ABSTRACT
Glycosylation is a widespread mechanism employed by both eukaryotes and bacteria to increase the functional diversity of their proteomes. The nontypeable Haemophilus influenzae glycosyltransferase HMW1C mediates unconventional N-linked glycosylation of the adhesive protein HMW1, which is encoded in a two-partner secretion system gene cluster that also encodes HMW1C. In this system, HMW1 is modified in the cytoplasm by sequential transfer of hexose residues. In the present study, we examined Kingella kingae and Aggregatibacter aphrophilus homologues of HMW1C that are not encoded near a gene encoding an obvious acceptor protein. We found both homologues to be functional glycosyltransferases and identified their substrates as the K. kingae Knh and the A. aphrophilus EmaA trimeric autotransporter proteins. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis revealed multiple sites of N-linked glycosylation on Knh and EmaA. Without glycosylation, Knh and EmaA failed to facilitate wild-type levels of bacterial autoaggregation or adherence to human epithelial cells, establishing that glycosylation is essential for proper protein function.
IMPORTANCE
This work emphasizes the importance of glycosylation for proper function of bacterial proteins. Here we show that the Kingella kingae Knh and the Aggregatibacter aphrophilus EmaA trimeric autotransporter proteins are N-glycosylated by novel homologues of the Haemophilus influenzae HMW1C glycosyltransferase, highlighting the first examples of trimeric autotransporters that are modified by HMW1C-like enzymes. In the absence of glycosylation, Knh and EmaA lack adhesive activity. This work has relevance to our understanding of bacterial pathogenicity and expression of potential vaccine antigens.
INTRODUCTION
Glycosylation provides a means by which a cell can further specify a particular function for a protein. Once thought to be unique to eukaryotic organisms, glycosylation of proteins is now recognized to be common in prokaryotes (1). Modification of bacterial proteins promotes proper folding (2), adhesive activity (3), solubility (4), antigenic variation (5), and protection against proteases (6). There are two major forms of protein glycosylation: O-linked, in which the modification is attached to the side chain oxygen of a serine or threonine residue, and N-linked, in which the modification is attached to the amide nitrogen of an asparagine residue. Rarely, proteins can be glycosylated on residues other than serine, threonine, or asparagine (7).
O-linked glycosylation in bacteria generally takes place in the cytoplasm and involves a series of glycosyltransferases that sequentially build polysaccharide chains on the acceptor protein. These glycosyltransferases are not membrane bound and are commonly able to transfer only a single specific activated saccharide molecule (8). In a limited number of cases, O-glycosylation has been shown to occur in the periplasm. Specifically, the O-linked modification of pili in Pseudomonas aeruginosa and Neisseria meningitidis involves an inner membrane bound oligosaccharyltransferase (9). At present, there is no recognized consensus sequence for modification by O-linked glycosylation, though existing evidence suggests that there may be a structural element in the acceptor peptide that directs glycosylation (2).
The best-studied mechanism of bacterial protein N-linked glycosylation is the pgl system used by the Gram-negative pathogen Campylobacter jejuni. In this system, undecaprenyl-linked sugars are used to assemble branching heptasaccharide chains attached to a lipid anchor on the cytoplasmic face of the bacterial inner membrane. Once assembled, the chains are flipped across the membrane into the periplasm and are transferred to proteins by an inner membrane-bound oligosaccharyltransferase. Glycans are attached to the substrate protein at the consensus site Asn-Xaa-Ser/Thr, where Xaa can be any amino acid except proline (10). The pgl system mimics the N-linked glycosylation system found in eukaryotic cells, in which branching sugar chains are built on the cytoplasmic face of the endoplasmic reticulum, flipped into the lumen, and then transferred to substrate proteins by a membrane-bound oligosaccharyltransferase at the same Asn-Xaa-Ser/Thr consensus site (10).
Nontypeable Haemophilus influenzae (NTHi) uses a glycosylation system with elements of both the N-linked and O-linked processes. In this system, the N-linking glycosyltransferase HMW1C transfers glucose and galactose to the acceptor protein HMW1 using the activated UDP forms of the sugars (11). This transfer occurs in the cytoplasm, and sugar chains are built on HMW1, similar to O-linked glycosylation. However, the sugars are added at the Asn-Xaa-Ser/Thr N-linked glycosylation consensus site (12). HMW1 is modified at at least 31 sites with either mono- or dihexoses, and HMW1C is responsible for this modification (3, 11, 12).
HMW1 is an adhesive protein that mediates interaction between bacterial cells and human epithelial cells and is encoded by a two-partner secretion system gene cluster that also encodes HMW1B (an outer membrane pore-forming protein that facilitates the translocation of HMW1 to the bacterial cell surface) and HMW1C (13, 14). Without glycosylation, HMW1 is released from the bacterial cell surface and is unable to facilitate adherence (3). A two-partner secretion system genetic locus with similar organization is found in enterotoxigenic Escherichia coli (ETEC). This locus contains the etpB, etpA, and etpC genes, with the HMW1C homologue EtpC being responsible for modifying the adhesive protein EtpA in a manner similar to HMW1C modification of HMW1 (15).
Due to the unique nature of HMW1C-mediated glycosylation, we wondered if this system is used by Gram-negative pathogenic species other than NTHi and ETEC. In this study, we identified a number of HMW1C homologues in other species using bioinformatic approaches. Interestingly, these HMW1C homologues were not always encoded by a genetic locus that also encodes a potential acceptor protein as in NTHi and ETEC. To look specifically at HMW1C homologues that are not encoded near a gene for a potential acceptor protein, we examined Kingella kingae and Aggregatibacter aphrophilus. Our results demonstrate that the HMW1C homologues in these species modify adhesive proteins with mono- and dihexoses and establish that modification is needed for normal function of the acceptor protein.
RESULTS
Kingella kingae and Aggregatibacter aphrophilus have HMW1C homologues.
An NCBI standard protein BLAST search using the HMW1C polypeptide sequence as the query sequence and default parameters revealed 21 bacterial species encoding an HMW1C homologue with identity over 50%. From these results, we chose to investigate the homologues in K. kingae and A. aphrophilus, in particular because of their high homology to HMW1C (Fig. 1), the fact that they are encoded in the genome away from any gene encoding an obvious acceptor protein, and the relevance of K. kingae and A. aphrophilus to human health. The K. kingae homologue is encoded by a gene located between an upstream gene encoding a putative protein-disulfide reductase and a downstream gene encoding a putative 2-C-methyl-d-erythritol 4-phosphate cytidylytransferase, gene products that are predicted cytoplasmic proteins and are not suspected to be targets of glycosylation. Similarly, the A. aphrophilus homologue is encoded between genes encoding predicted cytoplasmic proteins, namely, an upstream gene encoding the putative ribosomal protein S12 methyltransferase and a downstream gene encoding a putative low-molecular-weight protein tyrosine phosphatase. The fact that the flanking genes do not encode proteins that are targets of glycosylation or are involved in glycosylation suggests that any phenotypes observed when the hmw1C homologue genes are interrupted are not due to polar effects. The K. kingae and A. aphrophilus HMW1C homologues are referred to here as HMW1CKk, encoded by hmw1CKk, and HMW1CAa, encoded by hmw1CAa.
FIG 1 .
HMW1CKk and HMW1CAa are highly homologous to HMW1C. The amino acid sequences of the K. kingae strain 269-492 and A. aphrophilus strain NJ8700 HMW1C homologues are aligned with the HMW1C sequence from nontypeable H. influenzae strain R2846. The plus signs below the sequences denote conserved residues. The three conserved residues involved in binding UDP are highlighted in gray.
Previous work identified residues in HMW1C that are needed to bind UDP-hexose, including lysine 467, asparagine 547, and aspartic acid 551. Aligning HMW1C to HMW1CKk and HMW1CAa revealed that these residues are conserved, suggesting that the homologues may be capable of binding and transferring UDP-hexose (Fig. 1).
HMW1CKk is required for K. kingae adherence to human epithelial cells and autoaggregation.
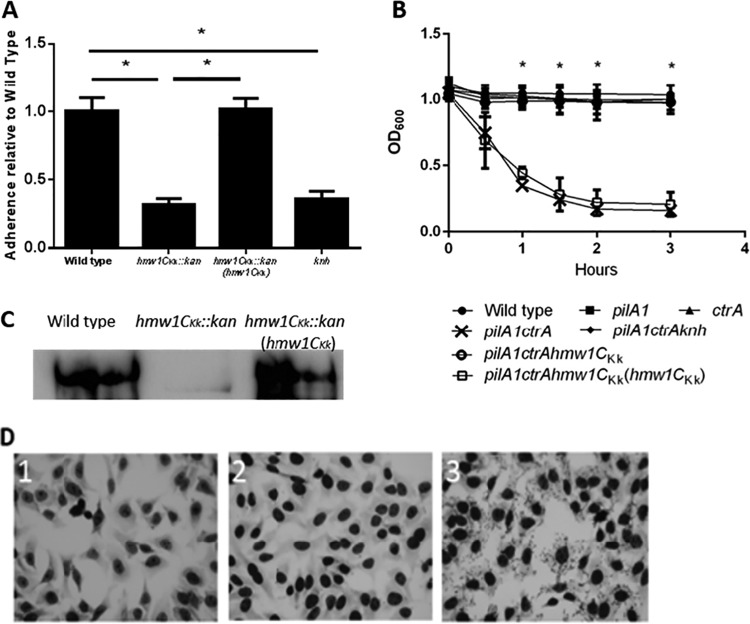
K. kingae derivatives with an interruption of hmw1CKk or reversion of the interrupted hmw1CKk gene were compared with the wild-type strain for their ability to adhere to cultured human epithelial cells. The interruption of hmw1CKk resulted in a marked reduction in adherence (Fig. 2). Wild-type levels of adherence were restored when the interrupted hmw1CKk was reverted to the wild-type sequence (Fig. 2A).
FIG 2 .
Expression of HMW1CKk is required for adherence and autoaggregation. (A) Mean adherence of K. kingae with the wild-type hmw1CKk gene, an interrupted hmw1CKk gene, and the interrupted hmw1CKk gene reverted to the wild type. (B) Mean autoaggregation of K. kingae. The pilA1 mutant lacks type IV pili, the ctrA mutant lacks the polysaccharide capsule, the knh mutant lacks the trimeric autotransporter protein Knh, and the hmw1CKk mutant lacks HMW1CKk. Graphs in panels A and B show data from biological replicates performed in triplicate. Error bars represent standard errors. An asterisk denotes a P value of <0.05 obtained from a paired t test. (C) Western blot of the formic acid-treated outer membrane fraction of wild-type K. kingae (lane 1), K. kingae with an interrupted hmw1CKk gene (lane 2), and K. kingae with the interrupted hmw1CKk gene reverted to the wild type (lane 3) using an antiserum raised against the Knh protein. (D) Qualitative adherence assay using E. coli strain BL21 expressing HMW1CKk (frame 1), Knh (frame 2), or HMW1CKk and Knh (frame 3).
A number of bacterial species are capable of autoaggregation (adherence of bacteria to themselves). To assess whether K. kingae autoaggregates, we performed tube settling assays, measuring optical density at 600 nm (OD600) over time. We found that K. kingae is capable of autoaggregation when the polysaccharide capsule (CtrA) and type IV pili (PilA1) are lacking, suggesting that these two surface factors mask one or more other surface factors responsible for autoaggregation. Interruption of hmw1CKk in a capsule-deficient, type IV pilus-deficient mutant abolished autoaggregation, and reversion of the interrupted hmw1CKk gene reversed this phenotype. Interestingly, elimination of the trimeric autotransporter Knh by interruption of the knh gene abolished the ability of K. kingae to autoaggregate, suggesting that Knh may be a substrate of HMW1CKk. These results are illustrated in Fig. 2B, where it can be seen that the curves for the wild type and the pilA1, the ctrA, the pilA1 ctrA knh, and the pilA1 ctrA hmw1CKk mutants overlap and the curves for the pilA1 ctrA and pilA1 ctrA hmw1CKk(hmw1CKk) mutants are indistinguishable.
HMW1CKk glycosylates the trimeric autotransporter Knh.
In previous work on K. kingae, we established that type IV pili and the trimeric autotransporter Knh are responsible for mediating bacterial adherence to human epithelial cells (16, 17). In an effort to determine the mechanism by which interruption of hmw1CKk interferes with K. kingae adherence, we examined the PilA1 major subunit of type IV pili and the Knh protein in the wild-type, mutant, and revertant strains by Western analysis. There was no difference in mobility of the PilA1 protein (data not shown). However, Western analysis using Knh antiserum demonstrated that Knh migrated at a lower molecular mass and was less abundant in the hmw1CKk mutant than in the wild-type strain (Fig. 2C). Both species of Knh were excised from a Coomassie blue-stained gel of outer membrane fractions from K. kingae and K. kingae lacking HMW1CKk and were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a hybrid LTQ Orbitrap Elite mass spectrometer.
We obtained 57% coverage of the Knh protein from the wild-type strain. With this coverage, we identified 32 sites of glycosylation (Table 1). All 32 of these sites were modified with at least a single hexose, and four of the 32 sites were modified with either a single hexose or a dihexose (Table 2). Potential sites of glycosylation were sorted by comparing the expected and observed m/z ratios. Sites for which the expected and observed m/z ratios were within 1 ppm of each other were considered true sites of modification. All hexose modifications were on asparagine residues, though only 81% of these sites were in the recognized Asn-Xaa-Ser/Thr consensus sequence for N-linked glycosylation. In contrast, there was no evidence of glycosylation of the Knh protein from the hmw1CKk mutant (for which we obtained 50% coverage).
TABLE 1 .
Glycopeptides identified with single hexose modification in Knh
| Modified sequencea | Asparagine position(s) | Calculated m/z |
|---|---|---|
| STDGKPNTTNTTDADINK | 218 | 685.6502 |
| STDGKPNTTNTTDADINK | 218, 221 | 739.6678 |
| FIAGDNLNLTQTGSNFTYSLNK | 603, 610 | 2,741.2953 |
| DGINAGNATISNVK | 640, 643 | 930.4338 |
| DTLTPNGDPSNVGNPVTK | 665 | 1,986.9384 |
| DTLTPNGDPSNVGNPVTK | 670 | 1,986.9376 |
| DGSGSPVNASNVTSQISAIK | 785, 788 | 1,128.54 |
| GVDNVTLLTNGTTTTVTNK | 801, 807 | 1,056.039 |
| VNVTTTPTNTPDANNVTINEAGK | 844, 845 | 899.0979 |
| KVNVTTTPTNTPDANNVTINEAGK | 845, 849 | 941.7963 |
| VVAPTNSTVAGNTFLTAK | 859, 865 | 705.695 |
| NINITQTGSTITVATK | 912, 914 | 1,985.0025 |
| DNVTFNNVNTTTMTVGSKPDNAVNFK | 929 | 3,151.4489 |
| TNAPTTALNITSADGK | 967, 974 | 949.9575 |
| GTLENTTSVANADGKGNAATGIGTEVTK | 1171 | 2,838.3720 |
| SNVSYNVAVDNK | 1267 | 898.402 |
| TTTLDVSTEPMTANNNTPAGK | 1329, 1330 | 2,486.1227 |
| AQGENASVVNPGGTVDMK | 1371, 1376 | 1,049.478 |
| INVSTNTTTGANIYDVSVNTGK | 1497 | 865.0836 |
| INVSTNTTTGANIYDVSVNTGK | 1501 | 865.0836 |
| SLTINSTTGAIDVK | 1522 | 1,742.8663 |
| GDLTVAGNTTVK | 1638 | 669.3434 |
| NFTVNPNSTVNMGGNK | 1649 | 936.4256 |
Underlined residues represent hexosylated sites.
TABLE 2 .
Glycopeptides identified with dihexose modification in Knh
| Modified sequencea | Asparagine position | Calculated m/z |
|---|---|---|
| SNVSYNVAVDNK | 1267 | 979.4284 |
| AQGENASVVNPGGTVDMK | 1371 | 705.3196 |
| GDLTVAGNTTVK | 1638 | 750.3698 |
| NFTVNPNSTVNMGGNK | 1649 | 1,017.452 |
Underlined residues represent dihexosylated sites.
For samples from the wild-type and hmw1CKk mutant strains, control database searching for O-linked glycosylation on serine or threonine residues showed nothing above the false discovery rate.
Coexpression of Knh and HMW1CKk in E. coli is sufficient to facilitate bacterial adherence to epithelial cells.
To further demonstrate the dependence of Knh function on glycosylation, we expressed both Knh and HMW1CKk in the nonadherent E. coli strain BL21. When Knh or HMW1CKk was expressed alone, the bacteria remained nonadherent (Fig. 2D). However, when Knh and HMW1CKk were coexpressed, the bacteria were capable adhering to human epithelial cells, establishing that Knh is capable of facilitating adherence only when glycosylated.
Mutating hmw1CKk decreases the amount of Knh on the bacterial cell surface.
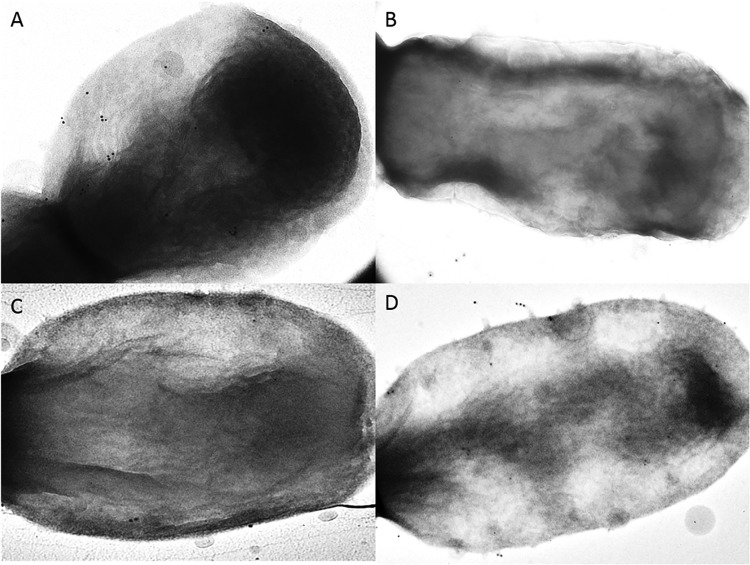
In an effort to assess whether HMW1CKk influences surface structures on K. kingae, we immunogold labeled Knh on the the wild-type and hmw1CKk mutant strains and examined them by transmission electron microscopy. In order to avoid interference by type IV pili, we used strains lacking type IV pili. As shown in Fig. 3, we saw labeling on the surface of the type IV pili mutant (Fig. 3A) that disappeared when the knh gene was interrupted (Fig. 3B). Labeling was less abundant in the hmw1CKk mutant strain (Fig. 3C) and became more abundant when hmw1CKk was reverted to the wild type (Fig. 3D). The average number of gold particles on the type IV pili mutant strain was 19.1. When the knh gene was interrupted, there was an average of 1.1 particles per cell; when hmw1CKk was interrupted, there was an average of 6.7 particles per cell; and when hmw1CKk was reverted to the wild type, there was an average of 11.1 particles per cell. Bacteria were also examined without labeling, as Knh fibers can be detected on the surface. Observations made in this way corresponded with the immunogold-labeling experiments, as fewer fibers were seen on the surface of the hmw1CKk mutant (data not shown).
FIG 3 .
HMW1CKk is required for Knh expression on the surface of K. kingae. Images show immunogold-labeled Knh on the surface of K. kingae ΔpilA1 ΔctrA (A), K. kingae knh::kan (B), K. kingae hmw1CKk::kan (C), and K. kingae hmw1CKk::kan (hmw1CKk) (D). All strains lacked polysaccharide capsule and type IV pili.
HMW1CAa is necessary for A. aphrophilus autoaggregation and adherence to human epithelial cells.
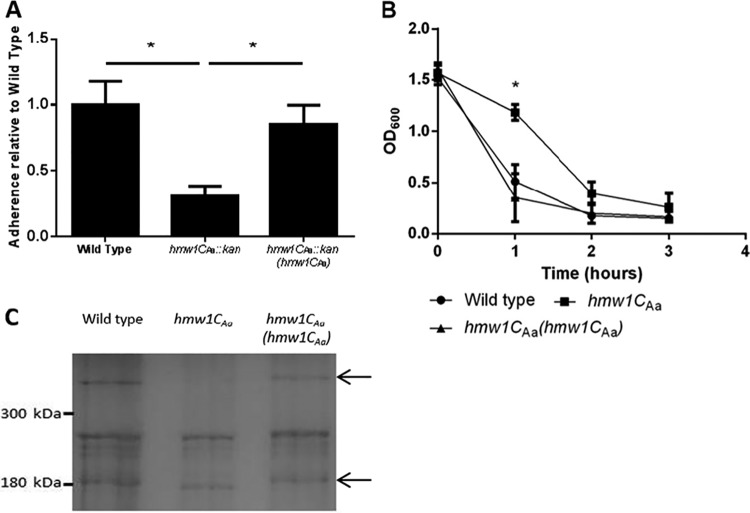
To expand our knowledge of species that use HMW1C-mediated glycosylation, the hmw1CAa gene in A. aphrophilus was interrupted, and adherence by this strain was compared with adherence by the wild-type strain. The interruption of hmw1CAa resulted in a statistically significant reduction in adherence (Fig. 4A). Wild-type levels of adherence were restored when the interrupted hmw1CAa was reverted to the wild-type sequence (Fig. 4A).
FIG 4 .
HMW1CAa expression is required for A. aphrophilus adherence and autoaggregation. (A) Mean adherence of A. aphrophilus with the wild-type hmw1CAa gene, an interrupted hmw1CAa gene, and the interrupted hmw1CAa gene reverted to the wild type. (B) Mean autoaggregation of A. aphrophilus with the wild-type hmw1CAa gene, an interrupted hmw1CAa gene, and the interrupted hmw1CAa gene reverted to the wild type. The graphs in panels A and B show data from biological replicates performed in triplicate. Error bars represent standard errors. An asterisk denotes a P value of <0.05 obtained from a paired t test. (C) Commassie blue-stained 7.5% SDS-PAGE gel of the outer membrane fractions of A. aphrophilus with the wild-type hmw1CAa gene, an interrupted hmw1CAa gene, and the interrupted hmw1CAa gene reverted to the wild type.
Experiments with A. aphrophilus have demonstrated that this species autoaggregates rapidly in liquid culture in the absence of agitation (18). To determine if interruption of hmw1CAa affects the ability of A. aphrophilus to autoaggregate, the OD600 was measured over the course of 8 h for isogenic strains containing the wild-type, interrupted, and reverted forms of the hmw1CAa gene. As shown in Fig. 4, the hmw1CAa mutant initially autoaggregated at a lower rate but was able to attain the same degree of autoaggregation as the wild-type strain over the course of the experiment. Reversion of the interrupted hmw1CAa to the wild type restored the rate of autoaggregation to wild-type levels (Fig. 4B).
HMW1CAa glycosylates the trimeric autotransporter EmaA.
Adherence factors have not been described previously for A. aphrophilus. To determine the mechanism by which interruption of hmw1CAa interferes with adherence in this species, we examined outer membrane fractions from isogenic strains of A. aphrophilus with wild-type, interrupted, or reverted hmw1CAa on a Coomassie blue-stained SDS-PAGE gel (Fig. 4C). We noticed a band at approximately 180 kDa that had increased mobility in the strain with an interrupted hmw1CAa and a band larger than 300 kDa that disappeared in the strain with an interrupted hmw1CAa (Fig. 4C). These bands were excised and analyzed by LC-MS/MS on a hybrid LTQ Orbitrap Elite mass spectrometer.
In the samples from A. aphrophilus, both the 180-kDa band and the >300-kDa band were identified as the trimeric autotransporter protein EmaA, with the 180-kDa band corresponding to the monomer form and the >300-kDa band likely representing a multimer. Coverage of 82% of the EmaA sequence in the sample from wild-type A. aphrophilus revealed six sites of glycosylation with a single hexose (Table 3). All six of the identified sites were asparagine residues and were in the Asn-Xaa-Ser/Thr consensus sequence for N-linked glycosylation. We obtained 23% coverage of the EmaA protein from the strain with an interrupted hmw1CAa gene and detected no modifications. Peptides obtained from the mutant corresponded to the peptides that were modified in the wild-type sample.
TABLE 3 .
Glycopeptides identified in EmaA
| Peptide sequencea | Asparagine position | Calculated m/z |
|---|---|---|
| INLNNTLDLGSSGSIK | 687 | 904.96 |
| VSGTSPITVNK | 819 | 632.83 |
| TTNNGVDDYAVSFNGTEAAK | 834 | 1,118.49 |
| AAVAGTPVNGANGTDGKDGVATVQNVVDALNK | 907 | 1,062.53 |
| VSLGGDNGNTTEK | 1140 | 727.33 |
| DGANASITVAQGK | 1592 | 697.34 |
Underlined residues represent hexosylated sites.
EmaA facilitates autoaggregation and bacterial adherence to host cells.
The identification of EmaA as the substrate of HMW1CAa and the phenotypes demonstrated by an hmw1CAa mutant suggested that EmaA is involved in A. aphrophilus adherence to human epithelial cells and autoaggregation. To determine if EmaA does indeed mediate these functions, we insertionally inactivated the emaA gene with a kanamycin resistance cassette. The resulting mutant was unable to adhere to human epithelial cells or autoaggreate (Fig. 5). The dependence of bacterial adherence and autoaggreation on EmaA was confirmed by reverting the emaA gene to the wild type by removing the kanamycin resistance cassette (Fig. 5).
FIG 5 .
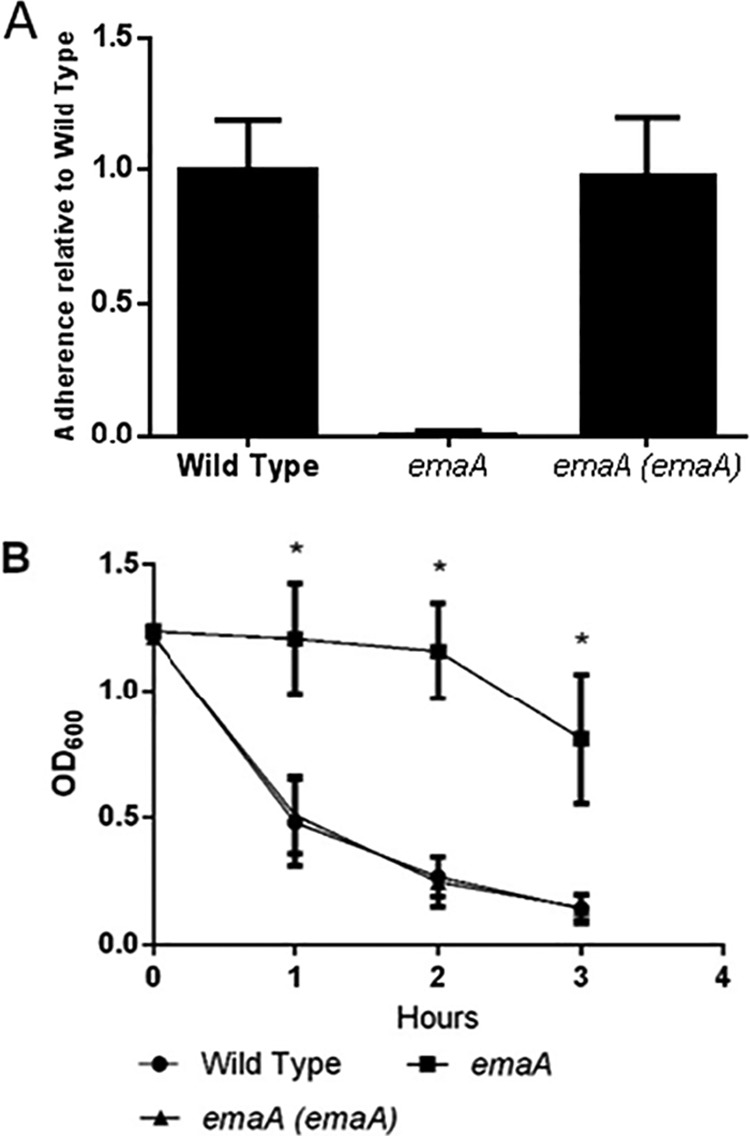
EmaA is an adhesin and involved in autoaggregation. (A) Mean adherence to human epithelial cells by A. aphrophilus with the wild-type emaA gene, an interrupted emaA gene, and the interrupted emaA gene reverted to the wild type. (B) Mean autoaggregation of A. aphrophilus with the wild-type emaA gene, an interrupted emaA gene, and the interrupted emaA gene reverted to the wild type. Each graph shows data from biological replicates performed in triplicate. Error bars represent standard errors. An asterisk denotes a P value of <0.05 obtained from a paired t test.
DISCUSSION
In this study, we describe two homologues of the H. influenzae HMW1C protein that are functional glycosyltransferases. Unlike HMW1C, HMW1CKk in K. kingae and HMW1CAa in A. aphrophilus are not encoded in the same genetic locus as a two-partner secretion system. Rather, these enzymes modify type V secretion proteins encoded by genes at distant locations in the genome. The acceptor protein for HMW1CKk was identified as the trimeric autotransporter protein Knh, and the acceptor protein for HMW1CAa was found to be EmaA, also a trimeric autotransporter.
Autotransporters are a family of adhesive proteins characterized by a C-terminal domain that forms an outer membrane beta-barrel pore and facilitates surface localization of the N-terminal passenger domain of the protein. A number of autotransporters are modified by O-glycosylation, including Ag43, AIDA-I, and TibA of E. coli. Glycosylation of these proteins affects stability (AIDA-I and TibA), oligomerization (TibA), and adhesive activity (AIDA-I and TibA) (19–21). In the case of trimeric autotransporters, the protein must trimerize in order to form a fully functional pore. To date all known trimeric autotransporters have adhesive properties, and only one has been identified as being glycosylated, namely, the Aggregatibacter actinomycetemcomitans EmaA protein (22).
Knh is a trimeric autotransporter adhesin expressed by the pediatric pathogen Kingella kingae and has been shown in previous work to be essential for bacterial adherence to human epithelial cells (17). Our data in this study show that the ability of Knh to mediate adherence and autoaggregation is dependent on expression of hmw1CKk.
Interestingly, only 81% of the identified modified residues in Knh were in the N-linked glycosylation consensus sequence. This lack of sequence specificity is reminiscent of O-linking glycosyltransferases, for which no glycosylation consensus sequence has been identified, leading to the hypothesis that O-linked glycosylation sites are specified by structural cues (2). It is possible that there is a structural component to the selection of modification sites on Knh. Recent work showed that the HMW1C homologue from Actinobacillus pleuropneumoniae is able to modify asparagine residues outside the NXS/T consensus sequence, including NXA and NXV, albeit with low efficiency (23). This variation is much more limited than the sites identified in Knh, which included NXG, NXV, NXF, NXP, and NXN.
Glycosylation can protect against protease activity, stabilize protein structure, and facilitate interaction between a protein and a binding partner. Any of these mechanisms could explain the phenotypes that we observed when Knh was not glycosylated. The Western blot of outer membrane fractions revealed that Knh was present in the outer membrane of the strain with the interrupted hmw1CKk gene, albeit at reduced levels. To further investigate the relationship between the amount of Knh on the bacterial surface and glycosylation, we used transmission electron microscopy. We observed surface fibers on wild-type K. kingae and significantly fewer fibers on a derivative with an interrupted hmw1CKk gene. When the knh gene was deleted, we observed no surface fibers. This result is consistent with the observation that the hmw1CKk mutant is deficient in adherence and autoaggregation, similar to a knh mutant (17). The observed decrease of Knh on the surface of the hmw1CKk mutant could be a result of either a decrease in efficiency in trafficking to the outer membrane or a change in protein stability.
The A. aphrophilus EmaA protein is also a trimeric autotransporter adhesin. The contribution of EmaA to bacterial adherence and/or autoaggregation had not been characterized prior to this study. By interrupting the emaA gene, we showed that both adherence to human epithelial cells and autoaggregation are dependent on expression of EmaA. In the presence of HMW1CAa, EmaA is modified with a monosaccharide at at least six sites. All six of these sites were at the NXS/T N-linked glycosylation consensus motif, similar to observations with HMW1C and HMW1 and different from HMW1CKk and Knh. The strain lacking HMW1CAa displayed reduced adherence to cultured epithelial cells and delayed autoaggregation. Unlike the strain lacking EmaA, the strain lacking HMW1CAa was still capable of a low level of adherence to human epithelial cells, suggesting that unmodified EmaA is still functional, though to a lesser extent than glycosylated EmaA.
The EmaA protein in A. aphrophilus is 69% identical to the EmaA protein in Aggregatibacter actinomycetemcomitans, a protein that has been studied extensively and facilitates adherence to collagen (24). Interestingly, A. actinomycetemcomitans EmaA is O-glycosylated in a process involving enzymes used in the biosynthesis of the O-polysaccharide of LPS (23). The proposed modification is a nonasaccharide rather than the monosaccharide that we identified on A. aphrophilus EmaA. It is interesting that A. actinomycetemcomitans does not contain an HMW1C homologue.
In summary, this work describes the first examples of an HMW1C-like enzyme that glycosylates a protein that is not encoded in the same gene cluster as the enzyme itself and is not a member of a two-partner secretion system. Furthermore, Knh and EmaA are the first examples of trimeric autotransporters that are N-glycosylated. To date, all known targets of HMW1C-facilitated modification are members of type V secretion systems and function as adhesins, with adhesive activity being dependent on HMW1C-mediated glycosylation. As adherence to host tissue is a critical first step in infection, the ability to interrupt HMW1C-mediated glycosylation may have therapeutic potential.
MATERIALS AND METHODS
Strains and culture conditions.
The bacterial strains are listed in Table 4. K. kingae strains were cultured at 37°C with 5% CO2 on chocolate agar supplemented with 50 µg/ml kanamycin, 1 µg/ml erythromycin, or 0.75 µg/ml chloramphenicol, as needed. A. aphrophilus strains were cultured at 37°C with agitation (225 rpm) in tryptic soy broth (TSB) supplemented with NAD (3.5 µg/ml) and lysed horse blood (1:1,000 dilution), using 50 µg/ml kanamycin for selection as necessary.
TABLE 4 .
Bacterial strains
| Strain | Description | Reference |
|---|---|---|
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE441 thi-1 gyrA96 relA | |
| 269–492 (KK03) | Clinical isolate, spontaneous spreading, corroding colony variant of 269-492 | 16 |
| KK03 hmw1CKk::kan | Interruption of hmw1CKk with kanamycin resistance cassette | This study |
| KK03 hmw1CKk::kan(hmw1CKk) | Reversion of interrupted hmw1CKk to wild type | This study |
| KK03 crtA::erm | Interruption of ctrA with erythromycin resistance cassette | This study |
| KK03 pilA1::cm | Interruption of pilA1 with chloramphenicol resistance cassette | This study |
| KK03 ctrA::erm pilA1::cm | Interruption of ctrA with erythromycin resistance cassette and pilA1 with chloramphenicol resistance cassette | This study |
| KK03 ctrA::erm pilA1::cm hmw1CKk::kan | Interruption of ctrA with erythromycin resistance cassette, pilA1 with chloramphenicol resistance cassette, and hmw1CKk with kanamycin resistance cassette | This study |
| KK03 ctrA::erm pilA1::cm knh::kan | Interruption of ctrA with erythromycin resistance cassette, pilA1 with chloramphenicol resistance cassette, and knh with kanamycin resistance cassette | 17 |
| KK03 ctrA::erm pilA1::cm hmw1CKk::kan(hmw1CKk) | Interruption of ctrA with erythromycin resistance cassette and pilA1 with chloramphenicol resistance cassette and reversion of interrupted hmw1CKk to wild type | This study |
| CCUG 11575 | Clinical isolate of A. aphrophilus | Culture collection of the University of Gothenburg |
| CCUG 11575 hmw1CAa::kan | Interruption of hmw1CAa with kanamycin resistance cassette | This study |
| CCUG 11575 hmw1CAa::kan(hmw1CAa) | Reversion of interrupted hmw1CAa to wild type | This study |
| CCUG 11575 emaA::kan | Interruption of emaA with kanamycin resistance cassette | This study |
| CCUG 11575 emaA::kan(emaA) | Reversion of the interrupted emaA to wild type | This study |
| E. coli BL21 pACYC HMW1CKk + pBAD | E. coli expressing HMW1CKk | This study |
| E. coli BL21 pACYC + pBAD Knh | E. coli expressing Knh | This study |
| E. coli BL21 pACYC HMW1CKk + pBAD Knh | E. coli expressing HMW1CKk and Knh | This study |
Strain construction.
Disruption of hmw1CKk (the K. kingae hmw1C homologue) was achieved via random transposon mutagenesis as described earlier and was identified by nucleotide sequencing (16). All other gene disruptions in K. kingae and A. aphrophilus were generated as described previously (17). Briefly, plasmid-based gene disruption constructs were made in E. coli, linearized, and introduced into the appropriate species via natural transformation. Transformants were recovered after plating on medium containing the appropriate antibiotic. Correct localization of the interruption was confirmed by PCR and nucleotide sequencing.
Reversions of gene disruptions were generated by PCR amplifying a wild-type copy of the gene and using the wild-type gene to replace the disrupted copy via natural transformation. Transformants were screened for loss of resistance to the appropriate antibiotic, and sequence integrity was confirmed by nucleotide sequencing.
Homologue identification.
Homologues of HMW1C were identified by using the NCBI BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with the nontypeable Haemophilus influenzae HMW1C protein (GenBank no. ADO96126.1) as the query sequence. Results were organized by percent similarity and were assessed for relevance of the host organism to human health. Identity and similarity were determined using the EMBOSS Needle program (http://www.ebi.ac.uk/Tools/psa/emboss_needle/).
Adherence assays.
Quantitative adherence assays were performed as previously described using Chang epithelial cells (ATCC CCL 20.2) (16). Briefly, approximately 6.5 × 106 CFU of bacteria were inoculated onto a monolayer of epithelial cells in a 24-well tissue culture plate. Bacteria were allowed to adhere to the cells for 30 min. Subsequently, monolayers were rinsed with phosphate-buffered saline (PBS) to remove nonadherent bacteria. Adherent bacteria were released from the monolayer using trypsin-EDTA and were quantitated by plating dilutions on chocolate agar plates. CFU counts of adherent bacteria were compared to CFU counts of the original inoculum to determine the percentage of bacteria that were adherent.
Qualitative adherence assays were performed as quantitative adherence assays with the following modifications: human epithelial cells were seeded onto glass coverslips in a 24-well tissue culture plate; coverslips were removed and stained with Giemsa stain; stained coverslips were observed at a magnfication of ×400 with a light microscope.
For adherence assays with K. kingae, human epithelial cells were fixed with 2% glutaraldehyde in 0.1 M sodium phosphate buffer prior to the assay to prevent disruption of the monolayer by the K. kingae RTX toxin.
Autoaggregation assays.
For autoaggregation assays with K. kingae, bacteria were grown overnight on chocolate agar at 37°C with 5% CO2. The next day, the bacteria were resuspended in tubes containing brain heart infusion broth to an OD600 of 1.0. Tubes were allowed to stand at room temperature, and OD600 was measured after 30, 60, 90, 120, and 180 min.
For autoaggregation assays with A. aphrophilus, bacteria were grown overnight with agitation in TSB supplemented with NAD and heme. The following day, cultures were removed from agitation and allowed to stand at room temperature, and the OD600 was measured hourly for 3 h.
Statistical analysis.
Statistical analyses were done using the GraphPad Prism 6 software. Values were compared using the unpaired t test. P values of less than 0.05 were considered statistically significant.
Isolation of outer membrane proteins.
Bacteria were resuspended in 10 mM HEPES (pH 7.5) and sonicated until the suspension cleared. Suspensions were then centrifuged for 2 min at 21,000 × g and 4°C to remove any intact cells. The supernatant was centrifuged again in a Beckman Coulter Optima MAX-TL ultracentrifuge using a TLA-5 rotor for 1 h at 100,000 × g and 4°C to pellet membranes. The membrane pellet was treated with 1% Sarkosyl to solubilize the inner membrane fraction, and the solution was centrifuged again for 1 h at 100,000 × g and 4°C to pellet the outer membrane fraction.
Formic acid treatment of outer membrane proteins.
Outer membrane fractions were incubated with 75% formic acid overnight, at 25°C in the dark. Samples were then lyophilized three times to remove the formic acid, and the dried protein was resuspended in 1.5 M Tris (pH 8.8).
SDS-PAGE.
Samples were boiled with Laemmli SDS-PAGE loading buffer for 5 min and were then loaded and resolved on 7.5% SDS-PAGE gels.
In-gel digestion.
Coomassie-stained samples were excised from gels, cut into 1-mm cubes, destained with 50% methanol–1.25% acetic acid, reduced with 5 mM dithiothreitol (Thermo), and alkylated with 20 mM iodoacetamide (Sigma). Gel pieces were then washed with 20 mM ammonium bicarbonate (Sigma) and dehydrated with acetonitrile (Fisher). Trypsin (Promega) (5 ng/ml in 20 mM ammonium bicarbonate) was added to the gel pieces, and proteolysis was allowed to proceed overnight at 37°C. Peptides were extracted with 0.3% trifluoroacetic acid (J.T. Baker) and then 50% acetonitrile. Extracts were combined, and the volume was reduced by vacuum centrifugation.
Mass spectrometry analysis.
Tryptic digests were analyzed by LC-MS/MS on a hybrid LTQ Orbitrap Elite mass spectrometer (Thermo, Fisher Scientific San Jose, CA) coupled with a nanoLC Ultra (Eksigent). Peptides were separated by reverse phase high-performance liquid chromatography (RP-HPLC) on a nanocapillary column (75 µm [inside diameter] by 15 cm; Reprosil-pur; 3 μm silica, 120 Å pore diameter; Dr. Maisch, Germany) in a nanoflex chip system (Eksigent). Mobile phase A consisted of 1% methanol (Fisher)–0.1% formic acid (Thermo), and mobile phase B consisted of 1% methanol–0.1% formic acid–80% acetonitrile. Peptides were eluted into the mass spectrometer at 300 nl/min, with each RP-LC run comprising a 90-min gradient from 10 to 25% B in 65 min and 25 to 40% B in 25 min. The mass spectrometer was set to repetitively scan m/z from 300 to 1,800 (R = 240,000 for LTQ-Orbitrap Elite) followed by data-dependent MS/MS scans on the twenty most abundant ions, with a minimum signal of 1,500, dynamic exclusion with a repeat count of 1, repeat duration of 30 s, exclusion size of 500, duration of 60 s, isolation width of 2.0, normalized collision energy of 33, and waveform injection and dynamic exclusion enabled. The Fourier transform MS (FTMS) full-scan AGC (automatic gain control) target value was 1e6, and the MSn (multistage mass spectrometry) AGC was 1e4. The FTMS full-scan maximum fill time was 500 ms, and the ion trap MSn fill time was 50 ms; microscans were set at one. FT preview mode, charge state screening, and monoisotopic precursor selection were all enabled, with rejection of unassigned and 1+ charge states.
Database searching.
The tandem mass spectra were extracted using ProteoWizard (v3.0.5047). Charge state deconvolution and deisotoping were not performed. All MS/MS samples were analyzed using SEQUEST (Thermo, Fisher Scientific; version 1.0). SEQUEST was set up to search the UniProt Kingella sp. complete proteome database (2014-03-06; 10,238 entries) or Aggregatibacter aphrophilus complete proteome database (2014-07-01; 2,407 entries) appended with common contaminants, assuming a full tryptic digestion with the possibility of two missed cleavages. SEQUEST was searched with a fragment ion mass tolerance of 1 Da, and a parent ion tolerance of 15 ppm S-carbamamidomethyl of cysteine was specified in SEQUEST as a fixed modification. Oxidation of methionine and hexose on asparagine were specified in SEQUEST as variable modifications.
Immunogold labeling and transmission electron microscopy.
Bacteria were grown overnight on chocolate agar at 37°C and resuspended in PBS to on OD600 of 0.8. Samples were then fixed using 1% paraformaldyhde at room temperature for 30 min. Following fixing, samples were washed twice with Tris-buffered saline (TBS). Samples were blocked for 30 min using 2% naive guinea pig serum and 0.1% bovine serum albumin (BSA) in PBS. Following blocking, samples were incubated with guinea pig serum raised against Knh (1:250) for 1 h. Samples were then washed with PBS and incubated with goat polyclonal antibody raised against guinea pig IgG and conjugated to 10-nm gold particles for 1 h. Following washing in PBS, bacteria were resuspended in 0.2 M ammonium acetate. Samples were negatively stained with uranyl acetate, and transmission electron microscopy was performed using a FEI-Technai 12 microscope.
GenBank Accession number.
The sequence of hmw1CAa has been deposited in GenBank under accession no. KR131724.
ACKNOWLEDGMENTS
We thank Jessica R. McCann for her thoughtful discussions and her assistance in the preparation of the manuscript.
This work was supported by NIH grant R01-DC02873 to J. W. St. Geme III.
Footnotes
Citation Rempe KA, Spruce LA, Porsch EA, Seeholzer SH, Nørskov-Lauritsen N, St Geme JW III. 2015. Unconventional N-linked glycosylation promotes trimeric autotransporter function in Kingella kingae and Aggregatibacter aphrophilus. mBio 6(4):e01206-15. doi:10.1128/mBio.01206-15.
REFERENCES
- 1.Szymanski CM, Wren BW. 2005. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol 3:225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- 2.Charbonneau ME, Côté JP, Haurat MF, Reiz B, Crépin S, Berthiaume F, Dozois CM, Feldman MF, Mourez M. 2012. A structural motif is the recognition site for a new family of bacterial protein O-glycosyltransferases. Mol Microbiol 83:894–907. doi: 10.1111/j.1365-2958.2012.07973.x. [DOI] [PubMed] [Google Scholar]
- 3.Grass S, Buscher AZ, Swords WE, Apicella MA, Barenkamp SJ, Ozchlewski N, St Geme JW III. 2003. The Haemophilus influenzae HMW1 adhesin is glycosylated in a process that requires HMW1C and phosphoglucomutase, an ezyme involved in lipooligosaccharide biosynthesis. Mol Microbiol 48:737–751. doi: 10.1046/j.1365-2958.2003.03450.x. [DOI] [PubMed] [Google Scholar]
- 4.Marceau M, Nassif X. 1999. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J Bacteriol 181:656–661PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doig P, Kinsella N, Guerry P, Trust TJ.. 1996. Characterization of a post-translational modification of Campylobacter flagella: identification of a sero-specific glycosyl moitey. Mol Microbiol 19:379–387. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann JL, O’Gaora P, Gallagher A, Thole JE, Young DB. 1996. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J 15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- 7.Hofsteenge J, Müller DR, de Beer T, Löffler A, Richter WJ, Vliegenthart JF. 1994. New type of linkage between a carbohydrate and a protein: C-glycosylation of a specific tryptophan residue in human RNase. Biochemistry 33:13524–13530. doi: 10.1021/bi00250a003. [DOI] [PubMed] [Google Scholar]
- 8.Iwashkiw JA, Vozza NF, Kinsella RL, Feldman MF. 2013. Pour some sugar on it: the expanding world of bacterial protein O-linked glycosylation. Mol Microbiol 89:14–28. doi: 10.1111/mmi.12265. [DOI] [PubMed] [Google Scholar]
- 9.Faridmoayer A, Fentabil MA, Mills DC, Klassen JS, Feldman MF. 2007. Functional characterization of bacterial oligosaaccharyltransferases involved in O-linked protein glycosylation. J Bacteriol 189:8088–8098. doi: 10.1128/JB.01318-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol 32:1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 11.Grass S, Lichti CF, Townsend RR, Gross J, St Geme JW III. 2010. The Haemophilus influenzae HMW1C protein is a glycosyltransferase that transfers hexose residues to asparagine sites in the HMW1 adhesin. PLoS Pathog 6:e1000919. doi: 10.1371/journal.ppat.1000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross J, Grass S, Davis AE, Gilmore-Erdmann P, Townsend RR, St Geme JW III. 2008. The Haemophilus influenzae HMW1 adhesin is a glycoprotein with an unusual N-linked carbohydrate modification. J Biol Chem 283:26010–26015. doi: 10.1074/jbc.M801819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Geme JW III, Falkow S, Barenkamp SJ. 1993. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A 90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Geme JW III, Grass S. 1998. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplamsic intermediate and requires the HMWB and HMWC proteins. Mol Microbiol 27:617–630. doi: 10.1046/j.1365-2958.1998.00711.x. [DOI] [PubMed] [Google Scholar]
- 15.Fleckenstein JM, Roy K, Fischer JF, Burkitt M. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect Immun 74:2245–2258. doi: 10.1128/IAI.74.4.2245-2258.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehl-Fie TE, St Geme JW III. 2007. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J Bacteriol 189:430–436. doi: 10.1128/JB.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porsch EA, Kehl-Fie TE, St Geme JW III. 2012. Modulation of Kingella kingae adherence to human epithelial cells by type IV pili, capsule, and a novel trimeric autotransporter. mBio 3:e00372-12. doi: 10.1128/mBio.00372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khairat O. 1940. Endocarditis due to a new species of Haemophilus. J Pathol Bacteriol 50:497–505. doi: 10.1002/path.1700500312. [DOI] [Google Scholar]
- 19.Sherlock O, Dobrindt U, Jensen JB, Munk Vejborg R, Klemm P. 2006. Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein. J Bacteriol 188:1798–1807. doi: 10.1128/JB.188.5.1798-1807.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benz I, Schmidt MA. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA-I adhesin. Mol Microbiol 40:1403–1413. doi: 10.1046/j.1365-2958.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 21.Côté JP, Charbonneau ME, Mourez M. 2013. Glycosylation of the Escherichia coli TibA self-associating autotransporter influences the conformation and functionality of the protein. PLoS One 8:e80739. doi: 10.1371/journal.pone/0080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang G, Mintz KP. 2010. Glycosylation of the collagen adhesion EmaA of Aggregatibacter actinomycetemcomitans is dependent upon the lipopolysaccharide biosynthetic pathway. J Bacteriol 192:1395–1404. doi: 10.1128/JB.01453-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naegeli A, Michaud G, Schubert M, Lin CW, Darbre T, Reymond JL, Aebi M. 2014. Substrate specificity of cytoplasmic N-glycosyltransferase. J Biol Chem 289:24521–24532. doi: 10.1074/jbc.M114.579326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mintz KP. 2004. Identification of an extracellular matrix protein adhesion, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology 150:2677–2688. doi: 10.1099/mic.0.27110-0. [DOI] [PubMed] [Google Scholar]