Abstract
Introduction
Few results from programmes based on combination prevention methods are available. We propose to analyse the degree of protection provided by postexposure prophylaxis (PEP) for consensual sexual activity at healthcare clinics, its compensatory effects on sexual behaviour; and the effectiveness of combination prevention methods and pre-exposure prophylaxis (PrEP), compared with exclusively using traditional methods.
Methods and analysis
A total of 3200 individuals aged 16 years or older presenting for PEP at 5 sexually transmitted disease (STD)/HIV clinics in 3 regions of Brazil will be allocated to one of two groups: the PEP group—individuals who come to the clinic within 72 h after a sexual exposure and start PEP; and the non-PEP group—individuals who come after 72 h but within 30 days of exposure and do not start PEP. Clinical follow-up will be conducted initially for 6 months and comprise educational interventions based on information and counselling for using prevention methods, including PrEP. In the second study phase, individuals who remain HIV negative will be regrouped according to the reported use of prevention methods and observed for 18 months: only traditional methods; combined methods; and PrEP. Effectiveness will be analysed according to the incidence of HIV, syphilis and hepatitis B and C and protected sexual behaviour. A structured questionnaire will be administered to participants at baseline and every 6 months thereafter. Qualitative methods will be employed to provide a comprehensive understanding of PEP-seeking behaviour, preventive choices and exposure to HIV.
Ethics and dissemination
This study will be conducted in accordance with the resolution of the School of Medicine Research Ethics Commission of Universidade de São Paulo (protocol no. 251/14). The databases will be available for specific studies, after management committee approval. Findings will be presented to researchers, health managers and civil society members by means of newspapers, electronic media and scientific journals and meetings.
Keywords: HIV, Post-Exposure Prophylaxis, Pre-Exposure Prophylaxis, combination HIV prevention, prevention and control, Brazil
Strengths and limitations of this study.
Brazil has a tradition of universal access to antiretrovirals and human rights-based prevention programmes, which will enable a deeper understanding of the potential results coming from introducing new prevention methods and strategies.
The study design will enable a more consistent analysis on the degree of protection from prophylaxis before and after sexual exposure in the context of healthcare clinics, thus contributing to prevention programmes.
Combination prevention methods and strategies have varied according to the context and over the course of time, which make strategies for obtaining reliable information more difficult. Moreover, the retention rate for the study may be impaired because of the profile of the study population.
Introduction
The emergence of new prevention methods has increased the optimism towards achieving a more effective control over the AIDS epidemic,1 2 as these methods have shown the potential to promote significant reductions in the number of new infections in the regions and social groups that are most affected by the disease.3 An important part of this optimism relates to the development of methods based on using antiretrovirals (ARV-based prevention),4 which may avoid acquisition of infection when used before5 6 or after exposure to HIV,7–9 and also avoid transmission of the virus in sexual intercourse among serologically discordant couples3 10–12 (treatment as prevention, TasP). As a result, the present set of methods13 makes it possible to use preventive measures at different times which lead to HIV transmission, thereby creating a virtuous circle in which people may not come into contact with the virus during sexual intercourse (ie, through using male and female condoms, safer sexual practices and anti-HIV tests for sexual agreements); or, when they do come into contact with the virus, occurrence of infection might be avoided (ie, pre-exposure and postexposure prophylaxes); or, if they become infected, they might not transmit the virus (ie, TasP).
This new reality has encouraged the recommendation that prevention methods should be made available together with structural interventions.13–15 This would make it possible to address key points relating to reduction of vulnerabilities and increase in the number of people who are included in prevention programmes, who would come to be able to access and choose the method that best suits their needs, or even to be able to combine different methods in specific situations. The use of diverse methods has already been observed among social segments that are most exposed to HIV,16–18 and these individuals have changed their behaviour as a way of adapting the need to prevention to their daily routines and sexual practices. This relates to the fact that the choice of prevention methods is based on a wide range of factors,19–22 such as knowledge on how to use each method and its benefits and risks, the perception of the risks involved in sexual relationships and the context in which they occur. Thus, choices can sometimes mean the adoption of less-effective methods for a specific situation or even the abandonment or exchange of those proven to be the most effective.
However, it must be highlighted that although efficacy studies have shown that prevention methods provide a significant degree of protection,5–7 23 many of them, and especially those of biomedical nature, have still not been fully analysed within the context of healthcare services and the daily routine of social groups that are most exposed to the risk of infection.8 13 24 Thus, important questions remain regarding the risks and benefits of the new methods, the way in which they are routinely offered and used by individuals, as well as their real effects on the epidemic.
The present article describes the design of a pragmatic clinical trial that will be conducted in public healthcare clinics in five Brazilian cities and analyses the combination prevention methods, included pre-exposure and postexposure prophylaxes.
The fragility of the evidence relating to new methods
So far, postexposure prophylaxis (PEP) programmes implemented in different countries25–28 have been analysed in a single efficacy study,7 which presented limitations because of its small sample size. The main evidence supporting implementation of PEP programmes relies on basic research using animal models. Findings from a meta-analysis study29 show that non-humans receiving PEP had a reduction in the risk of infection of 89% (odds 0.11; 95% CI 5 to 23). In similar situations, the use of PEP led to a reduced risk of HIV infection, such as newborns who received ARV within the first 72 h of life;30 healthcare professionals who suffered occupational accidents;31 and victims of sexual violence.9 32 33
Thus, there is controversy regarding the impact of PEP programmes on public health. An analysis conducted in eastern Australia34 reported that a low number of cases of infection was avoided (1–9 cases) over 6 years of programme, probably due to the high proportion of users with sexual intercourse not taking place with HIV-positive partners. Other studies25 33–37 have shown that knowledge of PEP and the demand for it continued to present low rates over the course of time and were restricted to a smaller number of social groups that would be potential users of the PEP. Even the individuals who could benefit after being exposed to HIV tended not to seek the prophylaxis.7 38 This also occurred in situations in which the individuals had received information and had the medications for starting to use prophylaxis in case of exposure.7 The main reasons were failure to identify the risk, a stable partnership and fear of adverse events. Healthcare professionals also tended to downplay the risk of infection through sexual intercourse, when evaluating the reports of individuals who were seeking the prophylaxis, thereby interfering with timely prescription.19 On the other hand, a positive relationship between knowledge and greater demand for prophylaxis has been noted,38 thus corroborating studies that have shown that communication strategies lead to increased demand for and better use of PEP.39
The rates of adherence to medication and clinical follow-up have presented great variation among the studies conducted.8 25 36 40 41 A meta-analysis42 of 17 studies found a summary measure of 77% (95% CI 68% to 87%). This variation has been explained in terms of the type of exposure, individual characteristics and type of treatment. Lower adherence rates have tended to be seen among the victims of sexual violence24 40 42 and because of occurrences of adverse effects or the use of triple regimens.8 Individuals identified as presenting a greater risk of infection7 and those who participate in counselling sessions present greater chances of adherence.43
One of the fears regarding PEP relates to loss of sexual inhibition, such that high-risk sexual practices occur more often and prophylaxis is repeatedly sought. However, studies on this have shown conflicting results.38 41 44–46 In San Francisco (USA), in 2004, participation in counselling sessions reduced high-risk sexual practices among PEP users by 73%.44 In Australia,38 an analysis on a cohort of men who have sex with men (MSM) found that, between 2001 and 2007, the HIV incidence rate among PEP users became three times greater. This may have been related to the persistence of higher frequency of high-risk sexual practices in this group, thus suggesting that seeking PEP might be a predictor of HIV infection. A qualitative study conducted in Australia45 indicated that the use of PEP was reported more as a means of making up for failings of safe sex practices than as a substitute for another prevention method. This coincides with rates of repeatedly seeking PEP of less than 20% in several studies.7 38 41 44
These results are contradicted by a study conducted in Amsterdam between 2000 and 2009,46 which found an increase in the HIV incidence rate and an HIV incidence rate that was four times greater among MSM who used PEP, in comparison with the MSM community in general. One explanation for this could be the generalised increase in higher risk sexual practices among the MSM population and the more pronounced risk among PEP users who previously had this characteristic of greater exposure.
These factors indicate that some PEP users have a profile that is appropriate for using new prevention methods, such as pre-exposure prophylaxis (PrEP), or for using combinations of the different existing methods.19 38 45 47 The safety and efficacy of PrEP have been analysed in controlled clinical trials involving serologically different heterosexual couples,6 young heterosexuals48 and MSM5 in different countries. In these studies, an oral daily dose of tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) reduced the risk of infection from 40% to 75%, according to the degree of compliance, which is currently the main concern about this method. In this regard, two studies enrolling African women49 50 failed to show differences between the groups that used and did not use prophylaxis possibly due to low adherence. A possible explanation is that the motivation to take the medication on a regular basis may vary according to the partnership and its HIV status.
On the other hand, recent studies have brought promising results among MSM and transgender women from high income countries. In an open-label trial in the USA,51 using PrEP for at least 4 days a week could avoid any infections over 72 weeks. Optimal treatment adherence was achieved in only 33% of the blood assessments. Another open study52 (PROUD study) conducted at sexual health clinics in England found an 86% (90% CI 62% to 96%) protection rate from HIV. The Ipergay study53 registered a similar percentage (86%; 95% CI 39.4% to 98.5%) among French and Canadian participants who used PrEP one day before and 48 h after a sexual exposure, which was called ‘PrEP on demand’.
Additional findings on PrEP included lower rates of adverse effects5 6 and viral resistance.5 6 48 In the iPrEx study, conducted with 2499 MSM from six countries, the active drug group showed a higher frequency (2% vs <1%, p 0.04) of moderate nausea and similar rates (12% vs 13%, p 0.51) of severe adverse effects were found in the active drug and placebo groups.5 A higher rate of adverse effects, including reduction in bone density, was observed among 1219 heterosexual women and men using TDF/FTC in Botswana.48 The Partners PrEP study,6 conducted with serodiscordant couples, identified viral resistance in two individuals who took the active drug but have not had a recent infection detected by anti-HIV serological tests.
There is also evidence that PrEP use has not led to any compensatory effect on sexual behaviour.5 54 55 An investigation carried out with 400 MSM in the USA54 showed decrease or stability of the unprotected anal intercourse and in the number of sexual partners among PrEP and control groups. Increase of condom use in anal sex was also observed in the iPrEx study.5
These findings have led the American regulatory agency to approve the use of a combination of TDF and FTC for HIV prophylaxis in 2012. This also led to a variety of recommendations2 4 13 15 56 57 for development of implementation studies with the purpose of building evidence on the effectiveness of this method in the context of health services. These recommendations have been followed by a slight increase in PrEP acceptability among the most exposed groups to HIV58 and serodiscordant couples.59 Nonetheless, the intention to use PrEP is still controversial.58 60 61 A study conducted with 404 homosexual individuals recruited on the internet in Thailand showed that only 35% referred to the intention to use PrEP after being informed about its efficacy.60 In order to support the WHO guidelines for PrEP, a systematic review61 found that stigma, disclosure of sexual orientation, the possibility of being regarded as HIV positive and the lack of trust in the health provider may affect the demand for PrEP.
Thus, if on the one hand new knowledge produced in the field of prevention has made it possible to discern more effective control over the epidemic, this has, on the other hand, given rise to a series of questions. To analyse these issues, a broader approach towards the various prevention methods offered in the daily routine of health services is required.
Objectives
The present study was designed to analyse four central issues: first, the effectiveness of PEP provided at public healthcare clinics in Brazil for consensual sexual activity; second, changes in sexual and preventive practices resulting from the provision of PEP; third, the effectiveness of combination prevention methods—including PrEP—that are provided routinely at healthcare clinics in Brazil; and fourth, the understanding of how most exposed individuals choose, access and use prevention methods.
Methodology
Study population
Prevention of HIV in Brazil is centred on behavioural and structural interventions,62 and the main strategy is the promotion of condom use. PEP was incorporated into prevention policies in 2010;28 and in 2013 the Ministry of Health announced that TasP would become universally available63 and that demonstration projects for PrEP would be implemented.
PEP has been provided through the public healthcare system, encompassing testing and counselling centres, HIV/AIDS outpatient clinics and accredited emergency and walk-in services. Among these services, five centres in different cities and regions of Brazil were selected to participate in this study as follows: Fortaleza (northeastern region); Porto Alegre and Curitiba (southern region); and São Paulo and Ribeirão Preto (southeastern region).
Among the cities selected, São Paulo and Fortaleza presented the lowest incidence rates for AIDS between 2011 and 2014 (23.1 and 26.8 per 100 000 inhabitants-year, respectively), although it needs to be highlighted that over this period São Paulo recorded the largest number of cases of this disease in Brazil (9333). These two cities are characterised by the predominance of HIV transmission through homosexual and bisexual intercourse (±40%), while Porto Alegre presents the highest incidence of AIDS in Brazil between the state capitals (91.1 cases per 100 000 inhabitants-year). In Ribeirão Preto, the only non-coastal city among those selected, HIV transmission due to heterosexual intercourse accounted for almost 70%, with greater relative participation of the category of injection drug use (±10%). Curitiba has an incidence rate of 27.0 per 100 000 inhabitants-year and its epidemiological profile presents all characteristics from the other cities, with 35.1% of cases among homosexuals and bisexuals and 55.3% of cases among heterosexuals. Except for Fortaleza and Curitiba, which presented a stabilising tendency, the cities presented a slight decline in the AIDS incidence rate.
In São Paulo and Curitiba (table 1), PEP is provided routinely at testing and counselling centres and at HIV/AIDS outpatient clinics in the other cities. These services were implemented in 2008 and 2011 and the majority of individuals presenting for PEP are homosexual and heterosexual men. Female sex workers are the third most frequent group in Sao Paulo. The clinics in Porto Alegre, São Paulo, Ribeirão Preto and Fortaleza are referral services for clinical follow-up on patients who started the prophylaxis at emergency services. All of these clinics have treatment for sexually transmitted diseases (STDs), multiprofessional teams and counselling and serological follow-up regarding HIV infection, syphilis and hepatitis B and C. Activities to promote adherence to clinical follow-up and drug use are implemented in Porto Alegre, São Paulo and Fortaleza and to contact absent users in Porto Alegre. All clinics provide male and female condoms and only three services offer counselling on using a hierarchy of sexual risk behaviours for HIV and test as prevention.
Table 1.
Characteristics of participating sites (The Combine! Project)
| Characteristics | Sites* |
||||
|---|---|---|---|---|---|
| Porto Alegre | São Paulo | Ribeirão Preto | Fortaleza | Curitiba | |
| Availability of PEP (year) | 2010 | 2010 | 2011 | 2008 | 2009 |
| Type of health service where PEP is available | |||||
| STD/HIV clinic | x | x | x | ||
| Emergency service | x | x | x | ||
| VCT centre | x | x | |||
| Most attended groups | |||||
| Homosexual men | 1st | 1st | 2nd | 1st | 2nd |
| Bisexual men | |||||
| Heterosexual men | 2nd | 1st | 2nd | 1st | |
| Heterosexual women | 3rd | 2nd | 3rd | 3rd | |
| Sex workers | 3rd | 3rd | |||
| Service seeking (%) | |||||
| Spontaneous | 78.6 | 51.6 | 53.5 | 70.0 | 80.0 |
| Referred by a health provider | 21.4 | 48.4 | 46.5 | 30.0 | 20.0 |
| Services and inputs | |||||
| Treatment for STDs | x | x | x | x | x |
| Treatment for hepatitis | x | x | x | x | |
| Hepatitis vaccination | x | x | x | x | x |
| Male condom | x | x | x | x | x |
| Female condom | x | x | x | x | x |
| Pre-exposure prophylaxis | x | ||||
| Counselling on hierarchy of sexual risk | x | x | x | ||
| Counselling on using the test to establish a sexual agreement with the partner | x | x | |||
| Adherence to antiretroviral therapy and follow-up | x | x | x | ||
| Follow-up time (days) | |||||
| 1st consultation | 30 | 7–10 | 20 | 30–45 | 30–45 |
| 2nd consultation | 60 | 30 | 45 | 90 | 90 |
| 3rd consultation | 90 | 90 | 90 | 180 | 180 |
| 4th consultation | 120 | 180 | 180 | Absent | Absent |
| Contact absent users | x | ||||
*City of Porto Alegre- Serviço de Atendimento Especializado DST/AIDS, Vila dos Comerciários; city of São Paulo—Centro de Referência e Treinamento de DST e Aids; city of Ribeirão Preto—Centro de Referência em Especialidades Central; city of Fortaleza—Hospital São José de Doenças Infecciosas; and city of Curitiba—Centro de Orientação e Aconselhamento.
PEP, postexposure prophylaxis; STD, sexually transmitted disease; VCT, voluntary counselling and testing.
Design
The study is structured into three components, which will bring together quantitative and qualitative methodologies. In the first component, the protective effect of PEP will be evaluated on the basis of the assumption that there will be a reduction in the risk of HIV infection among individuals who use prophylaxis, in comparison with those who are exposed and do not use it.
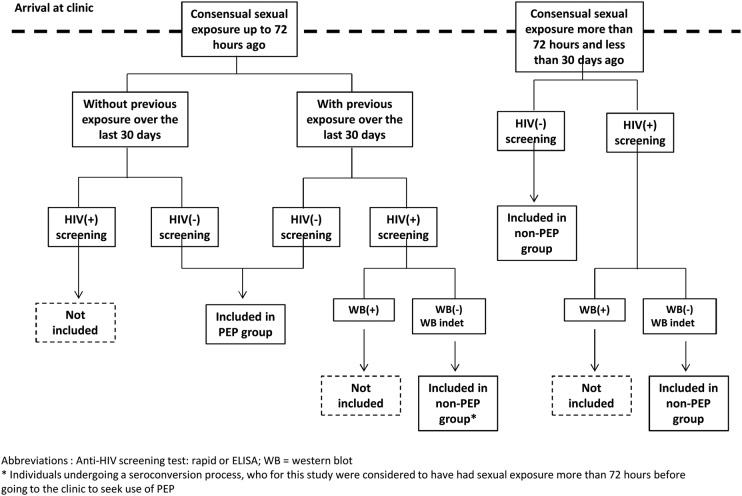
Therefore, individuals without HIV infection who are sexually exposed (figure 1) and attended healthcare clinics will be followed by 6 months after being allocated to one of two groups, in accordance with the detailed criteria in figure 2, which can be summarised thus:
PEP group: individuals who come to the clinic in less than 72 h after sexual exposure and start PEP use.
Non-PEP group: individuals who come to the clinic in more than 72 h and less than 30 days after exposure and do not start PEP.
Figure 1.

Criteria for postexposure prophylaxis use in Brazil.
Figure 2.
Inclusion flow chart (PEP, postexposure prophylaxis).
The individuals who do not become infected with HIV will then be involved in the second component, in which, over an 18-month period, the effect of the PEP programme on sexual practices and on repeated use of this prophylaxis will be analysed. It is assumed that after attending the PEP programme, there will be fewer or an equal number of occurrences of unprotected sexual practices than in the period preceding attendance at the clinic. For this, the sexual and preventive practices of individuals participating will be investigated for periods of 6 months and then compared with the 6 months prior to the use of PEP.
In this second component, the degree of protection resulting from using prevention methods, including PrEP, will also be examined, once these methods are routinely offered in the healthcare clinics. The assumption is that linkage to HIV services among individuals with higher exposure leads to increased protected sexual practices, whereas the use of combination methods and/or PrEP has a greater degree of protection when compared with the exclusive use of traditional methods. As a result, there will be fewer occurrences of unprotected insertive sex and the HIV incidence rate will be lower among individuals who used a combination of methods and/or PrEP, without this implying an increase in the incidence of syphilis and hepatitis B and C.
For this analysis, individuals involved in this second component will be regrouped according to their reports of the prevention methods used as follows: (1) combination prevention methods, characterised by the use of at least two of the following methods: male/female condoms, sexual practices without vaginal or anal penetration, use of anti-HIV tests and viral load for sexual agreements, PEP and TasP; (2) exclusive use of traditional methods: condoms and/or sexual practices without vaginal or anal penetration and (3) PrEP, independent of association with another method.
The third component comprises qualitative studies aimed at understanding how prevention methods are chosen, accessed and used, with emphasis on ARV-based prevention methods, through the inter-relationships of three dimensions: clinic organisation; perceptions and practices of healthcare professionals; and motivations and perceptions of people who are more exposed to HIV. It starts from the following assumptions: provision of PEP and other prevention methods is influenced by the rationality that guides the organisation and the healthcare practices in the clinic; PEP is sought because of a need to complement individual prevention strategies; and the use of different prevention methods is moderated by the context, course of life and sexual life and perceived prevention requirements.
Eligibility and recruitment
All individuals who come to the clinics seeking PEP or who are sexually exposed will be invited to participate in the project, taking into consideration the following eligibility criteria:
16 years of age or over;
Reporting sexual exposure (figure 1) during the past 30 days, with an indication for PEP;
A negative anti-HIV test confirmed at the time when the clinic is sought, or a positive anti-HIV serological test with a negative/indeterminate western blot, for individuals with exposure that occurred more than 72 h and less than 30 days ago;
Not having sought PEP for the purpose of assisted reproduction or due to occupational exposure or sexual violence;
Having started PEP at the clinic or having been referred for clinical follow-up soon after the initial attendance at an emergency service, provided that information on serological status, sexual exposure and prescribed therapy is available.
The inclusion limit of 30 days after the sexual exposure aimed to certify that the individuals included were not already infected with HIV at the time of the exposure. Viral load and western blot will be used to detect individuals undergoing seroconversion.
Interventions
Attendance for PEP
The procedures for PEP will follow the standards of the Ministry of Health,28 which envisage clinical and serological follow-up for 6 months, defined thus: (1) initial attendance consisting of epidemiological and clinical screening and assessment; diagnostic tests for HIV (rapid test), syphilis and hepatitis B and C (conventional tests); prescription of therapy and dispensing of medications (28 days), with guidance regarding adherence; and (2) follow-up: four consultations (between the 1st and 3rd weeks, between the 4th and 6th weeks, in the 12th week and in the 24th week) for clinical assessments, management of adverse events, reinforcement of adherence and serological tests (for HIV, performed between the 4th and 6th weeks and in the 24th week; for syphilis, in the 12th week; and for hepatitis B and C, in the 24th week).
The prophylaxis, for a 28-day period, will involve prescribing the drugs zidovudine (AZT), lamivudine (3TC) and TDF or AZT, 3TC and lopinavir/ritonavir (LPV/r). For cases with an HIV-positive partner who is using ARV, the therapeutic regimen will be evaluated in accordance with the regimen used by the partner.
Individuals who come more than 72 h after exposure will undergo the same procedures, except for prescription of ARV.
Provision of preventive measures
Provision of preventive measures will occur in accordance with the current practices in the clinics and will involve making supplies; HIV, syphilis and hepatitis testing; STD treatment and hepatitis B vaccination.
The main technique to be used in interventions will be counselling, which will be structured starting from identification of the individual risks and structural factors that influence the use of safe practices. Routine approaches during regular and specific attendance in the 1st, 6th, 12th and 18th months are envisaged.
The individuals included in the cohort who will not have been actively using PrEP will receive guidance to return to the clinic if they are sexually exposed to HIV again, in order to analyse the pertinence of using PEP.
Provision of PrEP
Individuals involved in the second component may decide, together with a healthcare professional, to use PrEP, taking into consideration the individual risks and benefits, which include: belonging to a group with a higher prevalence of HIV; regular sexual intercourse with partners in groups with a greater prevalence of HIV; sexual intercourse with serologically different partners without treatment for HIV infection; history of or intention to continue with sexual intercourse presenting a greater risk of infection, without consistent use of other prevention method; use of recreational drugs; recurrent use of PEP; willingness to continue use of PrEP; and renal function compatible with use of TDF/FTC.
The follow-up consultations for PrEP users will take place in the first 30 and 60 days and then at maximum intervals of up to 3 months. The consultations will involve counselling, provision of condoms and other prevention methods, investigation for STDs and serological status regarding HIV and hepatitis, management of adverse events, monitoring of renal function and guidance regarding pregnancy and breast feeding. Specific guidance regarding acute infection will be given and whenever signs or symptoms are reported, the serological and viral load tests will be repeated. Counselling will be used to reinforce adherence. TDC/FTC will be used for prophylaxis.
Follow-up and censoring
For the first component, clinical and serological data will be collected from participants included in non-PEP and PEP groups during the inclusion period and on the 30th day, and in the 3rd and 6th months. Information on sexual and preventive behaviour within 6 months prior to study inclusion will be obtained at initial follow-up and at the sixth month follow-up.
For the second component, additional reassessment will be carried on in the 12th, 18th and 24th months of follow-up. Information on sexual practices, use of prevention methods and serological tests for HIV, syphilis and hepatitis B and C will be obtained in the reassessment.
Individuals who miss consultations and visits will be contacted in accordance with the procedures established at each clinic. Those who continue to be absent after 6 months will be considered to have been lost to follow-up.
Censoring of observations in the first component will be performed in the event of positive anti-HIV findings; at the time of clinical closure of the case (performing the envisaged serological test); or on the date of the last visit, for the cases that are lost to follow-up. In the second component, censoring will take place at the end of the observations (24th month follow-up) or at the time of a positive anti-HIV test or loss to follow-up.
Follow-up, clinical treatment and rapid tests will be registered in the medical records and performed by health workers at the clinics. Western blot and viral load testing will be performed by a reference laboratory at each site and supervised by Instituto Adolfo Lutz.
Primary outcomes
In the first component, the main outcome will be occurrence of HIV infection, as verified through screening laboratory tests (ELISA or rapid test) and confirmatory tests (viral load, western blot, immunofluorescence or a second rapid test).
In the second component, the outcomes will be reports of unprotected sex, categorised as anal or vaginal insertive and/or receptive sex without condom use or in case of a condom break, or absence of any ARV-based prevention methods (TasP, PrEP or PEP). The type of partner (stable/casual) and testing for HIV will be used to grade this risk (higher or lower); the incidence of infection due to HIV, syphilis and hepatitis B and C; and reports of occurrences of sexually transmittable diseases.
Secondary outcomes
In the first component, the frequency of adverse events associated with ARV will be considered, measured from the records of clinical events in the medical files or from laboratory tests. Partial or full non-compliance with the prophylaxis will be defined as non-use of medications over a 28-day period or failure to undergo serological tests for HIV, syphilis and hepatitis for conclusion of the case.
The following will be considered in the second component: coming to the clinic again to seek PEP; proportions of the prevention methods according to choice; non-attendance at the clinic for the educational interventions that had been arranged; and non-adherence to PrEP, characterised according to reported frequency (days) of non-use of medications, non-attendance at appointments or non-attendance to collect medications.
Instruments and variables
Information on the use of PEP will be obtained from the medical records, for which the minimum information will be standardised using a specific form, consisting of the following: demographic characteristics; sexual exposure (time of occurrence, sexual practice and sexual partner); previous exposure over the preceding 30 days; factors increasing the risk of HIV transmission during the exposure (STDs and bleeding); serological tests; start of treatment; treatment regimen; adverse events; and adherence to medication. Data will be collected from medical records by trained professionals who are members of the study team.
Information on sexual behaviour will be obtained by applying a structured questionnaire that contains information on sexual identity, sexual practices, strategies and prevention methods used according to type of sexual partnership, use of recreational drugs, experience of situations of violence, degree of information about and perception of risk, and paid sex, among other information. Data on the reasons for loss of follow-up will be obtained through telephone contact, using a short version of the questionnaire. The interviews and clinical consultations will be conducted simultaneously and by trained professionals and are expected to provide counselling on prevention strategies.
Data gathering will be carried out online, using the Research Electronic Data Capture (REDCap) platform.64 Consistency and integrity will be insured by checking all records. For the purpose of quality control, at least 5% of the medical records will be checked.
Qualitative studies
The qualitative analysis will encompass ethnographic observations and in-depth interviews with healthcare providers and users and will be conducted by experienced qualitative researchers. Ethnographic observation will provide a detailed and systematic description of the routine healthcare of the services, all registered in field diaries. Additionally, clinical consultations and counselling sessions will be recorded without the presence of an observer. Ethnographic observation will envisage the ways in which users deal with the clinics; work organisation and processes; professional practices; and users’ and professionals’ decision-making processes relating to prophylactic treatment and the choice of prevention methods, specially PrEP and PEP.
In-depth interviews with different types of healthcare professionals working at the reception, screening and clinical attendance, along with the unit managers, are intended. Professional practice, perceptions relating to users, notions regarding prevention methods (with emphasis on ARV-based prevention methods), response to situations regarded as non-adherance to prevention and the users’ right to choose and not use prevention methods will be explored.
The interviews envisaged with users will be categorised as groups of homosexual men; heterosexual men; sex workers; heterosexual women; and transvestites. The interviews will address knowledge about PEP; context of sexual exposure; demand for and use of PEP; risk management; reasons for choosing methods; and preventive strategies. Twenty interviews will be conducted with participants who did not adhere to PEP.
The observations and interviews are part of the first and second components. Using pretested guides, they will be conducted during 15 days in the routine of each service. The number of interviews will be adjusted using the criterion of saturation of information and meanings for each group of interviewees.
Sample
The initial sample was estimated to be 3200 individuals, divided into 1600 in the PEP sexual group and 1600 in the non-PEP group, with an estimated loss from follow-up of 26.3% (420). This sample enables detection of a relative risk of 2.3, with a significance level of 5% and test power of 80%. It has been estimated that 650 individuals will choose to use PrEP. Basic demographic information will be registered for individuals who refuse to participate.
Analysis
To analyse effectiveness, the outcomes of interest will be compared between the PEP and non-PEP groups, and these will be calculated as rates and risks (with 95% CIs). Individuals with multiple exposures will be included repeatedly in the PEP and non-PEP groups, in accordance with the inclusion criteria defined for each of them. Associations between exposures and outcomes will be analysed by means of ratios and relative risk, using generalised linear models to control for possible confounding effects. Stratified analyses will be performed for the variables of interest, such as the time elapsed between exposure and the start of the therapy, adherence to prophylaxis and occurrences of adverse events. The amount of treatments needed in order to prevent transmission will be estimated, taking into account the intention of treatment. For the group with PEP, the prevalence of abandonment of ARV treatment and the factors associated with this event will also be estimated.
To analyse the effects of the programme on sexual practices, information from before attendance at the clinic was sought will be compared with the observations in the 6th, 12th, 18th and 24th months, taking into account the whole population and the groups of interest (ie, prevention methods, sexual orientation and sex). Repeated measurement models will be used to analyse occurrences of events over the course of the follow-up, for the entire cohort and for comparisons between groups of interest.
Qualitative analysis
Recordings will be fully transcribed and checked for accuracy. NVivo software will be used to analyse interviews and observations, which will be interpreted by study team members with expertise in qualitative research. A method of interpretation of meanings,65 based on hermeneutic principles, will be adopted. In the cases of testimonies, this method will seek to interpret the context, motivations and logic of what was said, the actions and interactions between the participants and the healthcare institution. In the case of ethnographic observation, this method will seek to interpret the logic and implied meanings in the actions and to compare these actions at the level of intentions and idealisations with their context.
Use of the data will be managed by a committee of investigators at the participating sites, who will make decisions regarding analysis proposals and partnerships with other institutions that are interested in having access to the information generated by the project.
Limitations and risks of the study
The main risks of the study relate to selecting and retaining study participants. Although the PEP programme in Brazil was implemented more than 4 years ago, prophylaxis seeking at clinics remains relatively low. It is possible that only a low proportion of this clientele has sexual intercourse with people who are known to be HIV positive, leading to a lower incidence in study groups. The participants may also omit information or report higher exposure in order to obtain treatment and have access to ARV therapy outside of the criteria established by the Ministry of Health. The rates of loss of follow-up may be high because of the characteristics of the population and changes over the course of life. The absence of external data may cause difficulty in interpreting information on sexual disinhibition. Errors in recording the information may occur, given that it is generated through clinical practice and the daily routine at these clinics.
Dissemination
All individuals who express their agreement to participate in the study will sign a free and informed consent form, which will also authorise access to the clinical records produced within the clinic. Patients who choose to use PrEP will be informed about the risks of ARV. The field observations and in-depth interviews will have specific informed consent forms, signed by the manager of the clinic, the professionals involved and the users participating.
As a matter of priority, infected individuals will be followed up at clinics participating in the study, which have outpatient services. If this is not possible, or if the individual so chooses, a referral will be made to the network of the healthcare system. Access to prevention supplies will be guaranteed for the participating volunteers, even after the project has been concluded, if this method is proven to be effective.
Conclusion
ARV-based prevention has inaugurated a new era in dealing with the AIDS epidemic. This study will contribute to generate new evidence on the best attainable conditions for offering these methods within the daily routine of healthcare programmes and services, thereby indicating advantages and disadvantages in relation to the promotion and exclusive use of traditional methods.
Footnotes
Contributors: AG, MTC, MFP, OL, EMZ, DLE, RA, KW, MME, GC, DF, VSA and TK drew up the project and are participating in its implementation. EAC, EA, MGC, FRA and JCVS reviewed the final version of the project and are participating in its implementation. All authors have read and approved the final version of this manuscript.
Funding: Brazilian Ministry of Health; public call SVS-MS 01-2012; proposal no. 027941/2012; and National Council for Scientific and Technological Development; public call MCTI/CNPQ/UNIVERSAL 14/2014. The TDF/FTC will be donated by Gilead Sciences
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This study is in accordance with resolution no. 466/2012 of the National Research Ethics Commission and was approved by the Research Ethics Committee of the School of Medicine, University of São Paulo, under protocol no. 251/14.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Data sharing statement: Use of the data will be managed by a committee of investigators at the participating sites, who will make decisions regarding analysis proposals and partnerships with other institutions that are interested in having access to the information generated by the project. Data collection forms are available and can be obtained from AG.
References
- 1.The International AIDS Society. The International AIDS Conference, The University of California, San Francisco. The Washington D.C Declaration: turning the tide together: a declaration to end the AIDS epidemic Washington DC: 2012. http://www.2endaids.org/about.html (accessed 14 Apr 2014). [Google Scholar]
- 2.Padian N, McCoy SI, Karim SS et al. . HIV prevention transformed: the new prevention research agenda. Lancet 2011;378:269–78. 10.1016/S0140-6736(11)60877-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer-Rath G, Over M. Investigating the impact of treatment on new HIV infection. PLoS Med 2012;9:1–92. [Google Scholar]
- 4.World Health Organization. The strategic use of antiretroviral: to help end the HIV epidemic. Geneva, 2012. [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2787–99. 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Donnell D, Ndase P et al. , Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechter M, do Lago RF, Mendelsohn AB et al. . Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acquir Immune Defic Syndr 2004;35:519–25. 10.1097/00126334-200404150-00010 [DOI] [PubMed] [Google Scholar]
- 8.Bryant J, Baxter L, Hird S. Non-occupational postexposure prophylaxis for HIV: a systematic review. Health Technol Assess 2009;13:1–60. 10.3310/hta13140 [DOI] [PubMed] [Google Scholar]
- 9.Baber TJ, Benn PD. Postexposure prophylaxis for HIV following sexual exposure. Curr Opin HIV/AIDS 2010;5:322–6. 10.1097/COH.0b013e32833a5e6c [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnell D, Baeten JM, Kiarie J et al. . Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010;375:2092–8. 10.1016/S0140-6736(10)60705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Programmatic update: antiretroviral treatment as prevention (TasP) of HIV and TB. Geneva, 2012. [Google Scholar]
- 13.Chang LW, Serwadda D, Quinn TC et al. . Combination implementation for HIV prevention: moving form clinical trial evidence to population-level effect. Lancet 2013;13:65–76. 10.1016/S1473-3099(12)70273-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joint United Nation Programme on HIV/AIDS. Combination HIV prevention: tailoring and coordinating biomedical, behavioral and structural strategies to reduce new HIV infection. A UNAIDS discussion paper Geneva, 2010. [Google Scholar]
- 15.Kurth AE, Celum C, Baeten JM et al. . Combination HIV prevention: significance, challenges and opportunities. Curr HIV/AIDS Rep 2011;8:62–72. 10.1007/s11904-010-0063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConnell JJ, Bragg L, Shiboski S et al. . Sexual seroadaptation: lessons for prevention and sex research from a cohort of HIV-positive men who have sex with men. PLoS ONE 2010;5:e8831 10.1371/journal.pone.0008831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin F, Crawford J, Prestage GP et al. . Unprotected anal intercourse, risk reduction behaviours, and subsequent HIV infection in a cohort of homosexual men. AIDS 2009;23:243–52. 10.1097/QAD.0b013e32831fb51a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart GJ, Elford J. Sexual risk behaviour of men who have sex with men: emerging patterns and new challenges. Curr Opin Infect Dis 2010;23:39–44. 10.1097/QCO.0b013e328334feb1 [DOI] [PubMed] [Google Scholar]
- 19.Cohen SE, Liu AY, Bernstein KT et al. . Preparing for HIV pre-exposure prophylaxis: lessons learned from post-exposure prophylaxis. Am J Prev Med 2013;44:S80–5. 10.1016/j.amepre.2012.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prestage G, Brown G, Down IA et al. . It's hard to know what is a risky or not a risky decision: gay men's beliefs about risk during sex. AIDS Behav 2013;17:1352–61. 10.1007/s10461-012-0180-7 [DOI] [PubMed] [Google Scholar]
- 21.Kippax S, Campbell D, van de Ven P et al. . Cultures of sexual adventurism as markers of HIV seroconversion: a case control study in a cohort of Sydney gay men. AIDS Care 1998;10:677–88. 10.1080/09540129848307 [DOI] [PubMed] [Google Scholar]
- 22.Vieira EM, Villela WV, Réa MF et al. . Alguns aspectos do comportamento sexual e prática de sexo seguro em homens do Município de São Paulo. Cad Saude Publica 2000;16:997–1009. 10.1590/S0102-311X2000000400018 [DOI] [PubMed] [Google Scholar]
- 23.Weller SC, Davis-Beaty K. Condom effectiveness in reducing heterosexual HIV transmission (Review). Cochrane Collaboration 2007. http://apps.who.int/rhl/reviews/CD003255.pdf (accessed 14 Apr 2014). [Google Scholar]
- 24.Curran JW, Crosby RA. Pre-exposure prophylaxis for HIV: who will benefit and what are the challenges? Am J Prev Med 2013;44:S163–66. 10.1016/j.amepre.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 25.Lunding S, Katzenstein TL, Kronborg G et al. . The Danish PEP registry: experience with the use of postexposure prophylaxis (PEP) following sexual exposure to HIV from 1998 to 2006. Sex Transm Dis 2010;37:49–52. 10.1097/OLQ.0b013e3181b6f284 [DOI] [PubMed] [Google Scholar]
- 26.Smith DK, Grohskopf LA, Black RJ et al. . Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep 2005;54:1–20. [PubMed] [Google Scholar]
- 27.Rey D, Bendiane MK, Moatti JP et al. . Post-exposure prophylaxis after occupational and non-occupational exposures to HIV: an overview of the policies implemented in 27 European countries. AIDS Care 2000;12:695–701. 10.1080/09540120020014228 [DOI] [PubMed] [Google Scholar]
- 28.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Programa Nacional de DST e Aids. Recomendações para terapia antirretroviral em adultos infectados pelo HIV-2008. Brasília: Suplemento III: tratamento e prevenção, 2010. [Google Scholar]
- 29.Irvine C, Egan KJ, Shubber Z et al. . Efficacy of HIV postexposure prophylaxis: systematic review and meta-analysis of nonhuman primate studies. Clin Infect Dis. 2015;60:S165–9. 10.1093/cid/civ069 [DOI] [PubMed] [Google Scholar]
- 30.Sperling RS, Shapiro DE, Coombs RW et al. . Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med 1996;335:1621–9. 10.1056/NEJM199611283352201 [DOI] [PubMed] [Google Scholar]
- 31.Cardo DM, Culver DH, Ciesielski CA et al. . A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med 1997;337:1485–90. 10.1056/NEJM199711203372101 [DOI] [PubMed] [Google Scholar]
- 32.Shoptaw S, Rotheram-Fuller E, Landovitz RJ et al. . Non-occupational post exposure prophylaxis as a biobehavioral HIV-prevention intervention. AIDS Care 2008;20:376–81. 10.1080/09540120701660353 [DOI] [PubMed] [Google Scholar]
- 33.Landovitz RJ, Currier JS. Postexposure prophylaxis for HIV infection. N Engl J Med 2009;361:1768–75. 10.1056/NEJMcp0904189 [DOI] [PubMed] [Google Scholar]
- 34.Poynten IM, Smith DE, Cooper DA et al. . The public health impact of widespread availability of nonoccupational postexposure prophylaxis against HIV. HIV Med 2007;8:374–81. 10.1111/j.1468-1293.2007.00483.x [DOI] [PubMed] [Google Scholar]
- 35.Mehta SA, Silvera R, Bernstein K et al. . Awareness of post-exposure HIV prophylaxis in high-risk men who have sex with men in New York City. Sex Transm Infect 2011;87:344–8. 10.1136/sti.2010.046284 [DOI] [PubMed] [Google Scholar]
- 36.Tissot F, Erard V, Dang T et al. . Nonoccupational HIV post-exposure prophylaxis: a 10-year retrospective analysis. HIV Med 2010;11:584–92. 10.1111/j.1468-1293.2010.00826.x [DOI] [PubMed] [Google Scholar]
- 37.Balbuena SF, Belza MJ, Castilla J et al. . Awareness and use of nonoccupational HIV post-exposure prophylaxis among people receiving rapid HIV testing in Spain. HIV Med 2013;14:252–7. 10.1111/j.1468-1293.2012.01056.x [DOI] [PubMed] [Google Scholar]
- 38.Poynten IM, Jin F, Limin M et al. . Nonoccupational postexposure prophylaxis, subsequent risk behaviour and HIV incidence in a cohort of Australian homosexual men. AIDS 2009;23:1119–26. 10.1097/QAD.0b013e32832c1776 [DOI] [PubMed] [Google Scholar]
- 39.Minas B, Laing S, Jordan H et al. . Improved awareness and appropriate use of non-occupational post-exposure prophylaxis (nPEP) for HIV prevention following a multi-modal communication strategy. BMC Public Health 2012;12:906 10.1186/1471-2458-12-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong K, Hughes CA, Plitt S et al. . HIV non-occupational postexposure prophylaxis in a Canadian province: treatment completion and follow-up testing. Int J STD AIDS 2010;21:617–21. 10.1258/ijsa.2008.008482 [DOI] [PubMed] [Google Scholar]
- 41.Armishaw J, Hoy JF, Watson KM et al. . Non-occupational post-exposure prophylaxis in Victoria, Australia: responding to high rates of re-presentation and low rates of follow-up. Int J STD AIDS 2011;22:714–18. 10.1258/ijsa.2011.011174 [DOI] [PubMed] [Google Scholar]
- 42.Oldenburg CE, Bärnighausen T, Harling G et al. . Adherence to post-exposure prophylaxis for non-forcible sexual exposure to HIV: a systematic review and meta-analysis. AIDS Behav 2014;18:217–25. 10.1007/s10461-013-0567-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bentz L, Enel P, Dunais B et al. . Evaluating counseling outcome on adherence to prophylaxis and follow-up after sexual HIV-risk exposure: a randomized controlled trial. AIDS Care 2010;22:1509–16. 10.1080/09540121.2010.484457 [DOI] [PubMed] [Google Scholar]
- 44.Martin JN, Roland ME, Neilands TB et al. . Use of postexposure prophylaxis against HIV infection following sexual exposure does not lead to increases in high-risk behaviour. AIDS 2004;18:787–92. 10.1097/00002030-200403260-00010 [DOI] [PubMed] [Google Scholar]
- 45.Körner H, Hendry O, Kippax S. Safe sex after post-exposure prophylaxis for HIV: intentions, challenges and ambivalences in narratives of gay men. AIDS Care 2006;18:879–87. 10.1080/09540120500307909 [DOI] [PubMed] [Google Scholar]
- 46.Heuker J, Sonder GJ, Stolte I et al. . High HIV incidence among MSM prescribed postexposure prophylaxis, 2000–2009: indications for ongoing sexual risk behaviour. AIDS 2012;26:505–12. 10.1097/QAD.0b013e32834f32d8 [DOI] [PubMed] [Google Scholar]
- 47.Zablotska IB, Prestage G, Holt M et al. . Australian gay men who have taken nonoccupational postexposure prophylaxis for HIV are in need of effective HIV prevention methods. J Acquir Immune Defic Syndr 2011;58:424–8. 10.1097/QAI.0b013e318230e885 [DOI] [PubMed] [Google Scholar]
- 48.Thigpen MC, Kebaabetswe PM, Paxton LA. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–34. 10.1056/NEJMoa1110711 [DOI] [PubMed] [Google Scholar]
- 49.Damme LV, Corneli A, Ahmed K et al. . Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012;367:411–22. 10.1056/NEJMoa1202614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Microbicide Trials Network. Understanding the results of VOICE. Fact Sheet—results. http://www.mtnstopshiv.org/node/2003 (accessed 24 Nov 2013).
- 51.Grant RM, Anderson PL, McMahan V et al. , iPrEx study team. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014;14:820–9. 10.1016/S1473-3099(14)70847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormack S, Dunn D. Pragmatic open-label randomised trial of preexposure prophylaxis: the PROUD study. Paper presented at: Conference on retroviruses and opportunistic infections 2015. [Google Scholar]
- 53.Molina J, Capitant C, Spire B et al. . On demand PrEP with oral TDF-FTC in MSM: results of the ANRS Ipergay trial. Paper presented at: Conference on retroviruses and opportunistic infections 2015. [Google Scholar]
- 54.Liu AY, Vittinghoff E, Chillag K et al. . Sexual risk behavior among HIV-uninfected men who have sex with men participating in a tenofovir preexposure prophylaxis randomized trial in the United States. J Acquir Immune Defc Syndr 2013;64:87–94. 10.1097/QAI.0b013e31828f097a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mugwanya KK, Donnell D, Celum C et al. . Sexual behavior of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis 2013;13:1021–8. 10.1016/S1473-3099(13)70226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease and Control. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep 2011;60:65–8. [PubMed] [Google Scholar]
- 57.The Consensus Committee, Southern African HIV Clinicians Society. Southern African guidelines for the safe use of pre-exposure prophylaxis in men who have sex with men who are at risk for HIV infection. SAJHIVMED 2012;2:40–55. [Google Scholar]
- 58.Krakower DS, Mimiaga MJ, Rosenberger JG et al. . Limited awareness and low immediate uptake of pre-exposure prophylaxis among men who have sex with men using an internet social networking site. PLoS ONE 2012;7:e33119 10.1371/journal.pone.0033119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks RA, Landovitz RJ, Kaplan RL et al. . Sexual risk behaviors and acceptability of HIV pre-exposure prophylaxis among HIV-negative gay and bisexual men in serodiscordant relationships: a mixed methods study. AIDS Patient Care STDS 2012;26:87–94. 10.1089/apc.2011.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sineath RC, Finneran C, Sullivan P et al. . Knowledge of and interest in using preexposure prophylaxis for HIV prevention among men who have sex with men in Thailand. J Int Assoc Provid AIDS Care 2013;12:227–31. 10.1177/2325957413488184 [DOI] [PubMed] [Google Scholar]
- 61.Kennedy C, Fonner V. Pre-exposure prophylaxis for men who have sex with men: a systematic review. World Health Organization, 2014. [Google Scholar]
- 62.Parker R. Grassroots activism, civil society mobilization, and the politics of the global AIDS epidemic. Brown J World Aff 2011;17:21–37. [Google Scholar]
- 63.Brasil. Ministério da Saúde. Protocolo clínico e diretrizes terapêuticas para adultos vivendo com HIV/aids. Brasília: Ministério da Saúde, 2013. [Google Scholar]
- 64.Harris PA, Taylor R, Thielke R et al. . Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverman D. Analyzing talk and text. In: Denzin N, Lincoln Y, eds. Handbook of qualitative research. 4th edn California: Sage Publication, 2011:821–34. [Google Scholar]