Abstract
Objectives
Socially disadvantaged people experience greater risk for illnesses that may contribute to premature death. This study aimed to evaluate the impact of treatable illnesses on mortality among adults living in precarious housing.
Design
A prospective cohort based in a community sample.
Setting
A socially disadvantaged neighbourhood in Vancouver, Canada.
Participants
Adults (N=371) living in single room occupancy hotels or recruited from the Downtown Community Court and followed for median 3.8 years.
Main outcome measures
Participants were assessed for physical and mental illnesses for which treatment is currently available. We compared cohort mortality rates with 2009 Canadian rates. Left-truncated Cox proportional hazards modelling with age as the time scale was used to assess risk factors for earlier mortality.
Results
During 1269 person-years of observation, 31/371 (8%) of participants died. Compared with age-matched and sex-matched Canadians, the standardised mortality ratio was 8.29 (95% CI 5.83 to 11.79). Compared with those that had cleared the virus, active hepatitis C infection was a significant predictor for hepatic fibrosis adjusting for alcohol dependence and age (OR=2.96, CI 1.37 to 7.08). Among participants <55 years of age, psychosis (HR=8.12, CI 1.55 to 42.47) and hepatic fibrosis (HR=13.01, CI 3.56 to 47.57) were associated with earlier mortality. Treatment rates for these illnesses were low (psychosis: 32%, hepatitis C virus: 0%) compared with other common disorders (HIV: 57%, opioid dependence: 61%) in this population.
Conclusions
Hepatic fibrosis and psychosis are associated with increased mortality in people living in marginal conditions. Timely diagnosis and intervention could reduce the high mortality in marginalised inner city populations.
Keywords: PUBLIC HEALTH, INFECTIOUS DISEASES
Strengths and limitations of this study.
Participants were recruited from marginalised housing sites and a Downtown Community Court to better capture the population living in inner city conditions.
While comprehensive follow-up assessments facilitated understanding of complex multimorbidities, longitudinal trajectories of illness progression and mortality risk may offer additional insights.
Self-report of treatment has the advantage of capturing adherence, but accuracy and precision may be affected by participants’ memory or understanding of their own care.
Since illness measures were assessed at baseline only, it is not possible to draw conclusions of the impact of the course of illness on mortality.
Paucity of literature limits our ability to generalise findings broadly to communities with different access to treatment, healthcare systems or origins of social marginalisation. Based on existing literature, our findings may be generalised to other marginally housed adults in Canada.
Introduction
Virtually the same healthcare is available to all persons in Canada, at no direct cost to patients; yet, financial and social disadvantage continues to be a risk for increased morbidity and mortality.1 Those with greatest disadvantage, who are homeless or housed in marginal conditions, such as single room occupancy (SRO) hotels, shelters or rooming houses, have increased mortality and decreased life expectancy.2 Homeless and marginalised populations face accumulating health challenges associated with poverty, substance use, and physical and mental illness.3 Vila-Rodriguez et al4 found that individuals living in SRO hotels in the Downtown Eastside (DTES) neighbourhood of Vancouver faced high mortality rates and suffered from compounding physical and mental multimorbidities associated with poor psychosocial functioning. Mental illness, infectious disease, substance use and substance dependence were common.4
Mental illness is a rising cause of morbidity and premature mortality worldwide, especially during reproductive years.5 Globally, mental and behavioural disorders account for 7.4% of total disability-adjusted life years, with schizophrenia, depression, and drug and alcohol use disorders among the main causes.5 A recent meta-analysis demonstrated a significant pooled mortality risk of 2.54 (95% CI 2.35 to 2.75) for psychosis (schizophrenia was primarily studied) and 1.71 (95% CI 1.54 to 1.90) for depression.6 Acute and chronic illness, suicide, and accidents contribute to premature mortality among those living with mental illness.6 7 However, a Danish study of homeless adults found that comorbid psychiatric and substance use disorders were associated with increased mortality, but psychiatric illness alone was not,8 calling for further study of the impact of mental illness in socially marginalised populations.
Infection, including HIV, hepatitis C virus (HCV) and hepatitis B virus (HBV), contributes significant disease burden across the lifespan.5 Chronic untreated HIV, HCV and HBV can manifest as debilitating immune-compromise, hepatic fibrosis and failure, and ultimately premature death. Progression of hepatic fibrosis can be monitored by surrogate blood measures that predict all-cause mortality.9–11 Globally, cirrhosis and liver cancer secondary to HBV, HCV and/or alcohol had a growing impact on morbidity and mortality over the past several decades.5 Ten years ago in Canada, HIV—but not HCV-positive serology—was found to be associated with all-cause mortality among inner city residents, and deaths among HCV-positive individuals were related to HIV, drug use and liver disease.12
Substance use and dependence can also lead to acute and chronic disease and earlier death, largely due to risks associated with injection drug use and polydrug use.13 14 Heroin15 and cocaine16 are among the most associated with premature death. Psychiatric illness, liver disease, accidental injury, overdose and infectious exposure via unsafe injection practices are among the fatal hazards of substance use.13 17 Harm reduction initiatives such as needle exchange and safe injection facilities mitigate some of these risks and reduce mortality.18 19
Although research on the health of homeless people is advancing, the impacts of potentially treatable illnesses on mortality risk are not well understood. As part of a longitudinal cohort study of health and disease in DTES residents living in marginal conditions, we examined the relationship between all-cause mortality and selected treatable disorders. We expected to replicate and extend the finding that marginally housed residents would have increased mortality rates compared with the general Canadian population.2 4 We hypothesised that psychotic and mood disorders would be associated with increased vulnerability for earlier death. We also examined the impact of certain infectious diseases, liver disease, and substance use and dependence disorders on premature death. Understanding the current risk factors for mortality for this high-risk population may contribute to improved outcomes through patient and provider education and system-level improvements.
Methods
Participants
Recruitment was from SRO hotels and the Downtown Community Court (DCC) in the DTES neighbourhood of Vancouver, Canada. Approximately 3800 people live in subsidised, SRO housing in this neighbourhood, and the DCC processes 2500 cases per year. Owing to the time requirements of the comprehensive baseline assessment, recruitment occurred from 13 November 2008 to 27 August 2012. During this period, there was no evidence of meaningful changes in healthcare service delivery or availability of new treatments that could impact the interpretation of findings. Adults were eligible for inclusion if they were able to communicate in English and provide written informed consent. The study design included monthly follow-up interviews including standardised health measures for up to 5 years.
Mortality
For participants who died during the study, Coroner's reports were requested, healthcare providers interviewed, and medical and mental health-related hospital records for the year prior to death were obtained to determine cause of death.
Illness and mortality risk
Mental and physical illnesses were identified to assess their impact on mortality risk. The focus was on potentially treatable disorders. A comprehensive baseline assessment of physical and mental health, substance use, and healthcare utilisation was carried out.4 Psychosis, mood disorders and substance dependence diagnoses were assessed using a series of standardised interviews administered over two-to-three sessions by a research assistant, followed by a clinical interview and mental status examination by a psychiatrist, and cognitive testing supervised by a neuropsychologist.4 Health records were obtained for all previous mental health-related hospitalisations, from as long ago as the 1960s. All available clinical information was reviewed by study psychiatrists (WGH and FV-R) in a Best Estimate Clinical Evaluation and Diagnosis process modified to make psychiatric diagnoses according to Diagnostic and Statistical Manual for Mental Disorders-TR Fourth Edition (DSM-IV TR) criteria.
Positive serology indicated HCV exposure; viral DNA detection by qPCR indicated current HCV infection. HIV exposure was identified by antibody detection. HBV exposure was identified by core antibody detection. Hepatic fibrosis was determined using the surrogate serological measure aspartate aminotransferase-to-Platelet Ratio Index (APRI).9 Substance use was reported for the 4 weeks prior to assessment employing the Drug Timeline Followback approach. The substance-related Composite Harm Score (CHS) was calculated as previously described.20 Crack pipe and needle sharing were also reported for the month prior to baseline assessment.
Participant engagement in treatment was assessed by self-report of the number of days taking each medication during the 4 weeks prior to baseline interview. Medications recorded included methadone maintenance therapy for opioid dependence, antiretroviral medication for HIV infection and antipsychotic medication for psychotic illness. A history of any treatment for HCV was obtained.
Statistical analysis
The sample size for analysis of treatable illnesses as risk factors was estimated from a preliminary report indicating higher than expected mortality in this cohort.4 The indirect method of standardisation was used to determine the standardised mortality ratio (SMR) for this cohort. The SMR was calculated as the ratio of the observed number of deaths to the number of deaths expected if the cohort experienced the same age-specific and sex-specific mortality rates as the general Canadian population in 2009. The Boice-Monson method was used to calculate the 95% CI.
The impact of mental illness (psychosis and mood disorder), addiction (stimulant, opioid, and alcohol dependence, injection drug use, and CHS) and physical illness (HIV, HCV and hepatic fibrosis) on mortality risk was assessed using left-truncated Cox proportional hazards modelling with age as the time scale. This survival analysis approach accounts for deaths before or after study participation and the effect of ageing on mortality risk. Loss to follow-up, study exit, and the time between birth and study entry were censored. The proportional hazard assumption for Cox models was verified by plotting Schoenfeld residuals against the time scale and visual and statistical assessment. In the case of non-proportionality (Schoenfeld residual global test p<0.05), we used a change-point estimate approach21 22 to account for distinct effects on mortality at younger and older ages. The change point was selected by visual inspection of Schoenfeld residual plots, which showed an inflection between 44 and 59 years of age and an inflection point at age 55. Thus, we selected 55 years of age as the change point. Other change points were examined, but results were similar.
The Kaplan-Meier method was used to plot the effect of statistically significant predictors on mortality. Multiple logistic regression was used in secondary analyses to evaluate factors associated with hepatic fibrosis. Variables were considered for inclusion in the final adjusted models if they fit the scientific framework, were associated with mortality risk (p values ≤0.10) and changed unadjusted effect coefficients by more than 10%. Interactions between mortality risk factors were examined.
Each variable had <5.0% missing data, except HBV (5.9% missing). Participants with missing data were compared with the rest of the cohort. Complete cases were used for each regression analysis. Significance was set at p<0.05. Data were analysed using JMP (V.11) and R (V.3.0.3).
Role of the funding source
The funders had no roles in the design or implementation of the study, and did not contribute to the writing of the report. All authors had full access to the data.
Results
Participants
Of the 515 potentially eligible, 371 (72%) SRO tenants and DCC participants met inclusion criteria and agreed to enrol. As of 1 June 2014, participants were followed for a median of 3.8 years (IQR 1.9–5.0 years), including 60/371 (16%) who were lost to follow-up (table 1). Reasons for loss of contact included moving away from Vancouver and living in a jail or treatment facility. Participants were mostly middle-aged males, many of who had been homeless (no fixed address) for at least one period in their life. Substance use was prevalent across the cohort, with tobacco, crack cocaine and cannabis among the most commonly used. Half the cohort injected drugs, while few reported sharing needles. Conversely, pipe sharing was common among those using crack cocaine. Participants from DCC (N=65) were followed for less time (1.9 years, IQR 1.9–1.9 years) than SRO participants (N=306; 4.3 years, IQR 3.1–5.0 years) at the time of analysis. Eighty per cent of DCC participants were living in an SRO hotel at study entry and were more likely to report a previous history of homelessness (83% vs 65%) and opioid dependence (54% vs 39%) compared with participants recruited directly from SRO hotels (data not shown).
Table 1.
Participant baseline characteristics and mortality
| Characteristic | Value |
|---|---|
| Age, N=371 | 44 (37–51) |
| Female sex | 81/371 (22%) |
| Monthly income*, N=363 | 875 (635–1075) |
| Monthly disposable income*, N=363 | 500 (260–700) |
| Welfare | 261/367 (71%) |
| History of homelessness | 252/370 (68%) |
| Visit with family physician (6 months)† | 311/369 (84%) |
| Mortality | |
| Mortality | 31/371 (8%) |
| Age at death, N=31 | 47 (23–72) |
| Causes of death | |
| Physical disease | 16/31 (52%) |
| Accidental drug overdose | 7/31 (23%) |
| Trauma | 4/31 (13%) |
| Suicide | 2/31 (6%) |
| Undetermined | 2/31 (6%) |
| Mental illness | |
| Psychotic disorder | 175/371 (47%) |
| Mood disorder | 105/371 (28%) |
| Substance dependence | 355/371 (96%) |
| Stimulant dependence | 305/371 (82%) |
| Opioid dependence | 202/371 (54%) |
| Alcohol dependence | 67/371 (18%) |
| Physical illness | |
| HIV exposure | 63/359 (18%) |
| Hepatitis B exposure | 141/349 (40%) |
| Current hepatitis B infection | 5/141 (4%) |
| Hepatitis C exposure | 244/357 (68%) |
| Current hepatitis C infection | 180/244 (74%) |
| HIV/HCV co-infection | 43/244 (18%) |
| Hepatic fibrosis (APRI>0.7)‡ | 73/353 (21%) |
| Substance use§ | |
| Any injection drug use | 194/366 (53%) |
| Composite Harm Score, N=364 | 2806 (1847–4010) |
| Any tobacco use | 336/364 (92%) |
| Any crack cocaine use | 210/364 (58%) |
| Any powder cocaine use | 86/364 (24%) |
| Any cannabis use | 173/364 (48%) |
| Any heroin use | 127/364 (35%) |
| Any methamphetamine use | 91/374 (25%) |
| Any alcohol use | 180/366 (49%) |
| Users sharing crack pipes | 108/207 (52%) |
| Users sharing needles | 3/194 (2%) |
Median and IQR reported for continuous variables. Number and proportion reported for categorical variables.
*Canadian dollars.
†Reported for first 6 months of study follow-up.
‡APRI, surrogate measure of hepatic fibrosis.
§Reported for month prior to baseline.
APRI, aspartate aminotransferase-to-Platelet Ratio Index; HCV, hepatitis C virus.
Mortality
During 1269 person-years of observation, 31/371 (8%) of participants died. Coroner's reports were obtained for 14 participants who died outside of hospital. Deaths were attributed to physical disease, accidental drug overdose, trauma (ie, motor vehicle or blunt trauma) and suicide. Cocaine and opioids together were implicated in four of the seven deaths attributed to accidental drug overdose (see online supplementary table S1). Deaths occurred in participants recruited directly from SRO hotels (30/306) and from the DCC (1/65).
The crude mortality rate was 24 deaths per 1000 person-years. The mean age at death was 47 (range 23–72). Overall, 25/31 (81%) of decedents were male, similar to remaining participants. Compared with age-matched and sex-matched Canadian population data, the SMR was 8.29 (95% CI 5.83 to 11.79). Deaths were over-represented in the younger (20–39) and middle (40–59) age groups at 10.73 (CI 3.46 to 33.25) and 10.54 (CI 7.12 to 15.59) times the national rates, while in the older (60+) group, the SMR was elevated but not different (2.76; CI 0.89 to 8.56) from the national rate.
Illness prevalence
Physical and mental illness were prevalent at baseline assessment (table 1). Psychiatric disorders were common. Psychotic illness included schizophrenia, schizoaffective, bipolar with psychosis, depression with psychosis, delusional disorder, postanoxic or interferon-related psychosis, substance-induced psychosis and psychosis not otherwise specified. HCV exposure was prevalent, including several participants who were unaware of being seropositive until being tested as part of the study (12/244, 5%). Many had persistent HCV infection, and evidence for hepatic fibrosis was present in a substantial minority.
Impact of illness on mortality risk
Survival analysis was employed to evaluate the association between illnesses amenable to treatment and mortality risk (table 2). Notably, baseline HIV or HCV exposure, and opioid or stimulant dependence were not associated with increased risk of mortality.
Table 2.
Survival analysis of illnesses amenable to treatment as risk factors of earlier mortality
| Univariate regression models |
||||
|---|---|---|---|---|
| Factor | N | HR (95% CI) | Log-rank p value |
Schoenfeld p value |
| Mental illness | ||||
| Psychotic disorder | 371 | 1.18 (0.56 to 2.47) | 0.660 | 0.008 |
| Mood disorder | 371 | 0.88 (0.39 to 2.00) | 0.767 | 0.147 |
| Stimulant dependence | 371 | 0.90 (0.37 to 2.22) | 0.819 | 0.852 |
| Opioid dependence | 371 | 1.18 (0.56 to 2.45) | 0.665 | 0.644 |
| Alcohol dependence | 371 | 2.05 (0.93 to 4.53) | 0.075 | 0.996 |
| Physical illness | ||||
| HIV exposure | 359 | 1.42 (0.59 to 3.41) | 0.431 | 0.118 |
| HCV exposure | 357 | 1.32 (0.52 to 3.35) | 0.558 | 0.765 |
| Current HCV infection | 244 | 1.00 (0.36 to 2.78) | 0.993 | 0.427 |
| Hepatic fibrosis (APRI>0.7)* | 353 | 3.42 (1.63 to 7.17) | <0.001 | 0.259 |
| Substance use† | ||||
| Any injection drug use | 366 | 1.45 (0.65 to 3.21) | 0.365 | 0.619 |
| Composite Harm Score | 364 | 1.10 (0.99 to 1.23) | 0.076 | 0.921 |
*APRI, surrogate measure of hepatic fibrosis.
†Reported for month prior to baseline.
APRI, aspartate aminotransferase-to-Platelet Ratio Index; HCV, hepatitis C virus.
The effect of psychosis on mortality interacted with age as determined by a significant Schoenfeld residual global test (table 2). For participants younger than the change-point age 55, psychosis was significantly associated with earlier death, but not in those 55 or older (figure 1). The probability that an individual with psychosis would survive to age 50 is 68% compared with 94% for those without the diagnosis among marginally housed individuals.
Figure 1.
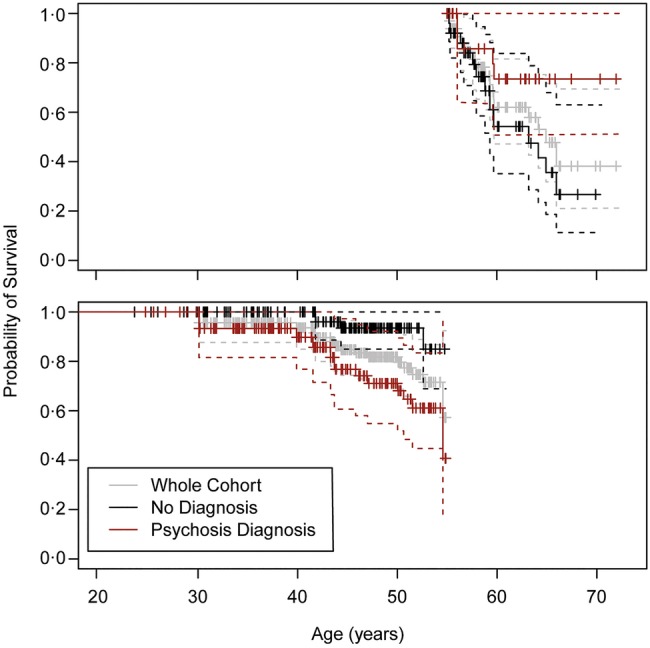
Kaplan-Meier curves for the probability of survival by age among DTES residents (Above) >55 years old (N=82) and (Below) <55 years old (N=289) with psychotic illness as compared to those without the diagnosis. Survival curves coloured grey for population average, red for those with psychosis, and black for those without psychosis.
Surpassing the threshold APRI value of 0.7 (suggesting hepatic fibrosis) was also significantly associated with earlier mortality (figure 2). The probability that an individual with hepatic fibrosis in this cohort would survive to age 50 is 34% compared with 89% for those without evidence of fibrosis. HCV infection was not independently associated with earlier mortality. However, active HCV infection was associated with evidence of fibrosis. As compared with those that had cleared the infection, current HCV infection was a significant predictor of above threshold APRI, adjusting for age and alcohol dependence (table 3).
Figure 2.
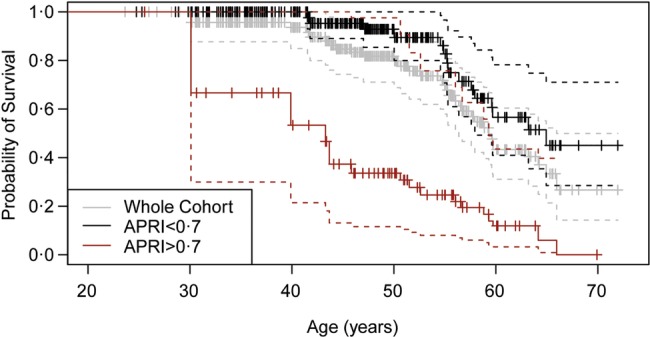
Kaplan-Meier curves for the probability of survival by age among DTES residents with evidence of hepatic fibrosis (APRI>0.7) as compared to those with APRI<0.7. (N=353) Survival curves coloured grey for population average, red for those with APRI>0.7, and black for those with APRI<0.7.
Table 3.
Logistic regression analysis of factors associated with hepatic fibrosis
| Unadjusted models |
Adjusted model (n=239) |
||||
|---|---|---|---|---|---|
| Factor | N | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Female sex | 353 | 0.73 (0.07 to 1.39) | 0.354 | – | – |
| Age at entry | 353 | 1.05 (1.02 to 1.08) | <0.001 | 1.04 (1.01 to 1.08) | 0.015 |
| Current HCV infection | 240 | 2.57 (1.23 to 5.92) | 0.017 | 2.96 (1.37 to 7.08) | 0.009 |
| Any alcohol use | 350 | 1.15 (0.68 to 1.95) | 0.593 | – | – |
| Alcohol dependence | 353 | 1.74 (0.93 to 3.18) | 0.074 | 1.90 (0.83 to 4.29) | 0.122 |
| CHS | 345 | 1.07 (0.99 to 1.15) | 0.096 | – | – |
CHS, Composite Harm Score; HCV, hepatitis C virus.
For participants younger than 55 years of age, hepatic fibrosis and psychosis were significantly associated with earlier mortality (table 4). Neither CHS nor alcohol dependence met inclusion criteria for the adjusted model. Interactions between predictors were not significant (p=0.37).
Table 4.
Unadjusted and adjusted survival analysis of risk factors of earlier mortality for younger (age <55, N=289) and older (age ≥55, N=82) DTES residents
| Unadjusted models |
Adjusted model (n=267) |
||||
|---|---|---|---|---|---|
| Factor | N | HR (95% CI) | Log-rank p value | HR (95% CI) | Log-rank p value |
| Age <55 | |||||
| Psychotic disorder | 289 | 3.78 (1.03 to 13.84) | 0.032 | 8.12 (1.55 to 42.47) | 0.013 |
| Hepatic fibrosis* | 273 | 8.90 (2.83 to 27.93) | <0.001 | 13.01 (3.56 to 47.57) | <0.001 |
| Alcohol dependence | 289 | 2.91 (0.95 to 8.96) | 0.051 | – | |
| CHS | 282 | 1.05 (0.90 to 1.24) | 0.509 | – | |
| Age ≥55 | |||||
| Psychotic disorder | 82 | 0.36 (0.10 to 1.31) | 0.107 | – | |
| Hepatic fibrosis* | 80 | 2.14 (0.79 to 5.82) | 0.128 | – | |
| Alcohol dependence | 82 | 1.83 (0.57 to 5.91) | 0.304 | – | |
| CHS | 82 | 1.12 (0.96 to 1.30) | 0.150 | – | |
*APRI>0.7, surrogate measure of hepatic fibrosis.
APRI, aspartate aminotransferase-to-Platelet Ratio Index; CHS, Composite Harm Score; DTES, Downtown Eastsid.
Treatment rates
A large majority of participants reported seeing a family physician during the first 6 months of study follow-up (table 1). However, baseline treatment rates were low for illnesses associated with earlier mortality in this cohort. Treatment for psychosis and hepatic fibrosis associated with active HCV infection was less common than treatment for HIV and opioid dependence (figure 3). Of those who died, 15/31 (48%) were psychotic at the last visit before death; available data indicated only 2/12 (17%) with psychosis were receiving antipsychotic drug treatment.
Figure 3.
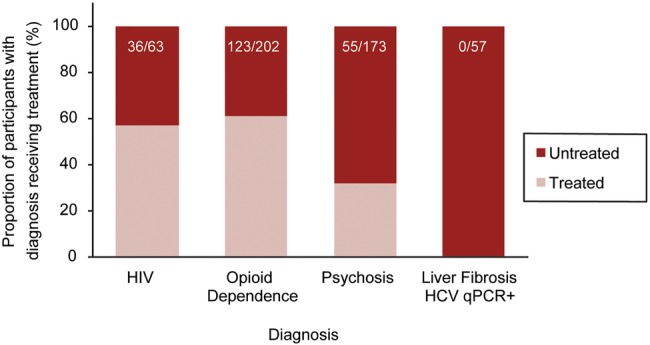
Stacked bar plot comparing baseline treatment rates for those with HIV infection, opioid dependence, psychosis, and liver fibrosis (APRI>0.7) with current HCV infection (qPCR+). Proportions of diagnosed participants receiving treatment (n/N) are reported here.
Discussion
Adults living in marginalised conditions have significantly greater all-cause mortality rates than other Canadians. We found a greater than eightfold increase in mortality rate. Deaths were over-represented in participants younger than 60. The excess mortality in the present cohort is comparable or greater than other reports of marginally housed individuals,1 3 8 except one cohort study of homeless women in Toronto.23 Causes of death included overdose and acute and chronic diseases. Mental and physical multimorbidities were common, many of which are complex but potentially treatable. We found that having psychosis and/or hepatic fibrosis (APRI>0.7) were significantly associated with earlier mortality in participants <55 years old.
Individuals with psychosis may face a greater than eightfold increase in mortality risk compared with those without psychosis, adjusting for hepatic fibrosis. The associated mortality risk is high compared with a meta-analysis that demonstrated a pooled mortality risk of 2.54 (95% CI 2.35 to 2.75) for psychosis.6 The majority of the studies included in the meta-analysis focused on inpatient schizophrenia, while other psychotic illnesses and untreated psychosis were less represented.6 Psychosis itself is a devastating biological insult in addition to the effects of co-occurring social factors, including poverty and social connectedness, access to quality healthcare, and behavioural and lifestyle factors.24
Biochemical evidence of hepatic fibrosis was also associated with earlier death across the cohort and was strongly associated with current HCV infection. Prior to age 55, hepatic fibrosis conferred a 13-fold increase in premature mortality, adjusting for the effects of psychosis. These findings are consistent with other studies indicating that persistent untreated HCV infection can lead to hepatic fibrosis and shorten life expectancy.9–12 25 Progressive fibrosis compromises liver function and ultimately can result in liver failure and death.10 11 Historically, HIV infection and needle sharing were associated with high rates of mortality in urban Vancouver.12 However, low rates of HIV and needle sharing and the high rate of antiretroviral treatment seen here may reflect the impact of harm reduction initiatives and increased access to highly active antiretroviral therapy.18 19 26
Many participants also suffered from a substance use and/or mood disorder, but neither illness was associated with premature death. We did not identify a significant association between premature mortality and specific substances or a composite score of drug-related harm. Though an increased CHS may be associated with some types of psychosis,20 possible effects on mortality may be complicated by multimorbidities.
The majority of participants saw a family physician during the first 6 months of the study. This is consistent with high primary care and emergency department utilisation seen among homeless populations.27 Despite being connected to primary care, less than one-third of participants with psychosis were receiving pharmacological treatment for their illness. Antipsychotic medications reduce the risk of suicide, the leading cause of premature death in people with schizophrenia.28 Low treatment rates may be attributed to financial barriers associated with lack of supplemental insurance for prescription medications.29 Limited psychiatric care access and challenges in diagnosis and comorbidity management may also contribute.
Successful treatment of HCV infection with antiviral therapy reduces all-cause mortality.25 Here we show that none of the participants with HCV infection and evidence of hepatic fibrosis were receiving antiviral treatment at baseline. As of 2009, the American Association for the Study of Liver Diseases guidelines outlined that persons who inject drugs and/or have psychiatric illness should be considered for treatment with monitoring and the support of a multidisciplinary team.30 Despite advancements in oral treatment options, several obstacles to effective care remain.31 32 Barriers include limited access to care, cost of treatment, education about illness, low perceived need for treatment and physician expertise in managing comorbidities.32 Treatment may also be postponed due to other pressing health concerns.32 Specific to our study, patient access to medications available for HCV did not differ from access to medications for HIV (both supported by provincial programmes). However, the new generation of orally active HCV medications was not available during the time frame reported, and concern about psychiatric side effects of the older drugs in an at-risk population may have limited utilisation. Costs are now an issue, as the new, orally active drugs are available only at a high cost to patients in British Columbia. In this study, several participants with HCV exposure at baseline screening were unaware of their status. Despite inconsistencies in treatment accessibility, high-risk populations have demonstrated an interest in knowing their HCV status,33 supporting increased community-based HCV screening for inner city populations. Finally, while an active public awareness campaign supported by the health authorities has been in place for HIV testing and treatment, HCV has not received similar prominence as a public health concern. This is reflected in the previously described low lifetime rates of treatment for HCV (9%) in the cohort, relative to the high rate of successful viral suppressive treatment for HIV (89%).4 These findings underscore the need to address barriers to detection and treatment of HCV to prevent fibrosis and premature mortality.
There are several limitations to the study. Treatment self-report has the advantage of capturing adherence, but accuracy and precision may be affected by participants’ memory or understanding of their own care. Follow-up was limited to median 3.8 years; this is similar to other survival reports6 12 25 but should be considered when interpreting results, as different, additional exposures could take longer to influence mortality. This is particularly true for the participants recruited from the DCC, as the period of follow-up was shorter. Also, since illness measures were assessed at baseline only, it is not possible to draw conclusions of the impact of the course of illness on mortality. Longitudinal trajectories of illness progression and mortality risk may offer further insights into health risks and care prioritisation. The comprehensive follow-up allows us to understand complex multimorbidities in this cohort; however, the paucity of literature about the health of marginally housed adults limits our ability to evaluate the generalisability of these results in communities that differ in treatment availability, healthcare system and the origins of social marginalisation. Based on Hwang et al,2 our findings may be generalisable to adults in living in substandard housing in Canada. Further study is required to assess generalisability to other cultural and societal contexts.
Conclusion
People living in substandard housing face a high standardised mortality rate, even in a system providing free access to healthcare. Identifiable, treatable risk factors included psychosis (especially in younger people) and biochemical evidence for hepatitis C-related liver damage. Timely diagnosis and intervention could reduce the high mortality in marginalised inner city populations.
Footnotes
Twitter: Follow Fidel Vila-Rodriguez at @NINETLAB
Contributors: WGH, DJL, AET, AMB, RMP, AR and MK designed the study. FV-R, WJP, GWM, OL, VL and KS collected data. JSM contributed to interpretation of data. AAJ, GNS and WGH performed data analysis and drafted the final report. TB was the study coordinator. All authors read and approved the final draft.
Funding: The study was funded by the Canadian Institutes for Health Research (CBG-101827, MOP-137103), and the British Columbia Mental Health and Substance Use Services (an Agency of the Provincial Health Services Authority). AR is supported by a New Investigator Award from the Canadian Institutes for Health Research (201109MSH-261306-183304). WGH was supported by the Jack Bell Chair in Schizophrenia.
Competing interests: WGH has received consulting fees or sat on Advisory Boards for In Silico, Eli Lilly, Roche, Lundbeck and Otsuka. AMB has received consulting fees or sat on Advisory Boards for Bristol-Myers Squibb, Eli Lilly and Roche. RMP has received speaking and Advisory Board fees from AstraZeneca, Bristol-Myers Squibb, Janssen, Otsuka, Pfizer and Sunovion. MK has received grant support from Bell Canada, CIHR, the Mental Health Commission of Canada, the Canadian Center of Substance Abuse, and the Innerchange Foundation. JSM has received grant support from Abbott, Biolytical, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. GWM has received speaking or consulting fees, or sat on Advisory Boards for Apotex, AstraZeneca, Bristol-Myers Squibb, Janssen, Lundbeck, Otsuka, Pfizer, and Sunovion, and research grant support from Janssen. AR has received Advisor Board fees from Hofmann-La Roche.
Ethics approval: Ethics Board of the University of British Columbia.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Researchers interested in accessing data for meta-analyses or other studies should contact WGH.
References
- 1.Phipps S. The impact of poverty on health. Ottawa, ON: Canadian Institute for Health Information, 2003. [Google Scholar]
- 2.Hwang SW, Wilkins R, Tjepkema M et al. . Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow-up study. BMJ 2009;339:b4036 10.1136/bmj.b4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014;384:1529–40. 10.1016/S0140-6736(14)61132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vila-Rodriguez F, Panenka WJ, Lang DJ et al. . The Hotel study: multimorbidity in a community sample living in marginal housing. Am J Psychiatry 2013;170:1413–22. 10.1176/appi.ajp.2013.12111439 [DOI] [PubMed] [Google Scholar]
- 5.Murray CJL, Vos T, Lozano R et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 6.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41. 10.1001/jamapsychiatry.2014.2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RT, Kiely DK, Bharel M et al. . Geriatric syndromes in older homeless adults. J Gen Intern Med 2012;27:16–22. 10.1007/s11606-011-1848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielson SF, Hjorthoj CR, Erlangsen A et al. . Psychiatric disorders and mortality among people in homeless shelters in Denmark: a nationwide register-based cohort study. Lancet 2011;377:2205–14. 10.1016/S0140-6736(11)60747-2 [DOI] [PubMed] [Google Scholar]
- 9.Wai C-T, Greenson JK, Fontana RJ et al. . A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 10.Rosen HR. Chronic hepatitis C infection. NEJM 2011;364:2429–38. 10.1056/NEJMcp1006613 [DOI] [PubMed] [Google Scholar]
- 11.Vergara M, Guillermina B, Dalmau B et al. . Usefulness of indirect noninvasive methods in predicting progression to cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 2015;27:826–33. 10.1097/MEG.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 12.Grebely J, Raffa JD, Lai C et al. . Impact of hepatitis C virus on all-cause and liver-related mortality in a large community-based cohort of inner city residents. J Viral Hepat 2011;18:32–41. 10.1111/j.1365-2893.2010.01279.x [DOI] [PubMed] [Google Scholar]
- 13.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012;379:55–70. 10.1016/S0140-6736(11)61138-0 [DOI] [PubMed] [Google Scholar]
- 14.Begg S, Vos T, Barker B et al. . The burden of disease and injury in Australia 2003. Canberra: Australian Institute of Health and Welfare, 2007. [Google Scholar]
- 15.Webb L, Oyefeso A, Schifano F et al. . Cause and manner of death in drug-related fatality: an analysis of drug-related deaths recorded by coroners in England and Wales in 2000. Drug Alcohol Depend 2003;72:67–74. 10.1016/S0376-8716(03)00191-1 [DOI] [PubMed] [Google Scholar]
- 16.Hayden A, Hayashi K, Dong H et al. . The impact of drug use patterns on mortality among polysubstance users in a Canadian setting: a prospective cohort study. BMC Public Health 2014;14:1153–9. 10.1186/1471-2458-14-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffin PO, Galea S, Ahern J et al. . Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–98. Addiction 2003;98:739–47. 10.1046/j.1360-0443.2003.00376.x [DOI] [PubMed] [Google Scholar]
- 18.Marshall BDL, Milloy M-J, Wood E et al. . Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: a retrospective population-based study. Lancet 2011;377:1429–37. 10.1016/S0140-6736(10)62353-7 [DOI] [PubMed] [Google Scholar]
- 19.Hyshka E, Strathdee S, Wood E et al. . Needle exchange and the HIV epidemic in Vancouver: lessons learned from 15 years of research. Int J Drug Policy 2012;23:261–70. 10.1016/j.drugpo.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones AA, Vila-Rodriguez F, Panenka WJ et al. . Personalized risk assessment of drug-related harm is associated with health outcomes. PLoS ONE 2013;8:e79754 10.1371/journal.pone.0079754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quandt RE. The estimation of the parameter of a linear regression system obeying two separate regimes. J Am Stat Assoc 1958;53:873–80. 10.1080/01621459.1958.10501484 [DOI] [Google Scholar]
- 22.Hinkley DV. Inference in two-phase regression. J Am Stat Assoc 1971;66:736–43. 10.1080/01621459.1971.10482337 [DOI] [Google Scholar]
- 23.Cheung AM, Hwang SW. Risk of death among homeless women: a cohort study and review of the literature. CMAJ 2004;170:1243–7. 10.1503/cmaj.1031167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol 2014;10:425–48. 10.1146/annurev-clinpsy-032813-153657 [DOI] [PubMed] [Google Scholar]
- 25.Backus LI, Boothroyd DB, Phillips BR et al. . Sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509–16. 10.1016/j.cgh.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Montaner JSG, Lima VD, Barrios R et al. . Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010;376:532–9. 10.1016/S0140-6736(10)60936-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SW, Chambers C, Chiu S et al. . A comprehensive assessment of health care utilization among homeless adults under a system of universal health insurance. Am J Public Health 2013;103:S294–301. 10.2105/AJPH.2013.301369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman AF, Lieberman JA, Dixon LB et al. , American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia second edition. Am J Psychiatry 2004;161(2 Suppl):1–56. 10.1176/appi.ajp.161.1.1 [DOI] [PubMed] [Google Scholar]
- 29.Mulvale G, Hurley J. Insurance coverage and the treatment of mental illness: effect on medication and provider use. J Ment Health Policy Econ 2008;11:177–99. [PubMed] [Google Scholar]
- 30.Ghany MG, Strader DB, Thomas DL et al. . Diagnosis, management and treatment of hepatitis C: an update. Hepatology 2009;49:1335–74. 10.1002/hep.22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015;385:1124–35. 10.1016/S0140-6736(14)62401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donepudi I, Paredes A, Hubbard S et al. . Utility of evaluating HCV in an uninsured population. Dig Dis Sci 2015;60:1092–7. 10.1007/s10620-014-3416-8 [DOI] [PubMed] [Google Scholar]
- 33.Norton BL, Voils CI, Timberlake SH et al. . Community-based HCV screening: knowledge and attitudes in a high risk urban population. BMC Infect Dis 2014;14:74–82. 10.1186/1471-2334-14-74 [DOI] [PMC free article] [PubMed] [Google Scholar]


