factors responsible for macrophage dysfunction. Conditioned supernatant from either WT S. aureus or a Δagr mutant strain grown under planktonic or biofilm conditions was harvested and TCA precipitated for protein isolation in triplicate. Relative protein concentrations in sample sets were compared by SWATH-MS. (A) Sixty-eight (44%) of 153 identified proteins were significantly more enriched in WT than Δagr mutant biofilm, with the largest percentage associated with metabolism and virulence. Eighty-five (56%) of 153 identified proteins were significantly more enriched in Δagr mutant than WT biofilm, with the largest percentage of proteins falling into the functional category of cell wall proteins. (B) Direct comparison of significantly expressed proteins in WT and Δagr mutant biofilms by functional category, with more-refined groups of metabolism and virulence shown. (C) Functional-association network of proteins identified by SWATH-MS as significantly more enriched in WT biofilm-conditioned supernatant than in planktonic and Δagr mutant biofilm-conditioned supernatants. For all comparisons, statistical significance was assessed with a Z test (***, P < 0.05).
FIG 4 .

LukA and Hla secretion is enhanced in S. aureus biofilms. (A) LukA and Hla levels in S. aureus biofilm- and planktonic culture-conditioned media were assessed by Western blot assays. (B) Quantitation of Hla levels in conditioned medium from WT S. aureus biofilms and planktonic bacteria. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments.
LukAB and Hla play significant roles in biofilm-induced macrophage dysfunction.
Biofilm-conditioned medium from both ΔlukA and ΔlukB mutant strains elicited minimal macrophage death, in contrast to WT biofilm-conditioned medium (Fig. 5B). Likewise, the phagocytic block induced by the ΔlukA and ΔlukB mutant strains was less pronounced than that induced by WT biofilm-conditioned medium, although phagocytosis did not return to baseline levels (Fig. 5A). Both the ΔlukA and ΔlukB mutant phenotypes could be complemented, providing direct evidence of the involvement of LukAB in the modulation of macrophage survival and phagocytosis (Fig. 5A and B). In contrast, while serine proteases were also shown to be elevated by SWATH-MS, biofilm-conditioned medium from serine protease mutants (Δspl or a Δaur spl sspAB scpA quad mutant), as well as another leukocidin mutant (ΔlukD), behaved similarly to WT biofilm-conditioned medium (data not shown).
FIG 5 .
LukAB and Hla play significant roles in biofilm-induced macrophage dysfunction. (A and B) Bone marrow-derived macrophages were exposed to fresh or conditioned medium from WT S. aureus, ΔlukA mutant, ΔlukB mutant, or chromosomally complemented ΔlukAB mutant (ΔlukAB::lukAB) biofilms. After a 3-h treatment period, phagocytosis of fluorescent microspheres (A) and viable macrophages (B) were quantitated by confocal microscopy. (C and D) Bone marrow-derived macrophages were exposed to fresh or conditioned medium from S. aureus WT biofilm plus Hla Ab (α-Hla), Δhla mutant, plasmid-complemented Δhla mutant [Δhla(pHla)], and constitutively expressed hla (hlaon) biofilms. After a 3-h treatment period, phagocytosis of fluorescent microspheres (C) and viable macrophages (D) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (*, P < 0.05; ***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least three independent experiments.
Since LukAB did not account for the entire macrophage dysfunction phenotype and SWATH-MS identified increased Hla levels in WT biofilm-conditioned medium (see Table S1 in the supplemental material), we next examined the contribution of Hla to macrophage dysfunction. Further justification for investigating Hla stemmed from the vast literature on Hla regulation by the agr quorum-sensing system (41, 52–54), the finding that conditioned medium from Δagr mutant biofilms was less effective at inducing macrophage dysfunction (Fig. 2), and the fact that Hla secretion was significantly increased during biofilm growth (Fig. 4). Hla inserts itself into host cell membranes and oligomerizes to form pores, leading to cell death (41, 55). Indeed, macrophage survival was significantly improved following exposure to Δhla mutant biofilm-conditioned medium compared to WT biofilm-conditioned medium, which was complementable (Fig. 5D). The effects of Δhla on macrophage phagocytosis were less pronounced but still reached statistical significance (Fig. 5C). Furthermore, blockade of Hla activity in WT biofilm-conditioned medium with an Hla-neutralizing antibody (Ab) phenocopied the findings with Δhla mutant biofilm-conditioned medium (Fig. 5C and D). The specificity of the Hla Ab was demonstrated by its ability to inhibit the effects of purified Hla on macrophage survival and viability (see Fig. S3 in the supplemental material). Additional evidence to support the action of Hla was provided by the ability of biofilm-conditioned medium from a S. aureus strain that constitutively expresses hla (hlaon) to induce significant macrophage death and inhibit phagocytosis (Fig. 5C and D).
The expression of both Hla and LukAB was markedly greater in conditioned medium from WT biofilms than in conditioned medium from planktonic bacteria (Fig. 4). Therefore, to assess whether LukAB and Hla act cooperatively to effect macrophage activity, ΔlukA and ΔlukB mutant biofilm-conditioned media were treated with an Hla-neutralizing Ab (Fig. 6). Interestingly, negating the action of Hla in ΔlukA and ΔlukB mutant biofilm-conditioned media significantly improved macrophage phagocytosis over that in supernatants where Hla was active (Fig. 6A). Similar findings were obtained with a ΔlukAB Δhla double mutant (Fig. 6A). Hla blockade in ΔlukA and ΔlukB mutant biofilm-conditioned media had no additional effect on macrophage survival, which was not unexpected since viability had nearly been restored with each of the single mutants to levels observed in fresh medium (Fig. 6B). When macrophages were treated with ΔlukAB mutant biofilm-conditioned medium (which still produces Hla) in combination with purified LukAB or a point mutant that lacks lytic activity (LukABE323A) (56), only bioactive LukAB returned both phagocytic inhibition and cytotoxicity to levels observed in WT biofilm-conditioned medium (see Fig. S4 in the supplemental material). Collectively, these results demonstrate that LukAB acts in concert with Hla to induce macrophage dysfunction.
FIG 6 .

S. aureus Hla and LukAB act in concert to promote macrophage dysfunction. Bone marrow-derived macrophages were exposed to fresh or conditioned medium from S. aureus WT or isogenic ΔlukA mutant, ΔlukB mutant, and ΔlukAB hla double mutant biofilms plus Hla Ab (α-Hla). After a 3-h treatment period, phagocytosis of fluorescent microspheres (A) and viable macrophages (B) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least three independent experiments.
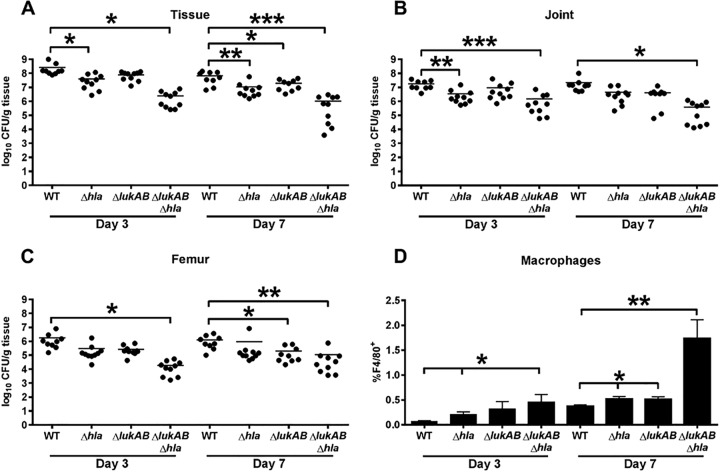
LukAB and Hla are important for S. aureus biofilm formation in vivo.
Previous work in our laboratory demonstrated that augmentation of macrophage proinflammatory activity is critical for biofilm clearance in vivo (15). To determine whether the identified functional role for LukAB and Hla in mediating macrophage dysfunction in vitro would impact biofilm formation in vivo, we utilized a murine model of S. aureus orthopedic implant biofilm infection (57–59). Similar to our in vitro studies revealing cooperation between LukAB and Hla, the ΔlukAB Δhla double mutant displayed the greatest reduction in bacterial burdens in the knee joint, surrounding soft tissue, and femur at days 3 and 7 postinfection, in contrast to ΔlukAB or Δhla mutants (Fig. 7A to C). Furthermore, macrophage infiltrates were significantly increased in mice infected with the ΔlukAB Δhla double mutant (Fig. 7D), although they represent a minor population in this model of orthopedic implant biofilm infection, which is dominated by myeloid-derived suppressor cells (58, 59). The observed increase in macrophages may result from less cytotoxicity by the combined loss of LukAB and Hla, supporting our in vitro studies. Taken together, these results identify LukAB and Hla as important virulence factors in the modulation of bacterial persistence and macrophage infiltrates during S. aureus biofilm formation in vivo.
FIG 7 .
LukAB and Hla are important for S. aureus biofilm formation in vivo. Shown are the bacterial burdens associated with the soft tissue surrounding the knee (A), knee joint (B), and femur (C) of mice infected with WT S. aureus and isogenic Δhla, ΔlukAB, and ΔlukAB hla mutant strains at days 3 and 7 postinfection (n = 10 mice per strain for each time point). Results are expressed as the number of CFU per gram of tissue to correct for differences in tissue sample size. (D) Quantitation of F4/80+ macrophages infiltrating the soft tissue of mice infected with WT S. aureus and isogenic Δhla, ΔlukAB, and ΔlukAB hla mutant strains. Significant differences are denoted by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired two-tailed Student t test). Results are combined from two independent experiments.
DISCUSSION
S. aureus subverts the host immune response by numerous mechanisms, including increased resistance to cationic antimicrobial peptides, impairment of phagocyte recruitment, interference with Ab-mediated opsonization and complement activation, and resistance to intracellular killing (13). In addition, biofilm formation further protects S. aureus from the host innate immune response, representing a communal virulence determinant (4, 25, 60). We have previously demonstrated that biofilm formation shields S. aureus from Toll-like receptor detection and interferes with macrophage activation in vivo (4, 15). Here we explored the genetic basis of how biofilm growth prevents macrophage phagocytosis. Our earlier study showed that macrophages are capable of phagocytosing bacteria from mechanically disrupted, but not intact, biofilms, suggesting that the size of the biofilm and/or the density of its matrix represents a physical obstacle, a phenomenon referred to as “frustrated phagocytosis” (4, 61, 62). Here we extend these findings to demonstrate that S. aureus biofilms also secrete proteinaceous factors that actively inhibit macrophage phagocytosis and induce cell death. Interestingly, this proteinaceous component was evident mainly in intact biofilms, as conditioned medium from mechanically disrupted biofilms or planktonic cultures grown to early or late stationary phase failed to prevent phagocytosis to the same extent, although the bacterial numbers and secreted protein concentrations were similar (data not shown; Fig. 1D; see Fig. S2 in the supplemental material). The preferential ability of intact S. aureus biofilms to inhibit macrophage phagocytosis suggested the involvement of quorum-sensing mechanisms that are enriched during biofilm growth and dissipate once the biofilm structure has been disrupted. This was confirmed by the finding that biofilm-mediated macrophage dysfunction, in particular, cell death, was less pronounced following exposure to conditioned medium from a Δagr mutant biofilm. These observations, combined with the fact that conditioned medium from PAS-treated biofilms maintained inhibitory activity whereas lysostaphin-treated biofilms did not, strongly implied the importance of the ability of active protein secretion by the biofilm (based on proteinase K sensitivity) to inhibit macrophage phagocytosis and induce cell death.
The identification of candidate proteins responsible for inducing macrophage death and inhibiting phagocytosis was facilitated by a relatively new quantitative mass spectrometry technique, namely, SWATH-MS (48). After generating a protein library from our combined sample sets (i.e., WT biofilm, Δagr mutant biofilm, WT planktonic, and Δagr mutant planktonic), comparisons were performed to identify the most abundant proteins unique to WT biofilm-conditioned medium. While this list included some proteins undoubtedly released as a result of cell lysis (i.e., metabolic enzymes and ribosomal subunits), we focused on known secreted toxins and proteases that were also detected. Of the proteins on this list, LukB and its partner component LukA were shown to have a significant impact on macrophage phagocytosis and viability. LukAB is unique among leukocidins for its ability to either remain cell wall associated or be released into the extracellular milieu (49–51). A recent study has shown that human leukocytes are exquisitely sensitive to the cytolytic actions of LukAB because of its specificity for CD11b (63) mediated by the binding of a specific glutamic acid residue (323A) (56). While murine leukocytes are less sensitive to LukAB (63, 64), this toxin was still implicated in S. aureus pathogenesis in a murine renal abscess model (50), which was confirmed in the present study by using a murine S. aureus orthopedic implant biofilm model. Therefore, although human cells display a greater sensitivity to LukAB, it is clear from our report and work by others that this bicomponent leukotoxin is also active toward murine leukocytes.
In addition to LukAB, Hla also significantly contributed to biofilm-associated murine macrophage death and phagocytosis. The toxic effects of Hla are well known, and while a recent publication has demonstrated the cytoprotective effects of S. aureus Hla within phagosomes (65), it is important to note that this scenario is not applicable to our studies, given that our phagocytosis assay utilized microspheres and not viable bacteria. This strategy was employed to avoid confounding effects of toxins secreted by live planktonic S. aureus if they were used to measure macrophage phagocytosis, which could not be discriminated from biofilm-secreted molecules. Interestingly, the already potent cytolytic effects of S. aureus Hla were enhanced with the addition of purified bioactive LukAB. Furthermore, treatment of ΔlukA and ΔlukB mutant biofilm-conditioned media with an Hla-neutralizing Ab significantly dampened the macrophage phagocytic block. These results suggest a synergistic effect whereby the presence of LukAB enhances or accelerates Hla-mediated macrophage dysfunction, perhaps via enhanced binding, localization to the cell membrane, or regulation of intracellular signaling pathways.
Previous studies have demonstrated the importance of LukAB or Hla for S. aureus pathogenesis in murine models of renal abscess (50), pneumonia (66, 67), skin infection (68, 69), bacteremia (70, 71), peritonitis (69, 72, 73), and other localized infection models (41). However, it should be noted that a ΔlukAB single mutant displayed no attenuation of virulence in murine models of skin infection and bacteremia (64). In support of our in vitro findings, our study is the first to report that LukAB and Hla cooperate to regulate S. aureus virulence in a murine orthopedic implant biofilm infection model. While a ΔlukAB or Δhla single mutant displayed decreased bacterial burdens in some tissues, a ΔlukAB Δhla double mutant showed the largest reduction in bacterial numbers compared to mice infected with the isogenic WT strain. In further support of this synergistic effect, ΔlukAB Δhla mutant-infected mice displayed the greatest increase in macrophage infiltrates. While these in vivo data reveal an important synergistic role for LukAB and Hla during S. aureus biofilm infection, it remains unclear whether these toxins directly alter macrophage survival (i.e., via cell lysis) or indirectly tailor the immune response (i.e., elicit tissue damage resulting in altered cytokine signaling to promote macrophage phagocytosis and proinflammatory activity). We also found that biofilm-conditioned medium elicited similar cytotoxic effects on proinflammatory (classically activated) and profibrotic (alternatively activated) macrophages (data not shown). We further investigated whether biofilm-conditioned medium augmented macrophage CD11b expression, which binds LukAB, but found no evidence to support this possibility. Another potential mechanism to link the synergistic effects of Hla and LukAB is the zinc-dependent metalloproteinase ADAM10, since Hla is known to recognize ADAM10 on the host cell surface (74). Once bound, Hla augments ADAM10 activity (75), which could result in increased LukAB dissociation from the bacterial cell surface and, in turn, enhanced Hla activity. However, it should be noted that this interaction may provide an explanation for our in vivo findings but fails to inform the apparent synergistic effect in our in vitro assay, since macrophages were treated with biofilm-conditioned medium cleared of bacteria. The mechanism whereby LukAB and Hla influence biofilm formation in vivo is an area of active investigation in our laboratory.
While the effect of S. aureus biofilm-conditioned medium on macrophage viability was largely LukAB/Hla dependent, it appears that part of the phagocytic block was not. SWATH-MS identified other potential candidate proteins that could act in concert with Hla/LukAB to maximally impair macrophage phagocytosis, including pyrimidine biosynthetic enzymes, phosphotransferase proteins, pyruvate kinase, and histidine metabolic enzymes. Along these lines, it is important to recognize that biofilms represent a diverse bacterial population influenced by a myriad of complex gradients (e.g., nutrient, oxygen, pH), metabolic activity, and virulence potential (76–79). For example, while our studies utilized conditioned medium collected from static biofilms, a subpopulation of planktonic or “dispersed” cells is also present at the air-liquid interface. While this was a 2- to 3-log smaller cell population than that in the biofilm (see Fig. S5 in the supplemental material), it is probably naive to disregard its impact, particularly in light of recent studies demonstrating the secretory potential of biofilm-dispersed cells (80). On the basis of this evidence, we posit that S. aureus biofilms prevent macrophage phagocytosis in part by inducing cell death through LukAB and Hla production (Fig. 8). However, since the phagocytic block was still evident even when macrophage viability was restored to 100% following LukAB/Hla inactivation, this suggests that additional proteins act together with Hla/LukAB to maximally inhibit macrophage phagocytic activity (Fig. 8).
FIG 8 .

Proposed model of S. aureus biofilm-induced macrophage dysfunction. S. aureus biofilms produce an abundance of LukAB, which can associate with the cell surface as a toxin reservoir or be actively secreted into the extracellular milieu. Biofilms also secrete Hla, which acts synergistically with LukAB to elicit macrophage dysfunction. Other secreted proteins may also impact macrophage phagocytosis.
Collectively, this study demonstrates that S. aureus biofilms have evolved mechanisms to establish persistent infections in part by actively preventing macrophage phagocytosis and eliciting cell death that is mediated by the synergistic actions of LukAB and Hla. These findings not only identify a novel interaction for these secreted proteins but also highlight the layers of redundancy within the S. aureus virulence repertoire.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice (8 weeks of age) were obtained from Charles River Laboratories (Frederick, MD). This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
S. aureus strains.
MRSA clinical isolate USA300 LAC 13C (31–34) was cured of its 27-kb LAC-p03 plasmid encoding erythromycin resistance (81) by screening for spontaneous erythromycin sensitivity as previously described (35). The isogenic LAC 13C Δagr mutant strain was generated as previously reported (9). The isogenic LAC 13C Δhla mutant strain was constructed by insertion mutation by site-directed mutagenesis with the pE194 erythromycin resistance cassette (ermB) as previously described (72), with an hla-complemented strain (72) and an hla constitutively active strain (82) included to confirm the specificity of toxin action. The USA300 JE2 Nebraska Transposon Mutant Library (NTML) (83) ΔlukA ΔlukH, ΔlukB ΔlukG, ΔlukD, and Δspl mutants were moved to the USA300 LAC 13C background by transduction with bacteriophage φ11. In addition, allelic-replacement lukA and lukB mutants and complemented strains were used to confirm findings obtained with the transposon mutants (50). Importantly, all of the mutant strains utilized in these studies grew to comparable extents during the 6-day biofilm culture period and also secreted similar concentrations of extracellular proteins (see Fig. S2 in the supplemental material).
Preparation of biofilm-conditioned media and treatments.
S. aureus static biofilms were formed on two-well glass chamber slides (Nunc, Rochester, NY) or 12-well plates (Becton, Dickinson, Franklin Lakes, NJ) in RPMI 1640 medium supplemented with 1% Casamino Acids (CAA; Becton, Dickinson) as previously described (4, 84, 85). Spent medium was replaced daily, whereupon conditioned medium for experiments was collected from 6-day-old USA300 LAC biofilms 24 h following the last medium change and filtered (0.2 µm). Our prior studies have established that 6-day-old S. aureus biofilms propagated in RPMI 1640 are mature on the basis of the presence of tower structures and thickness (4, 15). For comparisons, planktonic culture-conditioned medium was prepared by growing an overnight culture of USA300 LAC to early (12 h) or late (18 h) stationary phase. As no significant differences in phagocytosis or viability were observed when macrophages were treated with conditioned medium from either early or late stationary phase cultures (data not shown), early stationary phase supernatant was utilized throughout these studies. In some experiments, conditioned medium was collected from biofilms that were mechanically disrupted by trituration. Specifically, fresh medium was added to 6-day-old USA300 LAC biofilms and following mechanical dispersal, conditioned medium was collected 24 h later and clarified by filtration (0.2 µm). Where indicated, biofilm-conditioned medium was treated with 10 µg/ml proteinase K for 1 h at 37°C to degrade proteins. Biofilms were also either exposed to 10 µg/ml PAS or mechanically disrupted and incubated with 50 µg/ml lysostaphin (both from Sigma, St. Louis, MO) for 24 h prior to collection to assess whether proteins released by active secretion or bacterial lysis, respectively, were responsible for macrophage dysfunction. In some experiments, fresh RPMI 1640 medium was spiked with 10 µg of purified S. aureus Hla (Sigma) or 25 µg of purified bioactive or inactive LukAB (56) to examine effects on macrophage phagocytosis and viability. For some experiments, biofilm-conditioned medium was incubated with rabbit anti-staphylococcal Hla antiserum or control rabbit serum (both from Sigma) for 30 min prior to macrophage treatment.
Macrophage phagocytosis and cell viability assay.
Mice were euthanized with an overdose of inhaled isoflurane, followed by cervical dislocation as a secondary method to ensure death prior to the harvesting of long bones to prepare bone marrow-derived macrophages as previously described (4, 86). Bone marrow-derived macrophages were labeled with 5 µM CellTracker Blue (CTB; Molecular Probes, San Diego, CA) as previously described (4, 86). CTB-labeled macrophages were added to sterile two-well glass chamber slides (5 × 106 cells/chamber) and allowed to adhere for 2 h. Next, macrophages were exposed to undiluted biofilm- or planktonic culture-conditioned medium for 2 h at 37°C, since a robust phenotype was observed under these conditions (see Fig. S1A in the supplemental material), whereupon 4.5 × 1010 green fluorescent microspheres (2.0 µm; Molecular Probes) were added for 1 h to assess phagocytic activity. Fluorescent microspheres were used instead of live S. aureus, since live bacteria actively secrete factors during planktonic growth that would have been impossible to differentiate from biofilm-derived molecules and pilot studies revealed that similar results were obtained with both reagents (see Fig. S1A). To test the effects of opsonization in this assay, beads were preincubated in RPMI 1640 medium with 10% fetal bovine serum for 30 min at 37°C and washed prior to macrophage addition. The extent of macrophage phagocytosis of fluorescent microspheres was similar with or without opsonization (see Fig. S1B). Macrophage viability was assessed by propidium iodide (PI) staining at the end of the 3-h incubation period. Macrophage phagocytosis and viability were assessed with a Zeiss 510 META laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) and quantitated by determining the number of phagocytic or PI+ macrophages in at least eight random fields of view (magnification, ×63) with ZEN 2009 software (Carl Zeiss). Data are expressed as either percent phagocytosis or viable macrophages, which reflects manual counts within a given experiment, or total phagocytosed beads or viable macrophages per ×63 field, where the total number of events within a given experiment was calculated by an ImageJ plugin (ImageJ 1.47v; Wayne Rasband, NIH, Bethesda, MD) based on an experimentally determined average pixel area and RGB color code specific to CTB-labeled macrophage overlap with microspheres or PI+ cells. Pilot studies confirmed that identical results were obtained by this method and manual counting (data not shown).
SWATH-MS proteomics and analysis.
Conditioned medium from WT and Δagr mutant S. aureus strains grown under biofilm or planktonic conditions as described above was harvested and treated with a protease inhibitor cocktail (Roche, Basel, Switzerland), and proteins were precipitated with 20% TCA. Relative protein concentrations were compared between groups by using three independent replicates per sample by SWATH-MS as previously described (48). A Z transformation, followed by a Z test, was performed on all of the positively identified proteins (>98% confidence) with two sample sets at a time (i.e., WT biofilm versus Δagr biofilm or WT biofilm versus WT planktonic) to assess significant differences in relative protein abundance as previously described (48). Identified proteins were functionally grouped by UniProt identifier utilizing David bioinformatic resource 6.7 (http://david.abcc.ncifcrf.gov/) (87). Protein-protein interactions were predicted by using STRING 9.05 (http://string-db.org) (88, 89).
Western blot assays.
Conditioned medium from WT S. aureus biofilm and planktonic cultures was sterile filtered and treated with a protease inhibitor cocktail (Roche). Samples were TCA precipitated overnight and suspended in 30 µl of Laemmli buffer, whereupon 5 µl of each sample was loaded onto a gel, transferred to a nitrocellulose membrane, and probed for S. aureus LukA and Hla.
ELISA.
Conditioned medium from WT S. aureus biofilm and planktonic cultures was sterile filtered (0.2 µm) and analyzed for Hla concentrations by direct enzyme-linked immunosorbent assay (ELISA). Briefly, to generate a standard curve, serial dilutions of purified S. aureus Hla (Sigma) were diluted in carbonate-bicarbonate buffer and incubated in 96-well ELISA plates overnight at 4°C along with experimental samples. The following day, wells were washed extensively with 1× phosphate-buffered saline (PBS)–0.5% Tween and incubated with a rabbit anti-Hla Ab, followed by an anti-rabbit IgG-horseradish peroxidase Ab (both from Sigma) for detection. Plates were developed with a 3,3′,5,5′-tetramethylbenzidine substrate (Becton, Dickinson), and the reaction was halted with stop solution prior to reading at 450 nm. Hla concentrations were normalized to total protein as measured by a Pierce bicinchoninic acid protein assay.
Mouse model of S. aureus orthopedic implant biofilm infection.
To simulate infectious complications in patients following surgical device placement, a mouse S. aureus orthopedic implant biofilm infection model was used as previously described (57, 58). Briefly, animals were anesthetized with ketamine-xylazine (100 and 5 mg/kg, respectively) and the surgical site was disinfected with povidone-iodine. A medial parapatellar arthrotomy with lateral displacement of the quadriceps-patella was performed to access the distal femoral trochlea. A burr hole in the femoral intercondylar notch extending into the intramedullary canal was created with a 26-gauge needle, whereupon a precut 0.8-cm orthopedic-grade Kirschner wire (0.6-mm diameter, Nitinol [nickel-titanium]; Custom Wire Technologies, Port Washington, WI) was inserted into the intramedullary canal, leaving ~1 mm protruding into the joint space. A total of 103 CFU of WT USA300 LAC or an isogenic Δhla, ΔlukAB, or ΔlukAB Δhla double mutant strain was inoculated at the implant tip. Animals received Buprenex (0.1 mg/kg subcutaneously; Reckitt Benckiser, Hull, United Kingdom) immediately after infection and 24 h later for pain relief. After this interval, all mice exhibited normal ambulation and no discernible pain behavior.
Flow cytometry.
To characterize macrophage infiltrates in inflamed soft tissues surrounding the knee joint during S. aureus biofilm infection, tissues were excised, dissociated with the rubber end of a plunger from a 3-ml syringe, and passed through a 35-µm filter (BD Falcon, Bedford, MA). The resulting filtrate was washed with 1× PBS, and cells were collected by centrifugation (300 × g, 10 min), whereupon red blood cells were lysed with BD Pharm Lyse (BD Biosciences, San Diego, CA), resuspended in 1× PBS, and incubated in Fc Block (BD Biosciences) to minimize nonspecific Ab binding. Cells were stained with CD45-allophycocyanin, Ly6G-phycoerythrin (PE), Ly6C-peridinin chlorophyll protein-Cy5.5, F4/80-PE-Cy7, and CD11b-eFluor 450, and viability was determined with a LIVE/DEAD Fixable Blue Dead Cell Stain (Life Technologies, Eugene, OR). All fluorochrome-conjugated Abs were purchased from BD Biosciences or eBioscience (San Diego, CA). An aliquot of cells was stained with isotype-matched control Abs to assess the degree of nonspecific staining. The number of events analyzed per sample ranged from 10,000 to 400,000. Analysis was performed with BD FACSDiva software with doublet exclusion performed, and cells were gated on the “live” CD45+ population.
Statistical analysis.
The significance of differences between experimental groups was determined with either an unpaired two-tailed Student t test or one-way analysis of variance (ANOVA), followed by Bonferroni’s multiple-comparison test, in GraphPad Prism 4 (GraphPad, La Jolla, CA). For all analyses, a P value of less than 0.05 was considered statistically significant.
SUPPLEMENTAL MATERIAL
Fluorescent microspheres can be used as a surrogate for live S. aureus in the macrophage phagocytosis assay. (A) Bone marrow-derived macrophages were incubated for 3 h with fresh medium or conditioned medium collected from WT biofilms, whereupon phagocytosis was assessed by measuring the uptake of planktonic S. aureus plus fluorescent microspheres (beads) or microspheres alone. (B) Beads were incubated in RPMI 1640 with or without 10% fetal bovine serum prior to their addition to bone marrow-derived macrophages to assess the impact of opsonization on phagocytic uptake. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments. Download
Bacterial counts and extracellular protein concentrations of S. aureus WT planktonic cells and biofilm and isogenic mutant biofilms are similar. (A) All of the S. aureus strains used in these studies grew to comparable extents after 6 days of culture in RPMI 1640 supplemented with 1% CAA, as indicated by the number of viable bacteria determined by quantitative culture with results expressed in CFU per milliliter. (B) Quantitation of proteins secreted by each strain. Data are representative of at least two independent experiments. Strains are abbreviated as follows: Δagr, accessory gene regulator mutant; Δhla, alpha-toxin mutant; Δhla(pHla), plasmid-complemented alpha-toxin mutant; hlaon, WT strain constitutively expressing alpha-toxin; ΔlukA, leukocidin AB component single mutant; ΔlukB, leukocidin AB component single mutant; ΔlukAB::lukAB, chromosomally complemented leukocidin AB double mutant; ΔlukAB Δhla, leukocidin AB and alpha-toxin double mutant. Download
Validation of S. aureus Hla action on macrophage dysfunction. Bone marrow-derived macrophages were incubated for 2 h with fresh medium alone or fresh medium with purified S. aureus Hla plus an isotype control or Hla Ab (α-Hla). After a 2-h treatment period, macrophage phagocytosis of fluorescent microspheres (A) and total viable macrophages (B) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (*, P < 0.05; ***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments. Download
Purified LukAB augments macrophage cytotoxicity. Bone marrow-derived macrophages were exposed to fresh or biofilm-conditioned medium from WT S. aureus or the isogenic ΔlukAB mutant plus purified LukAB or inactive LukABE323A. After a 3-h treatment period, the percentage of macrophages phagocytosing fluorescent microspheres (A) and percentage of viable macrophages (B) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments. Download
Characterization of S. aureus biofilm growth in 1% CAA. Total numbers of dispersed (supernatant) and biofilm-associated bacteria in RPMI 1640 supplemented with 1% CAA were assessed by serial dilution (CFU per well) throughout the 6-day biofilm maturation process. Download
Proteins found to be in greater abundance in WT S. aureus biofilm-conditioned medium than in Δagr mutant S. aureus biofilm-conditioned medium.
Proteins found to be in greater abundance in WT S. aureus biofilm-conditioned medium than in WT S. aureus planktonic-organism-conditioned medium.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Disease grants P01 AI083211 (Project 4 to T.K.), R01AI099394, and R01AI105129 (to V.J.T.); an American Heart Association predoctoral fellowship 14PRE20380400 (to T.D.S.); and training grant T32AI007180 (D.B.A.J.). We thank the Nebraska Center for Staphylococcal Research (CSR) and the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for use of the NTML, which was created in part with funds from the Department of Defense to K.W.B. and P.D.F.
We thank Amanda Angle for her contributions to Fig. S1A and Keer Sun, Jessica Snowden, Jill Poole, and Casey Gries for critical review of the manuscript. We also thank Jennifer Endres, Vijaya Vajjala, and Todd Widhelm for assistance with the NTML and Vinai Thomas and Jeff Bose for assistance with phage transduction. We acknowledge Jeff Bose for graciously providing the constitutively active Hla strain. Additionally, we thank Pawel Ciborowski and Jayme Horning in the University of Nebraska Medical Center Mass Spectrometry and Proteomics Core Facility for their invaluable assistance with the SWATH-MS analysis.
Footnotes
Citation Scherr TD, Hanke ML, Huang O, James DBA, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T. 2015. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. mBio 6(4):e01021-15. doi:10.1128/mBio.01021-15.
REFERENCES
- 1.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. 2012. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Negrate G. 2012. Viral interference with innate immunity by preventing NF-κB activity. Cell Microbiol 14:168–181. doi: 10.1111/j.1462-5822.2011.01720.x. [DOI] [PubMed] [Google Scholar]
- 3.Collette JR, Lorenz MC. 2011. Mechanisms of immune evasion in fungal pathogens. Curr Opin Microbiol 14:668–675. doi: 10.1016/j.mib.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanke ML, Kielian T. 2012. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Front Cell Infect Microbiol 2:62. doi: 10.3389/fcimb.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. 2012. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog 8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohde H, Burandt EC, Siemssen N, Frommelt L, Burdelski C, Wurster S, Scherpe S, Davies AP, Harris LG, Horstkotte MA, Knobloch JK, Ragunath C, Kaplan JB, Mack D. 2007. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 28:1711–1720. doi: 10.1016/j.biomaterials.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Kiedrowski MR, Horswill AR. 2011. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 10.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick F, Humphreys H, O’Gara JP. 2005. The genetics of staphylococcal biofilm formation—will a greater understanding of pathogenesis lead to better management of device-related infection? Clin Microbiol Infect 11:967–973. doi: 10.1111/j.1469-0691.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 12.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nizet V. 2007. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol 120:13–22. doi: 10.1016/j.jaci.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Rahman MM, McFadden G. 2011. Modulation of NF-kappaB signalling by microbial pathogens. Nat Rev Microbiol 9:291–306. doi: 10.1038/nrmicro2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. 2013. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol 190:2159–2168. doi: 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aderem A. 2003. Phagocytosis and the inflammatory response. J Infect Dis 187(Suppl 2):S340–S345. doi: 10.1086/374747. [DOI] [PubMed] [Google Scholar]
- 17.Underhill DM, Gantner B. 2004. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect 6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Underhill DM, Goodridge HS. 2012. Information processing during phagocytosis. Nat Rev Immunol 12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto M. 2006. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr Top Microbiol Immunol 306:251–258. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Head NE. 2002. Persistent infections and immunity in cystic fibrosis. Front Biosci 7:d442–d457. [DOI] [PubMed] [Google Scholar]
- 21.Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. 2007. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun 75:2612–2620. doi: 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 171:4329–4339. doi: 10.4049/jimmunol.171.8.4329. [DOI] [PubMed] [Google Scholar]
- 23.Kristian SA, Birkenstock TA, Sauder U, Mack D, Götz F, Landmann R. 2008. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis 197:1028–1035. doi: 10.1086/528992. [DOI] [PubMed] [Google Scholar]
- 24.Mittal R, Sharma S, Chhibber S, Harjai K. 2006. Effect of macrophage secretory products on elaboration of virulence factors by planktonic and biofilm cells of Pseudomonas aeruginosa. Comp Immunol Microbiol Infect Dis 29:12–26. doi: 10.1016/j.cimid.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Hanke ML, Angle A, Kielian T. 2012. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS One 7:e42476. doi: 10.1371/journal.pone.0042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadowska B, Wieckowska-Szakiel M, Paszkiewicz M, Rozalska B. 2013. The immunomodulatory activity of Staphylococcus aureus products derived from biofilm and planktonic cultures. Arch Immunol Ther Exp (Warsz) 61:413–420. doi: 10.1007/s00005-013-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. 2011. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, MAPK phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol 11:143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. 2011. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun 79:2267–2276. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiliopoulou AI, Kolonitsiou F, Krevvata MI, Leontsinidis M, Wilkinson TS, Mack D, Anastassiou ED. 2012. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase Staphylococcus epidermidis. FEMS Microbiol Lett 330:56–65. doi: 10.1111/j.1574-6968.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 30.Cerca F, Andrade F, França Â, Andrade EB, Ribeiro A, Almeida AA, Cerca N, Pier G, Azeredo J, Vilanova M. 2011. Staphylococcus epidermidis biofilms with higher proportions of dormant bacteria induce a lower activation of murine macrophages. J Med Microbiol 60:1717–1724. doi: 10.1099/jmm.0.031922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burlak C, Hammer CH, Robinson MA, Whitney AR, McGavin MJ, Kreiswirth BN, Deleo FR. 2007. Global analysis of community-associated methicillin-resistant Staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection. Cell Microbiol 9:1172–1190. doi: 10.1111/j.1462-5822.2006.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diep BA, Palazzolo-Ballance AM, Tattevin P, Basuino L, Braughton KR, Whitney AR, Chen L, Kreiswirth BN, Otto M, DeLeo FR, Chambers HF.. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 3:e3198. doi: 10.1371/journal.pone.0003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Saïd-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 34.Bubeck Wardenburg J, Palazzolo-Ballance A, Otto M, Schneewind O, DeLeo F. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis 198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bose JL, Lehman MK, Fey PD, Bayles KW. 2012. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Krishnan V, Macon K, Manne K, Narayana SV, Schneewind O. 2013. Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J Biol Chem 288:29440–29452. doi: 10.1074/jbc.M113.502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadykov MR, Bayles KW. 2012. The control of death and lysis in staphylococcal biofilms: a coordination of physiological signals. Curr Opin Microbiol 15:211–215. doi: 10.1016/j.mib.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wecke J, Lahav M, Ginsburg I, Kwa E, Giesbrecht P. 1986. Inhibition of wall autolysis of staphylococci by sodium polyanethole sulfonate “liquoid.” Arch Microbiol 144:110–115. doi: 10.1007/BF00414719. [DOI] [PubMed] [Google Scholar]
- 41.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bronner S, Monteil H, Prévost G. 2004. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 28:183–200. doi: 10.1016/j.femsre.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goerke C, Campana S, Bayer MG, Döring G, Botzenhart K, Wolz C. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect Immun 68:1304–1311. doi: 10.1128/IAI.68.3.1304-1311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao H, Dai M, Wang Y, Huang L, Yuan Z. 2012. Key genetic elements and regulation systems in methicillin-resistant Staphylococcus aureus. Future Microbiol 7:1315–1329. doi: 10.2217/fmb.12.107. [DOI] [PubMed] [Google Scholar]
- 47.Savage VJ, Chopra I, O’Neill AJ. 2013. Population diversification in Staphylococcus aureus biofilms may promote dissemination and persistence. PLoS One 8:e62513. doi: 10.1371/journal.pone.0062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haverland NA, Fox HS, Ciborowski P. 2014. Quantitative proteomics by SWATH-MS reveals altered expression of nucleic acid binding and regulatory proteins in HIV-1-infected macrophages. J Proteome Res 13:2109–2119. doi: 10.1021/pr4012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. 2010. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. 2013. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun 81:1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Recsei P, Kreiswirth B, O’Reilly M, Schlievert P, Gruss A, Novick RP. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet 202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 53.Peng H-L, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol 170:4365–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thay B, Wai SN, Oscarsson J. 2013. Staphylococcus aureus alpha-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS One 8:e54661. doi: 10.1371/journal.pone.0054661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DuMont AL, Yoong P, Liu X, Day CJ, Chumbler NM, James DB, Alonzo F III, Bode NJ, Lacy DB, Jennings MP, Torres VJ. 2014. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect Immun 82:1268–1276. doi: 10.1128/IAI.01444-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. 2010. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One 5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T. 2014. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol 192:3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T. 2015. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J Immunol 194:3861–3872 doi: 10.4049/jimmunol.1402689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. 2011. Protective role of IL-1beta against post-arthroplasty Staphylococcus aureus infection. J Orthop Res 29:1621–1626. doi: 10.1002/jor.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bainton DF, Takemura R, Stenberg PE, Werb Z. 1989. Rapid fragmentation and reorganization of Golgi membranes during frustrated phagocytosis of immobile immune complexes by macrophages. Am J Pathol 134:15–26. [PMC free article] [PubMed] [Google Scholar]
- 62.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun 70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DuMont AL, Yoong P, Day CJ, Alonzo F III, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koziel J, Chmiest D, Bryzek D, Kmiecik K, Mizgalska D, Maciag-Gudowska A, Shaw LN, Potempa J. 2015. The Janus face of α-toxin: a potent mediator of cytoprotection in staphylococci-infected macrophages. J Innate Immun 7:187–198 doi: 10.1159/000368048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bubeck Wardenburg J, Patel RJ, Schneewind O. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 68.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel AH, Nowlan P, Weavers ED, Foster T. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun 55:3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. 2012. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis 206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menzies BE, Kernodle DS. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun 64:1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Reilly M, de Azavedo JC, Kennedy S, Foster TJ. 1986. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog 1:125–138. [DOI] [PubMed] [Google Scholar]
- 73.Rauch S, DeDent AC, Kim HK, Bubeck Wardenburg J, Missiakas DM, Schneewind O. 2012. Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection. Infect Immun 80:3721–3732. doi: 10.1128/IAI.00442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilke GA, Bubeck Wardenburg J. 2010. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A 107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. 2011. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 77.Fey PD, Olson ME. 2010. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol 5:917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leibig M, Liebeke M, Mader D, Lalk M, Peschel A, Götz F. 2011. Pyruvate formate lyase acts as a formate supplier for metabolic processes during anaerobiosis in Staphylococcus aureus. J Bacteriol 193:952–962. doi: 10.1128/JB.01161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 80.Chua SL, Liu Y, Yam JK, Chen Y, Vejborg RM, Tan BG, Kjelleberg S, Tolker-Nielsen T, Givskov M, Yang L. 2014. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat Commun 5:4462. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy AD, Porcella SF, Martens C, Whitney AR, Braughton KR, Chen L, Craig CT, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. 2010. Complete nucleotide sequence analysis of plasmids in strains of Staphylococcus aureus clone USA300 reveals a high level of identity among isolates with closely related core genome sequences. J Clin Microbiol 48:4504–4511. doi: 10.1128/JCM.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bose JL, Daly SM, Hall PR, Bayles KW. 2014. Identification of the Staphylococcus aureus vfrAB operon, a novel virulence factor regulatory locus. Infect Immun 82:1813–1822. doi: 10.1128/IAI.01655-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-00512. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cassat JE, Lee CY, Smeltzer MS. 2007. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol Biol 391:127–144. doi: 10.1007/978-1-59745-468-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, Friedman DB, Heinrichs DE, Dunman PM, Skaar EP. 2010. Staphylococcus aureus Fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun 78:1618–1628. doi: 10.1128/IAI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang DW, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Snel B, Lehmann G, Bork P, Huynen MA. 2000. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res 28:3442–3444. doi: 10.1093/nar/28.18.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. 2013. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescent microspheres can be used as a surrogate for live S. aureus in the macrophage phagocytosis assay. (A) Bone marrow-derived macrophages were incubated for 3 h with fresh medium or conditioned medium collected from WT biofilms, whereupon phagocytosis was assessed by measuring the uptake of planktonic S. aureus plus fluorescent microspheres (beads) or microspheres alone. (B) Beads were incubated in RPMI 1640 with or without 10% fetal bovine serum prior to their addition to bone marrow-derived macrophages to assess the impact of opsonization on phagocytic uptake. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments. Download
Bacterial counts and extracellular protein concentrations of S. aureus WT planktonic cells and biofilm and isogenic mutant biofilms are similar. (A) All of the S. aureus strains used in these studies grew to comparable extents after 6 days of culture in RPMI 1640 supplemented with 1% CAA, as indicated by the number of viable bacteria determined by quantitative culture with results expressed in CFU per milliliter. (B) Quantitation of proteins secreted by each strain. Data are representative of at least two independent experiments. Strains are abbreviated as follows: Δagr, accessory gene regulator mutant; Δhla, alpha-toxin mutant; Δhla(pHla), plasmid-complemented alpha-toxin mutant; hlaon, WT strain constitutively expressing alpha-toxin; ΔlukA, leukocidin AB component single mutant; ΔlukB, leukocidin AB component single mutant; ΔlukAB::lukAB, chromosomally complemented leukocidin AB double mutant; ΔlukAB Δhla, leukocidin AB and alpha-toxin double mutant. Download
Validation of S. aureus Hla action on macrophage dysfunction. Bone marrow-derived macrophages were incubated for 2 h with fresh medium alone or fresh medium with purified S. aureus Hla plus an isotype control or Hla Ab (α-Hla). After a 2-h treatment period, macrophage phagocytosis of fluorescent microspheres (A) and total viable macrophages (B) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (*, P < 0.05; ***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments. Download
Purified LukAB augments macrophage cytotoxicity. Bone marrow-derived macrophages were exposed to fresh or biofilm-conditioned medium from WT S. aureus or the isogenic ΔlukAB mutant plus purified LukAB or inactive LukABE323A. After a 3-h treatment period, the percentage of macrophages phagocytosing fluorescent microspheres (A) and percentage of viable macrophages (B) were quantitated by confocal microscopy. Significant differences are denoted by asterisks (***, P < 0.001; unpaired two-tailed Student t test). Results are representative of at least two independent experiments. Download
Characterization of S. aureus biofilm growth in 1% CAA. Total numbers of dispersed (supernatant) and biofilm-associated bacteria in RPMI 1640 supplemented with 1% CAA were assessed by serial dilution (CFU per well) throughout the 6-day biofilm maturation process. Download
Proteins found to be in greater abundance in WT S. aureus biofilm-conditioned medium than in Δagr mutant S. aureus biofilm-conditioned medium.
Proteins found to be in greater abundance in WT S. aureus biofilm-conditioned medium than in WT S. aureus planktonic-organism-conditioned medium.