Abstract
Objectives
To compare noninvasive estimates of pulmonary artery pressure (PAP) obtained via echocardiography (ECHO) to invasive measurements of PAP obtained during right heart catheterization (RHC) across a wide range of PAP, to examine the accuracy of estimating right atrial pressure via ECHO (RAPECHO) compared to RAP measured by catheterization (RAPRHC), and to determine if adding RAPECHO improves the accuracy of noninvasive PAP estimations.
Animals
Fourteen healthy female beagle dogs.
Methods
ECHO and RHC performed at various data collection points, both at normal PAP and increased PAP (generated by microbead embolization).
Results
Noninvasive estimates of PAP were moderately but significantly correlated with invasive measurements of PAP. A high degree of variance was noted for all estimations, with increased variance at higher PAP. The addition of RAPECHO improved correlation and bias in all cases. RAPRHC was significantly correlated with RAPECHO and with subjectively assessed right atrial size (RA sizesubj).
Conclusions
Spectral Doppler assessments of tricuspid and pulmonic regurgitation are imperfect methods for predicting PAP as measured by catheterization despite an overall moderate correlation between invasive and noninvasive values. Noninvasive measurements may be better utilized as part of a comprehensive assessment of PAP in canine patients. RAPRHC appears best estimated based on subjective assessment of RA size. Including estimated RAPECHO in estimates of PAP improves the correlation and relatedness between noninvasive and invasive measures of PAP, but notable variability in accuracy of estimations persists.
Keywords: Tricuspid regurgitation, right heart catheterization, right atrial pressure, pulmonic regurgitation, dog
Introduction
In veterinary patients, pulmonary hypertension (PH) may be caused by heartworm disease, left-sided heart disease resulting in elevated pulmonary venous pressure, chronic pulmonary diseases, congenital cardiac diseases causing pulmonary overcirculation, thromboembolism, or may be idiopathic. 1–9 The gold standard test for diagnosis of PH is right heart catheterization (RHC) and direct measurement of pulmonary arterial pressure (PAP). In veterinary patients, procedural costs, associated risks and lack of availability often preclude performing RHC. While echocardiography (ECHO) serves as a preliminary screening tool in human patients, it is often the only diagnostic modality employed in veterinary medicine when confirming or excluding PH. Chronic moderate to severe PH, regardless of the underlying cause, results in characteristic echocardiographic changes to the right heart, including right ventricular (RV) concentric hypertrophy, RV dilation, changes in tricuspid and pulmonic blood flow and, potentially, RV systolic dysfunction and failure. 1,4,7,8,10–16 Anatomic and functional changes may occur as a result of the interaction of PH and other hemodynamic influences and can not be used alone to reliably estimate PH severity. Accordingly, an accurate noninvasive method of estimating actual PAP is preferable to diagnose PH in clinical patients.
Recent studies in veterinary patients have used analysis of the tricuspid regurgitation (TR) jet velocity-determined pressure gradient and pulmonic regurgitation (PR) jet velocity-determined pressure gradient, with and without addition of right atrial pressure (RAP) estimates, to estimate systolic PAP (sPAPECHO) and diastolic PAP (dPAPECHO), respectively. In recent reports, in humans with PH, the correlation between peak TR pressure gradient as an estimate of sPAP and invasively measured PAPRHC is moderate to poor with a high degree of variability.11,17–21 Other echocardiographic criteria that have been identified as possible predictors of PH in people and dogs include systolic time intervals, pulse-wave Doppler assessment of pulmonary outflow, tricuspid annular plane systolic excursion and tissue Doppler imaging. 1,4,7,15,22–29 These measurements add information to the echocardiographic assessment of PH patients, but the relatively straightforward analysis of PAP through assessment of TR and PR jets remains clinically popular even though the limitations of this type of assessment are widely acknowledged.
The benefits of including RAP obtained by ECHO (RAPECHO) in the TR or PR velocity-derived pressure gradient have also been investigated in the human literature, as the modified Bernoulli equation predicts the inclusion of RAPECHO should increase the accuracy of the sPAPECHO estimation. Despite its theoretical advantage, the reported effect of including RAPECHO is variable, with several studies failing to consistently show improved correlation when employing this approach. 18,21,30,31 In people, clinical assessment of jugular veins and echocardiographic assessment of inferior vena cava collapse are two methods commonly employed in estimating RAPECHO. In the latter, the patient is instructed to sniff vigorously to further decrease intrathoracic pressure and therefore minimize the influence of varying intrathoracic pressure seen during normal respiration. Both this method and jugular assessment are prone to inaccuracy, and many investigators suspect this partially explains why adding RAPECHO does not confer consistent benefit in PAP estimation. 16,18,21,30,32,33
In veterinary patients, noninvasively determining RAPECHO is even more challenging, owing to difficulties in assessing jugular veins and coordinating respiratory cycle during echocardiography. Identifying a method for accurate estimation of RAPECHO might increase the accuracy of noninvasively estimating PAP. This possibility has not been thoroughly evaluated in veterinary medicine. Right atrial (RA) size is presumably associated with RAP, and presents a potential tool for estimating RAPECHO in veterinary patients. Unlike the more spherical left atrium, the RA is ellipsoid in shape and its area and volume are difficult to repeatedly and objectively measure using 2-dimensional imaging methods. As a result, subjective assessment of RA size (RA sizesubj) is more commonly reported and used for interpretation. In theory, objective or subjective measures of RA enlargement might provide a method of estimating RAPECHO, which may then improve noninvasive estimations of PAP.
Even though ECHO estimates of PAP are routinely used in clinical veterinary medicine,1,2,5–8,28,29 there have been no veterinary studies examining the accuracy of echocardiographically estimated PAP in dogs. The objectives of this study were thus to compare noninvasive estimates of PAP obtained via ECHO to invasive measurements of PAP obtained by RHC in healthy beagles with experimentally-induced pulmonary hypertension. Additional study objectives were to examine the accuracy of estimating RAP via ECHO based on subjective and objective assessment of RA size, and to determine if adding estimated RAPECHO would improve the accuracy of echocardiographically-estimated PAPECHO. We hypothesized that a correlation between noninvasive estimations and invasive measurements would be present but poor. We also predicted that noninvasive estimation of RAPECHO would be inaccurate and that including estimated RAPECHO in PAP estimations would not improve the correlation with invasive measurements.
Materials and Methods
Study population and procedural overview
Fourteen intact female beagles were prospectively enrolled in this study between July 2010 and July 2012. The median weight of these animals was 8.5 kg (range 6.8 – 12 kg), and they were all one year of age. All animals were concurrently enrolled in a research project aimed at generating an acute embolization (EMB) model of PH, in which multiple polyvinyl alcohol microbeada injections into the right atrium were performed under general anesthesia. For additional details, see Bellofiore et. al. 2013.34 After general anesthesia was induced, an initial ECHO was immediately performed. There was a 30 minute delay between the initial ECHO and collection of RHC data. EMB was then performed and ean PAP (mPAPRHC) intermittently measured by RHC until a target mPAPRHC was >30 mmHg (achieved in 7/16 dogs), at which point the next data collection point was reached and RHC and ECHO were again performed. The ECHO measurement following EMB occurred immediately following the RHC once the recorded measurements remained static for a total of 10 minutes. Accordingly, two data collection points (normotensive at baseline and hypertensive after EMB) were obtained from each anesthetic procedure. For the purposes of this study, RHC data was compared with ECHO data only from the same data collection point (either when PAP pressures were normal at baseline or when they were increased following EMB). This provided comparison of RHC to ECHO across a range of normotensive and hypertensive PAP. Each subject contributed at least two data collection points, one at normal PAP and one at elevated PAP. Some animals underwent multiple EMB procedures on different dates and generated more than two data sets. In summary, the 14 dogs generated 33 pairs of measurements (RHC and ECHO) for analysis.
General anesthesia
General anesthesia was induced with intravenous propofol (10 mg/kg body weight) after intramuscular premedication (midazolam 0.1mg/kg and hydromorphone 0.1mg/kg). Following endotracheal intubation, the dogs were maintained during the procedure on isoflurane gas (1–3%) with 100% oxygen and were mechanically ventilated at 11 breaths/minute. Lactated Ringer’s intravenously at a rate of 10 ml/kg/hr. The femoral and external jugular veins were catheterized for access to the right heart, delivery of microbeadse, contrast injection for angiography and blood collection. Dogs receiving a single EMB were humanely euthanized after conclusion of data collection. Dogs scheduled to receive multiple EMB were either recovered from anesthesia or humanely euthanized at the end of the last procedure. All of the procedures were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin – Madison.
Echocardiography
Echocardiographyf was performed by a board certified cardiologist (HBK) on all animals both before and after EMB. Standard right parasternal long axis, right parasternal short axis and left apical views were obtained.35 Two-dimensional ECHO was used to evaluate chamber size and function, while color flow and spectral Doppler imaging were performed to assess valve regurgitation. The tricuspid valve was interrogated from multiple views, and continuous wave spectral Doppler imaging was used to measure peak TR velocity-determined pressure gradient for estimated sPAPECHO. Care was taken to align the TR jet as parallel to the plane of the ultrasound interrogation beams as possible. The pulmonic valve was similarly assessed and peak and end PR jet velocity-determined pressure gradients were measured for estimation of mPAPECHO and dPAPECHO, respectively. Views of the RA and RV were then optimized from the left-sided apical images for measurement of RA area. RA area was measured at the end of ventricular systole, when the RA was at its maximal size, in three separate ways. First, by tracing the inside border of the RA in this view, with RA area calculated; next by measuring RA height and width and using the standard equation for area of an ellipse [π *(RAh/2)*(RAw/2)]; and finally by using the standard equation for calculating the volume of an ellipse [(4/3) π *(RAh/2)* (RAh/2)*(RAw/2)].
Subjective assessment of RA size (RA sizesubj) was reported using the following scoring system: 0 = no enlargement, 1 = mild enlargement, 2 = moderate enlargement and 3 = severe enlargement, using multiple views from the right and left sides. Right atrial pressure (RAPECHO) was estimated subjectively using a method previously described by Kittleson, according to the following criteria: RAPECHO = 5 mmHg if the RA appears normal in size, RAPECHO = 10 mmHg if the RA appears enlarged but there is no evidence of right-sided heart failure present, and RAPECHO = 15 mmHg if the RA appears enlarged and there is evidence of right-sided heart failure present.36 All examinations and measurements were made by a board certified cardiologist (HBK). All measurements were made in triplicate from three consecutive cardiac cycles, if possible, and averaged.
Right heart catheterization
All animals underwent RHC using a 7.5 French Swan-Ganz catheter with data collected before and after EMB. A fluid-filled pressure catheter was advanced through a femoral vein to the pulmonary artery. Systolic, diastolic and mean PAP (sPAPRHC, dPAPRHC, mPAPRHC) were obtained; The catheter was then withdrawn into the RA, and RAPRHC measurements were obtained.
Embolization
A large bore catheter was advanced through an external jugular vein to the right atrium, where polyvinyl alcohol microbeadsa were repeatedly injected as previously described.34 Briefly, 2 mL of 700–900 μm beads were injected first, followed by one or more injections of 2–5 mL of 150–250 μm beads. Mean PAPRHC was intermittently measured and injections were continued until a goal mPAPRHC of >30 mmHg was reached.
Statistics
Right heart catheterization data, ECHO measurements (TR and PR), RA size calculations, RA size subj and RAPECHO were tested for normality using the D’Agostino and data was non-normally distributed, so non-parametric analyses were used for analysis. ECHO-estimated pressure gradients (sPAPECHO, dPAPECHO, mPAPECHO) were paired with the corresponding invasive RHC measurements and tested for correlation using a Spearman rho coefficient of correlation. If two indices were determined to be significantly correlated (p < 0.05), linear regression was then used to analyze their relationship. Linear regression equations were determined for all comparisons of invasive measurements and noninvasive estimations and assessed for goodness of fit (r2). RAPECHO was added to the PAPECHO estimates and these values were again paired with corresponding RHC measurements. Tests for correlation and linear regression were repeated to evaluate whether including RAPECHO impacted the correlation and/or linear regression. Bland-Altman analyses were also performed to assess the overall accuracy and relatedness of ECHO and RHC.
Receiver operating curves (ROC) were generated for select data sets to investigate the sensitivity and specificity of ECHO as a means of identifying animals with PH, as defined by a sPAPRHC > 30mmHg, mPAPRHC > 25mmHg or dPAPRHC > 10mmHg. The curves were also used to establish noninvasive cut-off values that can identify PH with maximal possible sensitivity. An additional ROC curve was generated to investigate cut-off values for noninvasive estimates that would predict invasive sPAPRHC > 60mmHg, since this value represents a degree of PH often considered as a clinical indication for medical treatment in veterinary medicine.
The association between various objective estimates of RA size and actual RAPRHC were analyzed using the Spearman rho correlation test. If two indices were determined to be significantly correlated (p <0.05), linear regression was then used to analyze their relationship. Animals were assigned one of three possible RAPECHO values (5 mmHg, 10mmHg or 15 mmHg) based on subjective assessment of atrial size.35 Since no animals were in right-sided congestive heart failure at the time of assessment, animals were allocated to two RAPECHO (5 mmHg and 10 mmHg) of the proposed three RAPECHO groups. The median values for invasive RAPRHC were compared between these groups using a Mann-Whitney U test (p < 0.05). For analysis of RA sizesubj, animals with a score of 2 (n=3, moderate RA enlargement) and 3 (n=2, severe RA enlargement) were combined into one group. Median values for RAPRHC were compared among the three RA sizesubj groups (no enlargement, mild enlargement, moderate/severe enlargement) using the Kruskal-Wallis test, followed by a post-hoc Dunn’s multiple comparisons test (p < 0.05).
Results
Pulmonary arterial pressure estimations
There were 33 data collection points available for analysis. In all cases, TR was of sufficient quality for interrogation. Only 21 of 33 data sets had PR of sufficient quantity and quality to permit interrogation. These 21 data sets were used to estimate mPAPECHO and dPAPECHO. A summary of invasive and noninvasive values at baseline and post-EMB are in Table 1.
Table 1. Summary of median and range for invasive measurements and noninvasive estimates of pulmonary artery pressure in 14 dogs at baseline and post-embolization.
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; dPAPRHC, diastolic pulmonary artery pressure obtained by right heart catheterization; mPAPRHC, mean pulmonary artery pressure obtained by right heart catheterization; RAPRHC, mean right atrial pressure obtained by right heart catheterization; TR, tricuspid regurgitation gradient; PR, pulmonic regurgitation gradient.
| Baseline (mmHg) (n) | Post-embolization (mmHg) (n) | |
|---|---|---|
| sPAPRHC | 17 (10–22) (17) | 42 (13–77) (16) |
| dPAPRHC | 7 (0–18) (17) | 24 (4–54) (16) |
| mPAPRHC | 10 (4–20) (17) | 30 (9–63) (16) |
| RAPRHC | 4 (0–5) (17) | 5 (1–10) (14) |
| Peak TR | 11.3 (6–16.9) (17) | 34.1 (5.7–75.5) (16) |
| Peak PR | 3.2 (1.1–8.9) (12) | 11.8 (8.4–18.9) (9) |
| End PR | 1 (.1–3.4) (12) | 4 (1.1–9.9) (9) |
Estimated sPAPECHO, based on peak TR velocity-determined pressure gradient; estimated mPAPECHO, based on peak PR velocity-determined pressure gradient; and estimated dPAPECHO, based on peak PR or end PR velocity-determined pressure gradient, were all significantly correlated with invasive measurements of sPAPRHC, mPAPRHC and dPAPRHC, respectively. Invasive dPAPRHC was better correlated with end PR velocity-determined pressure gradient than peak PR velocity-determined pressure gradient. When RAPECHO was included, the correlation improved in all cases. A similar improvement in goodness of fit was observed when RAPECHO was included in the analysis. Summary statistics are outlined in Table 2. Selected data sets are displayed in Figure 1.
Table 2. Correlation and linear regression equations for noninvasive estimates and invasive measurements of pulmonary artery pressure and right atrial size.
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; dPAPRHC, diastolic pulmonary artery pressure obtained by right heart catheterization; mPAPRHC, mean pulmonary artery pressure obtained by right heart catheterization; RAPECHO, right atrial pressure estimated by echocardiogram; RAPRHC, mean right atrial pressure obtained by right heart catheterization; TR, peak tricuspid regurgitation gradient; PR, pulmonic regurgitation gradient; RA, right atrium.
| Correlation (r) | 95% Confidence Interval | P value | Linear Regression (r2) | Regression Equation | |
|---|---|---|---|---|---|
| sPAPRHC vs. TR | 0.77 | 0.58–0.88 | <0.0001 | 0.61 | Y = 9.91 + 0.88x |
| sPAPRHC vs. (TR+RAPECHO) | 0.78 | 0.58–0.89 | <0.0001 | 0.66 | Y = 5.48 + 0.83x |
| mPAPRHC vs. peak PR | 0.68 | 0.35–0.87 | 0.0006 | 0.59 | Y = 7.20 + 1.82x |
| mPAPRHC vs. (peak PR + RAPECHO) | 0.69 | 0.36–0.87 | 0.0005 | 0.64 | Y = 1.40 + 1.34x |
| dPAPRHC vs. peak PR | 0.70 | 0.37–0.87 | 0.0004 | 0.57 | Y = 3.94 + 1.54x |
| dPAPRHC vs. (peak PR + RAPECHO) | 0.70 | 0.38–0.87 | 0.0004 | 0.61 | Y = −0.86 + 1.15x |
| dPAPRHC vs. end PR | 0.75 | 0.45–0.89 | 0.0001 | 0.48 | Y = 7.00 + 2.85x |
| dPAPRHC vs. (end PR + RAPECHO) | 0.79 | 0.54–0.92 | <0.0001 | 0.66 | Y = −3.95 + 2.00x |
| RAPRHC vs. RA area measured | 0.31 | −0.05–0 | 0.08 | N/A | N/A |
| RAPRHC vs. RA area ellipse method | 0.38 | 0.01–0.65 | 0.04 | 0.22 | Y = 1.66 + 5.90x |
| RAPRHC vs. RA volume ellipse method | 0.33 | −0.03–0.62 | 0.07 | N/A | N/A |
Figure 1. Noninvasive estimations versus invasive measurements of pulmonary hypertension.
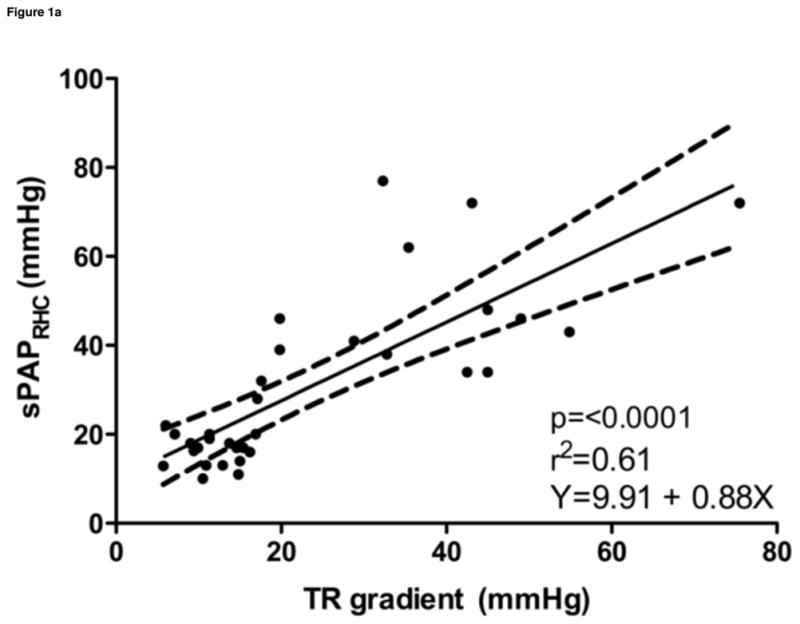
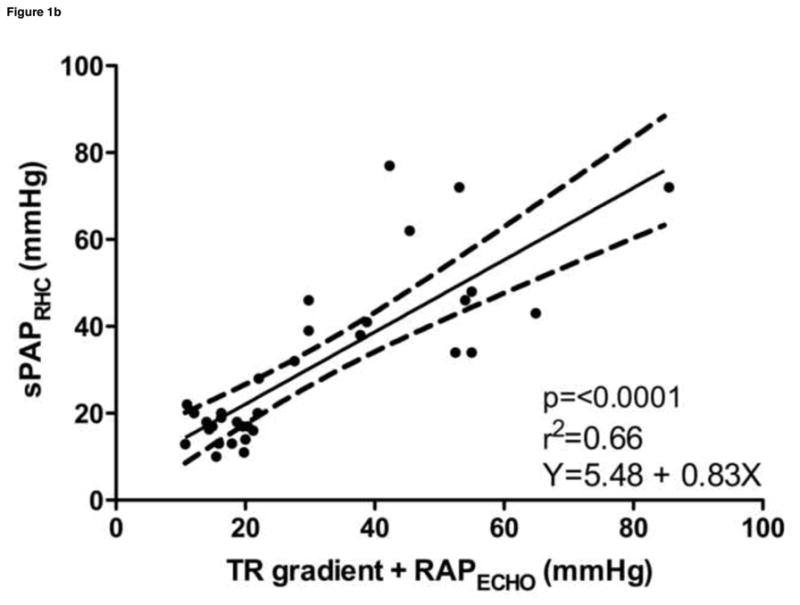
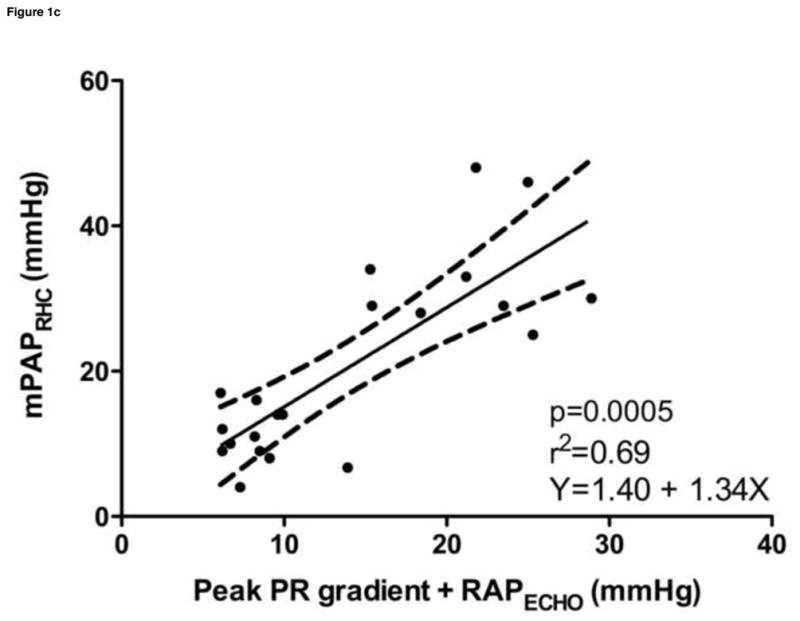
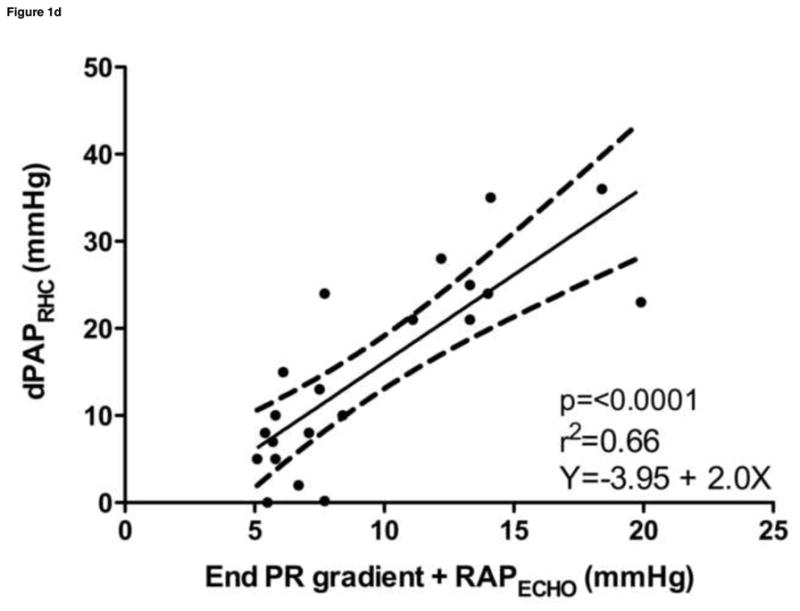
a. Systolic pulmonary artery pressure, b. Systolic pulmonary artery pressure plus the addition of estimated right atrial pressure, c. Mean pulmonary artery pressure plus the addition of estimated right atrial pressure, d. Diastolic pulmonary artery pressure plus the addition of estimated right atrial pressure
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; dPAPRHC, diastolic pulmonary artery pressure obtained by right heart catheterization; mPAPRHC, mean pulmonary artery pressure obtained by right heart catheterization; RAPECHO, right atrial pressure estimated by echocardiogram; TR, peak tricuspid regurgitation gradient; PR, pulmonic regurgitation gradient.
ROC analysis was used to determine optimum non-invasive cut-off values for predicting the presence of PH (sPAPRHC > 30mmHg and > 60mmHg, mPAPRHC > 25mmHg or dPAPRHC > 10mmHg) in these groups. ROC data is summarized in Table 3a and 3b.
Table 3a. Summarized ROC data for the prediction of pulmonary hypertension.
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; dPAPRHC, diastolic pulmonary artery pressure obtained by right heart catheterization; mPAPRHC, mean pulmonary artery pressure obtained by right heart catheterization; RAPECHO, right atrial pressure estimated by echocardiogram; TR, tricuspid regurgitation gradient; PR, pulmonic regurgitation gradient; Se, sensitivity; Sp, specificity; AUC, area under the curve; CI, confidence interval.
| Cut-off (mmHg) | Se/Sp | AUC | 95% CI | |
|---|---|---|---|---|
| Peak TR to predict sPAPRHC >30mmHg | 17.35 | 100/100 | 1.0 | 1.0–1.0 |
| Peak TR + RAPECHO to predict sPAPRHC >30mmHg | 24.85 | 100/100 | 1.0 | 1.0–1.0 |
| Peak PR + RAPECHO to predict mPAPRHC >25mmHg | 14.6 | 100/100 | 1.0 | 1.0–1.0 |
| End PR + RAPECHO to predict dPAPRHC >10mmHg | 7.3 | 84.6/87.5 | 0.92 | 0.81–1.0 |
Table 3b. Summarized ROC data for the prediction of sPAPRHC > 60mmHg.
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; RAPECHO, right atrial pressure estimated by echocardiogram; TR, tricuspid regurgitation gradient; Se, sensitivity; Sp, specificity; AUC, area under the curve; CI, confidence interval.
| Selected cut-offs to predict sPAPRHC > 60mmHg | ||||
|---|---|---|---|---|
| Cut-off (mmHg) | Se/Sp | AUC | 95% CI | |
| Peak TR gradient | 30.55 | 100/76.7 | 0.86 | 0.70–1.01 |
| Peak TR gradient + RAPECHO | 40.55 | 100/80 | 0.87 | 0.72–1.02 |
Bland-Altman analyses were performed for all data sets, and are summarized in Table 4 and Figure 2. Despite a tendency towards underestimation of PAPECHO, at higher pressures, PAPECHO overestimation was also present, resulting in 95% limits of agreement for bias that included zero in all cases.
Table 4. Summary of Bland-Altman data analyses.
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; dPAPRHC, diastolic pulmonary artery pressure obtained by right heart catheterization; mPAPRHC, mean pulmonary artery pressure obtained by right heart catheterization; RAPECHO, right atrial pressure estimated by echocardiogram; TR, peak tricuspid regurgitation gradient; PR, pulmonic regurgitation gradient; SD, standard deviation.
| Group | Bias | SD of Bias | 95% Limits of Agreement |
|---|---|---|---|
| sPAPRHC vs. TR | 7.18 | 12.00 | −16.33–30.70 |
| sPAPRHC vs. (TR + RAPECHO) | 0.36 | 11.53 | −22.24–22.97 |
| mPAPRHC vs. peak PR | 13.23 | 9.39 | −5.18–31.64 |
| mPAPRHC vs. (peak PR + RAPECHO) | 6.57 | 8.24 | −9.59–22.72 |
| dPAPRHC vs. peak PR | 7.89 | 7.79 | −7.38–23.16 |
| dPAPRHC vs. (peak PR + RAPECHO) | 1.22 | 6.97 | −12.53–14.88 |
| dPAPRHC vs. end PR | 12.35 | 9.39 | −6.05–30.75 |
| dPAPRHC vs. (end PR + RAPECHO) | 5.69 | 7.86 | −9.72–21.09 |
Figure 2. Selected Bland-Altman analyses.
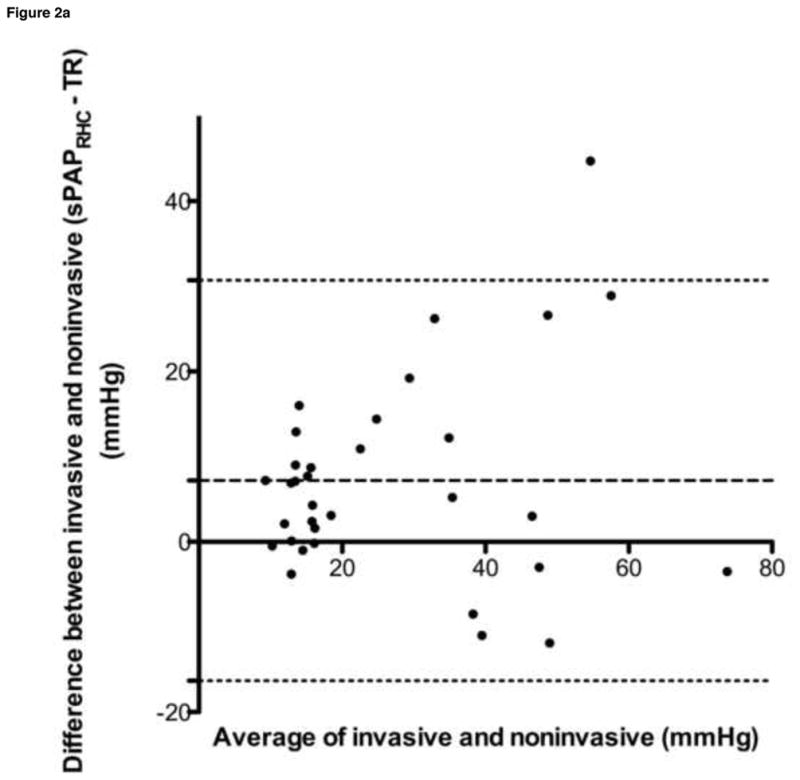
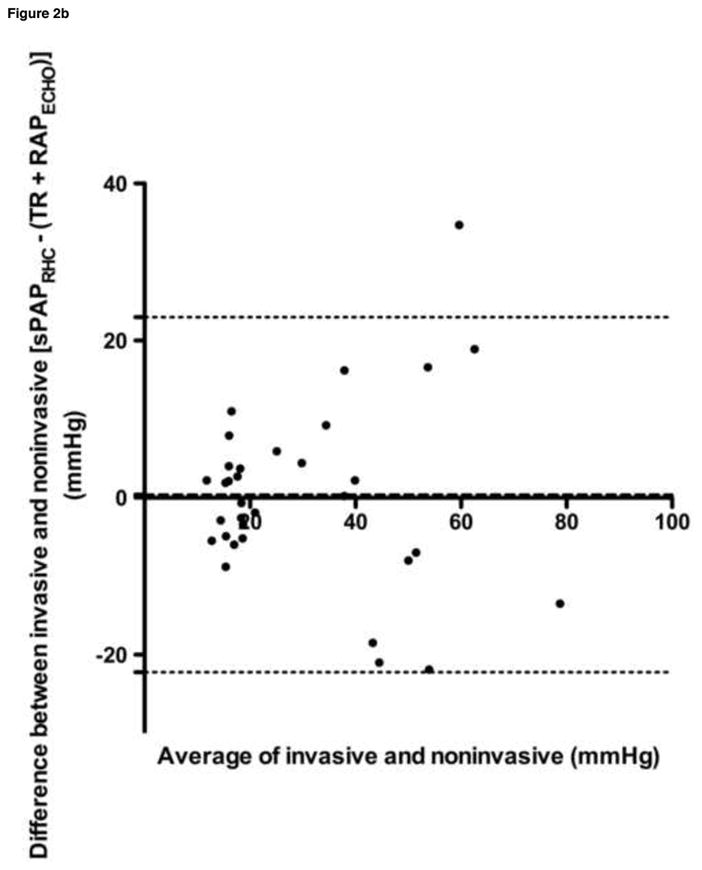
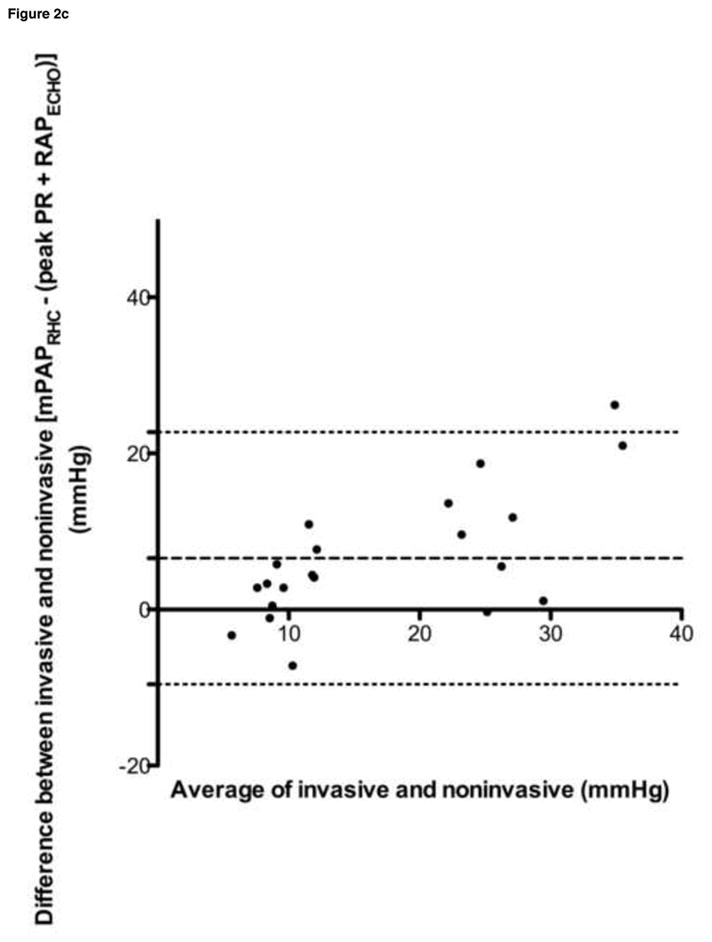
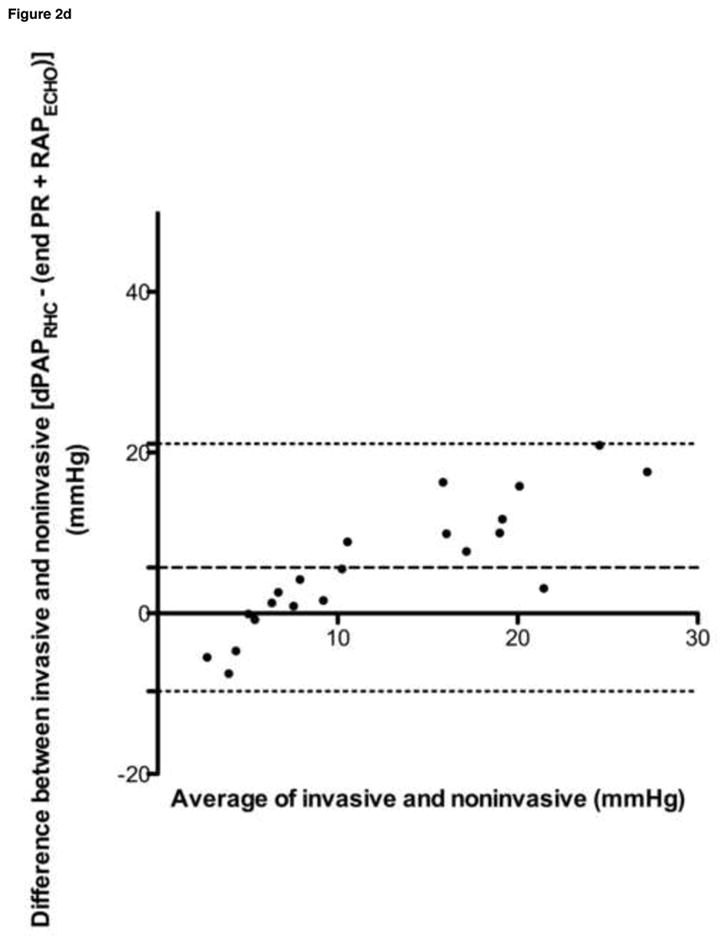
a. sPAPRHC vs. peak TR gradient, b. sPAPRHC vs. peak TR gradient plus RAPECHO, c. mPAPRHC vs. peak PR gradient plus RAPECHO, d. dPAPRHC vs. end PR gradient plus RAPECHO
sPAPRHC, systolic pulmonary artery pressure obtained by right heart catheterization; dPAPRHC, diastolic pulmonary artery pressure obtained by right heart catheterization; mPAPRHC, mean pulmonary artery pressure obtained by right heart catheterization; RAPECHO, right atrial pressure estimated by echocardiogram; TR, peak tricuspid regurgitation gradient; PR, pulmonic regurgitation gradient.
Right atrial pressure and size estimations
Mean RAP values (RAPRHC)were available in 31 of 33 data sets and RAP was objectively estimated using three different methods (Table 2) in these dogs. RAPRHC was significant, albeit weakly, correlated with RA size as estimated by the ellipse area method. RAPRHC was not significantly correlated with the RA tracing method or RA ellipse volume method. Median RAPRHC (4 mmHg, range: 0–6 mmHg) for dogs subjectively classified as RAPECHO of 5mmHg was mildly but significantly lower than median RAPRHC (5 mmHg, range: 1–10 mmHg) for dogs subjectively classified as RAPECHO of 10mmHg (p=0.015). There was a significant difference among the median RAPRHC in the three RA sizesubj groups (normal: 4 mmHg, range: 0–6; mild: 5 mmHg, range: 4–6; moderate/severe: 8 mmHg, range: 1–10 mmHg) (p=0.037). Post-test analysis failed to detect a significant difference between any two specific groups, likely due to the small number of animals in each group.
Discussion
The findings in this study suggest that despite a statistically significant correlation between noninvasive estimates and invasive measurements of PAP, the relationship between the predicted and actual values is highly variable as evidenced by moderate correlation coefficients with wide confidence intervals. The highest correlation coefficients for prediction of PAPRHC were observed when estimates of RAPECHO were included; however, the actual difference in correlation was negligible suggesting little additional value in including estimates of RAPECHO in this study. In evaluating the relationship between peak TR gradient and sPAPRHC, noninvasive estimates of PAP appear to have increased variance when PAP is higher. Our results are in agreement with many studies in human patients with PH. Several investigators have reported that using peak TR gradients as surrogates for invasive sPAPRHC measurements is prone to inaccuracy, both for the diagnosis of PH and for classifying its severity, 11,17–21 and another study identified no significant correlation between peak or end-diastolic PR gradients and mPAPRHC or dPAPRHC, respectively.37
Also similar to what has been reported in human patients, we observed both under and overestimation of actual PAPRHC in this study, with a tendency towards underestimation when using ECHO. 17,18,21 Accurate noninvasive estimation requires the presence and identification of a regurgitant jet (TR or PR), proper orientation of the ultrasound probe parallel to the jet, and generation of a flow profile of sufficient quality to permit proper identification of peaks. In the case of PR, identification of an early diastolic peak and end diastolic velocity are both necessary. Improper orientation or incomplete profiles will tend towards underestimation of actual PAP. Additionally, the modified Bernoulli equation applies specifically to blood flowing through a tube with no or negligible frictional losses, traversing across a constant area orifice. Changes in orifice size during the cardiac cycle and the effects of severe regurgitation into large cardiac chambers have been cited as possible causes of underestimation when applying the Bernoulli equation to TR flow. 18,21 Right ventricular systolic dysfunction, a possible component of severe PH, may also reduce TR velocity, leading to underestimation of true sPAP.
Overestimation of PAPRHC was also noted in our study. In the human literature, inaccuracy in estimating RAP by echo is well documented and discussed as the most likely source of overestimation, with underestimation of true RAP resulting in overestimation of PAPECHO. 16,18,30,32 In our patients, relatively large overestimations (in some cases > 15mmHg) remained even after RAPECHO was added to the peak TR velocity-determined pressure gradient. In those patients where RAP was invasively measured, the median RAPRHC was 4.0 mmHg (range 0–10), and minor discrepancies between actual mean RAPRHC and RAPECHO would not explain the degree of overestimation observed in several subjects. Alternatively, technical errors such as inaccurate identification of peak regurgitant velocity on the spectral trace or the presence of localized regions of accelerated velocity or vortices within a regurgitant jet are potential sources of this error. Although invasive measurements are considered the gold standard for determining PAPRHC, catheter tip location, catheter obstruction, or signal dampening along the length of the extension set could potentially reduce the measured PAPRHC and predispose to overestimation.
Although ECHO and RHC were performed within 30 minutes of one another in this study, variation in hemodynamic parameters during the course of general anesthesia could also result in apparent under- or overestimation. Commonly, one might expect patients to develop progressive hypothermia and hypotension during the course of anesthesia. Echocardiogram was performed prior to RHC in animals at the first data collection point, when PAP was normal, potentially resulting in overestimation of noninvasive estimations among this data. After EMB, which generated elevated PAP, RHC was performed prior to ECHO, potentially resulting in underestimation of noninvasive estimations.
If the modified Bernoulli equation is applicable in the estimation of pressure differential across the tricuspid valve in patients with TR, adding RAPECHO to the TR velocity-derived pressure differential should provide a more accurate prediction of sPAPRHC. Our findings did not support this theoretical improvement. Reports from the human literature are conflicting, with several studies failing to consistently show improved correlation when employing this approach. 18,21,30,31 In this study, no objective method of assessing RA size proved highly correlated to RAPRHC, although there was a significant but weak correlation between RA area, as determined by the ellipse method. Ultimately, RA sizesubj or RAPECHO, the two methods employing subjective assessment of RA size, were significantly associated with RAPRHC. The discriminatory ability of these predictors may be limited by the small range of RAP (RHC and subjective) seen in this study. This suggests that RA size may be better evaluated subjectively and that RA size increases with increasing mean RAPRHC. Although difficulties in accurately estimating RAPECHO have been cited as a potential source of error impacting non-invasively estimated PAPECHO, the difference between RAPECHO and actual RAPRHC was low in this study, with a median difference of 1 mmHg (range 0–9mmHg). This difference likely has minimal impact on noninvasive estimations, particularly in the case of sPAPECHO, where pressures are much higher than those of the right atrium.
An unexpected finding in our study was that a noninvasively estimated sPAPECHO > 17.4 mmHg (TR peak velocity > 2.1 m/sec) proved 100% sensitive and 100% specific for identifying animals with an sPAPRHC > 30 mmHg, while a noninvasively estimated sPAPECHO > 30.6 mmHg (TR velocity > 2.8 m/sec) proved 100% sensitive and 76.76% specific for identifying animals with an invasive sPAPRHC > 60 mmHg (Table 3a and 3b). These are much lower cut-off values than are typically used in veterinary medicine to diagnose PH or estimate severity, and are consistent with the tendency for noninvasive estimations to underestimate actual sPAPRHC. These lower than expected cut-off points may suggest that ECHO assessment leads to underdiagnosis of PH or misclassification of the severity of PH in many canine patients, at least in acute PH situations. The clinical significance of this error, if present in the actual canine population affected by more chronic PH, is unknown.
This study had several limitations. First, measurements were not performed simultaneously, so observed differences could be related to temporal variation in PAP. Significant hourly variation in PAP has been shown in awake human patients with PH,17 possibly due to variation in total pulmonary resistance associated with severe PH. Although this potential source of error was minimized in our animals by performing measurements within 30 minutes of each other for the pre-EMB and immediately following RHC measurement stabilization for 10 minutes for post-EMB, systemic blood pressure was not closely monitored and controlled during the procedure. General anesthetic agents, particularly inhalant gases, are known to reduce systemic and pulmonary pressures. In addition, these animals were receiving 100% inhaled oxygen, which can impact systemic and pulmonary arterial tone and pressure. Although these factors may affect PAP, it is difficult to predict the effect of change of PAP over time on RHC and ECHO at any given time point. Second, measurements were performed on a population of dogs with acutely generated PH, via EMB. This model likely differs from that of chronic PH, where compensatory cardiac remodeling occurs. The impact of chronic versus acute cardiac changes on the predictive value of noninvasive estimates is unknown. Right ventricular systolic failure may play a role in either situation, resulting in a tendency towards underestimation of actual PH based on TR velocity. Right atrial size may not increase with an acute increase in RAP and chronicity may be necessary to increase RA size in the presence of chronic PH. Comparatively, Oyama et. al. showed that an acute increase in left atrial pressure, as seen with acute mitral valve insufficiency, was not associated with a significant increase in left atrial size when assessed by ECHO.38 This limitation of RA size assessment in the acute setting, in addition to the small range of RAP observed after EMB, are clear limitations for the clinical translation of the observed results. Finally, the range of PAPRHC in this acute model of PH was limited, with all PAP < 77 mmHg. The predictive value of noninvasive measures at higher pressures and in naturally occurring disease remains unknown.
Conclusions
The study reported here documented a correlation between ECHO estimates of PAP and RAP and invasively measured values. Our results also confirmed a host of previous studies showing inconsistent predictive value of individual noninvasive measurements to predict invasive measurements of PAP and RAP. The results of this study suggest that employing more diagnostic modalities than ECHO alone may add important clinical information to the evaluation of canine patients with PH. Additional studies investigating ECHO measures of RV and pulmonary artery structure and function as indicators of existence and severity of PH are needed.
Acknowledgments
The authors gratefully acknowledge funding support from NIH R01HL105598 (NCC) American Heart Association (MLB) and Department of Radiology (CJF).
Abbreviation Table
- ECHO
echocardiography
- EMB
embolization
- dPAPECHO
diastolic pulmonary artery pressure estimated by echocardiography
- dPAPRHC
diastolic pulmonary artery pressure obtained by right heart catheterization
- mPAPECHO
mean pulmonary artery pressure estimated by echocardiography
- mPAPRHC
mean pulmonary artery pressure obtained by right heart catheterization
- PAP
pulmonary artery pressure
- PAPECHO
pulmonary artery pressure obtained by echocardiography
- PAPRHC
pulmonary artery pressure obtained by right heart catheterization
- PH
pulmonary hypertension
- PR
pulmonic regurgitation
- RA
right atrium
- RA sizesubj
subjective right atrial size obtained by echocardiography
- RAP
right atrial pressure
- RAPECHO
right atrial pressure estimated by subjective right atrial size obtained by echocardiography
- RAPRHC
mean right atrial pressure obtained by right heart catheterization
- RHC
right heart catheterization
- RV
right ventricular
- sPAPECHO
systolic pulmonary artery pressure estimated by echocardiography
- sPAPRHC
systolic pulmonary artery pressure obtained by right heart catheterization
- TR
tricuspid regurgitation
Footnotes
Contour SE Microspheres, Boston Scientific, Natick, MA, USA
Siemens/Acuson Cypress Plus Ultrasound, ultrasound probe 3–7 MHz, Siemens Medical Solutions, Erlangen D91052, Germany
Conflict of Interest:
None
References
- 1.Kellum HB, Stepien RL. Sildenafil citrate therapy in 22 dogs with pulmonary hypertension. J Vet Intern Med. 2007;21(6):1258–1264. doi: 10.1892/07-006.1. [DOI] [PubMed] [Google Scholar]
- 2.Bach JF, Rozanski EA, MacGregor J, Betkowski JM, Rush JE. Retrospective evaluation of sildenafil citrate as a therapy for pulmonary hypertension in dogs. J Vet Intern Med. 2006;20(5):1132–1135. doi: 10.1892/0891-6640(2006)20[1132:reosca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Hirano Y, Kitagawa H, Sasaki Y. Relationship between pulmonary arterial pressure and pulmonary thromboembolism associated with dead worms in canine heartworm disease. J Vet Med Sci. 1992;54(5):897–904. doi: 10.1292/jvms.54.897. [DOI] [PubMed] [Google Scholar]
- 4.Kellihan HB, MSRLS Pulmonary hypertension in canine degenerative mitral valve disease. Journal of Veterinary Cardiology. 2012;14(1):149–164. doi: 10.1016/j.jvc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Chiavegato D, Borgarelli M, D’Agnolo G, Santilli RA. Pulmonary hypertension in dogs with mitral regurgitation attributable to myxomatous valve disease. Veterinary radiology & ultrasound: the official journal of the American College of Veterinary Radiology and the International Veterinary Radiology Association. 2009;50(3):253–258. doi: 10.1111/j.1740-8261.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- 6.Serres FJ, Chetboul V, Tissier R, Sampedrano CC, Gouni V, Nicolle AP, Pouchelon JL. Doppler echocardiography-derived evidence of pulmonary arterial hypertension in dogs with degenerative mitral valve disease: 86 cases (2001–2005) J Am Vet Med Assoc. 2006;229(11):1772–1778. doi: 10.2460/javma.229.11.1772. [DOI] [PubMed] [Google Scholar]
- 7.Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in West Highland white terriers with chronic pulmonary disease. J Vet Intern Med. 2006;20(4):912–920. doi: 10.1892/0891-6640(2006)20[912:depoph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med. 1999;13(5):440–447. doi: 10.1892/0891-6640(1999)013<0440:ccodwd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Kellihan HB. Pulmonary hypertension in dogs: diagnosis and therapy. Veterinary Clinics of North America. 2010:1–20. doi: 10.1016/j.cvsm.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chrandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97(8):612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 12.Sciomer S, Magrì D, Badagliacca R. Noninvasive assessment of pulmonary hypertension: Doppler–echocardiography. Pulmonary Pharmacology & Therapeutics. 2007;20(2):135–140. doi: 10.1016/j.pupt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Posteraro A, Salustri A, Trambaiolo P, Amici E, Gambelli G. Echocardiographic estimation of pulmonary pressures. Journal of Cardiovascular Medicine. 2006;7(7):545–554. doi: 10.2459/01.JCM.0000234773.81077.68. [DOI] [PubMed] [Google Scholar]
- 14.Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock EP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998;113(3):576–583. doi: 10.1378/chest.113.3.576. [DOI] [PubMed] [Google Scholar]
- 15.Bossone E, Bodini BD, Mazza A, Allegra L. Pulmonary arterial hypertension: the key role of echocardiography. Chest. 2005;127(5):1836–1843. doi: 10.1378/chest.127.5.1836. [DOI] [PubMed] [Google Scholar]
- 16.Forfia P, Roberts J. Diagnosis and assessment of pulmonary vascular disease by Doppler echocardiography. Pulm Circ. 2011;1(2):160. doi: 10.4103/2045-8932.83446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139(5):988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 18.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homma A, Anzueto A, Peters JI, Susanto I, Sako E, Zabalogoitia M, Bryan CL, Levine SM. Pulmonary artery systolic pressures estimated by echocardiogram vs cardiac catheterization in patients awaiting lung transplantation. J Heart Lung Transplant. 2001;20(8):833–839. doi: 10.1016/s1053-2498(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 20.Attaran RR, Ramaraj R, Sorrell VL, Movahed MR. Poor correlation of estimated pulmonary artery systolic pressure between echocardiography and right heart catheterization in patients awaiting cardiac transplantation: results from the clinical arena. TPS. 2009;41(9):3827–3830. doi: 10.1016/j.transproceed.2009.06.201. [DOI] [PubMed] [Google Scholar]
- 21.Brecker SJ, Gibbs JS, Fox KM, Yacoub MH, Gibson DG. Comparison of Doppler derived haemodynamic variables and simultaneous high fidelity pressure measurements in severe pulmonary hypertension. Br Heart J. 1994;72(4):384–389. doi: 10.1136/hrt.72.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uehara Y. An attempt to estimate the pulmonary artery pressure in dogs by means of pulsed Doppler echocardiography. J Vet Med Sci. 1993;55(2):307–312. doi: 10.1292/jvms.55.307. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto M, Miyatake K, Kinoshita N, Sakakibara H, Nimura Y. Analysis of blood flow in pulmonary hypertension with the pulsed Doppler flowmeter combined with cross sectional echocardiography. Br Heart J. 1984;51(4):407–415. doi: 10.1136/hrt.51.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Duran R, Larman M, Trugeda A, Vazquez De Prada JA, Ruano J, Torres A, Figueroa A, Pajaron A, Nistal F. Comparison of Doppler-determined elevated pulmonary arterial pressure with pressure measured at cardiac catheterization. Am J Cardiol. 1986;57(10):859–863. doi: 10.1016/0002-9149(86)90627-2. [DOI] [PubMed] [Google Scholar]
- 25.Hatle L, Angelsen BA, Tromsdal A. Noninvasive estimation of pulmonary artery systolic pressure with Doppler ultrasound. Br Heart J. 1981;45(2):157–165. doi: 10.1136/hrt.45.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry GW, Katayama H, Lores ME, Lucas CL, Ferreiro JI. Intraluminal pulsed Doppler evaluation of the pulmonary artery velocity time curve in a canine model of acute pulmonary hypertension. Chest. 1991;100(2):474–479. doi: 10.1378/chest.100.2.474. [DOI] [PubMed] [Google Scholar]
- 27.Tahara M, Tanaka H, Nakao S, Yoshimura H, Sakurai S, Kashima T. Hemodynamic determinants of pulmonary valve motion during systole in experimental pulmonary hypertension. Circulation. 1981;64(6):1249–1255. doi: 10.1161/01.cir.64.6.1249. [DOI] [PubMed] [Google Scholar]
- 28.Serres F, Chetboul V, Gouni V, Tissier R, Sampedrano CC, Pouchelon J-L. Diagnostic value of echo-Doppler and tissue Doppler imaging in dogs with pulmonary arterial hypertension. J Vet Intern Med. 2007;21(6):1280–1289. doi: 10.1892/07-064.1. [DOI] [PubMed] [Google Scholar]
- 29.Pariaut R, Saelinger C, Strickland KN, Beaufrère H, Reynolds CA, Vila J. Tricuspid annular plane systolic excursion (TAPSE) in dogs: reference values and impact of pulmonary hypertension. J Vet Intern Med. 2012;26(5):1148–1154. doi: 10.1111/j.1939-1676.2012.00981.x. [DOI] [PubMed] [Google Scholar]
- 30.Currie PJ, Seward JB, Chan KL, Fyfe DE, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6(4):750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 31.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6(2):359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 32.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 33.Nageh MF, Kopelen HA, Zoghbi WA, Quiñones MA, Nagueh SF. Estimation of mean right atrial pressure using tissue Doppler imaging. Am J Cardiol. 1999;84(12):1448–51. doi: 10.1016/s0002-9149(99)00595-0. [DOI] [PubMed] [Google Scholar]
- 34.Bellofiore A, Roldán-Alzate A, Besse M, Kellihan HB, Consigny DW, Francois CJ, Chesler NC. Impact of acute pulmonary embolization on arterial stiffening and right ventricular function in dogs. Ann Biomed Eng. 2012 doi: 10.1007/s10439-012-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, Moses BL. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7(4):247–252. doi: 10.1111/j.1939-1676.1993.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 36.Kittleson MD, Kienle RD. Pulmonary arterial and systemic arterial hypertension. Small Animal Cardiovascular Medicine. 2014:433–449. [Google Scholar]
- 37.Lanzarini L, Fontana A, Lucca E, Campana C, Klersy C. Noninvasive estimation of both systolic and diastolic pulmonary artery pressure from Doppler analysis of tricuspid regurgitant velocity spectrum in patients with chronic heart failure. Am Heart J. 2002;144(6):1087–1094. doi: 10.1067/mhj.2002.126350. [DOI] [PubMed] [Google Scholar]
- 38.Oyama MA, Sisson DD, Bulmer BJ, Constable PD. Echocardiographic estimation of mean left atrial pressure in a canine model of acute mitral valve insufficiency. J Vet Intern Med. 2004;18(5):667–672. doi: 10.1892/0891-6640(2004)18<667:eeomla>2.0.co;2. [DOI] [PubMed] [Google Scholar]


