Abstract
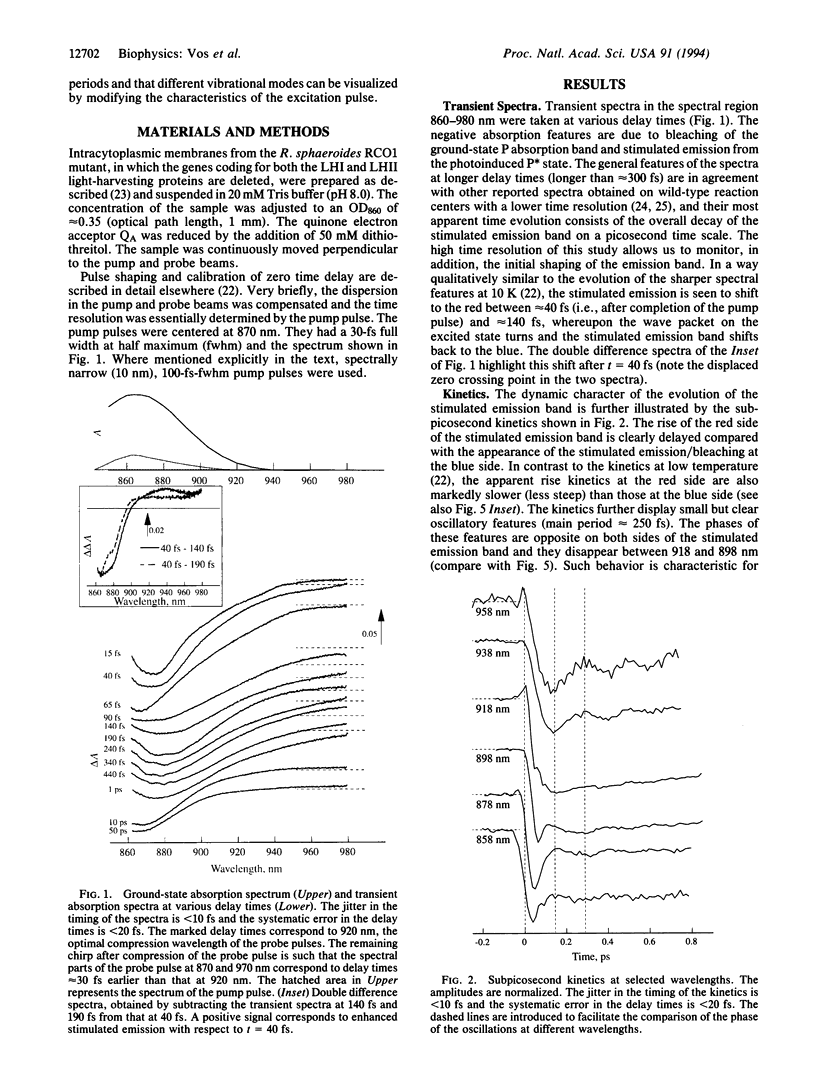
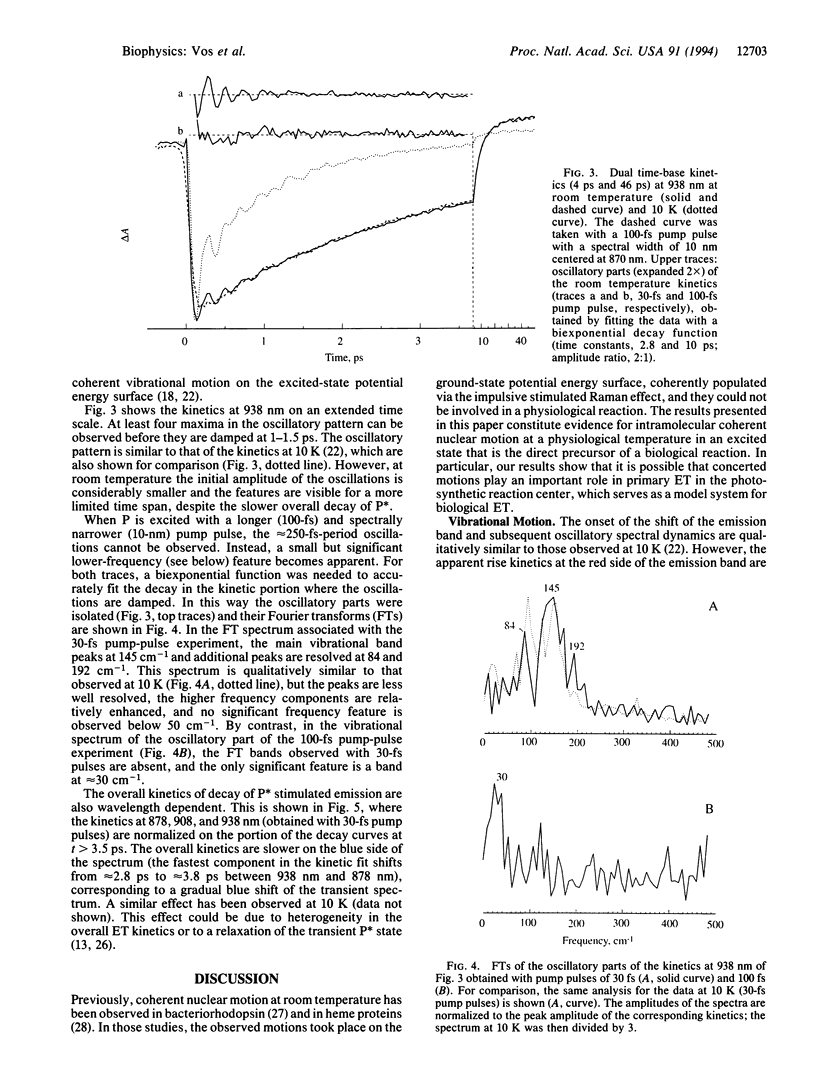
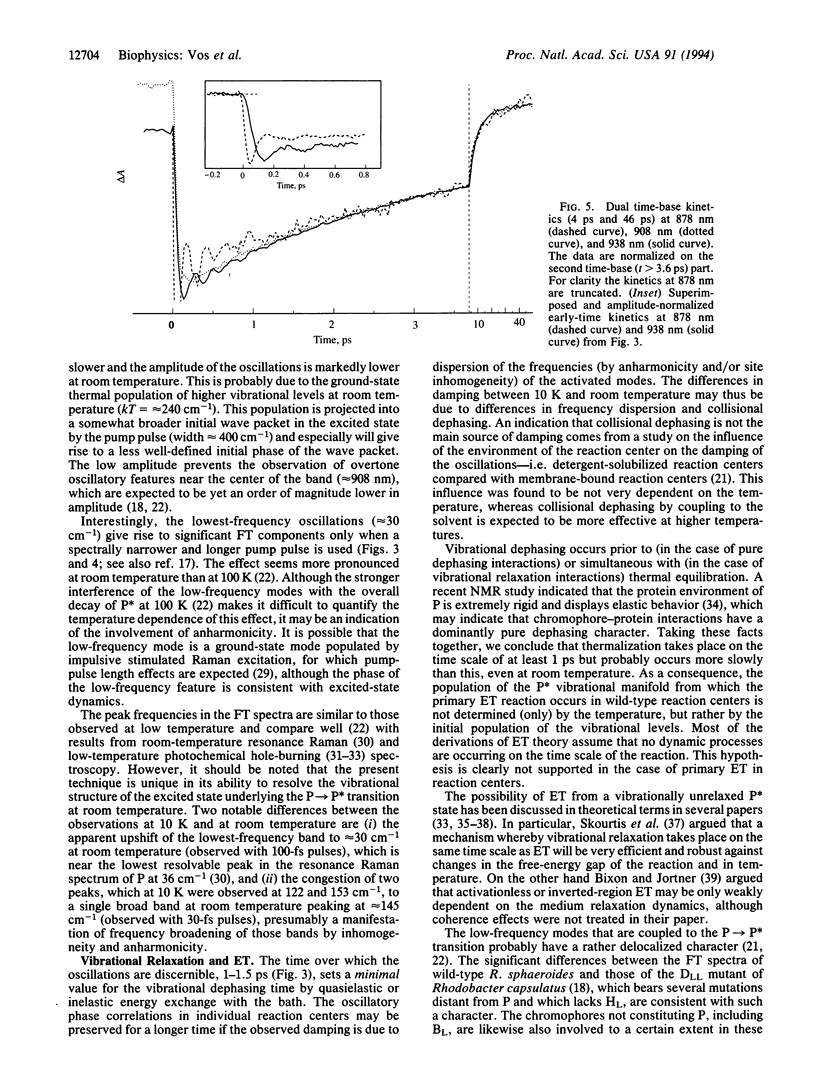
A room-temperature study is reported of the femtosecond spectral evolution of the stimulated emission band of the primary electron-transfer precursor P* in bacterial photosynthesis. The study was performed with membranes of the antenna-deficient RCO1 mutant of Rhodobacter sphaeroides. A time-dependent red shift, reflecting nuclear motion out of the Franck-Condon region of the excited state, is resolved. Analysis of oscillatory features persisting for > 1 ps in the kinetics revealed main frequencies of the activated motions at 30, 84, 145, and 192 cm-1. The oscillations occur on the time scale of primary electron transfer. Our results set a lower limit for the vibrational dephasing time in P* that is not compatible with the usual assumption in theoretical treatments of complete vibrational relaxation prior to electron transfer, even at room temperature.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt T., Schmidt S., Kaiser W., Lauterwasser C., Meyer M., Scheer H., Zinth W. The accessory bacteriochlorophyll: a real electron carrier in primary photosynthesis. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11757–11761. doi: 10.1073/pnas.90.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. K., DiMagno T. J., Chen L. X., Norris J. R., Fleming G. R. Mechanism of the initial charge separation in bacterial photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11202–11206. doi: 10.1073/pnas.88.24.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. G., Pollard K. M., Webb J. Antibodies to histones in systemic lupus erythematosus: prevalence, specificity, and relationship to clinical and laboratory features. Ann Rheum Dis. 1992 Jan;51(1):61–66. doi: 10.1136/ard.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Fischer M. R., de Groot H. J., Raap J., Winkel C., Hoff A. J., Lugtenburg J. 13C magic angle spinning NMR study of the light-induced and temperature-dependent changes in Rhodobacter sphaeroides R26 reaction centers enriched in [4'-13C]tyrosine. Biochemistry. 1992 Nov 17;31(45):11038–11049. doi: 10.1021/bi00160a013. [DOI] [PubMed] [Google Scholar]
- Gehlen J. N., Marchi M., Chandler D. Dynamics affecting the primary charge transfer in photosynthesis. Science. 1994 Jan 28;263(5146):499–502. doi: 10.1126/science.263.5146.499. [DOI] [PubMed] [Google Scholar]
- Holzapfel W., Finkele U., Kaiser W., Oesterhelt D., Scheer H., Stilz H. U., Zinth W. Initial electron-transfer in the reaction center from Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5168–5172. doi: 10.1073/pnas.87.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. R., Heer-Dawson M., Mattioli T. A., Hunter C. N., Robert B. Site-specific mutagenesis of the reaction centre from Rhodobacter sphaeroides studied by Fourier transform Raman spectroscopy: mutations at tyrosine M210 do not affect the electronic structure of the primary donor. FEBS Lett. 1994 Feb 14;339(1-2):18–24. doi: 10.1016/0014-5793(94)80376-5. [DOI] [PubMed] [Google Scholar]
- Jones M. R., Visschers R. W., van Grondelle R., Hunter C. N. Construction and characterization of a mutant of Rhodobacter sphaeroides with the reaction center as the sole pigment-protein complex. Biochemistry. 1992 May 12;31(18):4458–4465. doi: 10.1021/bi00133a011. [DOI] [PubMed] [Google Scholar]
- Jortner J. Dynamics of electron transfer in bacterial photosynthesis. Biochim Biophys Acta. 1980 Dec;594(4):193–230. doi: 10.1016/0304-4173(80)90001-4. [DOI] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. An assessment of the mechanism of initial electron transfer in bacterial reaction centers. Biochemistry. 1991 Jan 22;30(3):609–613. doi: 10.1021/bi00217a003. [DOI] [PubMed] [Google Scholar]
- Kirmaier C., Holten D., Bylina E. J., Youvan D. C. Electron transfer in a genetically modified bacterial reaction center containing a heterodimer. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7562–7566. doi: 10.1073/pnas.85.20.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Vos M. H. Femtosecond biology. Annu Rev Biophys Biomol Struct. 1992;21:199–222. doi: 10.1146/annurev.bb.21.060192.001215. [DOI] [PubMed] [Google Scholar]
- Moser C. C., Keske J. M., Warncke K., Farid R. S., Dutton P. L. Nature of biological electron transfer. Nature. 1992 Feb 27;355(6363):796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- Nagarajan V., Parson W. W., Davis D., Schenck C. C. Kinetics and free energy gaps of electron-transfer reactions in Rhodobacter sphaeroides reaction centers. Biochemistry. 1993 Nov 23;32(46):12324–12336. doi: 10.1021/bi00097a008. [DOI] [PubMed] [Google Scholar]
- Nagarajan V., Parson W. W., Gaul D., Schenck C. Effect of specific mutations of tyrosine-(M)210 on the primary photosynthetic electron-transfer process in Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7888–7892. doi: 10.1073/pnas.87.20.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve A. P., Cherepy N. J., Franzen S., Boxer S. G., Mathies R. A. Rapid-flow resonance Raman spectroscopy of bacterial photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11207–11211. doi: 10.1073/pnas.88.24.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A. K., Stocker J. W., Alden R. G., Causgrove T. P., Peloquin J. M., Boxer S. G., Woodbury N. W. Biochemical characterization and electron-transfer reactions of sym1, a Rhodobacter capsulatus reaction center symmetry mutant which affects the initial electron donor. Biochemistry. 1992 Oct 27;31(42):10345–10355. doi: 10.1021/bi00157a024. [DOI] [PubMed] [Google Scholar]
- Vos M. H., Jones M. R., Hunter C. N., Breton J., Lambry J. C., Martin J. L. Coherent dynamics during the primary electron-transfer reaction in membrane-bound reaction centers of Rhodobacter sphaeroides. Biochemistry. 1994 Jun 7;33(22):6750–6757. doi: 10.1021/bi00188a002. [DOI] [PubMed] [Google Scholar]
- Vos M. H., Lambry J. C., Robles S. J., Youvan D. C., Breton J., Martin J. L. Direct observation of vibrational coherence in bacterial reaction centers using femtosecond absorption spectroscopy. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8885–8889. doi: 10.1073/pnas.88.20.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]
- Zhu L, Li P, Huang M, Sage JT, Champion PM. Real time observation of low frequency heme protein vibrations using femtosecond coherence spectroscopy. Phys Rev Lett. 1994 Jan 10;72(2):301–304. doi: 10.1103/PhysRevLett.72.301. [DOI] [PubMed] [Google Scholar]



