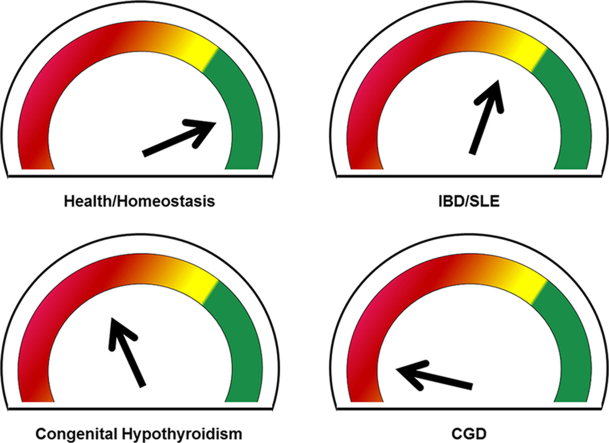
Abstract
Maintaining the redox balance between generation and elimination of reactive oxygen species (ROS) is critical for health. Disturbances such as continuously elevated ROS levels will result in oxidative stress and development of disease, but likewise, insufficient ROS production will be detrimental to health. Reduced or even complete loss of ROS generation originates mainly from inactivating variants in genes encoding for NADPH oxidase complexes. In particular, deficiency in phagocyte Nox2 oxidase function due to genetic variants (CYBB, CYBA, NCF1, NCF2, NCF4) has been recognized as a direct cause of chronic granulomatous disease (CGD), an inherited immune disorder. More recently, additional diseases have been linked to functionally altered variants in genes encoding for other NADPH oxidases, such as for DUOX2/DUOXA2 in congenital hypothyroidism, or for the Nox2 complex, NOX1 and DUOX2 as risk factors for inflammatory bowel disease. A comprehensive overview of novel developments in terms of Nox/Duox-deficiency disorders is presented, combined with insights gained from structure–function studies that will aid in predicting functional defects of clinical variants.
Keywords: NADPH oxidase, Chronic granulomatous disease, Inflammatory bowel disease, Hypothyroidism, NOX, DUOX, Genetic disease, Reactive oxygen species (ROS)
Graphical abstract
Highlights
-
•
Deficiency in reactive oxygen species is often caused by NADPH oxidase variants.
-
•
Overview of chronic granulomatous disease and alterations in the Nox2 complex.
-
•
CGD iPS cells as promising novel tools.
-
•
Risk for inflammatory bowel disease by Nox2 complex, Nox1 and Duox2 mutations.
-
•
Loss-of-function variants in DUOX2 and DUOXA2 trigger congenital hypothyroidism.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- CD
Crohn's disease
- CFU-G
colony forming unit-granulocyte
- CGD
chronic granulomatous disease
- CH
congenital hypothyroidism
- CV
cardiovascular
- DSS
dextran sulfate sodium
- DUOX
dual oxidase
- DUOXA
DUOX maturation factor
- EB
embryoid body
- ER
endoplasmic reticulum
- ESC
embryonic stem cell
- FAD
flavin adenine dinucleotide
- FMD
flow-mediated arterial dilation
- FNR family
fumarate-nitrate reductase regulator protein family
- GEF
guanine nucleotide exchange factor
- GI tract
gastrointestinal tract
- GM-CSF
granulocyte-macrophage colony stimulating factor
- GWAS
genome-wide association study
- H2O2
hydrogen peroxide
- IBD
inflammatory bowel disease
- IDO
indolamine 2,3 dioxygenase
- IEC
intestinal epithelial cell
- IFN-γ
interferon gamma
- IL-1
interleukin-1
- iNOS
inducible nitric oxide synthetase
- iPSC
induced pluripotent stem cell
- IYD
iodotyrosine deiodinase
- KO
knockout
- LPO
lactoperoxidase
- LPS
lipopolysaccharide
- LTP
long term potentiation
- l-T4
l-thyroxine/levothyroxine
- MPO
myeloperoxidase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCF1/2/4
neutrophil cytosolic factor 1/2/4
- NF-κB
nuclear factor-kappa B
- NIS
NADPH domain insertion sequence
- NO
nitric oxide
- NOX1-5
NADPH oxidase 1-5
- NOXA1
NADPH oxidase activator 1
- NOXO1
NADPH oxidase organizer 1
superoxide
- OMIM
online Mendelian inheritance in man
- OxLDL
oxidized low-density lipoprotein
- phox
phagocyte oxidase
- PIOD
partial iodide organification defect
- PMA
phorbol 12-myristate-13-acetate
- PO
peroxidase
- ROS
reactive oxygen species
- SCH
subclinical hypothyroidism
- SIN
self-inactivating
- SLE
systemic lupus erythematosus
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- TG
thyroglobulin
- ThOX
thyroid oxidase
- TIOD
total iodide organification defect
- TLR
toll-like receptor
- TM
transmembrane
- TNBS
trinitrobenzene sulfonic acid
- TPO
thyroid peroxidase
- TSH
thyroid stimulating hormone
- TSHR
thyroid stimulating hormone receptor
- UC
ulcerative colitis
- VEOIBD
very early onset inflammatory bowel disease
- WBS
Williams–Beuren Syndrome
- ZFN
zinc finger nuclease
1. Introduction
Oxidative stress, the imbalance between the generation of reactive oxygen species (ROS) and the ability of antioxidant defense systems to scavenge ROS, has been recognized as a risk and contributing factor for various forms of pathophysiology, including inflammation and tissue injury, neurodegeneration, and carcinogenesis. Many enzyme systems can be the source of superoxide (O2•) or hydrogen peroxide (H2O2), the initial ROS produced, and even more proteins are involved in adduct reactions involving oxygen radicals, generating for instance hypochlorous acid or peroxynitrite. A similar variety exists in antioxidant systems. The overall redox balance is critical for propagation and termination of essential signaling pathways in cells and tissues, while specialized functions in certain cells such as phagocytes require a regulated burst of ROS. Undesirable consequences of increased ROS, due to deregulated ROS overproduction or failure of antioxidant systems, can be detected by changes in cellular responses such as increased apoptosis or cell proliferation, and even in cases of overall cellular adaptation by the appearance of oxidative modifications on DNA, proteins or lipids.
The dichotomy of ROS being vital signaling molecules in a plethora of physiological processes while also propagating disease often impedes a clear distinction between beneficial and harmful ROS, but a comprehensive study of genetic disorders can reveal the overall consequences for health when the redox balance is permanently altered. For example, sequence alterations in mitochondrial DNA, superoxide dismutases, catalase and glutathione synthetase usually augment ROS levels. In contrast, NADPH oxidases (Nox/Duox), the only enzyme family whose sole known purpose is the regulated generation of ROS, are downregulated or inactivated in genetic variants. NADPH oxidases have been associated with pathologically elevated ROS mainly by linking gene/protein expression profiles with ROS levels and oxidative modifications, but inferring a causal relationship of increased ROS with disease has been more challenging. The only potential gain-of-function variants of a gene directly required for terminal NADPH oxidase activation are certain CYBA (p22phox) polymorphisms that may increase Nox1-4 activity and confer an elevated risk for cardiovascular disease [1–4]. On the other hand, loss-of-function variants in genes required for formation and catalytic activity of active Nox/Duox complexes are increasingly recognized as risk factors, or as origin of inherited or spontaneous genetic diseases that are characterized by reduced or abolished ROS production. The NADPH oxidase family comprises seven members (Nox1-5, Duox1-2) in humans, all of which assemble as multimeric complexes regulated by protein–protein interactions and by the small GTPase Rac (Nox1-3), requiring phosphorylation, calcium flux or lipid binding to generate or H2O2 by catalyzing the transfer of electrons from NADPH to molecular oxygen. Their largely tissue-specific expression profiles correlate well with specific genetic diseases linked to Nox/Duox deficiency. Here, disorders associated with gene variants in NADPH oxidases including chronic granulomatous disease, inflammatory bowel disease and congenital hypothyroidism will be discussed in the context of functional consequences initiated by structural changes due to missense variants.
2. Chronic granulomatous disease – the new faces of the disease
2.1. Background
Chronic granulomatous disease (CGD) is a rare inherited immunodeficiency syndrome (frequency 1/200,000 to 1/250,000) characterized by mutations in one of the genes encoding the components of the Nox2 NADPH oxidase complex in phagocytic cells. In most patients, diagnosis occurs early in childhood due to recurrent and life-threatening infections with bacterial and fungal pathogens (mainly catalase-positive bacteria, e.g. Staphylococcus aureus, Burkholderia and Nocardia species, and fungi e.g. Aspergillus and Candida species). These infections cannot be contained due to deficient generation of superoxide by a functionally impaired or structurally labile (and often absent) NADPH oxidase in innate immune cells, as pathogens cannot be killed even when phagocytosed efficiently [5,6].
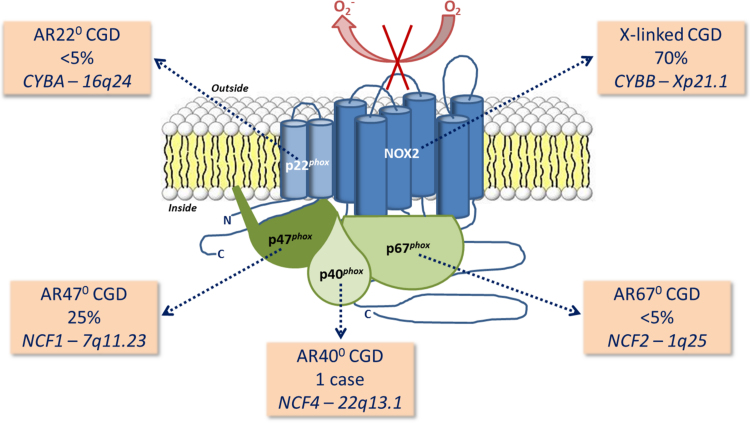
Understanding the composition of the multimeric phagocyte Nox2 oxidase was greatly aided by studies on neutrophils collected from CGD patients [7–9]. CGD is a genetically heterogeneous disease with all ethnic groups equally affected. The molecular basis of CGD is characterized by two types of transmission and four main genetic forms. The major genetic form of CGD is X-linked CGD caused by mutations in the CYBB gene (OMIM number 306400) encoding gp91phox (renamed Nox2) (Fig. 1). X-CGD represents about 70% of the total cases reported to date [10]. The other forms of CGD are autosomal recessive (AR), characterized by mutations in CYBA (OMIM number 233690), NCF1 (OMIM number 233700) and NCF2 (OMIM number 233710) encoding p22phox, p47phox and p67phox respectively [11]. Whereas AR-CGD220 and AR-CGD670 are extremely rare (less than 5% of cases), AR-CGD470 occurs with high frequency (about 25% of CGD cases) due to the presence of two NCF1 pseudogenes carrying the main mutation. Up to now only one case of AR-CGD in NCF4, encoding p40phox, was described [12]. Nox2 oxidase activity additionally requires activation of the small GTP-binding protein Rac, which was discovered concomitantly by Knaus et al. [13] and Abo et al. [14] in neutrophils. The importance of Rac2 was underlined by a case of severe immunodeficiency diverging from classical CGD in a 5-week-old child that was traced back to a dominant negative mutation in RAC2 [15,16]. For CYBB, CYBA, NCF1 and NCF2 many variants harboring deletions, frame shifts, missense, nonsense and splice site mutations have been identified and are accessible at the immunodeficiency (ID) bases (http://structure.bmc.lu.se/idbase/).
Fig. 1.
Molecular basis of chronic granulomatous disease. CGD is caused by alterations in CYBB, CYBA, NCF1, NCF2 or NCF4 encoding Nox2, p22phox, p47phox, p67phox and p40phox respectively. The main genetic form is X-linked CGD representing about 70% of total cases. Three autosomal recessive CGD forms, AR-CGD470, AR-CGD670, and AR-CGD220, represent the rest of the cases described, the AR-CGD470 being the most frequent form (25% of cases). Only one NCF4 variant has been described up to now.
2.2. Are absence of ROS and hyperinflammation paradoxical in CGD?
The link between absent or decreased ROS production in CGD and defective killing mechanisms including autophagy is well established, but in contrast to the prevailing notion of ROS initiating or exacerbating tissue damage, hyperinflammation is often documented in CGD patients. Initially, decreased degradation of phagocytosed material in the absence of ROS production was considered the cause of the observed proinflammatory phenotype. Thereby, phagocytosed microorganisms could accumulate in NADPH oxidase deficient phagocytes leading to persistent cell activation. In addition, ROS can induce neutrophil apoptosis of inflammatory cells limiting inflammation. Efferocytosis, the uptake of apoptotic cells, conducted by macrophages through phosphatidyl serine receptors, is also reduced in CGD [17]. The overall consequence will be unbalanced neutrophil necrosis, an increase of proteases and toxic oxygen-derived components, as well as release of proinflammatory cytokines, all contributing to local inflammation. CGD macrophages are also severely compromised in their ability to produce anti-inflammatory mediators due to a delay in apoptotic debris clearance [18].
Another factor that may explain hyperinflammation in tissues of CGD patients are alterations in intracellular signaling. ROS are essential for regulating signaling pathways and in particular the absence of ROS in CGD phagocytes favors proinflammatory responses [19,20]. Thus, ROS act as anti-inflammatory mediators that control gene expression, for example via NF-kB activation, thereby limiting the development of inflammatory disorders [21]. In addition, expression of certain innate immune receptors such as Toll-like receptor 5 that recognizes bacterial flagellin, or complement receptor, are reduced in CGD neutrophils [22]. However, how this decrease of immune receptors at the cell surface contributes to inflammatory manifestations in CGD patients remains unexplained.
The role of indolamine 2,3-dioxygenase (IDO) in CGD hyperinflammation is not yet resolved. IDO, mainly expressed in dendritic cells and monocytes, converts l-tryptophan into l-kynurenine, which acts as an anti-inflammatory agent by a poorly understood mechanism. l-kynurenine can induce cell death in pro-inflammatory Th17 and γδT cells, leading to immune tolerance in several autoimmune disorders such as chronic inflammatory bowel disease, rheumatoid arthritis, maternal tolerance or tolerance against malignant tumors [23–25]. IDO was also crucial for survival of CGD mice challenged with Aspergillus, suggesting that IDO activity depends on superoxide production [26]. However, several experimental findings in humans did not support these animal studies. First, human CGD macrophages exposed to IFN-γ or LPS degraded tryptophan like healthy donor cells, suggesting that superoxide is not essential for IDO activity [27]. IFN-γ induced normal levels of l-kynurenine in cultured monocytes, neutrophils and dendritic cells purified from CGD patients [28]. In addition, levels of l-kynurenine and other tryptophan metabolites were normal to elevated in CGD patients. While the kynurenine pathway is well-studied in mammals, bacteria such as Pseudomonas aeruginosa (commonly present in CGD patients) also use this pathway, which produces quinolone signaling molecules as virulence factors [29–31]. Recent studies show that IDO (KynA in bacteria) in P. aeruginosa is responsible for the production of kynurenines when in contact with phagocytes [32]. The production of kynurenines circumvents the innate immune response by scavenging released neutrophil superoxide, thereby promoting bacterial survival. Thus, KynA might be an interesting target to combat infections with P. aeruginosa. However, this observation suggests that l-kynurenine restores the immune tolerance in immune cells, while favoring bacterial virulence by its ROS scavenging ability. The exact role of IDO in hyperinflammation and immune tolerance still remains an open question.
Hyperinflammation in CGD can also be linked to defective autophagy, the major intracellular degradation process [33,34]. TLR activation, which is sometimes compromised in CGD, connects phagocytosis to the autophagy pathway in macrophages [35]. Upon TLR or Fcγ receptor stimulation Nox2-mediated ROS production seems to play a role in regulating the activation and recruitment of the autophagy machinery to phagosomes [36]. However, uric acid crystal-induced NLRP3 inflammasome activation in CGD monocytes generated a 4-fold increase in IL-1β secretion, indicating that IL-1β production is not dependent on Nox2 activity [37]. Rather, ROS seem to dampen inflammasome activation, possibly explaining the granuloma formation and hyperinflammation occurring in CGD patients. The link between autophagy and inflammasome activation in CGD has recently been further clarified [38]. ROS deficiency in CGD phagocytes caused autophagy dysfunction, which contributed to increased production of proinflammatory IL-1β. Two CGD patients treated with Anakinra®, an IL-1 receptor blocker, showed rapid and sustained improvement in colitis and this drug restored defective autophagy in CGD mice and human CGD phagocytic cells. These results open up the possibility of clinical trials in order to study the efficacy of IL-1 antagonists to ameliorate CGD granulomas or Crohn's-like disease (see Section 3, Inflammatory Bowel Disease).
2.3. Pathologies associated to CGD
Components of the phagocytic Nox2 complex are expressed in cells and tissues other than phagocytes, and thus inactivating mutations or deletions in these proteins (Nox2, p22phox, p47phox and p67phox) have pathophysiological consequences unrelated to immunodeficiency syndromes. Consequences of polymorphisms in CYBA, NCF1 or NCF2 will not be discussed here in detail, but are to some extent featured later in the context of inflammatory bowel disease. Many pathological effects of Nox2 complex deficiency were demonstrated in CYBB or NCF1 knockout mice, but we will mainly report results obtained in clinical studies involving CGD patients.
2.3.1. Nox2 deficiency and vascular diseases
The expression of several NADPH oxidases including Nox1, Nox2, Nox4 and Nox5 have been described in the vasculature, including the smooth muscle layer, the endothelium and the adventitia [39,40]. Superoxide is involved in the modulation of the arterial tone via rapid degradation of nitric oxide (NO), a well-known vasodilator. Data obtained in studies with Nox1 and Nox2 knockout mice suggest that these oxidases control vascular function via modulation of NO bioactivity [41,42]. In 2009, a multicenter study conducted with 25 X-CGD patients and 25 healthy subjects linked Nox2 deficiency with enhanced arterial dilatation [43]. Platelet Nox2 expression, urinary isoprostanes and oxLDL (both markers of oxidative stress) were reduced in X-CGD patients compared with healthy subjects. However, nitrite and nitrate levels were significantly higher in X-CGD patients and correlated with flow-mediated arterial dilation (FMD). FDM also correlated inversely with platelet Nox2 expression and isoprostanes. This was the first time that a ROS-generating pathway controlled by Nox2 was connected to arterial tone modulation in humans.
More than 10 years ago, Krotz et al. demonstrated that NADPH oxidase dependent formation enhanced platelet aggregation and platelet-dependent thrombosis in vitro [44]. Afterwards Violi et al. reported that soluble sCD40 L- and P (sP)-selectin, two markers of in vivo platelet activation, were reduced in X-CGD patients. Platelet isoprostane was downregulated, while platelet NO generation was enhanced. In addition, platelet Nox2 expression was directly associated with plasma levels of sCD40L and sP-selectin according to correlation analysis. Thus, this study provides the first evidence that in vivo platelet activation might be directly associated with Nox2 activity. Platelet Nox2 appears to be a novel target for anti-thrombotic treatment [45].
2.3.2. Nox2deficiency and brain diseases
Superoxide generation is required for hippocampal synaptic plasticity, especially long-term potentiation (LTP) and hippocampus-dependent memory [46]. Although the mitochondrial respiratory chain is considered the main ROS source in the brain, Nox2-deficient mice demonstrated impaired memory and synaptic deficit, pointing to a role of NADPH oxidase in these processes [47,48]. However, clinical studies in children with CGD were rather ambiguous. In the first study the cognitive function in a cohort of 23 CGD patients, most of them suffering from X-CGD, was assessed [49]. A 23% prevalence rate of cognitive deficits (IQ<70) was found in this selected CGD population, which is higher than the 1–3% low IQ prevalence in the general population. However, it is not clear whether this decrease in cognitive function reflects the sequelae of recurrent infections or the defect in superoxide generation. The second study compared the average cognitive ability of CGD patients with patients who had received a hematopoietic stem cell transplant to cure CGD [50]. Children with CGD (22 X-CGD), either treated conservatively with antimicrobial prophylaxis or curatively with hematopoietic stem cell transplantation, had normal IQ scores and cognitive ability was in the normal range. However, for both studies the low number of subjects impacted statistical significance. Furthermore, the applied WAS-I test may not be able to detect subtle cognitive deficits. These findings deserve further prospective studies with a larger cohort of X-CGD patients. In particular, it will be necessary to pay attention to the precise description of infections and their after effects, and to the adequate cognitive function test employed.
Expression of NADPH oxidase components was analyzed in brain tissues derived from human autopsies [51]. Particularly, Nox2 was overexpressed in microglia and infiltrating macrophages in patient tissues with initial multiple sclerosis lesions. This suggests that an inflammation-associated oxidative burst could play an important role in demyelination and tissue injury in multiple sclerosis and degenerative diseases. Indeed, the expression of Nox2 in microglia was inducible, and involved in motor neuron degeneration in a mouse model of amyotrophic lateral sclerosis [52,53]. However, it is not yet known if CGD patients with Nox2 deficiency are less prone to develop degenerative brain diseases than the general population.
2.3.3. p47phox deficiency, diabetes, renal and cardiovascular diseases
A recent clinical study conducted with 229 CGD patients at the National Institute of Health revealed that diabetes, renal and cardiovascular diseases occur more often and with greater severity in p47phox-deficient CGD patients than in Nox2-deficient CGD (or X-CGD) patients [54]. Six of 64 AR470-CGD patients developed Type 1 diabetes (~10% of cases) in contrast to none of the 165 X-CGD patients. Among the six diabetes patients, two patients presented with severe cardiovascular (CV) disease (coronary artery disease, myocardial infarction, cerebral aneurysms), which could be a consequence of diabetes. One AR470-CGD patient presented with pulmonary hypertension and mitral/aortic regurgitation without underlying diabetes. Despite comparable treatment with antifungal/nephrotoxic drugs AR470-CGD patients developed chronic kidney disease more frequently than X-CGD patients. In general, the residual superoxide production is higher in AR470 patients compared to those with X-CGD, which contributes to their improved survival [5,55]. However, the increased incidence of non-immune related disease may indicate distinct features of the AR470-CGD phenotype, likely beyond the generation of superoxide alone.
2.4. Rare variants in X-CGD are useful to decipher Nox2 structure and function
2.4.1. Functional domains of Nox2
As the Nox2 crystal structure has not yet been resolved, homology modeling offers the best tool for understanding structure–function relationships. The N-terminal half of the protein appears to be embedded in the plasma membrane and is structured into six α-helices, two cytosolic loops (named B and D), and three external loops (A, C, and E), and contains two non-identical hemes coordinated by four histidine residues located in the third and fifth transmembrane helices. Nox2 is glycosylated on asparagine residues in the C and E loops (Fig. 2). The B and D intracytosolic loops are essential for oxidase assembly and electron transfer in Nox2 [56–58]. The D loop might also participate in folding and interaction with p22phox (a 2 TM or 4 TM domain protein), as was recently shown for Nox4 [59]. The C-terminal half of Nox2 constitutes a cytosolic region required for catalysis and regulation of NADPH oxidase activity. The first three-dimensional homology model of the Nox2 C-terminal domain was based on sequence homology of this region with members of the FNR family [60]. This model provided an extremely useful structural image and indicated the presence of FAD and NADPH binding sites, thus the current terminology “dehydrogenase domain”. In 2000, a crystal structure of the NADPH binding domain of Nox2 was released in the Protein Data Bank (PDB 3A1F) by Sumimoto and colleagues. This unpublished structure confirmed homology of this domain with the FNR family.
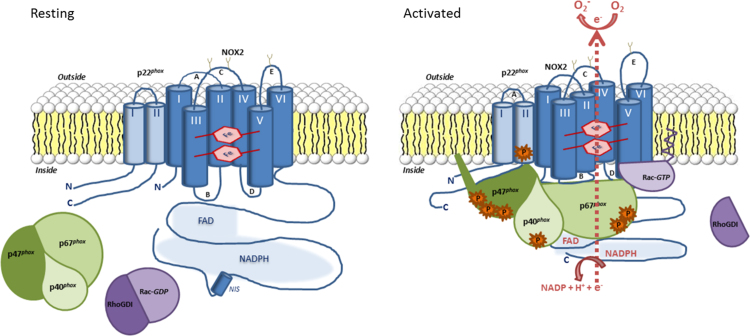
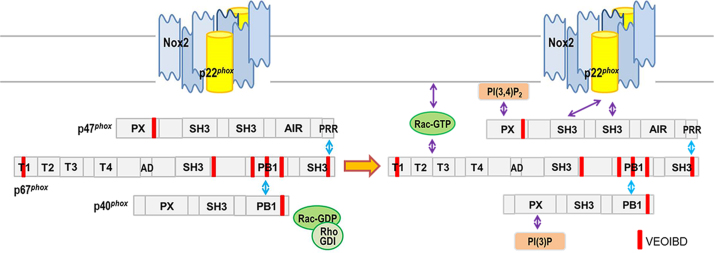
Fig. 2.
Molecular mechanisms of NADPH oxidase complex activation. The NADPH oxidase complex of phagocytic cells is dissociated in resting phagocytes. Cytochome b558 composed of Nox2 and p22phox is localized in the plasma membrane and the cytosolic factors p47phox, p67phox and p40phox form a complex in the cytoplasm. The small GTP-binding protein Rac associates with Rho-GDI in its inactive GDP form. Upon activation, signaling events cause phosphorylations and conformational changes of the NADPH oxidase subunits leading to their assembly. Activated Rac-GTP translocates, anchors in the membrane and binds to the NADPH oxidase complex. The fully assembled NADPH oxidase complex is able to trigger electron transfer from NADPH to FAD and hemes to reduce molecular oxygen into superoxide.
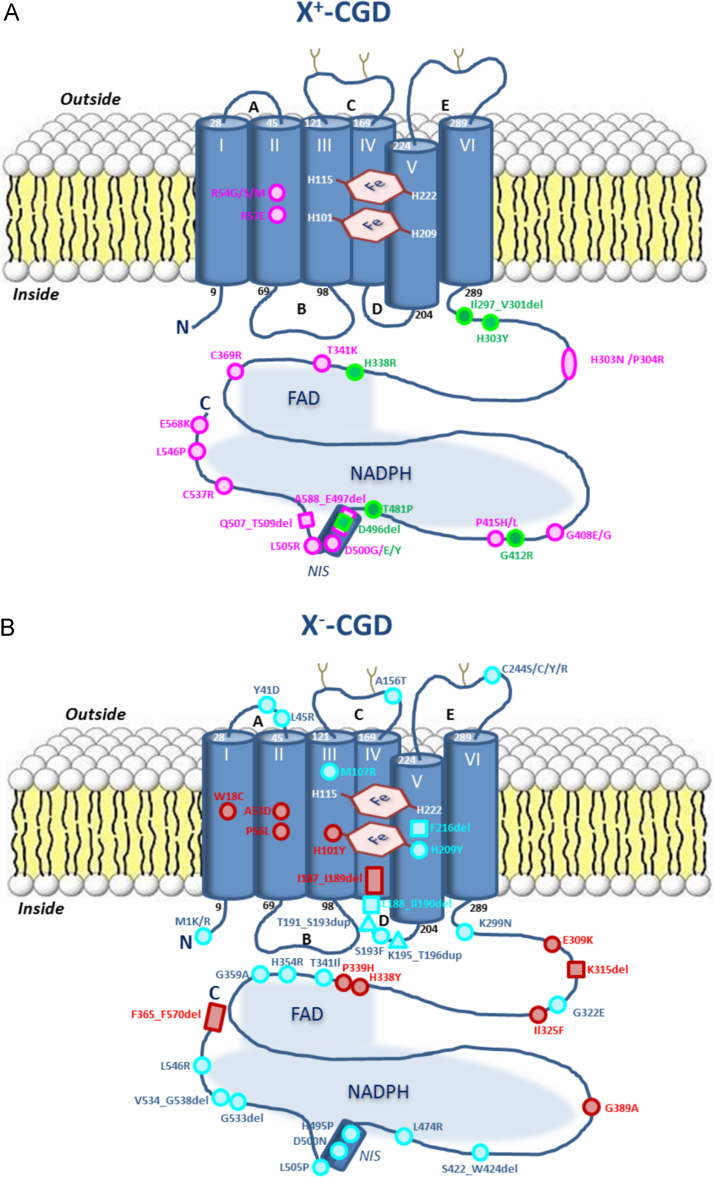
One approach for deciphering structure–function relationships in Nox2 is studying naturally occurring CYBB mutations causing X+-CGD. In this case mutated Nox2 is expressed normally, but is not catalytically active in neutrophils. This feature can be used to obtain insights into the importance of certain regions, but due to the scarcity of patient material, model systems are usually employed for detailed studies. In the CYBB KO PLB-985 cell model NADPH oxidase activity can be restored by transferring wild type Nox2 cDNA, while introduction of mutant Nox2 cDNA will mimic the phenotype of the patient's neutrophils [61,62]. To date about 26 X+-CGD mutations have been reported [10]. Most of them are missense mutations or small deletions, and are primarily located in the C-terminal cytosolic tail of Nox2, confirming the importance of this region in catalytic activity, but not in structural stability. Eighteen X+-CGD mutations were reproduced in the PLB-985 cell line by mutagenesis, stable transfection and clonal selection for functional studies [57,63–67] (Fig. 3A).
Fig. 3.
Localization of X+- and X−-CGD mutations in Nox2. The N-terminal part of the Nox2 protein is embedded in the plasma membrane and is structured into six α-helices, two cytosolic loops (B, D), and three external loops (A, C, E), and contains two non-identical hemes coordinated by four histidine (H) residues located in the third and fifth transmembrane helices. Nox2 is glycosylated on asparagines in C and E loop (ϒ). In the cytosolic “dehydrogenase domain” of Nox2, the FAD/NADPH-binding domains are illustrated as gray clouds. Variants causing X+-CGD are preferentially located in the C-terminal part of Nox2 (A). Green and pink circles and squares correspond to X+-CGD missense variants [10]. Pink circles are variants studied in the PLB-985 cell model [57,62,63,65–67,247]. Blue and red circles, squares and triangles correspond to X−-CGD missense variants, deletions or duplications respectively [10]. Red circles are additional variants studied in the PLB-985 cell model [67,78]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Nox2 contains an insertion of twenty amino acids in the dehydrogenase domain, which is absent in FNR family members (designated NOX-NIS for NOX-NADPH-domain Insertion Sequence, amino acid residues 484-504 in Nox2). As this insertion appears to be ideally positioned at the start of the NADPH binding site, Taylor et al. proposed that the insertion, modeled as an α-helix, may control access of NADPH to the binding site [60] (Fig. 3A). This would explain the need for an activation step in Nox enzymes in contrast to FNR-type enzymes, which are constitutively active. Three X+-CGD mutations-L505R, Q507_T509del and D500G-within this region were reproduced in the PLB-985 cell model to decipher the role of the NOX2-NIS [57,65,66]. Asp500 was essential for proper assembly of the Nox2 complex to facilitate the electron transfer from NADPH to FAD upon oxidase activation. The kinetic parameters of purified Nox2 L505R demonstrated that Leu505 affects the p67phox-dependent activation of the Nox2-p22phox heterodimer, thus controlling access of NADPH to its binding site. The short Q507_T509del Nox2 deletion partially inhibited the assembly of the oxidase and electron transfer from NADPH to FAD (diaphorase or iodonitrotetrazolium (INT) reductase) similar to the Nox2 L505R mutation. In addition, Nox2 was shown to be phosphorylated by protein kinase C in human neutrophils, thereby enhancing catalytic activity and assembly of the complex [68]. Recent results from our laboratory indicate that the insertion NOX-NIS is likely a dynamic switch for Nox2 activation by functionally linking to the Nox2 phosphorylation sites. This data highlights a new step in the Nox2 activation mechanism involving the NIS sequence [66].
Analyzing the X+-CGD mutations T341K, P415H, P415L and C537R in the PLB-985 cell model confirmed the location of the NADPH and FAD binding sites in the dehydrogenase domain of Nox2 [66] (Fig. 3A). Indeed, T341 is strictly conserved in Nox homologs and belongs to the putative FAD binding motif 338HPFT341. The Nox2 residues P415 and C537 are located in the 410GIGVTP415 and 535FLCGPE540 sequences respectively, which were proposed to be part of the active site pocket of the dehydrogenase domain with the first motif being the binding site of the NADPH isoalloxazine ring and the second motif forming the NADPH nicotinamide ring binding site. Indeed, the mutation of Thr341 to Lys inhibited the FAD incorporation into Nox2, whereas the Nox2 mutations P415H/L and C537R had no effect. All three mutations disturbed the diaphorase activity, but not the assembly of the NADPH oxidase complex. Thus, these mutations led to steric hindrance probably incompatible with the correct orientation of NADPH in respect to FAD within the active site. Another set of X+-CGD mutants, Nox2 C369R, G408E, G408R, and E568K, had common and global functional effects when expressed in the PLB-985 cell model. These mutants inhibited NADPH oxidase activity by affecting FAD incorporation and translocation of p47phox and p67phox to phagosomal membranes [66]. Nox2 Gly408 and Glu568 residues are probably located in sequences necessary to maintain the integrity of the FAD/NADPH binding domains. Due to its surface exposed location, Cys369 seems not to be critical for the overall structural integrity of Nox2, but it might be a docking site for cytosolic factors influencing modulation of the FAD environment during the oxidase activation process.
The second transmembrane passage of Nox2 is essential to maintain structural stability and electron transfer of the NADPH oxidase complex [67]. The preponderance of X-CGD mutations in a “hot spot” of five residues from Ala53 to Cys59, located in the putative second transmembrane α-helix, denotes the importance of this membrane region. Nox2 P56L and C59F mutations drastically reduced Nox2 expression, indicating that these residues are important for the structural stability of Nox2. The Nox2 A53D, R54G, R54M, and R54S mutations inhibited superoxide production, but did not affect spectral properties of the oxidized/reduced Nox2-p22phox complex, oxidase complex assembly, FAD binding, or diaphorase activity, suggesting that amino acids 53 and 54 are essential for electron transfer from FAD. In addition, the second transmembrane passage (and especially the Nox2 A57E mutation) negatively influenced the function of the first intracytosolic B-loop in terms of regulating diaphorase activity of Nox2. Indeed, Jackson et al. demonstrated that the B-loops of Nox2 and Nox4 provide an interface between the dehydrogenase domains and the transmembrane domains of Nox enzymes [69].
2.4.2. Structural domains of Nox2
The synthesis of the membrane-bound oxidase heterodimer (cytochrome b558) is a complex process, because it involves not only the presence of both subunits, Nox2 and p22phox, but also several maturation steps, including heme incorporation, heterodimer formation and glycosylation of Nox2 [70–72]. The association of Nox1-4 with p22phox seems to be a prerequisite for stabilization of the complex and localization of the heterodimer to specific membrane compartments [73,74]. The exact interaction surfaces and binding regions of Nox enzymes with p22phox are not yet known, although several residues and motifs have clearly functional relevance [75–77]. For Nox2, deciphering the molecular mechanism of cytochrome b558 synthesis was aided by focusing on decisive regions in CYBB-CGD disease variants termed X minus (X−). X−-CGD mutants are characterized by a partial defect of cytochrome b558 synthesis associated with no or diminished oxidase activity. The genetic defects in CYBB found in these variants (~36 different mutations) are often missense mutations, small deletions or insertions localized in the coding region (Fig. 3B) [10]. The clinical severity of these CGD variants is variable and likely correlates with the residual oxidase activity found in X−-CGD neutrophils derived from these patients. Ten X−-CGD mutations were reproduced in the KO PLB-985 cells in order to study their impact on different steps of the cytochrome b558 synthesis process [78]. These mutations were chosen in mutational hot-spot regions such as the D-loop, a cytosolic region close to the last transmembrane passage, the FAD-binding site, the extreme N-terminal region of Nox2 and isolated residues including W18C and G389A (Fig. 3B). Many of these residues are highly conserved in Nox1-4, suggesting a common and important role, possibly the interaction with p22phox. One group of mutations (Nox2 H338Y, P339H and F556-F570del) in the FAD-binding pocket of Nox2 led to loss of NADPH oxidase activity and was associated with variable levels of Nox2 expression, suggesting that the FAD domains are not only essential for electron transfer, but are also involved in the structural integrity of Nox2. Surprisingly, Nox2 H338Y and P339H mutants showed an abnormal accumulation of Nox2 in intracellular compartments, suggesting that these mutations disturbed Nox2 targeting or proper degradation. In another group of mutations (Nox2 W18C, E309K, K315del, I325F), decreased expression levels led to a proportional decrease in ROS generation. Thus, residues located in the first transmembrane passage, or in an intermediate region between the membrane domain and the dehydrogenase domain of Nox2, are involved in the structural stability and synthesis of cytochrome b558. For the Nox2 W18C, E309K and I325F mutants, an intracellular accumulation of the 65 kDa precursor of Nox2 was observed. Furthermore, a defect in dimer formation with p22phox, and thus in the final maturation of cytochrome b558, occurred. These results suggest that the first transmembrane passage and the intermediate region between the membrane and the cytosolic dehydrogenase domains of Nox2 are involved in association with p22phox.
In conclusion, functional studies of human defects, as demonstrated here for selected X-CGD variants, can identify essential sequences required for structural stability and/or functionality of Nox2. This approach establishes an example that can be used for the study of various clinical cases at the molecular level, which in turn may aid in predicting clinical outcome or treatment options.
2.5. Refined analysis of recent NCF1 and NCF2 variants
2.5.1. NCF1 and NCF1 pseudogenes
In contrast to the considerable heterogeneity found in most CGD variants, a common variant has been identified in approximately 90% of affected NCF1 alleles analyzed worldwide [11]. This variant is a GT deletion (ΔGT) in a GTGT tandem repeat, corresponding to the first four bases of exon 2 in NCF1 [79]. However, a few other alterations are present in the NCF1 gene [80]. AR470-CGD presents as a mild clinical form with higher cumulative survival and is usually diagnosed later than X-CGD or AR220-CGD [5,55]. Phagocytic AR470-CGD cells exhibit higher residual ROS generation underscoring the point that p47phox contributes to NADPH oxidase activity as an adapter, facilitating assembly of the oxidase, but is not as essential as properly formed cytochrome b558. Supporting this notion is the fact that the extremely rare AR670-CGD is associated with severe disease, as NCF2 (p67phox) is essential for optimal functioning of the Nox2 oxidase [81,82].
The NCF1 ΔGT mutation predominates as most normal individuals (>95%) have two NCF1 pseudogenes (ΦNCF1) on each allele. Both pseudogenes, located close to NCF1 at 7q11.23, exhibit the ΔGT deletion with more than 99% identity with the NCF1 gene. These ΦNCF1 are the best-conserved, unprocessed pseudogenes known [83]. The predominance of the ΔGT mutation arises from recombination events between NCF1 and the highly homologous pseudogenes ΦNCF1 [84,85]. At least three different cross-over points exist within the NCF1 gene cluster, indicating that autosomal AR470-CGD is genetically heterogeneous [86]. The biological relevance of the presence of ΦNCF1 is not yet clear. The ratio of NCF1/ΦNCF1 varies in different human tissues and in human populations [87]. An increased copy number of NCF1 was linked to protection for developing rheumatoid arthritis [88]. Extremely rare cases of CGD can be associated with Williams–Beuren syndrome (WBS) [89,90]. This neurodevelopmental disorder with multi-systemic manifestations is caused by a heterozygous segmental deletion at chromosomal band 7q11.23. This deletion can include the NCF1 gene and if an NCF1 mutation occurs simultaneously on the other allele by inheritance, the patient will present with both diseases. It has been postulated that WBS patients with two functional NCF1 genes were more susceptible to hypertension than WBS patients with only one functional NCF1 gene [91]. However, one of the two patients suffered from hypertension, indicating that factors other than vascular NADPH oxidase activity are likely involved in the development of hypertension [92]. A possible explanation is inclusion of the ELN locus in the classical WBS deletion, which codes for elastin, a protein essential for elasticity of the vasculature.
2.5.2. NCF2 gene
Most of the time gene variants lead to the absence of the mutated protein due to instability of the mRNA or synthesis of an unfolded and/or truncated protein. Very rare variants can abolish or reduce NADPH oxidase activity, but lead to normal (superscript +) or diminished (superscript −) expression of oxidase components in phagocytes [10,93]. Recently, an A67−-CGD patient with extremely late diagnosis of CGD was described. CGD was diagnosed in two brothers in their 50s, who harbored a splice variant in NCF2, generating several splice products of exons 11 and 12 [94]. Even though exons 11 and 12 of NCF2 seem to be necessary for optimal oxidase activity, the encoded p67phox protein remained partially expressed and had functional properties. The deletion of exons 11 and 12 does not affect any known functional domains, but is responsible for shortening the distance between the first SH3 domain (a putative Nox2 interaction domain) and the PB1 domain, a binding motif for p40phox.
One case of hypomorphic mutation in the activation domain of p67phox (12 amino acids, 199–210) was found in three CGD patients from two distinct families with mild clinical profiles [95]. The A202V change in p67phox led to a slight decrease of p67phox expression in neutrophils of CGD patients. Surprisingly, NADPH oxidase activity and translocation of p67phox to the phagosomal membrane varied depending on the stimulus. Upon PMA stimulation, but not with opsonized zymosan, ROS generation and p67phox translocation were reduced. This suggests that the conformation of the activation domain of p67phox is involved directly or indirectly in the binding to Nox2, thus affecting NADPH oxidase activity. The discrepancy between both stimuli might be linked to differential phosphorylation events of the activation domain of p67phox in response to each stimulus, but this remains to be resolved experimentally.
2.6. Induced pluripotent stem cells – potent cellular models for pathophysiological studies and therapeutic development
At the moment, the only cellular model mimicking CGD phagocytes is the KO PLB-985 cell line [62]. These cells can be differentiated into neutrophil-like cells, reproducing the X-CGD phenotype, and are very useful for structure–function relationship studies as described above. However, differentiated PLB-985 cells are still an imperfect model as they are lacking certain important neutrophil features. Induced pluripotent stem cells (iPSCs) represent a revolution in the field of stem cell research, permitting the establishment of cellular models of pathologies. A cocktail of four transcription factors is able to reprogram murine and human somatic cells to a pluripotent state similar to embryonic stem cells (ESCs) [96,97]. iPSCs can be differentiated into many cell types, their use is not hampered by ethical issues, and they are useful cell models for physiological studies, toxicity screening and cell therapy among others. Most importantly, iPSCs derived from somatic cells of patients, can be differentiated to produce cellular models of the disease that are very useful for drug development, and in the future for regenerative medicine after correction of the genetic defect [98,99].
Since 2011, four teams have modeled several genetic forms of CGD from iPSCs (Table 1). The first CGD cellular model was obtained from mouse fibroblasts isolated from X-CGD mice and reprogrammed into X-CGD iPSCs [100]. The hematopoietic differentiation involved the formation of embryoid bodies (EBs), culture on collagen IV to isolate FLK1+ cells, followed by co-culture on OP9 stromal cells in the presence of hematopoietic cytokines. Finally, X-CGD neutrophils obtained as CFU-G (colony forming unit-granulocytes) colonies in 24–31 days with a purity of around 71% were unable to produce ROS after PMA stimulation. Gene correction of the EBs with self-inactivating (SIN) lentiviral vectors encoding a codon-optimized gp91phox transgene successfully led to the generation of CFU-G containing neutrophils with restored NADPH oxidase activity [101]. This group demonstrated for the first time that X-CGD iPSC-derived neutrophils were a suitable tool to test new gene therapy approaches. Of importance, no difference in the differentiation process between control and X-CGD iPSC cells in the process of generating neutrophils was observed, suggesting that Nox2-derived ROS generation is not required. In the same year, a human model of X-CGD neutrophils was published [102,103]. The iPSCs were generated from mesenchymal stem cells isolated from an X-CGD patient and differentiation into neutrophils was obtained using a protocol developed for ESCs involving EB formation and OP9 co-culture [104]. Although purity was lower (30–40%) and the timing longer (34 days) than in - than in the previousthe previous work, neutrophils from X-CGD iPSCs were characterized for morphology, surface markers, phagocytosis and ROS production, confirming the successful generation of mature neutrophils mimicking the CGD neutrophils of the patient. Correction of the CGD phenotype was accomplished by genome editing using a zinc finger nuclease (ZFN) approach developed in 2009 with iPSCs [102,105]. Contrary to Mukherjee et al. who transduced EBs, iPSCs were directly transduced with ZFN, followed by selection of transduced iPS clones with one single allele AAVS1 locus insertion. This resulted in restored NADPH oxidase activity and a high level of Nox2 expression. In 2012, Jiang et al. produced the first X-CGD and AR47 human macrophages [106]. They used a co-culture-free protocol involving EBs formation and culture in suspension with hematopoietic cytokines. Monocytes emerged in the supernatant, which upon differentiation into mature macrophages were able to phagocytose zymosan particles and to secrete cytokines in response to stimulation. Thus, X-CGD and AR470-CGD macrophages could be modeled from iPSCs.
Table 1.
List of reported murine and human CGD iPSCs.
| Type | Cell model | Genetic form | Modeled variant | Ref | |
|---|---|---|---|---|---|
| Mukherjee et al. | Mouse | Neutrophils | X-CGD | CYBB knockout | [100] |
| Zou et al. | Human | Neutrophils | X0-CGD | CYBB: nonsense mutation: 458T>G in exon 5 | [102] |
| Jiang et al. | Human | Macrophages | X−-CGD | CYBB: point mutation in intron 1 (−11T>G) | [106] |
| X0-CGD | CYBB: large deletion including exon 1–3 | ||||
| AR470-CGD | NCF1: GT deletion in exon 2 | ||||
| Brault et al. | Human | Neutrophils and macrophages | X0-CGD | CYBB: nonsense mutation 469C>T in exon 5 | [107] |
| AR220-CGD | CYBA: deletion c.295_301delGTGCCCG in exon 5 | ||||
| AR470-CGD | NCF1: GT deletion in exon 2 |
Recently, our group optimized protocols to differentiate iPSCs from X0-, AR470- and AR220-CGD patients' fibroblasts into neutrophils and macrophages [107]. Unlike other protocols described for CGD modeling, iPSCs were directly differentiated using adapted and optimized protocols from Choi et al. for the production of neutrophils and macrophages separately [108,109]. The average production of CD34+ progenitors was 1.5×106 cells after 10 days of differentiation of 10×106 iPSCs. Extensive characterization of CGD iPSC-derived neutrophils confirmed the presence of primary, secondary and tertiary granules in the cytoplasm (Fig. 4A, B). Cells were terminally differentiated into about 3×105 neutrophils or 3×107 macrophages in 25–28 days. CGD neutrophils and macrophages exhibited an oxidase-negative phenotype characterized by the absence of NADPH oxidase activity related to the absence of Nox2 and p22phox expression in X0-CGD and AR220-CGD cells (Fig. 4C). CGD iPSC-derived macrophages were able to phagocytose opsonized S. aureus or zymosan particles and produced pro- and anti-inflammatory cytokines after stimulation. iPSC-derived macrophages expressed classical CD14, CD45, and CD11b antigen surface markers and were HLA-DR−, CCR7−, and MR+, specific to the M2c subtype of macrophages, which are regulatory macrophages involved in immunosuppression and wound healing/tissue repair [107].
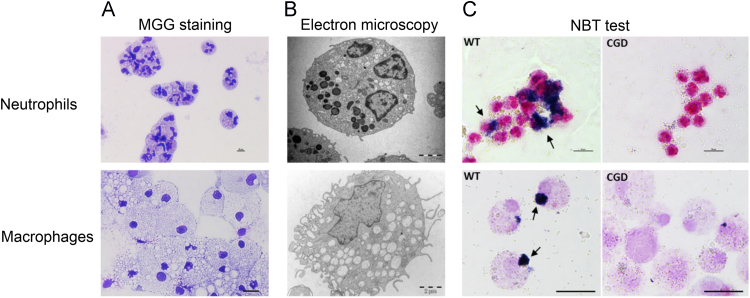
Fig. 4.
Phenotypic and functional characterization of neutrophils and macrophages derived from WT and CGD iPSCs. (A) MGG staining showing the characteristic morphology of neutrophils (upper panel, scale bar 10 µm) and macrophages (lower panel, scale bar 20 µm). (B) Electron microscopy shows the presence of cytoplasmic granules in neutrophils and vacuoles in macrophages (scale bar 2 µm). (C) NBT reduction assay on opsonized latex bead-activated WT or CGD iPSC-derived neutrophils (upper panel, scale bar 10 µm) and macrophages (lower panel, scale bar 20 µm). ROS-mediated NBT reduction is shown as blue formazan precipitates in WT neutrophils and macrophages (black arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Faithful cellular models of the three genetic forms of CGD (X0, AR220 and AR470) are now available for the development of new therapies for CGD, although an iPSC model for p67phox-deficient neutrophils and macrophages is still lacking. Today, scientific efforts are focusing on gene therapy to correct CGD using iPSCs or EBs. This will represent a source of “healthy” autologous cells for one-time treatment. However, safe iPSC reprogramming methods including xeno-free, feeder-free cell cultures, and flawlessly mastered gene therapy will be necessary to correct iPSCs derived from CGD patients before transplantation. “Safe made” autologous iPSCs are used already to treat age-related macular degeneration with iPSC-derived retinal pigment epithelium cells at the RIKEN Center for Developmental Biology in Kobe, Japan. This is the first human clinical trial for evaluating the efficiency of this approach.
3. Inflammatory bowel disease - reduced ROS as risk factor
Inflammatory bowel disease (IBD) is the common term for a group of chronic or recurring inflammatory conditions of the gut [110,111]. The main IBD disorders are Crohn's disease (CD), characterized by inflammatory patches and deep ulcers all along the digestive tract, and ulcerative colitis (UC), which affects the mucosal lining of the entire colon. The symptoms can be very similar, rangingfrom abdominal pain, diarrhea, and intestinal bleeding to weight loss, colon cancer and extra-intestinal manifestations. IBD is emerging as a global disease as incidence and prevalence are increasing with time, and the gap of affected populations in terms of economic, ethnic and racial differences is shrinking (www.cdc.gov). The annual incidence and overall prevalence is highest in Europe and North America [112], and in particular the incidence of pediatric IBD is rapidly increasing [113,114]. Very early onset (VEO)IBD, classified as disease in children <6 years of age according to the modified Paris classification [115], presents predominantly as pancolitis and occasionally perianal disease, with patients responding poorly to conventional anti-inflammatory and immunomodulatory therapy, resulting in increased morbidity and mortality [116]. VEOIBD patients often have an underlying rare genetic disorder causing primary immunodeficiency that cannot be detected in genome-wide association studies. Exome-targeted, candidate gene or whole-genome sequencing has identified several rare gain-of-function and loss-of-function variants in VEOIBD [116]. Considering the link between the intestinal immune response and the microbiota, IBD-associated shifts in the microbiome and virome, and the emergence of pro-inflammatory pathobionts in IBD [117,118], it is not surprising that dysfunction of NADPH oxidases is now a recognized risk factor for IBD.
3.1. Genetic susceptibility to IBD–CGD and beyond
The 35–40% prevalence of IBD with CD-like features in CGD patients places impairment of the Nox2 complex prominently as a risk factor for intestinal disease. Histologically this disease can be distinguished from CD by the presence of multiple granulomas in the lamina propria and large pigment-laden histiocytes [119–121]. The susceptibility for developing CGD-CD is associated with inactivating CYBB variants rather than with NCF1 variants or with the remaining ROS output in stimulated neutrophils [55,119]. What exactly determines the manifestation of gastrointestinal disease in CGD patients at some point in their lives is still unresolved. It is likely due to particular genetic CYBB variants in combination with other susceptibility loci and the presence of hereditary and environmental factors.
In contrast to the often polygenic nature of adult IBD, VEOIBD is considered a monogenic defect [116]. Several functionally altered variants in Nox2 complex components have been identified in VEOIBD [122–124]. Most of the variants are characterized by heterozygous single nucleotide polymorphisms (SNPs) in the coding sequence, occur in autosomal recessive genes coding for cytosolic Nox2 complex components and do not lead to severe immunodeficiency, indicating that the remaining ROS generation is above the threshold required for full onset of CGD [55]. While certain variants were only present in a single patient, some SNPs in NCF1 and NCF2 seemed common (up to 10%) in the analyzed VEOIBD cohort. Although only a single CYBB variant (rs141756032; NOX2 p.G364R) was identified [124], this missense mutation will likely cause a significant reduction in ROS production in male carriers due to its location directly adjacent to the second FAD binding site. Functional data of this variant are not available and this CYBB variant has not been associated with CGD (http://structure.bmc.lu.se/idbase/CYBBbase). An identified SNP in the CYBA promoter (rs72550704) is part of the Sp1 transcription factor consensus sequence [2], thus interfering with Sp1 binding and leading to a 20% reduction in p22phox expression.
The majority of recently identified NADPH oxidase-related variants occur in genes involved in Nox2 assembly and electron transfer (Fig. 5). A previously characterized NCF1 SNP (rs13447; p47phox R90H) [88] identified in 12/122 VEOIBD patients, is located in the phospholipid (PX) binding domain, leading to reduced ROS generation presumably due to altered membrane association. Five missense NCF2 mutations with varying prevalence were identified in a Canadian VEOIBD cohort [124]. These mutations are located either in the first tetratricopeptide repeat (T1) motif (rs147415774, p67phox R38Q), in the Phox and Bem1 (PB1) domain (rs17849502, p67phox H389Q, rs35012521, p67phox N419I), in the C-terminal SH3 binding domain (p67phox G501R) or in exon 15. These domains are required for protein interactions, namely for Rac-GTP binding (T1-4), for Vav1 guanine nucleotide exchange factor (GEF) and p40phox binding, and for p47phox binding, respectively [125]. Protein associations are required for retaining the p47phox–p67phox–p40phox complex in the cytosol in the dormant oxidase, and for reassembly of these components in their phosphorylated form with membrane-bound Nox2-p22phox and the GEF–Rac–GTP module when oxidase activation takes place. Functional analysis of NCF2 variants revealed decreased protein–protein associations when binding partners were immunoprecipitated after their overexpression in model cell lines. It would be important to determine the influence of p67phox sequence changes on Nox2 catalytic activity in a fully reconstituted system and in the presence of an appropriate receptor-mediated stimulus. Furthermore, one VEOIBD patient was identified with a NCF4 variant (rs141160114, p40phox R308Q), which negatively influenced p40phox PB1 binding to p67phox. A common SNP in NCF4 (rs4821544, intron 1) has been previously associated with ileal adult CD in genome-wide association studies (GWAS) [126,127]. Neutrophils isolated from CD patients carrying this NCF4 risk allele produced less superoxide when primed with granulocyte-macrophage colony-stimulating factor (GM-CSF) prior to stimulation with the chemoattractant fMLF [128]. This defect suggests that the NCF4 SNP alters regulation of Erk1/2-mediated p47phox phosphorylation [129]. NCF4 as a CD susceptibility gene could not be replicated in two other large scale studies [130,131]. Albeit the number of identified variants in VEOIBD associated with the Nox2 complex is still limited, a trend towards affecting mainly cytosolic components combined with reduced, but not substantial loss of ROS production is noticeable.
Fig. 5.
Missense mutations in cytosolic Nox2 complex components associated with VEOIBD without leading to CGD. NADPH oxidase activation triggers multiple phosphorylations and structural rearrangements, exposing domains masked by intramolecular interactions and permitting novel interactions required for assembly of the oxidase complex. Constitutive interactions are indicated by blue arrows, stimulus-induced interactions by purple arrows. Location of variants indicated in red. For domain explanation see text except: AIR (autoinhibitory region), PRR (proline-rich region), AD (activation domain). Adapted from [125]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Several NADPH oxidases (Nox1-3) require Rac activity for ROS generation, and thus variants in RAC1 (rs35761891, rs10951982) or RAC2 (rs739041, rs1476002) will likely influence oxidase function [122,124]. The observed changes in expression, i.e. upregulated Rac1 versus downregulated Rac2, may reflect involvement of Rac1 in oxidase regulation of cell types other than neutrophils, although alteration of Rac expression will affect many other signaling outputs, cytoskeletal rearrangements and mucosal barrier maintenance. Interestingly, several SNPs in NOS2 (inducible nitric oxide synthetase, iNOS) were identified in VEOIBD cohorts [132]. The most prominent NOS2 SNP (rs2297518, iNOS S608L) showed increased nitric oxide production in transfected cells. This is important in the context of NADPH oxidase function as nitrosylation of oxidase components inhibits Nox2-dependent ROS generation ([133], Hayes and Knaus, unpublished observations), once more reinforcing the link between genetic susceptibility to VEOIBD and decreased NADPH oxidase activity.
3.2. Mucosal NADPH oxidases as novel IBD susceptibility genes – NOX1 and DUOX2
Intestinal epithelial cells (IECs) are on the forefront of maintaining barrier function and mucosal homeostasis, and genetic alterations compromising their response to the gut environment can be considered the gateway for inflammatory processes. IECs express the NADPH oxidases Nox1 and Duox2, with the Duox2/DuoxA2 complex being highly responsive to microbial-induced upregulation. Expression of both Duox2 and DuoxA2 is upregulated by viral and bacterial infections and during inflammatory gut disease [118,134–136]. Grasberger and colleagues [137] used l-thyroxine supplemented duoxa−/− mice infected with Helicobacter felis to characterize the role of Duox2 in stomach infections. Mucosal colonization with H. felis was increased in duoxa−/− mice. This increased bacterial load resulted in enhanced shedding of bacterial antigens and virulence factors, leading to severe gastritis in duoxa−/− mice. These results suggest that the release of H2O2 by Duox2 at the apical surface of the gastric epithelium controls growth of H. felis in its niche, the overlying mucus layer [137].
In general, ROS generation by Nox1 can be considered the earliest engagement of an NADPH oxidase in the GI tract, followed by Duox2 upregulation and Nox2 activation in recruited neutrophils. If rare variants in ROS-generating enzymes associate with IBD development, one needs to consider NOX1 and DUOX2 as potential susceptibility genes. Indeed, we recently identified the first functionally altered NOX1 and DUOX2 variants in VEOIBD patients [138]. Two variants of X-linked NOX1 were identified in VEOIBD patients (Fig. 6). A patient with severe pancolitis harbored NOX1 p.P330S, a missense variant located directly upstream of the first FAD binding domain, which reduced ROS generation by 50–60%, likely by decreasing binding affinity for FAD. The second variant, NOX1 p.D360N (rs34688635), was discovered in two patients, both presenting with severe pancolitis. This sequence change occurred directly in the second FAD binding domain, leading to a 60–80% decrease in ROS production in a model cell line and in in vivo reconstituted crypts of Nox1 knockout mice. The localization of both Nox1 variants was not altered, but as suggested by our previous work [139], antimicrobial host defense was severely impaired. The same study linked two DUOX2 variants to VEOIBD. DUOX2 p.R1211C, a missense variant in the third intracellular loop (Nox D-loop) was present in one patient with recurrent pancolitis, perforation and colectomy. Reconstitution of the Duox2 R1211C-DuoxA2 complex in cells revealed intact localization patterns, but reduced H2O2 generation. Another patient with pancolitis harbored DUOX2 p.R1492C (rs374410986), an arginine to cysteine change in the highly conserved GRP sequence in the third NADPH binding domain. Despite proper localization and stimulus-dependent translocation, this variant showed a 5-fold decrease in H2O2 release and consequently a 50% increase in bacterial invasion. This study links for the first time NOX1 variants to human disease, and identifies the NADPH oxidases Nox1 and Duox2 as risk factors for IBD. Even though these variants are rare, the importance of Nox1 and Duox2 in mucosal host defense will likely reveal additional NOX1 and DUOX2 variants in the future, or even variants in genes required for assembly of functional oxidase complexes (CYBA, NOXA1, NOXO1, DUOXA2).
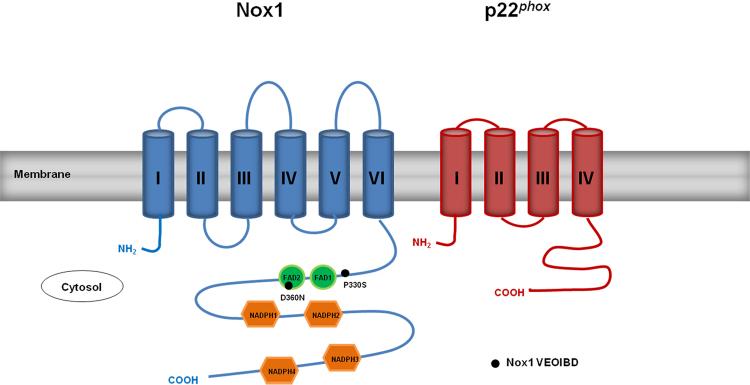
Fig. 6.
NOX1 variants identified as VEOIBD risk factor. Two functionally altered NOX1 variants were recently identified in VEOIBD patients, one located upstream of the first FAD binding domain, while the other is located within the second FAD domain (indicated by black dots). p22phox is represented as a 4 transmembrane (TM) domain protein, although models with 2 or 3 TM exist.
3.3. Challenges in NADPH oxidase IBD research – from man to mice
Understanding how NADPH oxidases contribute to the development of IBD is hampered by the scarcity of animal models reflecting human gut disease and the often weak correlation between phenotypes of genetically modified mice and IBD patients. The polygenic nature of the disease, the contribution of the microbial gut community of the host to development of pathogenesis, and diet/environment-related influences are likely responsible and need to be methodically altered to obtain better suited models. While mice deficient in cybb (Nox2) and ncf1 (p47phox) recapitulate many hallmarks of human CGD, they never develop CGD-CD. These mice were reported as either protected or more susceptible to dextran sulfate sodium (DSS)-induced colitis [140–142], while showing increased weight loss and injury in acute 2,4,6-trinitrobenzene sulfonic acid (TNBS)-mediated necrosis of the distal colon [38,143]. Deficiency in ncf4 (p40phox), an integral component of the Nox2 complex, was associated with exacerbated DSS colitis, which does not reflect the Nox2 knockout phenotype in the same model [140,144]. Similarly, the susceptibility to VEOIBD in male patients harboring the two inactivating X-linked NOX1 variants, which exhibited a 60–80% reduction of ROS generation in transduced crypts [138], seems not to be reflected in DSS or TNBS colitis models, where a protective phenotype for Nox1 deficiency was observed [145,146]. Only when combining Nox1 knockout with IL-10 deficiency in mice, a permanent colitis phenotype could be obtained [146]. For Duox2 conflicting data indicate higher susceptibility of patients with functionally inactivating DUOX2 variants for VEOIBD [138], while analysis of the general population of IBD patients indicated substantial upregulation of DUOX2 [118,147,148]. While Duox2-deficiency early in life predisposes to VEOIBD, presumably due to compromised mucosal host defense, the upregulation in adult IBD may correlate with the involvement of Duox2 in repair mechanisms or oxidative stress. This paradox and other inconsistencies will need to be addressed withdetailed studies in mice by varying the genetic background and environmental impact, and by introducing other susceptibility genes. The rather intimate connections between NADPH oxidase genes and prominent IBD susceptibility genes regulating immune recognition or autophagy are well documented and supported by patient data and cell-based assays, suggesting that modulation of the redox balance is indeed an important determinant in inflammatory intestinal disease, albeit predominantly not involving exacerbation of inflammation by oxidative stress, but by a decline in ROS production.
3.4. NADPH oxidase variants as risk factor for other diseases
Loss of or diminished Nox2 NADPH oxidase function is not only connected to the disorders presented here. A predisposition of CGD patients for developing mycobacterial infections has been recognized for some time [149–151], but recently, susceptibility to this disease in otherwise healthy subjects has been reported [152]. Two CYBB variants (Nox2 T178P, Nox2 Q231P) were linked to impaired macrophage NADPH oxidase function and mycobacterial disease, while ROS generation in neutrophils and monocytes was not affected. This observation reflects not only the critical role of ROS in the macrophage-mounted defense against Mycobacterium tuberculosis, but also how the context of a particular cell type can shape the (non)-functionality of gene variants. Furthermore, some CYBA variants in the promoter region (e.g. A-675T, A-930G) have been associated with hypertension in population-based or animal studies [1,3,153], although increased oxidative stress by p22phox overexpression has not been functionally characterized or attributed to a particular Nox family member.
The development of rheumatoid arthritis has been linked to a lower copy number of NCF1 [88]. This association has been replicated in p47phox mutant rodents [154–156] and has culminated in the successful use of oxidative burst stimulants for arthritis therapy [157]. A strong association of several NCF2 SNPs with systemic lupus erythematosus (SLE) susceptibility was reported [158–160]. One of these variants (rs17849502, p67phox H389Q) also conferred susceptibility to VEOIBD, as discussed earlier [123,158]. Similar to p67phox H389Q, some of the other lupus-associated NCF2 variants (rs13306575, p67phox R395W; rs35937854, p67phox A297V) are located in protein–protein interaction domains and may weaken association of oxidase components [125,159]. Functional consequences of these p67phox mutations have not been verified in model cell systems or in blood-derived cell types. A protective role for functional Nox2 in SLE was strengthened when cybb-deficient mice crossed with lupus-prone mice displayed exacerbated disease [161].
4. Hypothyroidism – spotlight on DUOX2/DUOXA2
Congenital hypothyroidism (CH), caused by thyroid hormone deficiency present at birth, is the most common congenital endocrine disorder. CH occurs in approximately 1/3000–4000 births [162] and can lead to delayed growth and neurodevelopmental disorders if untreated. With the ease and relative low cost of screening, most cases of congenital hypothyroidism are caught early and treated accordingly. The majority of CH cases result from defects in the development of the thyroid gland [163] with only 15–20% of cases being caused by thyroid dyshormonogenesis. The production of H2O2 by the NADPH oxidase Duox2 at the apical membrane of thyroid follicular cells is crucial for the organification of iodide, the rate-limiting step in thyroid hormone synthesis, which is catalyzed by thyroid peroxidase (TPO) [164]. Iodide is transported from the extracellular space to the follicular lumen in two stages: active transport across the basolateral plasma membrane and passive transport across the apical plasma membrane. Iodide is easily absorbed from the blood by thyrocytes, which contain a Na+/I− symporter on their plasma membrane that acts as an iodine trap and transports iodide into the cell. Once iodide is inside the cell, it is transported with thyroglobulin into the follicular lumen and oxidized at the apical membrane by Duox2-TPO. Pendrin, encoded by the PDS gene, was initially proposed as the potential iodide transporter responsible for transporting iodide across the apical plasma membrane [165,166]. However, a recent study by Twyffels et al. suggested that Anoctamin-1, and not pendrin, is responsible for the export of iodide into the follicular lumen [167].
Many loss-of-function variants have been identified in several of the genes implicated in thyroid dyshormonogenesis – thyroglobulin (TG) [168,169], thyroperoxidase (TPO) [170–172], the sodium-iodide symporter/NIS (SLC5A5) [173–175], pendrin (SLC26A4) [176,177], iodotyrosine deiodinase (IYD) [178,179], DUOX2 [180–196] and DUOXA2 [197–200]. All of these genes possess clearly characterized roles in the thyroid hormone synthesis pathway [201] with hypothyroidism due to mutations in the Duox2/DuoxA2 heterodimer being the most common form of thyroid dyshormonogenesis with an estimated prevalence of 1/44000 [189]. The importance of Duox2 in thyroid hormone synthesis grows ever more apparent with the identification of new DUOX2/DUOXA2 variants and their link to congenital hypothyroidism (CH), most of which ratherdisplay a partial iodide organification defect (PIOD) than a total iodide organification defect (TIOD).
4.1. Duox biochemistry and regulation
The NADPH oxidases Duox1 and Duox2 were originally identified in thyroid tissue resulting in their initial characterization as the thyroid oxidases, ThOX1 and ThOX2 [164,202]. However, expression of Duox1 and Duox2 proteins is not restricted to the thyroid. Since their initial identification, they have also been found on the mucosal surfaces of the trachea and bronchi [203], in airway epithelial cells [204,205] and in the colon [206]. Furthermore, recent studies reported that differential upregulation of Duox1 vs Duox2 occurs in selected tissue types or tumors [207,208]. The human Duox1 and Duox2 proteins are 1551 and 1548 amino acids in length, respectively, and share 83% sequence homology. In the thyroid, TPO oxidizes iodide in the presence of H2O2 generated by Duox2. Co-immunoprecipitation experiments revealed that Duox and TPO are located in close proximity on the plasma membrane, and that their association is upregulated by the protein kinase C (PKC) pathway and downregulated by protein kinase A (PKA) [209]. Duox activity seems to be regulated by the local H2O2 levels, stimulating Duox activity at low concentrations [210], while inhibiting it at high concentrations [211]. Low intracellular levels of H2O2 may support physiological functions such as signal transduction, but an excess may result in mutagenesis, carcinogenesis or apoptosis [212].
The original term dual oxidases for Duox enzymes transpired from an additional N-terminal domain that is homologous to peroxidases including myeloperoxidase (MPO), thyroid peroxidase (TPO) and lactoperoxidase (LPO) in addition to a Nox2 homology domain at the C-terminus [213].TheN-terminal domain shares approximately 20% sequence homology with MPO and as such has been labeled a peroxidase (PO)-like domain. Linking this extracellular PO-like domain and the Nox domain prototype conserved in all NADPH oxidases is an additional transmembrane domain and a cytosolic linker harboring two EF-hand motifs involved in calcium binding, reminiscent of those found in Nox5 [213]. Although peroxidases are heme-containing proteins, the PO-like domains of Duox1 and Duox2 lack the conserved histidine residues, which are present in all other peroxidases [214,215]. This suggests that the release of H2O2 observed for Duox proteins is associated with distinct intermolecular features of the Duox/DuoxA complex. Structurally, the PO-like domain sets Duox apart from the other members of the NADPH oxidase family, and this unique region could be directly involved in the conversion of superoxide to H2O2, even though it lacks classical peroxidase activity [216]. Expression and purification of thehuman and Caenorhabditis elegans Duox1 peroxidase domains (hDuox1 and CeDuox1, respectively) revealed that heme was associated with CeDuox1, but not with hDuox1 [216]. Both CeDuox1 and hDuox1 were examined for peroxidase and superoxide dismutase (SOD) activity. In comparison to LPO, hDuox1 exhibited no peroxidase activity, while only modest activity was observed for CeDuox1. The ability of Duox1 to act as SOD was also ruled out, as binding of metals required for SOD activity such as copper, zinc and manganese [217,218], was absent, and hDuox1 and CeDuox1 PO-like domains failed to react with superoxide [216]. Conversely, others reported that IFN-γ treatment of human tracheobronchial epithelial cells expressing Duox2 induced peroxidase activity that was inhibitable by sodium azide, suggesting heme peroxidase activity [219]. Despite their high level of sequence homology Duox1 and Duox2 are regulated by different phosphorylation pathways: Duox1 is activated by protein kinase A (Gs-PKA pathway), while Duox2 activation occurs through protein kinase C (Gq-phospholipase C (PLC) pathway) [220]. The transcriptional regulation of Duox1 and Duox2 by inflammatory cytokines in the lung epithelium suggests distinct roles for Duox1 and Duox2 proteins in host defense [205]. The Th-2 cytokines IL-4 and IL-13 increased Duox1 expression by up to 5-fold, while Duox2 was induced up to 25-fold, either with IFN-γ treatment (a Th-1 cytokine) or with the viral mimic polyinosine-polycytidylic acid (poly (I:C)), indicating a role for Duox2 in viral clearance, which was confirmed in several subsequent studies [136,205,221,222].
Both Duox1 and Duox2 are highly glycosylated with five putative N-glycosylation sites in the PO-like domains of both proteins (N94, N342, N354, N461 and N534 in Duox1; N100, N348, N382, N455 and N537 in Duox2) [223], and it appears that glycosylation is important for transport to the plasma membrane and cell surface expression of the active protein [223,224]. In a recent study by Wang et al. [193], a mutation in one of these glycosylation sites, N100D, was identified in a young Chinese patient. However, the patient was too young to assess the clinical outcome and type of CH. Deglycosylation experiments revealed two N-glycosylation states of both Duox1 and Duox2, corresponding to 190 and 180 kDa proteins [224]. Treatment of canine thyrocytes and Duox1/2-expressing Cos7 cells with N-glycosidase F or endoglycosidase H resulted in a drop in molecular mass to 160 kDa. Only the 190 kDa form of the protein was resistant to treatment with endoglycosidase H, indicating that this form is completely processed and capable of cell surface expression [224]. Early studies showed that many model cell lines when transfected with Duox cDNA were incapable of expressing active Duox at the plasma membrane [224], pointing to the presence of an unidentified component required for Duox maturation. Using data mining, Grasberger and Refetoff [225] identified two genes, DUOXA1 and DUOXA2, required for Duox maturation and escape from the endoplasmic reticulum (ER).
4.2. DUOX2 variants in hypothyroidism
To date, over 40 Duox2 mutations have been described that directly affect thyroid dyshormonogenesis, resulting in transient to severe congenital hypothyroidism (Fig. 7, Table 2). In an initial study by Moreno et al. [180], four DUOX2 variants were identified in a cohort of CH patients. One patient with severe and permanent CH carried a homozygous inactivating mutation in Duox2 R434X, while the other patients were heterozygous for inactivating mutations (Duox2 Q686X, R701X and S965fsX994) and showed a less severe, transient form of CH. It was concluded that biallelic inactivating mutations in Duox2 abolish the functional protein leading to the absence of proper thyroid hormone synthesis and permanent congenital hypothyroidism, while monoallelic inactivation of Duox2 result in the milder transient form of CH [180]. This initial hypothesis on biallelic vs monoallelic mutations and their outcome has since been challenged with the identification of patients with transient CH harboring compound heterozygous mutations in Duox2 [185,190]. It is conceivable that the transient or late-onset nature of CH in these patients is either due to the nature of the DUOX2 variant present, or to the pairing of a null mutation with a partially functional mutation, thereby alleviating the defect as in the Duox2 E327X/H678R mutant [190], or to patients harboring additional, gain-of-function variants or genetic modifiers that alter ROS generation. The VEOIBD patients harboring inactivating heterozygous DUOX2 variants showed normal thyroid function at diagnosis. One needs also to take into consideration that the entire Duox promoter region on chromosome 15q15 is prone to epigenetic modifications [226], which alter Duox/DuoxA expression levels.
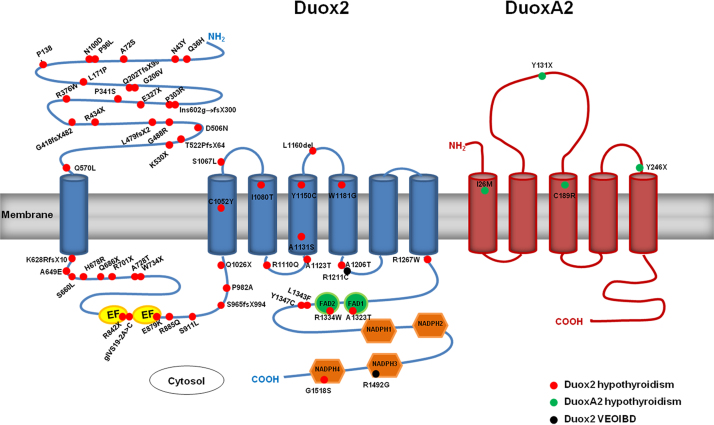
Fig. 7.
DUOX2 variants associated with hypothyroidism or VEOIBD. Mutations in Duox2 and DuoxA2 are prevalent in congenital hypothyroidism (CH). To date, over 40 DUOX2 variants and 4 DUOXA2 variants have been characterized. Duox2 CH mutations span the entire protein and are depicted as red dots, while DuoxA2 CH mutations are shown in green. Two DUOX2 variants, recently identified as risk factors for VEOIBD, are indicated by black dots. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Summary of Duox2 mutations in congenital hypothyroidism.
| Duox2 mutation | ROS production | Protein expression | Hypothyroidism (permanent/transient) | Literature |
|---|---|---|---|---|
| Q36H | N.D. | N.D. | Persistent | Varela et al. |
| N43Y | Decreased | N.D. | Permanent | Jin et al. |
| A72S | Decreased | N.D. | Permanent | Jin et al. |
| P96L | Decreased | N.D. | Permanent | Jin et al. |
| N100D | N.D. | N.D. | N.D. | Wang et al. |
| P138L | Mildly increased | N.D. | Permanent | Muzza et al. |
| L171P | Unchanged | N.D. | Permanent | Muzza et al. |
| Q202TfsX99 | Decreased | N.D. | Permanent | Jin et al. |
| G206V | Mildly decreased | N.D. | Permanent/transient | Jin et al. |
| P303R | Mildly decreased | N.D. | Permanent | Muzza et al. |
| ins602g→fsX300 | N.D. | N.D. | N.D. | Pfarr et al. |
| E327X | N.D. | N.D. | Transient | Kasahara et al. |
| P341S | Decreased | N.D. | N.D. | Muzza et al. |
| R376W | N.D. | N.D. | Persistent mild | Vigone et al. |
| G418fsX482 | N.D. | N.D. | Persistent | Varela et al. |
| R434X | N.D. | N.D. | Permanent | Moreno et al., Cangul et al. |
| L479fsX2 | N.D. | N.D. | Transient | Maruo et al., Yoshizawa et al. |
| G488R | Decreased | N.D. | Transient | Narumi et al., Jin et al., Yoshizawa et al. |
| D506N | N.D. | N.D. | N.D. | Pfarr et al. |
| T522PfsX64 | N.D. | N.D. | Permanent | Muzza et al. |
| K530X | N.D. | N.D. | Transient | Maruo et al. |
| Q570L | Decreased | N.D. | Permanent | Muzza et al. |
| K628RfsX10 | N.D. | N.D. | Transient | Maruo et al. |
| A649E | N.D. | N.D. | Transient | Maruo et al. |
| S660L | N.D. | N.D. | Permanent | Wang et al. |
| H678R | Mildly decreased | Unchanged | Permanent/transient | Maruo et al., Abe et al., De Marco et al., Narumi et al., Jin et al., Kasahara et al., Muzza et al. |
| Q686X | N.D. | N.D. | Transient | Moreno et al. |
| R701X | Mildly Decreased | Unchanged | Transient | Moreno et al., Muzza et al., De Marco et al. |
| A728T | Decreased | Decreased at cell surface | Transient | De Marco et al. |
| W734X | N.D. | N.D. | Permanent | Wang et al. |
| R842X | N.D. | N.D. | Persistent mild | Vigone et al. |
| gIVS19-2A>C | N.D. | N.D. | Persistent | Varela et al. |
| M866R | Abolished | N.D. | Permanent | Muzza et al. |
| E879K | Decreased | N.D. | Permanent/transient | Jin et al. |
| R885Q | Decreased | N.D. | Permanent/transient | Maruo et al., Jin et al. |
| S911L | Decreased | Decreased et cell surface | Mild persistent | Tonacchera et al. |
| S965fsX994 | Decreased | Decreased at cell surface | Permanent/transient/Subclinical | Moreno et al., Varela et al., De Marco et al. |
| P966SfsX29 | N.D. | N.D. | Muzza et al. | |
| P982A | Unchanged | Unchanged | Permanent/transient | De Marco et al., Muzza et al. |
| C1052Y | Decreased | Decreased at cell surface | Subclinical | Tonacchera et al., Muzza et al. |
| S1067L | Mildly increased | N.D. | Mild persistent/transient | Maruo et al., Muzza et al. |
| R1110Q | Decreased | N.D. | Transient | Ohye et al., Narumi et al., Wang et al., Jin et al. |
| I1080T | Decreased | N.D. | Late-onset/permanent | Narumi et al. |
| A1123T | Decreased | N.D. | Transient | Jin et al. |
| A1131S | N.D. | N.D. | Permanent | Wang et al. |
| Y1150C | Decreased | Decreased at cell surface | Permanent | De Marco et al., Muzza et al. |
| L1160del | Decreased | N.D. | Subclinical | Narumi et al. |
| W1181G | N.D. | N.D. | Transient | Wang et al. |
| A1206T | N.D. | N.D. | Transient | Wang et al. |
| R1267W | N.D. | N.D. | Transient | Wang et al. |
| A1323T | N.D. | N.D. | N.D. | Satoh et al. |
| R1334W | Decreased | N.D. | Normal thyroid at birth | Jin et al. |
| L1343F | N.D. | N.D. | Permanent | Satoh et al. |
| Y1347C | Decreased | Unchanged | Normal thyroid at birth | Abe et al. |
| G1518S | Abolished | Decreased at cell surface | Transient | Hoste et al. |
| E1546G | Decreased | N.D. | Transient | Muzza et al. |
| Permanent |
*N.D.=not determined.
More than 20 Duox2 mutations have been described in the PO-like domain. Several are nonsense mutations – E327X [190], R434X [180,192], K530X [185] – or frameshifts – G418fsX482 [182], L479SfsX2 [185], Q202RfsX93 [194] – which generate a premature stop codon resulting in a truncated Duox2 protein witha partial PO-like domain. Functionally, these mutations were deficient in H2O2 production. The Duox2 R434X mutant was associated with severe permanent CH in patients harboring the variant on both alleles [180,192]. Duox2 K530X was identified in two sisters from a Japanese cohort of patients with transient CH and was present as compound heterozygous Duox2 E876K:S1067L mutant [185]. Elevated TSH levels were recorded at birth, which returned to the normal range following two years of L-T4 supplementation. It is still unresolved why heterozygous Duox2 mutations cause either complete deficiency in H2O2 generation or result in transient CH. One might speculate that the closely related Duox1 enzyme, which is expressed in the thyroid at much lower levels [164], is capable of generating sufficient H2O2 to compensate for inadequate Duox2 activity. The recent development of Duox1 vs Duox2 specific antibodies might shed light on potential compensatory co-localization of Duox1 with TPO in patient tissue [227,228].
In addition to the peroxidase homology domain, a large number of Duox2 mutations have been described in the first cytosolic loop containing the EF-hand motifs. Two particular mutants, Duox2 R842X and E879K, reside within the EF-hand domains and were identified in patients with compound heterozygosity (Duox2 R376W/R842X, Duox2 K530X/E879K), leading to transient CH [181,185]. It is likely that mutations occurring in these domains reduce calcium binding and thus diminish Duox2 activity; however no functional studies were undertaken. De Marco et al. [188] described five Duox2 mutations within this cytosolic region (H678R, R701Q, A728T, S965fsX994 and P982A) in Italian patients with CH or subclinical hypothyroidism (SCH). Functional studies revealed that the Duox2 S965fsX994 and A728T mutants were defective in H2O2 production, while Duox2 H678R, R701Q and P982A were functional in model cell lines upon transfection. The Duox2 H678R mutant, located between the first transmembrane helix and the first EF hand domain, is a common variant found among the Japanese population and appears to occur with high frequency in transient CH [185,189,190,195]. Cell-based studies revealed that Duox2 H678R is a functional SNP retaining approximately 80% of its H2O2-generating capacity [189]. A case of pseudodominant inheritance (the inheritance of an autosomal recessive trait imitating an autosomal dominant pattern) in transient CH was reported in a Japanese family, where the patient and her two siblings presented with the compound heterozygous mutation Duox2 H678R/Y1347C [195]. The mother presented with SCH due to a biallelic Duox2 H678R mutation, while the father was symptom-free, harboring a monoallelic Duox2 Y1347C mutation. The replacement of Tyr1347 with Cys may affect FAD binding in Duox2. While expression levels of Duox2 Y1347C were comparable to wild type protein, this mutant generated 40% less H2O2, suggesting a partial defect in electron transfer [195]. One explanation for the occurrence of pseudodominant inheritance is a high carrier frequency for the autosomal recessive trait. Interestingly, Japanese individuals who are homozygous for Duox2 H678R are not necessarily affected by CH, since the frequency of the genotype (1/820) is higher than the prevalence of CH [189].
Although it is evident that Duox2 mutations play a prominent role in CH, the extent to which other factors (epigenetics, iodide status of the mother during pregnancy) may influence the onset of the disease is still uncertain. Wang et al. [193] reported two patients with the same monoallelic Duox2 mutation, A1206T, located in the last intracellular loop (termed D-loop in Nox). Both were diagnosed with CH after neonatal screening, however, upon treatment with L-T4 and subsequent withdrawal of this treatment, one patient was diagnosed with transient CH, while the other patient was diagnosed with permanent CH. Although TPO and TG mutations were not detected, it cannot be ruled out that a second loss-of-function mutation in the other allele may have occurred [193]. The first adult case of Duox2-associated CH was described by Ohye et al. [184], identifying a novel R1110Q mutation in the second intracellular domain of Duox2 (termed B-loop in Nox). The patient was homozygous for Duox2 R1110Q and, although biallelic mutations are believed to result in total organification defect, the onset of the disease did not occur until the patient was in her 40s and the perchlorate discharge test, performed at age 56, was suggestive of PIOD. Although no functional analysis was performed on Duox2 R1110Q, a previous study by von Lohneysen et al. [74] indicated that ROS production by Nox2 and Nox4 was dependent on the respective Nox B-loops. Thus, it is plausible that any mutation in this region of Duox2 could have a dramatic effect on the H2O2-generating ability of the protein. The Duox2 missense mutant R1334W, located in the second FAD-binding motif of Duox2, was identified in a cohort of Korean families, who presented with CH in conjunction with eutopic thyroid glands [194]. Functionally, this Duox2 mutation generated approximately 50% less H2O2 than wild type Duox2. Recently, a second Duox2 variant affecting the first FAD-binding domain has been identified [196]. This Duox2 A1323T mutation was found in association with a second Duox2 mutation (L1343F) on the same allele, and a heterozygous mutation for TSHR (R450H) on the second allele. This patient developed CH at the age of 20 months and required a dose of L-T4 usually administered to children with permanent CH to keep serum TSH levels within the normal range [196]. It is likely that the combination of Duox2 and TSHR variants contributed to the severity of the phenotype in this particular case.
4.3. DuoxA2 biochemistry and DUOXA2 variants in hypothyroidism
Both DUOX and DUOXA genes are located in a head-to-head orientation on chromosome 15, with each of the DUOXA genes sharing the core promoter region with their respective partner DUOX gene, enabling co-expression of Duox/DuoxA complexes [225]. Co-expression of DuoxA2 with Duox2 was critical for the exit of Duox2 from the ER and allowed surface expression of the protein [225]. Although the DuoxA proteins were initially characterized as ER-resident proteins allowing the transition of Duox from the ER to the Golgi [225], later studies showed that DuoxA1 and DuoxA2 could escape the ER and translocate as heterodimers with their matched Duox partner to various membrane compartments [227,229]. In both studies, Duox1 and Duox2 were co-expressed with DuoxA1, DuoxA2 and several DuoxA1 splice variants to explore their ability to form stable complexes, thereby facilitating targeting to the cell surface and catalytic activity. Of the four DuoxA1 splice variants investigated (DuoxA1-1, DuoxA1-2, DuoxA1-3 and DuoxA1-4), Duox1/DuoxA1-3 formed the preferred heterodimer for optimal Duox1 activity. Both DuoxA2 and DuoxA1-3 were capable of targeting Duox2 to the plasma membrane, albeit to different membrane subdomains. Dependent on the cellular context, H2O2 generation was somewhat more efficient for the Duox2/DuoxA2 complex [227,229]. The mismatched pairs, Duox2/DuoxA1-3 and Duox2/DuoxA1-4 generated low levels of superoxide, suggesting that forced formation of mismatched heterodimers leads to superoxide leakage due to incorrect complex formation [229]. A recent study using truncated and chimeric DuoxA1 and DuoxA2 constructs [230], revealed that in the Duox1/DuoxA1 complex, the C-terminus of DuoxA1 was crucial for H2O2 generation, whereas in Duox2/DuoxA2 the N-terminal domain of DuoxA2 was involved in promoting the catalytic activity of the complex. Therefore, if Duox2 is capable of forming a functional complex with both DuoxA1 and DuoxA2, it seems plausible that Duox2 can be present, for example in the thyroid, in two different active conformations, generating superoxide if it is matched with DuoxA1 and H2O2 when preferentially paired with DuoxA2. Ueyama et al. [231] recently identified the first extracellular loop of Duox proteins as an area that prevents superoxide leakage, with the Duox1 loop being more effective. This superoxide leakage seems to occur when the stabilization and maturation of the Duox/DuoxA complex is disrupted. Additionally, three N-glycosylation sites (N84, N109 and N121), located in the large first extracellular loop between transmembrane domains 2 and 3 have been identified in both DuoxA1 and DuoxA2, but their contribution to complex formation with Duox1/Duox2 is unknown [225].
As heterodimerization is essential for the correct localization and catalytic activity of the Duox2/DuoxA2 complex, it is not surprising that DUOXA2 variants have been identified in CH. So far, four mutations associated with congenital hypothyroidism have been identified in DuoxA2-I26M, Y131X, C189R and Y246X [197,199,200]. The first mutation, DuoxA2 Y246X, was identified in a Chinese patient with CH due to PIOD, leading to a truncated protein lacking the fifth transmembrane region and C-terminal cytoplasmic domain [200]. Cell-based studies suggested that the mutant DuoxA2 Y246X protein may undergo rapid turnover at the site of synthesis, leading to lower steady-state expression compared to the wild type protein. Analysis of DuoxA2 Y246X revealed diminished protein expression and loss of DuoxA2 function upon co-transfection of Duox2 in model cell lines. N-glycosylation of the mutant DuoxA2 protein was not altered, as determined by immunoblot analysis, suggesting that the remaining protein was correctly inserted into the membrane [200]. Although the patient was homozygous for the DuoxA2 Y246X nonsense mutation, a milder form of CH was observed, possibly indicating that DuoxA1 may compensate for DuoxA2. Co-expression of Duox2 with DuoxA1 supported this hypothesis, since Duox2 was shown to be partially rescued by DuoxA1, although superoxide instead of H2O2 was generated [200]. The Y246X mutation is described in 3 out of 4 studies examining DuoxA2 mutations [197,198,200], indicating that this specific variant may occur at a high frequency in Chinese cohorts with congenital hypothyroidism. Liu et al. [198] recently described a novel heterozygous missense DuoxA2 mutation, I26M, in a patient with mild transient CH, where the steady-state expression of the protein was comparable to wild type DuoxA2 despite complete deficiency in H2O2 generation. It is conceivable that replacement of DuoxA2 Ile26 with Met introduces an alternative start site, deleting the N-terminus of DuoxA2, which is important for activity of the Duox2/DuoxA2 complex.
Cysteine residues play a key role in the structure and function of proteins by promoting stability and in some cases by reversible deactivation mechanisms through the formation of disulfide bonds [232]. Mutations of certain Duox2 cysteine residues caused ER retention [232,233]. Similarly, mutating Cys579 in Duox1 and the corresponding Cys582 in Duox2 completely blocked trafficking to the membrane [234]. Examining the role of specific cysteine residues in the PO-like domain revealed an intramolecular disulfide bond between Cys124 in the PO-like domain and Cys1162 in the second extracellular loop of the Nox domain, which was crucial for structure and functionality of Duox2 [235]. The formation of this disulfide bond in the ER appears to be a key step in transporting the Duox2/DuoxA2 complex to the membrane. An additional pair of cysteines, Duox2 Cys568 and Cys582, also located in the PO-like domain, were part of intermolecular disulfide bridges with Cys167 and Cys233 in the extracellular loops of DuoxA2, once again promoting the stability of the overall complex [235]. Interestingly, Cys167 is conserved in both DuoxA1 and DuoxA2, possibly also permitting linkage of DuoxA1 to Duox2 as this complex is functionally active [227,229]. These studies did not address the importance of Cys189, although replacing this conserved cysteine with arginine (DuoxA2 C189R) caused mild CH in a patient [199]. Functional cell-based experiments revealed the absence of protein expression or ROS generation when DuoxA2 C189R was co-transfected with Duox2 in HeLa cells, suggesting that the Duox2 C189R protein is unstable and rapidly degraded, preventing complex formation [199].
Mutation or deletion of Duox2 and/or DuoxA2 in mice mimicked human disease by causing congenital hypothyroidism [236,237]. Grasberger et al. [237] reported that mice deficient in DuoxA1 and DuoxA2 (duoxa−/−) showed severe delays in postnatal development, impaired linear growth of long bones and considerably enlarged thyroid glands. These traits, taken together with decreased T4 and serum T3 levels, and markedly elevated TSH, were indicative of severe hypothyroidism in duoxa−/− mice. Daily L-T4 replacement treatment of these mice completely reversed this phenotype, with bone growth, weight gain, fertility and circulating levels of T3 in duoxa−/− mice indistinguishable from that of wild type littermates. Functionally, deletion of DuoxA in these mice led to a maturation defect of Duox proteins, with mice expressing both Duox proteins in the immature, ER-resident form. As expected, deficiency in H2O2 production in the thyroid tissue of duoxa−/− mice was observed, reiterating the necessity of DuoxA for functional expression of Duox and the role of Duox in thyroidal H2O2 generation [237]. Studies in duox2thyd/thyd mice harboring aDuox2 V674G mutation have provided a genetic model for studying Duox2 function in the thyroid [238]. Mice homozygous for the thyd mutation showed reduced weight (50% less) and thin bones. The thyroid glands of mutant mice were goitrous and contained few normal follicles, with serum T4 levels approximately 10-fold lower than wild type controls and significantly increased TSH levels [238]. Transfection of theDuox2 V674G mutant into a DuoxA2-containing model cell system showed complete loss of catalytic activity [236]. This loss of activity was due to a combination of factors such as decreased protein expression and insufficient targeting to the plasma membrane. However, when Val674 was mutated to either Leu or Thr, ROS production returned to levels comparable to Duox2 wild type, suggesting a need for bulkier aliphatic and/or hydrophobic amino acids at the Duox2 Val674 site [236]. Only Duox2, but not Duox1, is required for thyroid hormone synthesis, as Donko et al. [239] reported normal growth and serum thyroxin levels in Duox1 knockout mice.
Since the first description of DUOX2 variants as genetic cause of congenital hypothyroidism, it has become apparent that additional factors influence the degree of disease progression associated with individual mutations. In some cases, mutations in other genes associated with CH, such as TPO or TSHR, have been identified [189,194]; in others, the iodine status of the mother at the time of birth may have played a role in the nature of CH observed [190]. Similarly, epigenetic factors cannot be excluded in the determination of whether certain Duox2 mutations lead to permanent or transient congenital hypothyroidism in individual cases. Regardless of additional contributing factors, it is apparent that deficiency in ROS production due to the absence or inefficiency of Duox2 catalytic activity, results in the manifestation of congenital hypothyroidism.
5. Discussion
Pathologies causally linked to ROS deficiency due to loss-of-function mutations in genes encoding for various NADPH oxidase complexes demonstrate not only the importance of ROS for specialized functions (microbial killing, thyroid hormone synthesis), but also indicate hyperinflammation as result of reduced or absent ROS generation. This rather unexpected phenotype is connected to ROS due to their role as a key intracellular signaling molecule, controlling redox-regulated pathways and transcription factors, and altering the activity of kinases, phosphatases and GTPases by oxidative modification. Other enzymes capable of generating ROS as a byproduct of their principal functions will cause disease when disabled by mutational events, but variants of these enzymes often produce even more ROS, and directly associating increased ROS levels causally with the observed pathology is challenging. For example, mtDNA mutations in NADH dehydrogenase subunit 6 cause deficiency in the activity of the respiratory complex I (CI), leading to overproduction of ROS. These mutations were connected to an increased metastatic potential of tumor cells in mice [240], although other studies connected CI mutations to modulation of tumor proliferation via stabilization of hypoxia-inducible factor 1α and not by ROS involvement [241].
If disrupting the redox balance is linked to inflammatory disease, one might expect that genetic polymorphisms in antioxidant genes constitute a risk factor. Only very little information is currently available in this regard. For rheumatoid arthritis (RA), common polymorphisms in SOD2, SOD3 or CAT were either not associated with risk or severity of RA [242,243], or showed a lower mean disease activity score [243]. The same CAT promoter region polymorphism (C-262T) was proposed to be a risk factor for ulcerative colitis, although the patient cohort was rather limited [244]. The over 170 SOD1 variants associated with familial amyotrophic lateral sclerosis (ALS) may not lead to disease due to their loss of the enzyme's antioxidant function. Recent studies highlight changes in localization, folding and subsequent aggregation as unfavorable SOD1 alterations reflecting theclinical phenotypes of ALS [245,246]. In contrast to the genetic association of Nox2 and Duox2 oxidases to disease (CGD, hypothyroidism), which is reflected in experimental mouse models, the observed susceptibility to disease due to inherited or private polymorphisms or mutations in redox-associated genes will necessitate interrogating other risk genes or environmental factors, as IBD genetics indicate beyond doubt. Knowledge regarding the interplay between these factors is steadily increasing, and will inform future therapeutic options.
Conflict of interest
None.
Acknowledgments
This work was supported by Science Foundation Ireland (10/IN.1/B2988) (U.G.K.), the University Joseph Fourier, Faculty of Medicine, the Regional Clinical Research Department, DRCI, Grenoble University Hospital, the CGD Research Trust Grant award reference J4G/09/09 and the National Institutes of Health Grant N01-AI-30070 from the Immunodeficiency Network and the Primary Immunodeficiency Disease Consortium (M-J.S.). S.ON. was part of the MolCellBiol Programme, funded under the Programme for Research in Third-Level Institutions and co-funded under the European Regional Development Fund. Several authors of this review were supported by the European Cooperation in Science and Technology (COST Action BM1203/EU-ROS).
References
- 1.Moreno M.U., San Jose G., Fortuno A. A novel CYBA variant, the −675A/T polymorphism, is associated with essential hypertension. J. Hypertens. 2007;25:1620–1626. doi: 10.1097/HJH.0b013e3281ac211d. [DOI] [PubMed] [Google Scholar]
- 2.Moreno M.U., San Jose G., Orbe J. Preliminary characterisation of the promoter of the human p22(phox) gene: identification of a new polymorphism associated with hypertension. FEBS Lett. 2003;542:27–31. doi: 10.1016/s0014-5793(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y.W., Peng J., Liang B.Y. The A930G polymorphism ofP22phox (CYBA) gene but not C242T variation is associated with hypertension: a meta-analysis. PLoS ONE. 2013;8:e82465. doi: 10.1371/journal.pone.0082465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno M.U., Zalba G. CYBA gene variants as biomarkers for coronary artery disease. Drug News Perspect. 2010;23:316–324. doi: 10.1358/dnp.2010.23.5.1437711. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg J.M., van Koppen E., Ahlin A. Chronic granulomatous disease: the European experience. PLoS ONE. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland S.M. Chronic granulomatous disease. Hematol. Oncol. Clin. N. Am. 2013;27:89–99. doi: 10.1016/j.hoc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal A.W., Jones O.T., Webster D. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978;2:446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- 8.Segal A.W., Cross A.R., Garcia R.C. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N. Engl. J. Med. 1983;308:245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- 9.Ohno Y., Buescher E.S., Roberts R. Reevaluation of cytochrome b and flavin adenine dinucleotide in neutrophils from patients with chronic granulomatous disease and description of a family with probable autosomal recessive inheritance of cytochrome b deficiency. Blood. 1986;67:1132–1138. [PubMed] [Google Scholar]
- 10.Roos D., Kuhns D.B., Maddalena A. Hematologically important mutations: X-linked chronic granulomatous disease (third update) Blood Cells Mol. Dis. 2010;45:246–265. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos D., Kuhns D.B., Maddalena A. Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update) Blood Cells Mol. Dis. 2010;44:291–299. doi: 10.1016/j.bcmd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matute J.D., Arias A.A., Wright N.A. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114:3309–3315. doi: 10.1182/blood-2009-07-231498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaus U.G., Heyworth P.G., Evans T. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 14.Abo A., Boyhan A., West I. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J. Biol. Chem. 1992;267:16767–16770. [PubMed] [Google Scholar]
- 15.Ambruso D.R., Knall C., Abell A.N. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. USA. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams D.A., Tao W., Yang F. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- 17.Rieber N., Hector A., Kuijpers T. Current concepts of hyperinflammation in chronic granulomatous disease. Clin. Dev. Immunol. 2012;2012:252460. doi: 10.1155/2012/252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown J.R., Goldblatt D., Buddle J. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD) J. Leukoc. Biol. 2003;73:591–599. doi: 10.1189/jlb.1202599. [DOI] [PubMed] [Google Scholar]
- 19.Bylund J., MacDonald K.L., Brown K.L. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-kappa B. Eur. J. Immunol. 2007;37:1087–1096. doi: 10.1002/eji.200636651. [DOI] [PubMed] [Google Scholar]
- 20.Brown K.L., Bylund J., MacDonald K.L. ROS-deficient monocytes have aberrant gene expression that correlates with inflammatory disorders of chronic granulomatous disease. Clin. Immunol. 2008;129:90–102. doi: 10.1016/j.clim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Bjorkman L., Dahlgren C., Karlsson A. Phagocyte-derived reactive oxygen species as suppressors of inflammatory disease. Arthritis Rheum. 2008;58:2931–2935. doi: 10.1002/art.23941. [DOI] [PubMed] [Google Scholar]
- 22.Hartl D., Lehmann N., Hoffmann F. Dysregulation of innate immune receptors on neutrophils in chronic granulomatous disease. J. Allergy Clin. Immunol. 2008;121(375–382):e9. doi: 10.1016/j.jaci.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 23.Harden J.L., Egilmez N.K. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol. Investig. 2012;41:738–764. doi: 10.3109/08820139.2012.676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson T.S., Munn D.H. Host indoleamine 2,3-dioxygenase: contribution to systemic acquired tumor tolerance. Immunol. Investig. 2012;41:765–797. doi: 10.3109/08820139.2012.689405. [DOI] [PubMed] [Google Scholar]
- 25.Prendergast G.C., Smith C., Thomas S. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani L., Fallarino F., De Luca A. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 27.Jurgens B., Fuchs D., Reichenbach J. Intact indoleamine 2,3-dioxygenase activity in human chronic granulomatous disease. Clin. Immunol. 2010;137:1–4. doi: 10.1016/j.clim.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Ravin S.S., Zarember K.A., Long-Priel D. Tryptophan/kynurenine metabolism in human leukocytes is independent of superoxide and is fully maintained in chronic granulomatous disease. Blood. 2010;116:1755–1760. doi: 10.1182/blood-2009-07-233734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurnasov O., Jablonski L., Polanuyer B. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiol. Lett. 2003;227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- 30.Farrow J.M., 3rd, Pesci E.C. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J. Bacteriol. 2007;189:3425–3433. doi: 10.1128/JB.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chugani S., Greenberg E.P. LuxR homolog-independent gene regulation by acyl-homoserine lactones in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2010;107:10673–10678. doi: 10.1073/pnas.1005909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genestet C., Le Gouellec A., Chaker H. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic. Biol. Med. 2014;73:400–410. doi: 10.1016/j.freeradbiomed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Levine B., Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orvedahl A., Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16:57–69. doi: 10.1038/cdd.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanjuan M.A., Dillon C.P., Tait S.W. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 36.Huang J., Canadien V., Lam G.Y. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Veerdonk F.L., Smeekens S.P., Joosten L.A. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Luca A., Smeekens S.P., Casagrande A. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. USA. 2014;111:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 40.Lassegue B., San Martin A., Griendling K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung O., Schreiber J.G., Geiger H. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 42.Matsuno K., Yamada H., Iwata K. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 43.Violi F., Sanguigni V., Carnevale R. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]
- 44.Krotz F., Sohn H.Y., Gloe T. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- 45.Violi F., Pignatelli P. Platelet NOX, a novel target for anti-thrombotic treatment. Thromb. Haemost. 2014;111:817–823. doi: 10.1160/TH13-10-0818. [DOI] [PubMed] [Google Scholar]
- 46.Nayernia Z., Jaquet V., Krause K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kishida K.T., Hoeffer C.A., Hu D. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol. Cell. Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kishida K.T., Pao M., Holland S.M. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J. Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pao M., Wiggs E.A., Anastacio M.M. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:230–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- 50.Cole T.S., McKendrick F., Cant A.J. Cognitive ability in children with chronic granulomatous disease: a comparison of those managed conservatively with those who have undergone hematopoietic stem cell transplant. Neuropediatrics. 2013;44:230–232. doi: 10.1055/s-0033-1333875. [DOI] [PubMed] [Google Scholar]
- 51.Fischer M.T., Sharma R., Lim J.L. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135:886–899. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu L.J. Microglial voltage-gated proton channel Hv1 in ischemic stroke. Transl. Stroke Res. 2014;5:99–108. doi: 10.1007/s12975-013-0289-7. [DOI] [PubMed] [Google Scholar]
- 53.Wu D.C., Re D.B., Nagai M. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leiding J.W., Marciano B.E., Zerbe C.S. Diabetes, renal and cardiovascular disease in p47 phox−/− chronic granulomatous disease. J. Clin. Immunol. 2013;33:725–730. doi: 10.1007/s10875-013-9871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhns D.B., Alvord W.G., Heller T. Residual NADPH oxidase and survival in chronic granulomatous disease. N. Engl. J. Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biberstine-Kinkade K.J., Yu L., Dinauer M.C. Mutagenesis of an arginine- and lysine-rich domain in the gp91(phox) subunit of the phagocyte NADPH-oxidase flavocytochrome b558. J. Biol. Chem. 1999;274:10451–10457. doi: 10.1074/jbc.274.15.10451. [DOI] [PubMed] [Google Scholar]
- 57.Li X.J., Grunwald D., Mathieu J. Crucial role of two potential cytosolic regions of Nox2, 191TSSTKTIRRS200 and 484DESQANHFAVHHDEEKD500, on NADPH oxidase activation. J. Biol. Chem. 2005;280:14962–14973. doi: 10.1074/jbc.M500226200. [DOI] [PubMed] [Google Scholar]
- 58.Carrichon L., Picciocchi A., Debeurme F. Characterization of superoxide overproduction by the d-Loop(Nox4)-Nox2 cytochrome b(558) in phagocytes – differential sensitivity to calcium and phosphorylation events. Biochim. Biophys. Acta. 2011;1808:78–90. doi: 10.1016/j.bbamem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Lohneysen K., Noack D., Hayes P. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C terminus. J. Biol. Chem. 2012;287:8737–8745. doi: 10.1074/jbc.M111.332494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor W.R., Jones D.T., Segal A.W. A structural model for the nucleotide binding domains of the flavocytochrome b-245 beta-chain. Protein Sci. 1993;2:1675–1685. doi: 10.1002/pro.5560021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tucker K.A., Lilly M.B., Heck L., Jr. Characterization of a new human diploid myeloid leukemia cell line (PLB-985) with granulocytic and monocytic differentiating capacity. Blood. 1987;70:372–378. [PubMed] [Google Scholar]
- 62.Zhen L., King A.A., Xiao Y. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc. Natl. Acad. Sci. USA. 1993;90:9832–9836. doi: 10.1073/pnas.90.21.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu L., Cross A.R., Zhen L. Functional analysis of NADPH oxidase in granulocytic cells expressing a delta488-497 gp91(phox) deletion mutant. Blood. 1999;94:2497–2504. [PubMed] [Google Scholar]
- 64.Bionda C., Li X.J., van Bruggen R. Functional analysis of two-amino acid substitutions in gp91 phox in a patient with X-linked flavocytochrome b558-positive chronic granulomatous disease by means of transgenic PLB-985 cells. Hum. Genet. 2004;115:418–427. doi: 10.1007/s00439-004-1173-z. [DOI] [PubMed] [Google Scholar]
- 65.Li X.J., Fieschi F., Paclet M.H. Leu505 of Nox2 is crucial for optimal p67phox-dependent activation of the flavocytochrome b558 during phagocytic NADPH oxidase assembly. J. Leukoc. Biol. 2007;81:238–249. doi: 10.1189/jlb.0905541. [DOI] [PubMed] [Google Scholar]
- 66.Debeurme F., Picciocchi A., Dagher M.C. Regulation of NADPH oxidase activity in phagocytes: relationship between FAD/NADPH binding and oxidase complex assembly. J Biol Chem. 2010;285:33197–33208. doi: 10.1074/jbc.M110.151555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Picciocchi A., Debeurme F., Beaumel S. Role of putative second transmembrane region of Nox2 protein in the structural stability and electron transfer of the phagocytic NADPH oxidase. J. Biol. Chem. 2011;286:28357–28369. doi: 10.1074/jbc.M111.220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raad H., Paclet M.H., Boussetta T. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson H.M., Kawahara T., Nisimoto Y. Nox4 B-loop creates an interface between the transmembrane and dehydrogenase domains. J. Biol. Chem. 2010;285:10281–10290. doi: 10.1074/jbc.M109.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallach T.M., Segal A.W. Analysis of glycosylation sites on gp91phox, the flavocytochrome of the NADPH oxidase, by site-directed mutagenesis and translation in vitro. Biochem. J. 1997;321(Pt 3):583–585. doi: 10.1042/bj3210583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu L., Zhen L., Dinauer M.C. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J. Biol. Chem. 1997;272:27288–27294. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- 72.Yu L., Quinn M.T., Cross A.R. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. USA. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L., DeLeo F.R., Biberstine-Kinkade K.J. Biosynthesis of flavocytochrome b558. gp91(phox) is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J. Biol. Chem. 1999;274:4364–4369. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- 74.von Lohneysen K., Noack D., Wood M.R. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol. Cell. Biol. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawahara T., Ritsick D., Cheng G. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 76.Zhu Y., Marchal C.C., Casbon A.J. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J. Biol. Chem. 2006;281:30336–30346. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
- 77.von Lohneysen K., Noack D., Jesaitis A.J. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J. Biol. Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beaumel S., Grunwald D., Fieschi F. Identification of NOX2 regions for normal biosynthesis of cytochrome b558 in phagocytes highlighting essential residues for p22phox binding. Biochem. J. 2014;464:425–437. doi: 10.1042/BJ20140555. [DOI] [PubMed] [Google Scholar]
- 79.Noack D., Rae J., Cross A.R. Autosomal recessive chronic granulomatous disease caused by defects in NCF-1, the gene encoding the phagocyte p47-phox: mutations not arising in the NCF-1 pseudogenes. Blood. 2001;97:305–311. doi: 10.1182/blood.v97.1.305. [DOI] [PubMed] [Google Scholar]
- 80.Roos D., de Boer M., Koker M.Y. Chronic granulomatous disease caused by mutations other than the common GT deletion in NCF1, the gene encoding the p47phox component of the phagocyte NADPH oxidase. Hum. Mutat. 2006;27:1218–1229. doi: 10.1002/humu.20413. [DOI] [PubMed] [Google Scholar]
- 81.Han C.H., Freeman J.L., Lee T. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J. Biol. Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 82.Dang P.M., Cross A.R., Babior B.M. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558. Proc. Natl. Acad. Sci. USA. 2001;98:3001–3005. doi: 10.1073/pnas.061029698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gorlach A., Lee P.L., Roesler J. A p47-phox pseudogene carries the most common mutation causing p47-phox- deficient chronic granulomatous disease. J. Clin. Investig. 1997;100:1907–1918. doi: 10.1172/JCI119721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vazquez N., Lehrnbecher T., Chen R. Mutational analysis of patients with p47-phox-deficient chronic granulomatous disease: the significance of recombination events between the p47-phox gene (NCF1) and its highly homologous pseudogenes. Exp. Hematol. 2001;29:234–243. doi: 10.1016/s0301-472x(00)00646-9. [DOI] [PubMed] [Google Scholar]
- 85.Heyworth P.G., Noack D., Cross A.R. Identification of a novel NCF-1 (p47-phox) pseudogene not containing the signature GT deletion: significance for A47 degrees chronic granulomatous disease carrier detection. Blood. 2002;100:1845–1851. doi: 10.1182/blood-2002-03-0861. [DOI] [PubMed] [Google Scholar]
- 86.Hayrapetyan A., Dencher P.C., van Leeuwen K. Different unequal cross-over events between NCF1 and its pseudogenes in autosomal p47(phox)-deficient chronic granulomatous disease. Biochim. Biophys. Acta. 2013;1832:1662–1672. doi: 10.1016/j.bbadis.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Brunson T., Wang Q., Chambers I. A copy number variation in human NCF1 and its pseudogenes. BMC Genet. 2010;11:13. doi: 10.1186/1471-2156-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olsson L.M., Nerstedt A., Lindqvist A.K. Copy number variation of the gene NCF1 is associated with rheumatoid arthritis. Antioxid. Redox Signal. 2012;16:71–78. doi: 10.1089/ars.2011.4013. [DOI] [PubMed] [Google Scholar]
- 89.Kabuki T., Kawai T., Kin Y. A case of Williams syndrome with p47-phox-deficient chronic granulomatous disease. Nihon Rinsho Meneki Gakkai Kaishi. 2003;26:299–303. doi: 10.2177/jsci.26.299. [DOI] [PubMed] [Google Scholar]
- 90.Gilbert-Barness E., Fox T., Morrow G. Williams syndrome associated with Crohn disease, multiple infections, and chronic granulomatous disease. Fetal Pediatr. Pathol. 2004;23:29–37. doi: 10.1080/15227950490423016. [DOI] [PubMed] [Google Scholar]
- 91.Del Campo M., Antonell A., Magano L.F. Hemizygosity at the NCF1 gene in patients with Williams–Beuren syndrome decreases their risk of hypertension. Am. J. Hum. Genet. 2006;78:533–542. doi: 10.1086/501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stasia M.J., Mollin M., Martel C. Functional and genetic characterization of two extremely rare cases of Williams–Beuren syndrome associated with chronic granulomatous disease. Eur. J. Hum. Genet. 2013;21:1079–1084. doi: 10.1038/ejhg.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stasia M.J., Li X.J. Genetics and immunopathology of chronic granulomatous disease. Semin. Immunopathol. 2008;30:209–235. doi: 10.1007/s00281-008-0121-8. [DOI] [PubMed] [Google Scholar]
- 94.Roesler J., Segerer F., Morbach H. P67-phox (NCF2) lacking exons 11 and 12 is functionally active and leads to an extremely late diagnosis of chronic granulomatous disease (CGD) PLoS ONE. 2012;7:e34296. doi: 10.1371/journal.pone.0034296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roos D., van Buul J.D., Tool A.T.J. Two CGD families with a hypomorphic mutation in the activation domain of p67phox. J. Clin. Cell. Immunol. 2014;5:1–8. [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 97.Yu J., Vodyanik M.A., Smuga-Otto K. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 98.Inoue H., Nagata N., Kurokawa H. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh V.K., Kalsan M., Kumar N. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell. Dev. Biol. 2015;3:2. doi: 10.3389/fcell.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mukherjee S., Santilli G., Blundell M.P. Generation of functional neutrophils from a mouse model of X-linked chronic granulomatous disorder using induced pluripotent stem cells. PLoS ONE. 2011;6:e17565. doi: 10.1371/journal.pone.0017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santilli G., Almarza E., Brendel C. Biochemical correction of X-CGD by a novel chimeric promoter regulating high levels of transgene expression in myeloid cells. Mol. Ther. 2011;19:122–132. doi: 10.1038/mt.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou J., Sweeney C.L., Chou B.K. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117:5561–5572. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sweeney C.L., Merling R.K., Choi U. Generation of functionally mature neutrophils from induced pluripotent stem cells. Methods Mol. Biol. 2014;1124:189–206. doi: 10.1007/978-1-62703-845-4_12. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama Y., Suzuki T., Sakata-Yanagimoto M. Derivation of functional mature neutrophils from human embryonic stem cells. Blood. 2009;113:6584–6592. doi: 10.1182/blood-2008-06-160838. [DOI] [PubMed] [Google Scholar]
- 105.Hockemeyer D., Soldner F., Beard C. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang Y., Cowley S.A., Siler U. Derivation and functional analysis of patient-specific induced pluripotent stem cells as an in vitro model of chronic granulomatous disease. Stem Cells. 2012;30:599–611. doi: 10.1002/stem.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brault J., Goutagny E., Telugu N. Optimized generation of functional neutrophils and macrophages from patient-specific induced pluripotent stem cells: ex vivo models of X(0)-linked, AR22(0)- and AR47(0) – chronic granulomatous diseases. Biores. Open Access. 2014;3:311–326. doi: 10.1089/biores.2014.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi K.D., Vodyanik M.A., Slukvin I.I. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J. Clin. Investig. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi K.D., Vodyanik M., Slukvin I.I. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat. Protoc. 2011;6:296–313. doi: 10.1038/nprot.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abraham C., Cho J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Biasi F., Leonarduzzi G., Oteiza P.I. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 2013;19:1711–1747. doi: 10.1089/ars.2012.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Molodecky N.A., Soon I.S., Rabi D.M. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(46–54):e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 113.Benchimol E.I., Mack D.R., Nguyen G.C. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147:803–813 e7. doi: 10.1053/j.gastro.2014.06.023. quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 114.Hope B., Shahdadpuri R., Dunne C. Rapid rise in incidence of Irish paediatric inflammatory bowel disease. Arch. Dis. Child. 2012;97:590–594. doi: 10.1136/archdischild-2011-300651. [DOI] [PubMed] [Google Scholar]
- 115.Eszter Muller K., Laszlo Lakatos P., Papp M. Incidence and paris classification of pediatric inflammatory bowel disease. Gastroenterol. Res. Pract. 2014;2014:904307. doi: 10.1155/2014/904307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uhlig H.H., Schwerd T., Koletzko S. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147(990–1007):e3. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gevers D., Kugathasan S., Denson L.A. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haberman Y., Tickle T.L., Dexheimer P.J. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J. Clin. Investig. 2014;124:3617–3633. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marciano B.E., Rosenzweig S.D., Kleiner D.E. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–468. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 120.Alimchandani M., Lai J.P., Aung P.P. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. Am. J. Surg. Pathol. 2013;37:1365–1372. doi: 10.1097/PAS.0b013e318297427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barbato M., Ragusa G., Civitelli F. Chronic granulomatous disease mimicking early-onset Crohn's disease with cutaneous manifestations. BMC Pediatr. 2014;14:156. doi: 10.1186/1471-2431-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muise A.M., Walters T., Xu W. Single nucleotide polymorphisms that increase expression of the guanosine triphosphatase RAC1 are associated with ulcerative colitis. Gastroenterology. 2011;141:633–641. doi: 10.1053/j.gastro.2011.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muise A.M., Snapper S.B., Kugathasan S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology. 2012;143:285–288. doi: 10.1053/j.gastro.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 124.Dhillon S.S., Fattouh R., Elkadri A. Variants in nicotinamide adenine dinucleotide phosphate oxidase complex components determine susceptibility to very early onset inflammatory bowel disease. Gastroenterology. 2014;147(680–689):e2. doi: 10.1053/j.gastro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 125.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 126.Roberts R.L., Hollis-Moffatt J.E., Gearry R.B. Confirmation of association of IRGM and NCF4 with ileal Crohn's disease in a population-based cohort. Genes Immun. 2008;9:561–565. doi: 10.1038/gene.2008.49. [DOI] [PubMed] [Google Scholar]
- 127.Rioux J.D., Xavier R.J., Taylor K.D. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Somasundaram R., Deuring J.J., van der Woude C.J. Linking risk conferring mutations in NCF4 to functional consequences in Crohn's disease. Gut. 2012;61:1097. doi: 10.1136/gutjnl-2011-301344. author reply 1097-1098. [DOI] [PubMed] [Google Scholar]
- 129.Dang P.M., Elbim C., Marie J.C. Anti-inflammatory effect of interleukin-10 on human neutrophil respiratory burst involves inhibition of GM-CSF-induced p47PHOX phosphorylation through a decrease in ERK1/2 activity. FASEB J. 2006;20:1504–1506. doi: 10.1096/fj.05-5395fje. [DOI] [PubMed] [Google Scholar]
- 130.Glas J., Seiderer J., Pasciuto G. rs224136 on chromosome 10q21.1 and variants in PHOX2B, NCF4, and FAM92B are not major genetic risk factors for susceptibility to Crohn's disease in the German population. Am. J. Gastroenterol. 2009;104:665–672. doi: 10.1038/ajg.2008.65. [DOI] [PubMed] [Google Scholar]
- 131.Amre D.K., Mack D.R., Israel D. NELL1, NCF4, and FAM92B genes are not major susceptibility genes for Crohn's disease in Canadian children and young adults. Inflamm. Bowel Dis. 2012;18:529–535. doi: 10.1002/ibd.21708. [DOI] [PubMed] [Google Scholar]
- 132.Dhillon S.S., Mastropaolo L.A., Murchie R. Higher activity of the inducible nitric oxide synthase contributes to very early onset inflammatory bowel disease. Clin. Transl. Gastroenterol. 2014;5:e46. doi: 10.1038/ctg.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Selemidis S., Dusting G.J., Peshavariya H. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc. Res. 2007;75:349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 134.Hornsby M.J., Huff J.L., Kays R.J. Helicobacter pylori induces an antimicrobial response in rhesus macaques in a cag pathogenicity island-dependent manner. Gastroenterology. 2008;134:1049–1057. doi: 10.1053/j.gastro.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aerssens J., Camilleri M., Talloen W. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strengert M., Jennings R., Davanture S. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxid. Redox Signal. 2014;20:2695–2709. doi: 10.1089/ars.2013.5353. [DOI] [PubMed] [Google Scholar]
- 137.Grasberger H., El-Zaatari M., Dang D.T. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–1054. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hayes P., Dhillon S., O’Neill K. Defects in NADPH oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2015 doi: 10.1016/j.jcmgh.2015.06.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Corcionivoschi N., Alvarez L.A., Sharp T.H. Mucosal reactive oxygen species decrease virulence by disrupting campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe. 2012;12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bao S., Carr E.D., Xu Y.H. Gp91(phox) contributes to the development of experimental inflammatory bowel disease. Immunol. Cell. Biol. 2011;89:853–860. doi: 10.1038/icb.2011.4. [DOI] [PubMed] [Google Scholar]
- 141.Krieglstein C.F., Cerwinka W.H., Laroux F.S. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J. Exp. Med. 2001;194:1207–1218. doi: 10.1084/jem.194.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodrigues-Sousa T., Ladeirinha A.F., Santiago A.R. Deficient production of reactive oxygen species leads to severe chronic DSS-induced colitis in Ncf1/p47phox-mutant mice. PLoS ONE. 2014;9:e97532. doi: 10.1371/journal.pone.0097532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Campbell E.L., Bruyninckx W.J., Kelly C.J. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Conway K.L., Goel G., Sokol H. p40phox expression regulates neutrophil recruitment and function during the resolution phase of intestinal inflammation. J. Immunol. 2012;189:3631–3640. doi: 10.4049/jimmunol.1103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leoni G., Alam A., Neumann P.A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Investig. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Treton X., Pedruzzi E., Guichard C. Combined NADPH oxidase 1 and interleukin 10 deficiency induces chronic endoplasmic reticulum stress and causes ulcerative colitis-like disease in mice. PLoS ONE. 2014;9:e101669. doi: 10.1371/journal.pone.0101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lipinski S., Till A., Sina C. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J. Cell Sci. 2009;122:3522–3530. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 148.MacFie T.S., Poulsom R., Parker A. DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflamm. Bowel Dis. 2014;20:514–524. doi: 10.1097/01.MIB.0000442012.45038.0e. [DOI] [PubMed] [Google Scholar]
- 149.Lee P.P., Chan K.W., Jiang L. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr. Infect. Dis. J. 2008;27:224–230. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- 150.Barese C., Copelli S., Zandomeni R. X-linked chronic granulomatous disease: first report of mutations in patients of Argentina. J. Pediatr. Hematol. Oncol. 2004;26:656–660. doi: 10.1097/01.mph.0000139455.29962.be. [DOI] [PubMed] [Google Scholar]
- 151.Naidoo R., Jordaan N., Chan K.W. A novel CYBB mutation with the first genetically confirmed case of chronic granulomatous disease in South Africa. S. Afr. Med. J. 2011;101:768–769. [PubMed] [Google Scholar]
- 152.Bustamante J., Arias A.A., Vogt G. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat. Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zalba G., San Jose G., Beaumont F.J. Polymorphisms and promoter overactivity of the p22(phox) gene in vascular smooth muscle cells from spontaneously hypertensive rats. Circ. Res. 2001;88:217–222. doi: 10.1161/01.res.88.2.217. [DOI] [PubMed] [Google Scholar]
- 154.Olofsson P., Holmberg J., Tordsson J. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat. Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- 155.Hultqvist M., Olofsson P., Holmberg J. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc. Natl. Acad. Sci. USA. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hagenow K., Gelderman K.A., Hultqvist M. Ncf1-associated reduced oxidative burst promotes IL-33R+ T cell-mediated adjuvant-free arthritis in mice. J. Immunol. 2009;183:874–881. doi: 10.4049/jimmunol.0900966. [DOI] [PubMed] [Google Scholar]
- 157.Hultqvist M., Olofsson P., Gelderman K.A. A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006;3:e348. doi: 10.1371/journal.pmed.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jacob C.O., Eisenstein M., Dinauer M.C. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc. Natl. Acad. Sci. USA. 2012;109:E59–E67. doi: 10.1073/pnas.1113251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim-Howard X., Sun C., Molineros J.E. Allelic heterogeneity in NCF2 associated with systemic lupus erythematosus (SLE) susceptibility across four ethnic populations. Hum. Mol. Genet. 2014;23:1656–1668. doi: 10.1093/hmg/ddt532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cunninghame Graham D.S., Morris D.L., Bhangale T.R. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 2011;7:e1002341. doi: 10.1371/journal.pgen.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Campbell A.M., Kashgarian M., Shlomchik M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Toublanc J.E. Comparison of epidemiological data on congenital hypothyroidism in Europe with those of other parts in the world. Horm. Res. 1992;38:230–235. doi: 10.1159/000182549. [DOI] [PubMed] [Google Scholar]
- 163.De Felice M., Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr. Rev. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- 164.De Deken X., Wang D., Many M.C. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 165.Royaux I.E., Suzuki K., Mori A. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 166.Yoshida A., Taniguchi S., Hisatome I. Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J. Clin. Endocrinol. Metab. 2002;87:3356–3361. doi: 10.1210/jcem.87.7.8679. [DOI] [PubMed] [Google Scholar]
- 167.Twyffels L., Strickaert A., Virreira M. Anoctamin-1/TMEM16A is the major apical iodide channel of the thyrocyte. Am. J. Physiol. Cell Physiol. 2014;307:C1102–C1112. doi: 10.1152/ajpcell.00126.2014. [DOI] [PubMed] [Google Scholar]
- 168.Ieiri T., Cochaux P., Targovnik H.M. A 3′ splice site mutation in the thyroglobulin gene responsible for congenital goiter with hypothyroidism. J. Clin. Investig. 1991;88:1901–1905. doi: 10.1172/JCI115513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cangul H., Boelaert K., Dogan M. Novel truncating thyroglobulin gene mutations associated with congenital hypothyroidism. Endocrine. 2014;45:206–212. doi: 10.1007/s12020-013-0027-7. [DOI] [PubMed] [Google Scholar]
- 170.Cangul H., Darendeliler F., Saglam Y. A truncating TPO mutation (Y55X) in patients with hypothyroidism and total iodide organification defect. Endocr. Res. 2015;40:146–150. doi: 10.3109/07435800.2014.967354. [DOI] [PubMed] [Google Scholar]
- 171.Bikker H., den Hartog M.T., Baas F. A 20-basepair duplication in the human thyroid peroxidase gene results in a total iodide organification defect and congenital hypothyroidism. J. Clin. Endocrinol. Metab. 1994;79:248–252. doi: 10.1210/jcem.79.1.8027236. [DOI] [PubMed] [Google Scholar]
- 172.Abramowicz M.J., Targovnik H.M., Varela V. Identification of a mutation in the coding sequence of the human thyroid peroxidase gene causing congenital goiter. J. Clin. Investig. 1992;90:1200–1204. doi: 10.1172/JCI115981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fujiwara H., Tatsumi K., Miki K. Congenital hypothyroidism caused by a mutation in the Na+/I− symporter. Nat. Genet. 1997;16:124–125. doi: 10.1038/ng0697-124. [DOI] [PubMed] [Google Scholar]
- 174.Pohlenz J., Medeiros-Neto G., Gross J.L. Hypothyroidism in a Brazilian kindred due to iodide trapping defect caused by a homozygous mutation in the sodium/iodide symporter gene. Biochem. Biophys. Res. Commun. 1997;240:488–491. doi: 10.1006/bbrc.1997.7594. [DOI] [PubMed] [Google Scholar]
- 175.Pohlenz J., Rosenthal I.M., Weiss R.E. Congenital hypothyroidism due to mutations in the sodium/iodide symporter. Identification of a nonsense mutation producing a downstream cryptic 3′ splice site. J. Clin. Investig. 1998;101:1028–1035. doi: 10.1172/JCI1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Everett L.A., Glaser B., Beck J.C. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat. Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 177.Bogazzi F., Raggi F., Ultimieri F. A novel mutation in the pendrin gene associated with Pendred's syndrome. Clin. Endocrinol. 2000;52:279–285. doi: 10.1046/j.1365-2265.2000.00930.x. [DOI] [PubMed] [Google Scholar]
- 178.Moreno J.C., Klootwijk W., van Toor H. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N. Engl. J. Med. 2008;358:1811–1818. doi: 10.1056/NEJMoa0706819. [DOI] [PubMed] [Google Scholar]
- 179.Afink G., Kulik W., Overmars H. Molecular characterization of iodotyrosine dehalogenase deficiency in patients with hypothyroidism. J. Clin. Endocrinol. Metab. 2008;93:4894–4901. doi: 10.1210/jc.2008-0865. [DOI] [PubMed] [Google Scholar]
- 180.Moreno J.C., Bikker H., Kempers M.J. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N. Engl. J. Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 181.Vigone M.C., Fugazzola L., Zamproni I. Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum. Mutat. 2005;26:395. doi: 10.1002/humu.9372. [DOI] [PubMed] [Google Scholar]
- 182.Varela V., Rivolta C.M., Esperante S.A. Three mutations (p.Q36H, p.G418fsX482, and g.IVS19-2A>C) in the dual oxidase 2 gene responsible for congenital goiter and iodide organification defect. Clin. Chem. 2006;52:182–191. doi: 10.1373/clinchem.2005.058321. [DOI] [PubMed] [Google Scholar]
- 183.Pfarr N., Musholt T.J., Musholt P.B. Congenital primary hypothyroidism with subsequent adenomatous goiter in a Turkish patient caused by a homozygous 10-bp deletion in the thyroid peroxidase (TPO) gene. Clin. Endocrinol. 2006;64:514–518. doi: 10.1111/j.1365-2265.2006.02500.x. [DOI] [PubMed] [Google Scholar]
- 184.Ohye H., Fukata S., Hishinuma A. A novel homozygous missense mutation of the dual oxidase 2 (DUOX2) gene in an adult patient with large goiter. Thyroid. 2008;18:561–566. doi: 10.1089/thy.2007.0258. [DOI] [PubMed] [Google Scholar]
- 185.Maruo Y., Takahashi H., Soeda I. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J. Clin. Endocrinol. Metab. 2008;93:4261–4267. doi: 10.1210/jc.2008-0856. [DOI] [PubMed] [Google Scholar]
- 186.Tonacchera M., De Marco G., Agretti P. Identification and functional studies of two new dual-oxidase 2 (DUOX2) mutations in a child with congenital hypothyroidism and a eutopic normal-size thyroid gland. J. Clin. Endocrinol. Metab. 2009;94:4309–4314. doi: 10.1210/jc.2009-0426. [DOI] [PubMed] [Google Scholar]
- 187.Hoste C., Rigutto S., Van Vliet G. Compound heterozygosity for a novel hemizygous missense mutation and a partial deletion affecting the catalytic core of the H2O2-generating enzyme DUOX2 associated with transient congenital hypothyroidism. Hum. Mutat. 2010;31:E1304–E1319. doi: 10.1002/humu.21227. [DOI] [PubMed] [Google Scholar]
- 188.De Marco G., Agretti P., Montanelli L. Identification and functional analysis of novel dual oxidase 2 (DUOX2) mutations in children with congenital or subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2011;96:E1335–E1339. doi: 10.1210/jc.2010-2467. [DOI] [PubMed] [Google Scholar]
- 189.Narumi S., Muroya K., Asakura Y. Molecular basis of thyroid dyshormonogenesis: genetic screening in population-based Japanese patients. J. Clin. Endocrinol. Metab. 2011;96:E1838–E1842. doi: 10.1210/jc.2011-1573. [DOI] [PubMed] [Google Scholar]
- 190.Kasahara T., Narumi S., Okasora K. Delayed onset congenital hypothyroidism in a patient with DUOX2 mutations and maternal iodine excess. Am. J. Med. Genet. A. 2013;161A:214–217. doi: 10.1002/ajmg.a.35693. [DOI] [PubMed] [Google Scholar]
- 191.Yoshizawa-Ogasawara A., Ogikubo S., Satoh M. Congenital hypothyroidism caused by a novel mutation of the dual oxidase 2 (DUOX2) gene. J. Pediatr. Endocrinol. Metab. 2013;26:45–52. doi: 10.1515/jpem-2012-0082. [DOI] [PubMed] [Google Scholar]
- 192.Cangul H., Aycan Z., Kendall M. A truncating DUOX2 mutation (R434X) causes severe congenital hypothyroidism. J. Pediatr. Endocrinol. Metab. 2014;27:323–327. doi: 10.1515/jpem-2013-0314. [DOI] [PubMed] [Google Scholar]
- 193.Wang F., Lu K., Yang Z. Genotypes and phenotypes of congenital goitre and hypothyroidism caused by mutations in dual oxidase 2 genes. Clin. Endocrinol. 2014;81:452–457. doi: 10.1111/cen.12469. [DOI] [PubMed] [Google Scholar]
- 194.Jin H.Y., Heo S.H., Kim Y.M. High frequency of DUOX2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm. Res. Paediatr. 2014;82:252–260. doi: 10.1159/000362235. [DOI] [PubMed] [Google Scholar]
- 195.Abe K., Narumi S., Suwanai A.S. Pseudodominant inheritance in a family with nonautoimmune hypothyroidism due to biallelic DUOX2 mutations. Clin. Endocrinol. 2014 doi: 10.1111/cen.12622. [DOI] [PubMed] [Google Scholar]
- 196.Satoh M., Aso K., Ogikubo S. Hypothyroidism caused by the combination of two heterozygous mutations: one in the TSH receptor gene the other in the DUOX2 gene. J. Pediatr. Endocrinol. Metab. 2015;28:657–661. doi: 10.1515/jpem-2014-0078. [DOI] [PubMed] [Google Scholar]
- 197.Yi R.H., Zhu W.B., Yang L.Y. A novel dual oxidase maturation factor 2 gene mutation for congenital hypothyroidism. Int. J. Mol. Med. 2013;31:467–470. doi: 10.3892/ijmm.2012.1223. [DOI] [PubMed] [Google Scholar]
- 198.Liu S., Liu L., Niu X. A novel missense mutation (I26M) in DUOXA2 causing congenital goiter hypothyroidism impairs NADPH oxidase activity but not protein expression. J. Clin. Endocrinol. Metab. 2015;100:1225–1229. doi: 10.1210/jc.2014-3964. jc20143964. [DOI] [PubMed] [Google Scholar]
- 199.Hulur I., Hermanns P., Nestoris C. A single copy of the recently identified dual oxidase maturation factor (DUOXA) 1 gene produces only mild transient hypothyroidism in a patient with a novel biallelic DUOXA2 mutation and monoallelic DUOXA1 deletion. J. Clin. Endocrinol. Metab. 2011;96:E841–E845. doi: 10.1210/jc.2010-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zamproni I., Grasberger H., Cortinovis F. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2008;93:605–610. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Park S.M., Chatterjee V.K. Genetics of congenital hypothyroidism. J. Med. Genet. 2005;42:379–389. doi: 10.1136/jmg.2004.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dupuy C., Ohayon R., Valent A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J. Biol. Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 203.Geiszt M., Witta J., Baffi J. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 204.Forteza R., Salathe M., Miot F. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 205.Harper R.W., Xu C., Eiserich J.P. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 206.El Hassani R.A., Benfares N., Caillou B. Dual oxidase2 is expressed all along the digestive tract. Am .J. Physiol. Gastrointest. Liver Physiol. 2005;288:G933–G942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 207.Fletcher N.M., Saed M.G., Abuanzeh S. Nicotinamide adenine dinucleotide phosphate oxidase is differentially regulated in normal myometrium versus leiomyoma. Reprod. Sci. 2014;21:1145–1152. doi: 10.1177/1933719114522552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Choi H., Park J.Y., Kim H.J. Hydrogen peroxide generated by DUOX1 regulates the expression levels of specific differentiation markers in normal human keratinocytes. J. Dermatol. Sci. 2014;74:56–63. doi: 10.1016/j.jdermsci.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 209.Song Y., Ruf J., Lothaire P. Association of duoxes with thyroid peroxidase and its regulation in thyrocytes. J. Clin. Endocrinol. Metab. 2010;95:375–382. doi: 10.1210/jc.2009-1727. [DOI] [PubMed] [Google Scholar]
- 210.Corvilain B., Collyn L., van Sande J. Stimulation by iodide of H(2)O(2) generation in thyroid slices from several species. Am. J. Physiol. Endocrinol. Metab. 2000;278:E692–E699. doi: 10.1152/ajpendo.2000.278.4.E692. [DOI] [PubMed] [Google Scholar]
- 211.Cardoso L.C., Martins D.C., Figueiredo M.D. Ca(2+)/nicotinamide adenine dinucleotide phosphate-dependent H(2)O(2) generation is inhibited by iodide in human thyroids. J. Clin. Endocrinol. Metab. 2001;86:4339–4343. doi: 10.1210/jcem.86.9.7823. [DOI] [PubMed] [Google Scholar]
- 212.Song Y., Driessens N., Costa M. Roles of hydrogen peroxide in thyroid physiology and disease. J. Clin. Endocrinol. Metab. 2007;92:3764–3773. doi: 10.1210/jc.2007-0660. [DOI] [PubMed] [Google Scholar]
- 213.Edens W.A., Sharling L., Cheng G. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell. Biol. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Daiyasu H., Toh H. Molecular evolution of the myeloperoxidase family. J. Mol. Evol. 2000;51:433–445. doi: 10.1007/s002390010106. [DOI] [PubMed] [Google Scholar]
- 215.Donko A., Peterfi Z., Sum A. Dual oxidases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:2301–2308. doi: 10.1098/rstb.2005.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Meitzler J.L., Ortiz de Montellano P.R. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: insights into heme binding and catalytic activity. J. Biol. Chem. 2009;284:18634–18643. doi: 10.1074/jbc.M109.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pociot F., Lorenzen T., Nerup J. A manganese superoxide dismutase (SOD2) gene polymorphism in insulin-dependent diabetes mellitus. Dis. Markers. 1993;11:267–274. doi: 10.1155/1993/678310. [DOI] [PubMed] [Google Scholar]
- 218.Zelko I.N., Mariani T.J., Folz R.J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 219.Harper R.W., Xu C., McManus M. Duox2 exhibits potent heme peroxidase activity in human respiratory tract epithelium. FEBS Lett. 2006;580:5150–5154. doi: 10.1016/j.febslet.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 220.Rigutto S., Hoste C., Grasberger H. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009;284:6725–6734. doi: 10.1074/jbc.M806893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hill T., 3rd, Xu C., Harper R.W. IFNgamma mediates DUOX2 expression via a STAT-independent signaling pathway. Biochem. Biophys. Res. Commun. 2010;395:270–274. doi: 10.1016/j.bbrc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Grandvaux N., Mariani M., Fink K. Lung epithelial NOX/DUOX and respiratory virus infections. Clin. Sci. 2015;128:337–347. doi: 10.1042/CS20140321. [DOI] [PubMed] [Google Scholar]
- 223.Meitzler J.L., Ortiz de Montellano P.R. Structural stability and heme binding potential of the truncated human dual oxidase 2 (DUOX2) peroxidase domain. Arch. Biochem. Biophys. 2011;512:197–203. doi: 10.1016/j.abb.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.De Deken X., Wang D., Dumont J.E. Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp. Cell Res. 2002;273:187–196. doi: 10.1006/excr.2001.5444. [DOI] [PubMed] [Google Scholar]
- 225.Grasberger H., Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 226.Luxen S., Belinsky S.A., Knaus U.G. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 227.Luxen S., Noack D., Frausto M. Heterodimerization controls localization of Duox-DuoxA NADPH oxidases in airway cells. J. Cell Sci. 2009;122:1238–1247. doi: 10.1242/jcs.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ameziane-El-Hassani R., Talbot M., de Souza Dos Santos M.C. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc. Natl. Acad. Sci. USA. 2015;112:5051–5056. doi: 10.1073/pnas.1420707112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Morand S., Ueyama T., Tsujibe S. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hoste C., Dumont J.E., Miot F. The type of DUOX-dependent ROS production is dictated by defined sequences in DUOXA. Exp. Cell. Res. 2012;318:2353–2364. doi: 10.1016/j.yexcr.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 231.Ueyama T., Sakuma M., Ninoyu Y. The extracellular A-loop of dual oxidases affects the specificity of reactive oxygen species release. J. Biol. Chem. 2015;290:6495–6506. doi: 10.1074/jbc.M114.592717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Morand S., Agnandji D., Noel-Hudson M.S. Targeting of the dual oxidase 2N-terminal region to the plasma membrane. J. Biol. Chem. 2004;279:30244–30251. doi: 10.1074/jbc.M405406200. [DOI] [PubMed] [Google Scholar]
- 233.Ameziane-El-Hassani R., Morand S., Boucher J.L. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J. Biol. Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 234.Meitzler J.L., Hinde S., Banfi B. Conserved cysteine residues provide a protein–protein interaction surface in dual oxidase (DUOX) proteins. J. Biol. Chem. 2013;288:7147–7157. doi: 10.1074/jbc.M112.414797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Carre A., Louzada R.A., Fortunato R.S. When an intramolecular disulfide bridge governs the interaction of DUOX2 with its partner DUOXA2. Antioxid. Redox Signal. 2015 doi: 10.1089/ars.2015.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Donko A., Morand S., Korzeniowska A. Hypothyroidism-associated missense mutation impairs NADPH oxidase activity and intracellular trafficking of Duox2. Free Radic. Biol. Med. 2014;73:190–200. doi: 10.1016/j.freeradbiomed.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Grasberger H., De Deken X., Mayo O.B. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol. Endocrinol. 2012;26:481–492. doi: 10.1210/me.2011-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Johnson K.R., Marden C.C., Ward-Bailey P. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol. Endocrinol. 2007;21:1593–1602. doi: 10.1210/me.2007-0085. [DOI] [PubMed] [Google Scholar]
- 239.Donko A., Ruisanchez E., Orient A. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic. Biol. Med. 2010;49:2040–2048. doi: 10.1016/j.freeradbiomed.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 240.Ishikawa K., Takenaga K., Akimoto M. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 241.Iommarini L., Kurelac I., Capristo M. Different mtDNA mutations modify tumor progression in dependence of the degree of respiratory complex I impairment. Hum. Mol. Genet. 2014;23:1453–1466. doi: 10.1093/hmg/ddt533. [DOI] [PubMed] [Google Scholar]
- 242.El-Sohemy A., Cornelis M.C., Park Y.W. Catalase and PPARgamma2 genotype and risk of rheumatoid arthritis in Koreans. Rheumatol. Int. 2006;26:388–392. doi: 10.1007/s00296-005-0013-3. [DOI] [PubMed] [Google Scholar]
- 243.Bohanec Grabar P., Logar D., Tomsic M. Genetic polymorphisms modifying oxidative stress are associated with disease activity in rheumatoid arthritis patients. Dis. Markers. 2009;26:41–48. doi: 10.3233/DMA-2009-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Khodayari S., Salehi Z., Fakhrieh Asl S. Catalase gene C-262T polymorphism: importance in ulcerative colitis. J. Gastroenterol. Hepatol. 2013;28:819–822. doi: 10.1111/jgh.12141. [DOI] [PubMed] [Google Scholar]
- 245.Matus S., Valenzuela V., Medinas D.B. ER dysfunction and protein folding stress in ALS. Int. J. Cell Biol. 2013;2013:674751. doi: 10.1155/2013/674751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Prudencio M., Borchelt D.R. Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states. Mol. Neurodegener. 2011;6:77. doi: 10.1186/1750-1326-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stasia M.J., Bordigoni P., Martel C. A novel and unusual case of chronic granulomatous disease in a child with a homozygous 36-bp deletion in the CYBA gene (A22(0)) leading to the activation of a cryptic splice site in intron 4. Hum. Genet. 2002;110:444–450. doi: 10.1007/s00439-002-0720-8. [DOI] [PubMed] [Google Scholar]