Abstract
A variable poly-T polymorphism in the TOMM40 gene, which is in linkage disequilibrium with APOE, was recently implicated with increased risk and earlier onset age for late-onset Alzheimer’s disease in APOE ε3 carriers. To elucidate potential neurobiological mechanisms underlying this association, we compared the effect of TOMM40 poly-T variants to the effect of APOE, an established LOAD-risk modulator, on cerebrospinal fluid (CSF) amyloid beta (Aβ) and tau levels, in cognitively intact elderly subjects. APOE ε4 carriers showed significant reductions in Aβ 1-42 levels compared to non-ε4 carriers, but no differences were detected across TOMM40 variants. Neither Aβ 1-40 nor tau levels were affected by APOE or TOMM40.
Keywords: TOMM40 poly-T, APOE, cerebrospinal fluid, amyloid beta, T tau, P tau, Alzheimer’s disease
Introduction
The ε4 allele of the APOE gene is associated with earlier age of onset and increased risk for late-onset Alzheimer’s disease (LOAD) [1]. Recently, a variable length deoxythymidine homopolymer (poly-T), rs10542523, in the TOMM40 gene, which is in linkage disequilibrium with APOE, has also been reported to modulate risk and onset age of LOAD [2, 3]. Roses et al. [2, 4] have shown that APOE ε4 alleles are nearly exclusively linked to TOMM40 poly-T variants between 21 and 30 T residues in length (long variants; i.e., L), whereas APOE ε3 alleles may be linked to either short variants (20 or lower T residues in length; i.e., S) or very long variants (31 or over T residues in length; i.e., VL). In individuals carrying the ε3 allele, VL poly-T variants were found to associate with earlier LOAD onset age [2], including in APOE ε3/ε3 carriers, a genotype previously considered to confer neutral risk for LOAD [3]. In contrast, S poly-T variants are found to be associated with later LOAD onset age in ε3/ε4 and ε3/ε3 individuals [2, 3] and are considered to possess a protective effect.
TOMM40 encodes a protein, Tom40, which is the import channel of the mitochondrial outer membrane through which cytoplasmic peptides and proteins pass during mitochondrial biogenesis [5, 6]. Roses and colleagues [2] have suggested a number of mechanisms by which different TOMM40 poly-T variants may contribute to increased risk for LOAD, including exon skipping leading to malfunctioning Tom40 protein, and interference with APOE transcription, but none of these mechanisms have yet been established.
Several reports have linked reductions in cerebrospinal fluid (CSF) amyloid beta (Aβ) 1-42 and elevations in tau levels to increased LOAD risk in cognitively intact elderly [7, 8]. In addition, there is emerging evidence that SNPs associated with LOAD risk influence CSF Aβ levels [9, 10]. In particular, the APOE ε4 allele has been shown to associate with lower CSF Aβ 1-42 levels [11, 12]. These considerations prompted us to examine the relationship between TOMM40 poly-T variants (rs10542523) and CSF Aβ 1-42, in cognitively intact elderly. In contrast, as no effect of APOE was detected on tau levels [11], we did not predict to find effects of TOMM40 poly-T variants on tau levels.
Experimental Procedure
Subjects
This study was approved by the Institutional Review Boards of the Nathan S. Kline Institute for Psychiatric Research and the New York University School of Medicine. Participants in the study were volunteers from the NY/NJ area who responded to advertisements in local newspapers and flyers. Some study participants were recruited from our active Memory Education and Research Initiative program sponsored in part by Rockland County Department of Health. All subjects signed a consent form prior to being examined. Compensation of up to $450 was provided to the study participants. The total number of 133 participants completed the baseline visit; 51 of these subjects took part in the optional lumbar puncture procedure where a CSF sample for Aβ determination was collected. Out of these 51 subjects, a total of three subjects were excluded because they showed brain abnormalities in the MRI, and one had a Mini-Mental State Exam (MMSE) score below 28. These subjects were originally recruited for a study on the relationship between depression and Aβ levels, which will be reported separately. For this reason, 28 of the 47 remaining subjects were diagnosed with clinical depression, and 19 were controls. The average age of the whole sample was 67.1 (SD=6.2) and average years of education was 16.6 (SD=2.7).
Aβ Determination
Levels of Aβ were determined in two separate and independent laboratories. In one laboratory, levels of Aβ 1-40 and Aβ 1-42 were measured using monoclonal antibody 6E10 (specific to an epitope present on 1-16 amino acid residues of Aβ) and R209 and R226 in a double antibody sandwich ELISA [13]. The percent coefficients of variation ranged from 8 to 14 % (intra-assay) and 10 to 18% (inter-assay). In another laboratory, CSF Aβ 1-40 and Aβ 1-42 levels were analyzed by electrochemiluminescence technology (Meso Scale Discovery, Gaithersburg, Maryland, USA), using the MS6000 Human Abeta Ultra-Sensitive Kit, following the recommendations by the manufacturer [14]. CSF total tau (T tau) concentration was determined using a sandwich ELISA (Innotest hTAU-Ag, Innogenetics, Gent, Belgium) specifically constructed to measure all tau isoforms irrespectively of phosphorylation status, as previously described [15]. Tau phosphorylated at threonine 181 (P tau) was measured using a sandwich ELISA method (INNOTEST® PHOSPHO-TAU(181P), Innogenetics, Ghent, Belgium), as described previously in detail [16].
Description of the TOMM40 Polymorphic Assays
The TOMM40 determinations were performed By Polymorphic DNA Technologies, Inc. (Alameda, CA). Four polymorphisms were analyzed for each genomic sample: rs8106922, rs429358, rs7412, and rs10524523. The polymorphism rs10524523 is a homopolymer length polymorphism (polyT) located in an intronic region of TOMM40. In the human reference sequence, the number of T residues in the homopolymer, “N”, is 35, and the specific variation described by rs10524523 is a 19 base pair deletion, making the homopolymer T16 (N=16) the variant allele. In the Duke/Polymorphic DNA Technologies haplotyping work, other alleles of this homopolymer have been observed, with values of N ranging from 14 to 46 residues. For each genomic sample, PCR was used to amplify each polymorphism using 40 to 120 nanograms of genomic DNA per sample followed by bidirectional direct Sanger sequencing of the DNA templates on an ABI 3730xl sequencing platform and sequence data analysis.
Design and Analysis
The purpose of this study was to test whether APOE and TOMM40 poly-T variants were associated with changes in Aβ and tau levels. APOE was defined by two levels: subjects in the ε4 carriers group had at least one ε4 allele (n= 16: three subjects had ε2/ε4 and 13 subjects had ε3/ε4), whereas non-ε4 carriers had no ε4 alleles (n= 31: ten subjects had ε2/ε3 and 21 subjects had ε3/ε3). TOMM40 was also defined by two levels: short carriers had at least one S allele (n= 30: three subjects had an S-L combination, 11 had S-S, and 16 had S-VL), whereas non-short carriers had no S allele (n= 17: one subjects had an L-L combination, nine had L-VL, and seven had VL-VL). The short/non-short distinction was chosen to allow for factorial analysis and to search for potential interactions between APOE and TOMM40 effects. In fact, ε4 cases distribute unevenly across TOMM40 poly-T variants, as they nearly exclusively associate with L variants [4]. Thus, only grouping subjects in short/non-short TOMM40 poly-T lengths allowed us to have more sizeable subject numbers in each group. In our ANOVAs, we had 24 short carriers without ε4 (of the nine ε2/ε3 in this group, one was S-L, three were S-VL and five were S-S; of the 15 ε3/ε3, four were S-S and 11 were S-VL), seven non-short carriers without ε4 (the single ε2/ε3 was VL-VL; of the six ε3/ε3, all were VL-VL), six short carriers with ε4 (of the two ε2/ε4, one was S-L and one was S-VL; of the four ε3/ε4, one was S-L, two were S-S and one S-VL), and ten non-short carriers with ε4 (the single ε2/ε4 was L-LV; of the nine ε3/ε4, one was L-L and eight were L-VL).
First, we conducted a hierarchical multiple regression analysis to evaluate whether control variables such as age, years of education, MMSE, gender and depression were associated with changes in Aβ and tau levels, then we assessed the relationship with APOE and TOMM40. In addition to employing the discrete short/non-short distinction for TOMM40, in the regression analysis we also defined TOMM40 in terms of total length, expressed in T residues and calculated as the sum of individual allele lengths. Second, we conducted a series of 2 X 2 analyses of covariance (ANCOVAs) to study the effect of APOE and TOMM40 on Aβ and tau levels. As depressive symptoms may interfere with Aβ levels, we used the score on the Hamilton Depression Scale as a covariate. The homogeneity of regression assumption was tested in all cases and was not violated. Procedure. The study was conducted over 4 visits, generally each 1 week apart. The first three visits were conducted at the Nathan Kline Institute, Orangeburg, NY and at the Clinical & Translational Science Institute, NYU Langone Medical Center. On visit 1, subjects were explained the study procedures and informed of their rights for the purpose of obtaining informed consent. Medical and psychiatric history and vital signs were obtained. Participants also underwent a psychiatric evaluation, and global cognitive status was assessed using the MMSE and the Clinical Dementia Rating. Blood was also drawn for routine laboratory tests and for APOE and TOMM40 genotyping. At visit 2, participants received an MRI scan of the head to quantify the magnitude of vascular brain pathology. At visit 3, subjects underwent a comprehensive neuropsychological assessment. On visit 4 a lumbar puncture was performed by a neurologist under guided fluoroscopy at Corinthian Diagnostic Radiology, New York, NY. Subjects were asked to fast overnight prior to the lumbar puncture (LP) visit. After fasting, at 10 AM, 15 ml of clear CSF was collected into three polypropylene tubes using a fine 25G LP needle guided by fluoroscopy. Tubes were then immediately placed directly on ice for a maximum of 1 hour until samples were centrifuged at 4 degrees C at 1500 rpm for 10 minutes, then aliquots of 0.25 cc placed into 1.00 cc polypropylene cryogenic vials and labeled “A”, “B”, or “C”, and placed in Nunc 81-Cell Storage Boxes at −80 degrees C.
Results
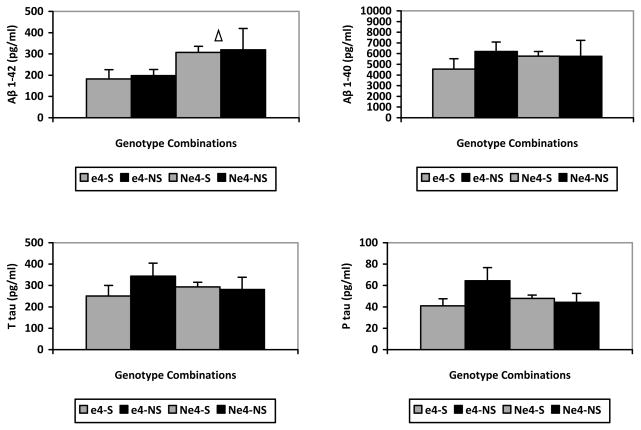
To ensure that Aβ determinations were consistent across laboratories, we conducted a bivariate correlation test on Aβ 1-40 and 1-42 values from both laboratories. Aβ 1-40 correlated significantly across the two labs [r=.536, p < .001] and so did Aβ 1-42 [r=.474, p = .001]. Because of these strong correlations, and to simplify the description of our results, we report only analyses performed on a single set of determinations. The results from our hierarchical linear regression analyses showed that, after controlling for age, years of education, MMSE, gender, and depression (using the Hamilton Depression Scale score), APOE status only affected two variables: Aβ 1-42 [β coefficient = −.385, p = .011] and the Aβ 42/40 ratio [β coefficient = −.560, p < .001], indicating that lower levels of Aβ 1-42 and Aβ 42/40 ratio are associated with the presence of APOE ε4. In contrast, we did not find either the presence of an S TOMM40 rs10524523 poly-T or total length to correlate significantly with the Aβ or tau measurements. To confirm these findings, we applied 2 X 2 ANCOVAs (using the Hamilton Depression Scale score as a covariate) to Aβ 1-40 and Aβ 1-42, the 42/40 ratio, and values of T tau and P tau. Consistent with our regression analysis, we observed a main effect of APOE on Aβ 1-42 levels [F(1,42 = 6.493, p = .015)] (see Figure, Top Left), showing lower levels of Aβ 1-42 with ε4, and a main effect on the ratio [F(1,42 = 22.174, p < .001)], showing lower ratio with ε4, but no main effect of carrying an S TOMM40 poly-T, or an interaction, on either (lowest p = .230). We did not detect an effect of APOE on Aβ 1-40 [F(1,42 = .187, p = .668)], T tau [F(1,42 = .060, p = .808)], or P tau [F(1,42 = .731, p = .397)] (see Figure). Similarly, we did not find a main effect of TOMM40, or an interaction, on any of these indexes (lowest p = .095). Finally, we observed no differences in Age or Years of Education across groups.
Figure.
Top Left: Aβ 1-42 levels in pg/ml for the four independent APOE/TOMM40 groups. Top Right: Aβ 1-40 levels in pg/ml for the four independent APOE/TOMM40 groups. Bottom Left: T tau levels in pg/ml for the four independent APOE/TOMM40 groups. Bottom Right: P tau levels in pg/ml for the four independent APOE/TOMM40 groups. e4-S: with APOE ε4 and Short TOMM40 poly-T; e4-NS: with APOE ε4 and no Short TOMM40 poly-T; Ne4-S: without APOE ε4 and with Short TOMM40 poly-T; Ne4-NS: without APOE ε4 and without Short TOMM40 poly-T; : significant difference (p=.015).
Discussion
Consistent with previous reports, the APOE ε4 allele was associated with reductions in CSF Aβ 1-42 levels [11, 12]. In contrast, we found no significant effect of TOMM40 rs10524523 poly-T variants on CSF Aβ levels, when contrasting subjects who carried S poly-T variants to non-carriers. The lack of a significant effect is consistent with previous findings that not all SNPs associated with increased risk for LOAD alter CSF Aβ levels [9]. Roses et al. [2] have suggested a number of mechanisms by which different TOMM40 poly-T variants may influence LOAD risk, e.g., exon skipping leading to malfunctioning Tom40 protein, interference with APOE transcription, and interactions between Tom40 and apoE isoforms. Of note is that none of the proposed mechanisms may necessarily involve an increase in brain amyloid deposits and correspondent lower amyloid CSF levels. Our results suggest that LOAD risk associated with TOMM40 poly-T variants, unlike with APOE, may not be accompanied by changes in CSF Aβ 1-42 levels. Future studies, using in vivo amyloid PET ligands, should evaluate the effect of poly-T variants on aggregated forms of amyloid in brain.
In our results, neither Aβ 1-40 nor tau levels were influenced by either APOE or TOMM40. The failure to detect elevations in CSF tau in cognitively intact ε4 carriers is consistent with a previous report [11]. An exploratory look at the bottom of our Figure may suggest that increased levels of CSF tau, which are considered deleterious, might be present when the protective effect of the S poly-T is missing, in association with the negative effect of APOE ε4. However, this difference is not significant in our results and would need to be tested in a larger sample.
Our study was somewhat limited by our sample size, which forced us to group subjects in aggregated categories (i.e., whether they possessed a S poly-T variant of TOMM40 or not). A restriction of this approach is that we were limited to testing whether the presence of a protective S poly-T influenced (specifically, in this case, increased) CSF Aβ 1-42 levels, and did not examine in greater detail TOMM40 poly-T sub-groups (e.g., L-L vs. L-VL), as defined by differences in length. However, we had an adequate sample size to detect the effect of APOE on Aβ 1-42 and a power analysis indicated that we had adequate power to detect comparable main effects of TOMM40 on Aβ 1-42. In summary, we found no evidence that longer TOMM40 poly-T variants and the APOE ε4 allele, which are both associated with increased risk for LOAD, have the same effect on CSF Aβ 1-42. Our results appear to indicate that increased LOAD risk associated with longer TOMM40 poly-T variants may not be based on an amyloid-dependent mechanism.
Acknowledgments
This study was supported in part by an NIHM Grant (R01 MH080405) to NP. The authors report no conflict of interest. We wish to thank Drs Michael W. Lutz, Ann M. Saunders and Allen D. Roses, Deane Drug Discovery Institute and Department of Medicine, Duke University, Durham, NC, for helping with the preparation of this manuscript and for supervising the TOMM40 polymorphic assays determination.
References
- 1.Blennow K, deLeon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Roses AD, Lutz MW, Amrine-Madsen H, Saunders A, Crenshaw DG, Sundseth SS, Huentelman MJ, Welsh-Bohmer KA, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. The Pharmacogenomics Journal. 2009;10:375–384. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caselli RJ, Saunders A, Lutz M, Huentelman M, Reiman E, Roses A. TOMM40, APOE, and age of onset of Alzheimer’s disease. Poster presented at the Alzheimer’s Association International Conference on Alzheimer’s Disease; Honolulu, HI. 2010. [Google Scholar]
- 4.Lutz MW, Crenshaw DG, Saunders AM, Roses AD. Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimers Dement. 2010;6:125–31. doi: 10.1016/j.jalz.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT. Dissection of the mitochondrial import and assembly pathway for human TOM40. Journal of Biological Chemistry; 2005;280:11535–11543. doi: 10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel K, Egan B, Lithgow T. Tom40, the import channel of the mitochondrial outer membrane, plays an active role in sorting imported proteins. EMBO J. 2003;10:2380–2386. doi: 10.1093/emboj/cdg229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauwe JSK, Wang J, Mayo K, Morris JC, Fagan AM, Holtzman DM, Goate AM. Alzheimer’s disease risk variants show association with cerebrospinal fluid amyloid-Beta. Neurogenetics. 2009;10:13–17. doi: 10.1007/s10048-008-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han MH, Schelleberg GD, Wang LS. Genome-wide association reveals genetic effects on human Aβ42 and τ protein levels in cerebrospinal fluids: A case control study. BMC Neurology. 2010;10:1471–2377. doi: 10.1186/1471-2377-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts Amyloid-Beta but not tau Alzheimer pathology in cognitively normal aging. Annals of Neurology. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K. APOE ε4 allele is associated with reduced cerebrospinal fluid levels of Aβ42. Neurology. 2004;62:2116–2118. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- 13.Mehta PD, Pirttila T, Patrick BA, Barshatzky M, Mehta SP. Amyloid beta protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;304:102–106. doi: 10.1016/s0304-3940(01)01754-2. [DOI] [PubMed] [Google Scholar]
- 14.Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid β-amyloid (1-42) in Alzheimer’s disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 15.Blennow K, Wallin A, Ågren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical diagnostic marker for axonal degeneration in Alzheimer’s disease? Mol Chem Neuropathology. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 16.Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van der Perre B, Sjögren M, Andreasen N, Blennow K. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]