Abstract
Background
It is unclear if achieving multiple risk factor (RF) goals through protocol-guided intensive medical therapy is feasible or improves outcomes in type 2 diabetes (T2DM).
Objectives
We sought to quantify the relationship between achieved RF goals in the BARI 2D (Bypass Angioplasty Investigation Revascularization 2 Diabetes) trial and cardiovascular events/survival.
Methods
We performed a nonrandomized analysis of survival/cardiovascular events and control of 6 RFs (nonsmoker, non-HDL-C <130 mg/dl, triglycerides <150 mg/dl, blood pressure [systolic <130 mm Hg; diastolic <80 mm Hg], hemoglobin A1c <7%) in BARI 2D. Cox models with time-varying number of RFs in control were adjusted for baseline number of RFs in control, clinical characteristics, and trial randomization assignments.
Results
In 2,265 patients (mean age 62 years, 29% women) followed for 5 years, the mean ± SD number of RFs in control improved from 3.5 ± 1.4 out of 6 at baseline to 4.2 ± 1.3 at 5 years, p < 0.0001. The number of RFs in control during the trial was strongly related to death (global p = 0.0010) and the composite of death, myocardial infarction and stroke (global p = 0.0035) in fully adjusted models. Participants with 0 to 2 RFs in control during follow-up had a 2-fold higher risk of death (hazard ratio [HR]: 2.0; 95% CI: 1.3 to 3.3, p = 0.0031) and a 1.7-fold higher risk of the composite endpoint (HR: 1.7; 95% CI 1.2 to 2.5, p = 0.0043), compared with those with 6 RFs in-control.
Conclusions
Simultaneous control of multiple RFs through protocol-guided intensive medical therapy is feasible and relates to cardiovascular morbidity and mortality in patients with coronary disease and T2DM.
Keywords: blood pressure, coronary heart disease, cholesterol, diabetes mellitus, hemoglobin A, glycosylated, smoking
Reduction in cardiovascular risk factors (RFs) has contributed to lower cardiovascular event rates in the United States (1). RF control and prognosis among individuals with type 2 diabetes mellitus (T2DM) have improved, but they remain at higher risk (2,3). Few prospective studies have addressed the effect of simultaneous control of multiple RFs in T2DM populations on cardiovascular outcomes (4,5). We hypothesized that achievement of multiple RF goals through protocol-guided intensive medical therapy is feasible and associated with improved survival and lower cardiovascular event rates among individuals with coronary heart disease (CHD) and T2DM in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial.
Methods
BARI 2D Design, Enrollment, and Follow-Up
The BARI 2D protocol and study results have been described (6–8). Briefly, this study enrolled participants with T2DM and angiographically documented stable CHD. Participants were randomized in a 2 × 2 factorial design simultaneously to cardiac treatment and glycemic control treatment strategies. The randomized cardiac treatment strategies entailed intensive medical therapy with revascularization within 4 weeks or intensive medical therapy with revascularization when clinically indicated. The randomized glycemic control strategies compared primarily insulin-sensitizing (IS) versus primarily insulin-providing (IP) treatments. The study was approved by the local institutional review boards and participants provided informed consent. The current post-hoc analysis includes 2,265 of the 2,368 BARI 2D patients (103 patients were missing RF information).
Target levels for RFs were adjusted as practice guidelines evolved. The final targets, collection frequency, and core laboratory status for key RFs in the BARI 2D protocol are shown in Table 1. Non-high-density lipoprotein cholesterol (HDL-C) rather than low-density lipoprotein cholesterol (LDL-C) was chosen for analysis based on pathophysiological and statistical considerations. Patients were followed until their 6-year visit or December 2008, whichever came earlier.
Table 1.
RF Target Levels and Collection Details.
| Risk Factor | Target | Collection Frequency | Core Laboratory |
|---|---|---|---|
| Systolic BP | <130 mm Hg | Monthly for first 6 months | No |
| Quarterly thereafter | |||
| Diastolic BP | <80 mm Hg | No | |
| Smoking status | Nonsmoker | Annually | No |
| HbA1c | <7% | Baseline; months 1, 3, 6, 20; and every 6 months thereafter | HbA1c core laboratory |
| Triglycerides | <150 mg/dl (<1.70 mmol/l) |
Baseline, 6 months, then annually | Lipid core laboratory |
| Non-HDL-C | <130 mg/dl (<3.37 mmol/l) Optional goal <100 mg/dl (<2.59 mmol/l) |
BP = blood pressure; HbA1c = glycosylated hemoglobin; non-HDL-C = non-high-density lipoprotein cholesterol; RF = risk factor.
RF Management
Cardiovascular RF management followed a detailed protocol (8) and included monitoring and regular feedback on smoking cessation, dietary and exercise advice, and protocol-guided pharmacologic management for dyslipidemia, hyperglycemia, and hypertension.
Of the 49,196 clinic visits in BARI 2D, 47,044 (95%) had up-to-date RF information for all 6 RFs. Visit information was carried forward up to 15 months. Clinic visits were included when all 6 RFs were measured or up to date, with participants contributing when they had available RF data.
RF Modeling
The number of RFs in control was modeled with 4 indicator variables (in control categories of: 0–2, 3, 4, and 5, with 6 as the reference). RFs were in control if they met the targets in Table 1. In a secondary exploratory analysis, we modeled a J-shaped relationship of blood pressure (BP) and glycosylated hemoglobin (HbA1c) with outcomes, as recent data suggest that overly tight control might be associated with harm (9,10). In this secondary analysis, systolic BP between 110 mm Hg and 140 mm Hg was in control and HbA1c between 6.5% and 7.5% was in control.
Values outside these ranges were considered out of control.
We analyzed the relationship between the number of RFs in control with all-cause death and with cardiovascular disease (CVD) events (composite endpoint of death, myocardial infarction [MI] or stroke).
Statistical Analysis
Baseline characteristics by the number of baseline RFs at goal were compared using analysis of variance (ANOVA) for continuous variables or chi-square tests for categorical variables. At trial initiation, RFs were intensively monitored and medication regimens intensified to achieve RF targets, resulting in a large initial change in RF control between baseline and year 1. We determined if subsequent RF control continued to improve, was maintained, or declined from year 1 to year 5. We quantified the initial changes (baseline to 1 year), and subsequent changes (after year 1) using a generalized logistic estimating equation with a continuous follow-up year and a baseline visit indicator. A significant coefficient for the baseline indicator indicated a significant first-year change. The sign and significance of the coefficient for year determined if there was continued improvement, maintenance or degradation over the 5 years of follow-up. Non-time-varying analyses used baseline or year 1 number of RFs in-control and time-varying RF in control during the trial were used in a separate analysis. We used Cox models to estimate the hazard ratios (HR) and verified the proportional hazard assumption. All Cox models included baseline angiographic information (number of total lesions, myocardial jeopardy index), baseline clinical and demographic information (abnormal left ventricular ejection fraction, prior revascularization, age, sex, race/ethnicity, country) and randomization assignment (IS vs. IP), prompt revascularization vs. medical therapy) and revascularization strata (CABG or PCI). A Wald test determined if the number of RFs in control was significant overall.
All analyses were conducted using SAS software, version 9.3 (Cary, North Carolina). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline Characteristics
The average age was 62 ± 9 years, with 29% women, 35% nonwhite, and a mean duration of T2DM of 10 years. Baseline RFs and comorbidities are detailed in Table 2. Younger individuals and participants outside North America had fewer RFs in control. Between 40% and 68% of individuals met individual RF targets, and only 7% met all 6 RF goals. (Table 3)
Table 2.
Baseline Characteristics
| All Participants |
Number of Risk Factors at Goal | p Value | ||||
|---|---|---|---|---|---|---|
| (N = 2,265) | 0–2 RFs (N = 536) |
3–4 RFs (N = 1,121) |
5–6 RFs (N = 608) |
|||
| Age (mean ± SD) | 62 ± 9 | 60 ± 8 | 63 ± 9 | 64 ± 9 | <0.0001 | |
| Female, % | 29 | 33 | 29 | 27 | 0.0730 | |
| Non-white race, % | 35 | 37 | 35 | 36 | 0.1576 | |
| Geographical region | <0.0001 | |||||
| United States (%) | 62 | 51 | 64 | 69 | ||
| Canada (%) | 15 | 14 | 16 | 17 | ||
| Europe, South/Central America (%) | 22 | 35 | 20 | 15 | ||
| Clinical Characteristics | ||||||
| History of MI (%) | 32 | 28 | 34 | 32 | 0.0359 | |
| History of heart failure (%) | 6 | 5 | 6 | 8 | 0.1484 | |
| Cerebrovascular accident (%) | 10 | 9 | 10 | 9 | 0.8491 | |
| Prior revascularization (%) | 24 | 22 | 24 | 25 | 0.5302 | |
| Number of coronary lesions (mean ± SD) | 5 ± 2 | 5 ± 2 | 5 ± 2 | 5 ± 2 | 0.0755 | |
| LVEF <50% (%) | 17 | 15 | 16 | 20 | 0.0889 | |
| Myocardial Jeopardy Index (mean ± SD) | 44 ± 24 | 46 ± 24 | 45 ± 24 | 43 ± 24 | 0.1797 | |
| Diabetes duration (mean ± SD) | 10 ± 9 | 10 ± 8 | 11 ± 9 | 10 ± 9 | 0.1530 | |
| History of insulin use (%) | 29 | 30 | 31 | 25 | 0.0186 | |
| Cardiovascular Risk Factors | ||||||
| Current cigarette smoker, % | 12 | 26 | 11 | 4 | <0.0001 | |
| BMI (mean ± SD) | 32 ± 6 | 32 ± 6 | 32 ± 6 | 31 ± 6 | 0.1368 | |
| Systolic BP (mean ± SD) | 132 ± 20 | 145 ± 20 | 132 ± 19 | 120 ± 14 | <0.0001 | |
| Diastolic BP (mean ± SD) | 75 ± 11 | 83 ± 11 | 74 ± 10 | 68 ± 8 | <0.0001 | |
| TC mg/dl (mean ± SD) | 169 ± 41 | 199 ± 40 | 170 ± 40 | 143 ± 25 | <0.0001 | |
| HDL-C mg/dl (mean ± SD) | 38 ± 10 | 37 ± 10 | 38 ± 10 | 39 ± 10 | 0.0002 | |
| LDL-C mg/dl (mean ± SD) | 96 ± 33 | 117 ± 35 | 96 ± 33 | 81 ± 22 | <0.0001 | |
| Triglyceride mg/dl (mean ± SD) | 181 ± 136 | 251 ± 167 | 185 ± 134 | 115 ± 57 | <0.0001 | |
| Non-HDL-C mg/dl (mean ± SD) | 131 ± 41 | 162 ± 38 | 132 ± 39 | 104 ± 22 | <0.0001 | |
| HbA1c % (mean ± SD) | 7.6 ± 1.6 | 8.5 ± 1.5 | 7.7 ± 1.6 | 6.8 ± 1.3 | <0.0001 | |
| Trial Strata (%) | ||||||
| Insulin sensitizing | 50 | 50 | 50 | 50 | 0.9919 | |
| Early revascularization | 50 | 45 | 48 | 56 | 0.0012 | |
| CABG | 32 | 36 | 33 | 29 | 0.0366 | |
To convert mg/dl to mmol/l, multiply by 0.02586 for cholesterol and 0.01129 for triglycerides. Percentages within categories shown in the table may differ from 100% due to rounding. BMI = body mass index; CABG = coronary artery bypass grafting; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; LVEF = left ventricular ejection fraction; MI = myocardial infarction; SD = standard deviation. Other abbreviations as in Table 1.
In the baseline table, the numbers of RFs in control are grouped as 0–2, 3–4, and 5–6 for ease of reading. In the analysis, the RFs are modeled as 0–2, 3, 4, 5, and 6 RFs in control
Table 3.
Achievement of Risk Factor Goals Over Time
| Risk Factor | Proportion of Participants With RF In Control (%)* |
Initial Change (Baseline to Year 1) p Value |
Subsequent Change (Year 1 to Year 5) p Value |
|||
|---|---|---|---|---|---|---|
| Baseline N = 2,265 |
Year 1 N = 2,137 |
Year 3 N = 1,949 |
Year 5 N = 1,060 |
|||
| Non-HDL-C | 54 | 70 | 79 | 82 | <0.0001 | <0.0001 |
| Triglycerides | 50 | 57 | 60 | 64 | 0.0005 | <0.0001 |
| Systolic BP | 49 | 56 | 62 | 62 | 0.0002 | 0.0009 |
| Diastolic BP | 68 | 69 | 73 | 77 | 0.59 | 0.0002 |
| Nonsmokers | 87 | 90 | 91 | 92 | 0.0013 | 0.14 |
| HbA1c | 40 | 51 | 48 | 46 | <0.0001 | <0.0001 |
| Meet all 6 goals | 7 | 12 | 15 | 15 | <0.0001 | 0.18 |
RF in control defined as: non-HDL-C <130 mg/dl, TG <150 mg/dl, SBP <130 mm Hg, DBP <80 mm Hg, HbA1c <7%, nonsmoker
TG = triglycerides. Other abbreviations as in Table 1.
Changes in Pharmacologic Therapy and Cardiovascular Risk Factor Control
The greatest change in medication use occurred within the first year (Table 4). Use of aspirin, lipid-lowering and antihypertensive drugs increased significantly over the first year and was maintained in follow-up. Changes in diabetes medications reflect the randomization to IP and IS strategies and use of medications outside their randomized strategy for glucose control.
Table 4.
Trial Medication Status
| Medication | Baseline N = 2,265 |
Year 1 N = 2,137 |
Year 3 N = 1,949 |
Year 5 N = 1,060 |
Initial Change (Baseline to Year 1) p Value |
Subsequent Change (Year 1 to Year 5) p Value |
|
|---|---|---|---|---|---|---|---|
| Lipid-lowering drugs (%)* | 79 | 98 | 99 | 97 | <0.0001 | 0.58 | |
| Statins (%) | 75 | 93 | 96 | 94 | <0.0001 | 0.13 | |
| Antihypertensive agents (%) | 95 | 99 | 99 | 98 | <0.0001 | 0.11 | |
| ACE or ARB (%) | 77 | 90 | 91 | 90 | <0.0001 | 0.26 | |
| Beta-blocker (%) | 73 | 87 | 87 | 87 | <0.0001 | 0.61 | |
| Aspirin (%) | 88 | 92 | 94 | 92 | <0.0001 | 0.78 | |
| Diabetes drugs† | |||||||
| IS Only (%) | 16 | 31 | 28 | 23 | <0.0001 | <0.0001 | |
| IP Only (%) | 30 | 43 | 44 | 45 | <0.0001 | 0.347 | |
| IS and IP (%) | 45 | 20 | 23 | 28 | <0.0001 | <0.0001 | |
| None (%) | 8 | 6 | 5 | 4 | <0.0001 | 0.02 | |
Lipid-lowering drugs include fibrates, niacin, bile acid sequestrants, omega-3 fatty acids, and cholesterol absorption inhibitors.
IS drugs included metformin and thiazolidinediones (TZDs). IP drugs included insulin and sulfonylurea. In BARI 2D, participants were randomized to initial IS or IP treatment strategies and were offered pharmacologic therapy if HbA1c values were >7%. Subsequently, patients could take drugs from the other arm of the trial if HbA1c values were >8%.
ACE = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ASA = aspirin; IP = insulin providing; IS = insulin sensitizing, TZD = thiazolidinediones.
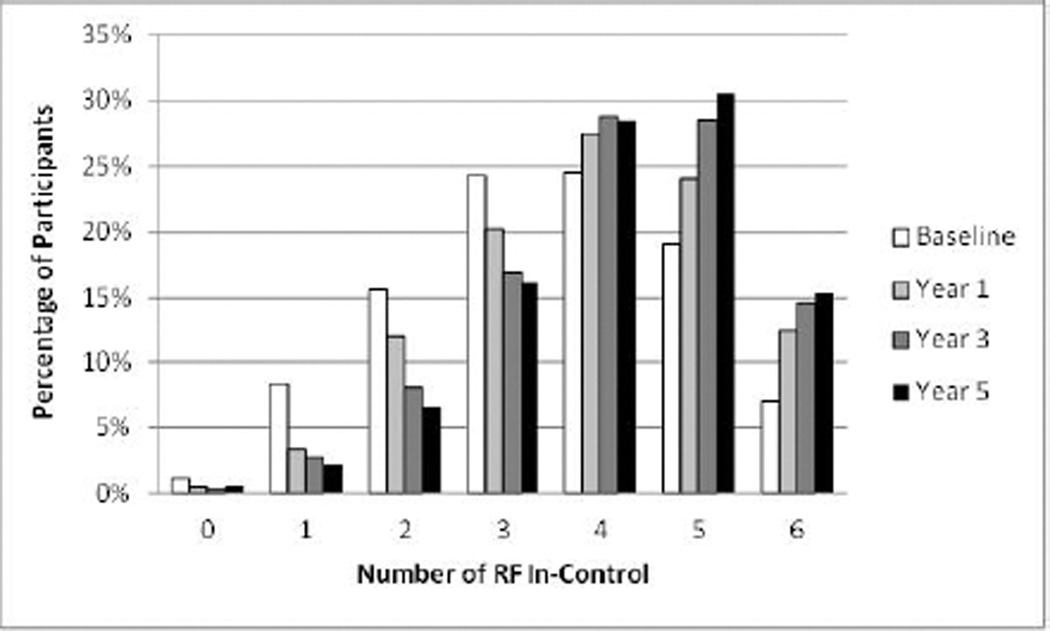
The mean ± SD number of RFs in-control increased from 3.5 ± 1.4 at baseline to 4.2 ± 1.3 after 5 years, p < 0.0001. Except for diastolic BP, the percent of participants at target increased between baseline and year 1 (Table 3). Improvements continued through year 5 except for smokers (maintained) and HbA1c (worsened). At 5 years, over 74% of patients had 4 or more RFs in control, but only 15% of individuals achieved control of all 6 RFs (Figure 1). Online Table 1 shows average values of RFs over time.
Figure 1. Distribution of the Number of RFs In Control: Baseline to Year 5.
The numbers of RFs in control are shown at baseline and for each year of the trial. Over time, the proportion of participants with 4 or more RFs in control increased while the proportion with fewer RFs in control declined.
RF = risk factor.
Clinical Outcomes
Mean ± SD follow-up time was 5.0 ± 1.4 years. The analysis includes 47,044 visits from 2,265 patients. There were 275 deaths, 254 incident fatal or nonfatal MIs (excluding 13 MIs prior to the first visit with all 6 RFs measured), 65 strokes, and 491 CVD events (excluding the previously mentioned 13 MIs). The 5-year Kaplan-Meier total mortality rate was 11% and the rate of CVD events was 22%.
Outcomes Related to RF Control at Baseline and Year 1
Among the 2,169 participants with baseline RF data, there was no relationship between the number of RFs in control at baseline and subsequent death (HRs between 0.8 and 1.1, p = 0.36) or CVD events (HRs between 1.0 and 1.3, p = 0.22). In contrast, RF control at year 1 was strongly related to both outcomes after adjusting for the number of RFs in control at baseline. Participants with 0 to 2 RFs in control had approximately twice the risk of death and 1.7× the risk of the composite outcome compared to participants with 6 RFs in-control (Table 5).
Table 5.
Hazard ratios for Death and CVD Events by Number of RFs In Control at Year 1 and Time-Varying*
|
Number of RFs at goal at Year 1 (N = 1,994) |
||||||
| Time to First Event After Year 1 | ||||||
| Death† | Death/MI/Stroke† | |||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| 0–2 | 2.1 | 1.2–3.7 | 0.0069 | 1.7 | 1.1–2.6 | 0.0199 |
| 3 | 1.7 | 1.0–2.8 | 0.0366 | 1.3 | 0.9–2.0 | 0.1589 |
| 4 | 1.1 | 0.7–1.8 | 0.7443 | 0.8 | 0.5–1.2 | 0.2118 |
| 5 | 1.1 | 0.7–1.8 | 0.7311 | 0.8 | 0.6–1.3 | 0.3929 |
| 6 | 1.0 | Reference | -- | 1.0 | Reference | -- |
| Global p value | 0.0056 | <0.0001 | ||||
|
Number of RFs at goal, time-varying RF control (N = 2,265) |
||||||
| Time to First Event After Randomization | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| 0–2 | 2.0 | 1.3 –3.3 | 0.0031 | 1.7 | 1.2, 2.5 | 0.0043 |
| 3 | 1.3 | 0.9–2.0 | 0.2092 | 1.2 | 0.8, 1.6 | 0.4071 |
| 4 | 1.1 | 0.8–1.7 | 0.6685 | 1.2 | 0.9–1.6 | 0.3239 |
| 5 | 0.8 | 0.5–1.2 | 0.2858 | 0.9 | 0.7–1.3 | 0.574 |
| 6 | 1.0 | Reference | -- | 1.0 | Reference | -- |
| Global p value | 0.0010 | 0.0035 | ||||
RF in control defined as: non-HDL-C <130 mg/dl; TG <150 mg/dl; SBP <130 mm Hg; DBP <80 mm Hg; HbA1c <7%; nonsmoker.
Cox models adjusted for: baseline number of RFs in control; number of total lesions; abnormal LVEF; myocardial jeopardy index; history of prior revascularization; age; sex; race/ethnicity; country; and trial strata. CI = confidence interval; CVD = cardiovascular disease; DBP = diastolic blood pressure; HR = hazard ratio; SBP = systolic blood pressure; TG = triglycerides. Other abbreviations as in Tables 1 and 2.
Outcomes Related to Time-Varying RFs In Control During the Trial
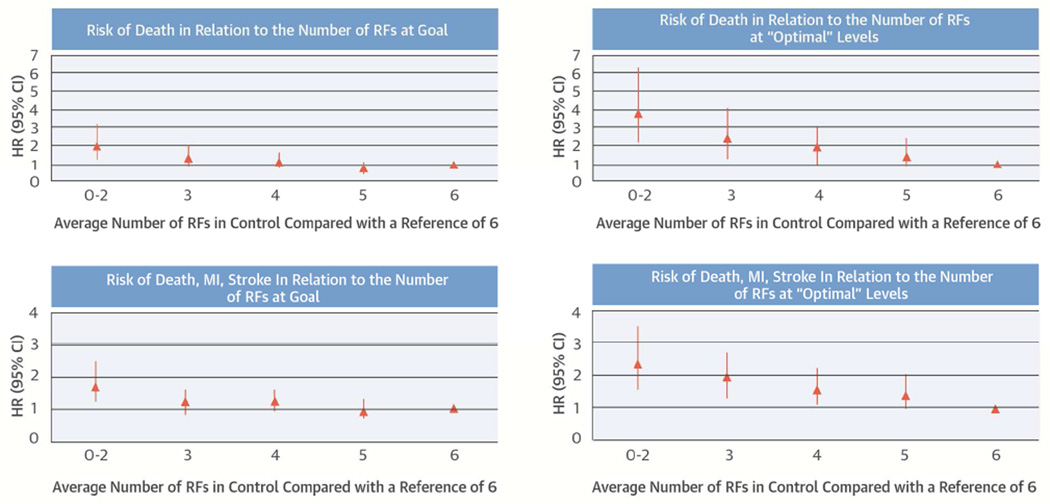
The number of RFs in control during the trial was strongly related to death (global p = 0.0010) and CVD event (global p = 0.0035) after adjusting for the number of baseline RFs in-control (Table 5). Participants with 0 to 2 RFs in control during follow-up were twice as likely to die as those with 6 RFs in control with similar results for CVD events. The model suggested a J-shape: participants with 6 RFs in control had nonsignificantly higher risks of death and the composite endpoint compared to individuals with 5 RFs in control.
Exploratory analysis to look for potential harms of intensive BP and glucose control
Table 6 shows hazard ratios as a function of the number of RFs in control, with systolic BP and HbA1c ranges modified to reflect less stringent control. The uptick in risk with 6 RFs in control compared to 5 RFs in control was no longer evident, suggesting that aggressive control of systolic BP or HbA1c is associated with increased risk. Hazard ratios associated with 0 to 2, 3, 4, and 5 RFs in control were consistently higher than in the main analysis (Central Illustration). Results were consistent with variations in the modified target ranges (Online Table 2). In analyses stratified by cardiac randomization group, those randomly assigned to revascularization within 4 weeks have a trend of larger benefit of RF control. However, the interaction between the treatment assignment and the number of RF in-control is not significant for either outcome (Online Table 3).
Table 6.
Hazard Ratios for Death and CVD Events by Number of RFs In Control/Within Target Range*
| Number of RFs In Control |
Death† | Death/MI/Stroke† | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| 0–2 | 3.8 | 2.2–6.5 | <0.0001 | 2.4 | 1.6–3.6 | <0.0001 |
| 3 | 2.4 | 1.4–4.1 | 0.0009 | 2.0 | 1.3–2.8 | 0.00011 |
| 4 | 1.9 | 1.1–3.0 | 0.0142 | 1.6 | 1.1–2.3 | 0.0163 |
| 5 | 1.5 | 0.9–2.4 | 0.1365 | 1.4 | 1.0–2.1 | 0.0709 |
| 6 | 1.0 | Reference | -- | 1.0 | Reference | -- |
| Global p value | <0.0001 | 0.0005 | ||||
RFs in-control defined as: non-HDL-C <130 mg/dl, TG <150 mg/dl, 110 mm Hg< SBP < 140 mm Hg, DBP <80 mm Hg, 6.5%< HbA1c <7.5%, nonsmoker. Note redefinition of target range for SBP and HbA1c in this exploratory analysis
Cox models adjusted for baseline number of RF in control, number of total lesions, abnormal LVEF, myocardial jeopardy index, history of prior revascularization, age, sex, race/ethnicity, country, and trial strata.
Central Illustration. Cardiac RF Control Improves Survival: Number of RFs in Control and Outcomes.
The number of RFs in control is plotted against mortality (A and B) and against CVD events (C and D). In panels A and C, RFs in control are defined on the basis of the BARI 2D protocol (main analysis). A J-shape is evident: individuals with 6 RFs in control have a numerically higher risk of events than those with 5 RFs in control. In panels B and D, “optimal ranges” are defined for systolic and diastolic BP and HbA1c. A J-shape is no longer evident and the risk gradient comparing 6 versus 0 to 2 RFs in control is steeper. BP = blood pressure; CVD = cardiovascular disease; HbA1c = glycosylated hemoglobin; HR = hazard ratio; MI = myocardial infarction; RF = risk factor.
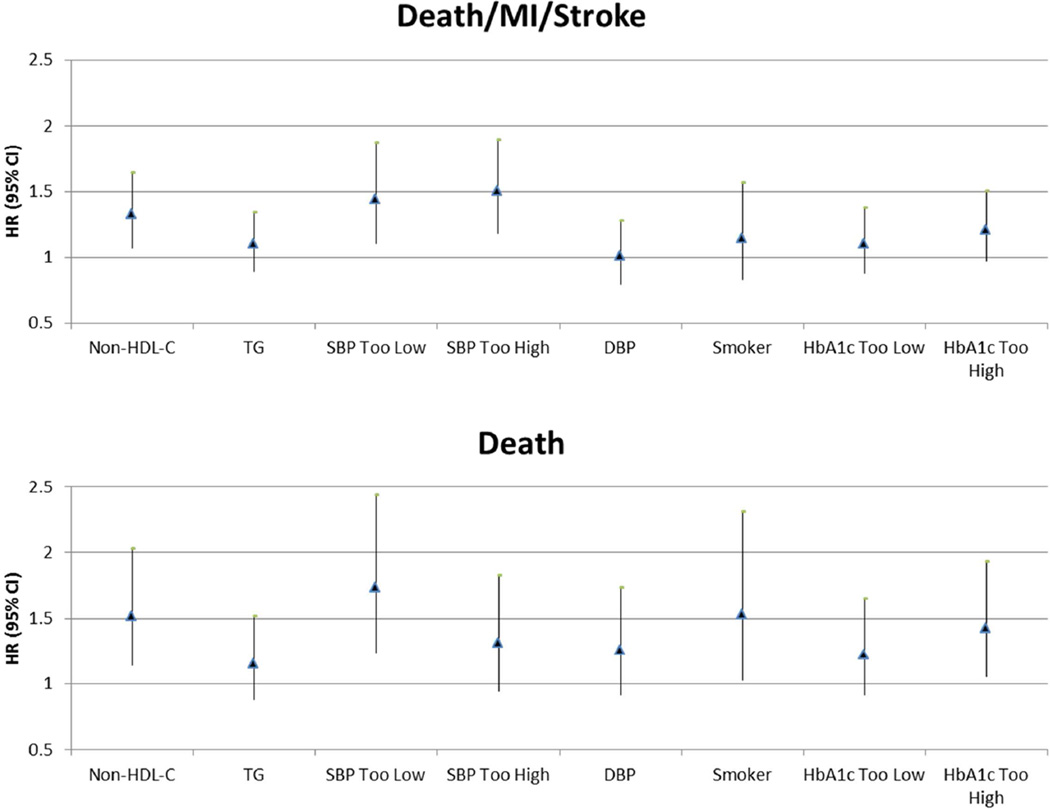
Figure 2 shows the adjusted effect of individual time-varying RF control status entered simultaneously into the same model on the outcomes of death and CVD events. Significant RFs for death included smoking, high non-HDL-C, systolic BP (too low), and HbA1c (too high). For CVD events, high non-HDL-C and systolic BP outside the target range (too low and too high) were significant predictors. When using a stepwise algorithm to identify the significant RFs, non-HDL-C and systolic BP outside the target range remained in the model (Online Table 4).
Figure 2. Hazard Associated With Individual RFs Out of Control/Out of Target Range.
Multivariable-adjusted hazard ratios (95% CI) are shown for individual RFs out of target range. RFs in control/in target range for this exploratory analysis were defined as: non-HDL-C <130 mg/dl, TG <150 mg/dl, 110 mm Hg< SBP <140 mm Hg, DBP <80 mm Hg, 65%< HbA1c <7.5%, nonsmoker. Cox models were adjusted for number of total lesions, abnormal LVEF, myocardial jeopardy index, history of prior revascularization, age, sex, race/ethnicity, country, and trial strata. DBP = diastolic blood pressure; HbA1c = glycosylated hemoglobin; LVEF = left ventricular ejection fraction; MI = myocardial infarction; non-HDL-C = high-density lipoprotein cholesterol; RF = risk factor; SBP = systolic blood pressure; TG = triglycerides.
Discussion
To our knowledge, this is the first study among people with T2DM and CHD to show a strong association between the number of RFs below predetermined target levels and clinical outcomes. These observational data suggest that individuals with CHD and T2DM require multiple RF interventions, including management of systolic BP and HbA1c, to avoid undertreatment and overtreatment.
RF control among persons with T2DM and CHD has improved, but treatment targets in effect during BARI 2D are often not achieved (3). The level of RF control at baseline in BARI 2D was comparable to that of a contemporary National Health and Nutrition Examination Survey cohort (3). Consistent with other recent trials that included patients with diabetes and CHD (4,5,11,12), BARI 2D data show that RF treatment goals are achievable using evidence-based, protocolguided therapy with dedicated personnel.
Prospective data on the benefits of multifactorial intervention in patients with diabetes are sparse. The Steno-2 study compared outcomes in patients with T2DM randomized to intensive management of multiple RFs versus usual care. Patients with intensively managed RFs had a 53% reduction in the 7-year risk for CVD events and a 46% reduction in mortality after post-trial follow-up to 13 years (4,5). The study was small (160 patients) and not designed to link observed benefits to achievement of specific treatment targets. Howard and colleagues observed benefits of tighter cholesterol and BP targets on carotid atherosclerosis in the Stop Atherosclerosis in Native Diabetics Study, but acknowledged a greater rate of adverse events associated with tighter BP control (13). Concerns were raised about increased mortality associated with “aggressive” treatment of hyperglycemia among patients with T2DM in the Action to Control Cardiovascular Risk in Diabetes Study (9). Long-term follow-up in the International Verapamil SR/Trandolapril Study suggested small, but significant increases in mortality among individuals with diabetes and CHD who achieved systolic BP <130 mm Hg compared with less stringent control (130 to 140 mm Hg) (14).
In the present study, the number of RFs in control at baseline was not related to study outcomes. In contrast, the number of RFs in control after 1 year of comprehensive medical intervention was strongly related to subsequent mortality and CVD events. Potential explanations for this observation include the potency of pharmacologic interventions initiated after randomization (statins and antihypertensive agents), which diminishes the prognostic value of baseline RFs and greater statistical power to show an effect of better RF control during follow-up when more participants have good RF control. Given that RF control at BARI 2D entry was comparable to the U.S. population with diabetes (3), these data suggest that, with appropriate resource allocation, similar improvements in prognosis could be achieved among people with diabetes in the general population.
Using BARI 2D treatment targets, individuals with 0 to 2 RFs under control had twice the risk of mortality and a 70% greater risk of death or CVD event during follow-up compared to those who had 6 RFs under control. These analyses also suggested a plateau of benefit at 5 RFs under control, with a small increase in risk among those who had 6 RF under control. Our exploratory analyses (including sensitivity analyses using 2 different ranges of “ideal” BP and HbA1c) suggested that over-control of systolic BP, but not HbA1c, could mediate this phenomenon.
Strengths and Limitations
BARI 2D represents a contemporary cohort of patients with T2DM, well characterized at baseline, with 5-year longitudinal assessment of RFs, and with adjudicated cardiovascular and mortality outcomes. Our statistical analysis has important strengths: first, it captured the cardiovascular and mortality risks associated with the number of RFs below target levels over the entire follow-up period; secondly, it assessed the risk associated with changes in RF status incorporating baseline RF status; thirdly, it adjusted for important confounders; and lastly, it explored the risk associated with BP and HbA1c within a target range.
We acknowledge some limitations. First, subjects enrolled in the BARI 2D study represent a selected population of individuals with T2DM, angiographically-documented stable CHD with revascularizable lesions, and myocardial ischemia followed at tertiary care centers. Secondly, while we express outcomes as a function of RF control, we are unable to distinguish benefits that accrued through pleiotropic effects of medications used to achieve RF control from benefits that accrued due to the actual level of each RF achieved. Finally, in our exploratory analysis, “over-control” of BP was associated with worse outcomes. Given the design of this post-hoc analysis, we are unable to distinguish between declines in BP due to intensified treatment as opposed to declines that occurred as a consequence of developing ill health. Our conclusion should thus be interpreted with caution and requires verification in specifically designed prospective trials.
Conclusion
Protocol-guided therapy with specific treatment targets can improve control of multiple RFs which relates to survival and future clinical events among patients with CHD and T2DM.
Supplementary Material
PERSPECTIVES.
Competency in Patient Care
In patients with type 2 diabetes mellitus (T2DM) and coronary artery disease, achievement of RF targets is related to cardiovascular events and mortality
Translational Outlook
Additional studies are needed to define optimal target levels for systolic blood pressure and glycated hemoglobin (HbA1c) for patients with T2DM.
Acknowledgments
Dr Bittner has research support from the NIH, Amgen, Bayer Healthcare, Janssen Pharmaceuticals, Pfizer and Sanofi-Aventis. She has served on advisory panels for Amgen and Eli Lilly. Dr. Farkouh has grant support from Eli Lilly and other research support from Boston Scientific, Bristol-Myers Squibb, Cordis, Eli Lilly, and Sanofi-Aventis. Dr. Rutter has grant support from Glaxo-Smith Kline, Eli Lilly and Company, and Novo Nordisk.
Sources of Funding:
BARI 2D was funded by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804, R21 HL121495). BARI 2D receives significant supplemental funding provided by: GlaxoSmithKline; Lantheus Medical Imaging Inc. (formerly Bristol-Myers Squibb Medical Imaging Inc.); Astellas Pharma US Inc.; Merck & Co Inc.; Abbott Laboratories Inc.; and Pfizer Inc. Generous support is given by Abbott Laboratories Ltd., MediSense Products; Bayer Diagnostics; Becton, Dickinson and Company; J.R. Carlson Labs; Centocor Inc.; Eli Lilly and Company; LipoScience Inc.; Merck Sante; Novartis Pharmaceuticals Corporation; and Novo Nordisk Inc. A full listing of all sponsors is in the Supplementary Appendix at http://www.NEJM.org (N Engl J Med. 2009;360:2503–15).
Abbreviations
- BP
blood pressure
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HbA1c
glycosylated hemoglobin
- HDL
high-density lipoprotein
- HR
hazard ratio
- IP
intraperitoneal
- MI
myocardial infarction
- RF
risk factor
- T2DM
type 2 diabetes mellitus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00006305
Disclosures: Drs. Bertolet, Feliz, Redmond, Ramanathan, Sperling and Ms. Goldberg have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 6.Brooks MM, Frye RL, Genuth S, et al. Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.BARI 2D Study Group. Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albu J, Gottlieb SH, August P, et al. Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Modifications of coronary risk factors. Am J Cardiol. 2006;97:41G–52G. doi: 10.1016/j.amjcard.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACCORD Study Group. Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barzilay JI, Howard AG, Evans GW, et al. Intensive blood pressure treatment does not improve cardiovascular outcomes in centrally obese hypertensive individuals with diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Blood Pressure Trial. Diabetes Care. 2012;35:1401–1405. doi: 10.2337/dc11-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden WE, O'Rourke RA, Teo KK, et al. COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 12.Farkouh ME, Domanski M, Sleeper LA, et al. FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 13.Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299:1678–1689. doi: 10.1001/jama.299.14.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.