Abstract
Preterm neonates are surviving with a milder spectrum of motor and cognitive disabilities that appear to be related to widespread disturbances in cell maturation that target cerebral gray and white matter. Whereas the preterm brain was previously at high risk for destructive lesions, preterm survivors now commonly display less severe injury that is associated with aberrant regeneration and repair responses that result in reduced cerebral growth. Impaired cerebral white matter growth is related to myelination disturbances that are initiated by acute death of pre-myelinating oligodendrocytes (preOLs), but are followed by rapid regeneration of preOLs that fail to normally mature to myelinating cells. Although immature neurons are more resistant to cell death than mature neurons, they display widespread disturbances in maturation of their dendritic arbors and synapses, which further contributes to impaired cerebral growth. Thus, even more mild cerebral injury involves disrupted repair mechanisms in which neurons and preOLs fail to fully mature during a critical window in development of neural circuitry. These recently recognized distinct forms of cerebral gray and white matter dysmaturation raise new diagnostic challenges and suggest new therapeutic strategies to promote brain growth and repair.
Keywords: Hypoxia-ischemia, prematurity, brain injury, white matter, gray matter, myelination, oligodendrocyte, astrocyte, glia, neuron
Neurodevelopmental disabilities continue to be a leading cause of morbidity in survivors of premature birth1-11 and persists at very high rates throughout life.12 Although improved neonatal intensive care has reduced the mortality of preterm neonates, 5-10% of preterm survivors continue to have major motor deficits, including cerebral palsy (CP), and more than half have significant cognitive, behavioral or sensory deficits. This broad range of disabilities is consistent with widely distributed brain abnormalities or problems with brain connectivity.13 Preterm children with IQ in the normal range, often display processing deficits related to attention and executive functions that include cognitive flexibility, inhibitory control and working memory,3,14,15 as well as deficits in visually-based information processing and language.16-21 Frequently, these cognitive and behavior problems persist to young adulthood.8-10,19,20,22,23
Given this broad spectrum of disabilities, how should neuro-rehabilitation be approached to improve the outcome for these children? This review will address this challenging and unresolved question by examining unexpected recent changes in our understanding of the pathogenesis of brain injury in preterm neonates. Whereas preterm infants were previously at high risk for destructive white and gray matter degeneration, preterm survivors now commonly display less severe injury that does not appear to involve pronounced glial or neuronal loss. Nevertheless, these milder forms of injury are associated with reduced cerebral growth. Recent human and experimental studies support that this impaired cerebral growth involves abnormal maturation of neurons and glia rather than cell death. These complex and disparate cellular responses result in large numbers of cells that fail to fully mature during a critical window in the development of neural circuitry. These recently recognized forms of cerebral gray and white matter dysmaturation raise new diagnostic challenges and suggest new therapeutic directions centered on reversal of the processes that promote dysmaturation.
I. Pathogenesis of White Matter Injury
The Changing Spectrum of White Matter Injury in Contemporary Preterm Survivors
In prior decades, preterm infants were at much higher risk for destructive brain lesions that resulted in cystic necrotic white matter injury (WMI) and secondary cortical and subcortical gray matter degeneration. Whereas cystic lesions were previously the major form of WMI in preterm survivors, the incidence has markedly declined.24-27 In several recent series, focal cystic lesions were detected by MRI in less than 5% of cases.24-29 More commonly, a milder spectrum of WMI is seen, which is characterized by two distinct forms of injury (Figure 1). The minor component of WMI is comprised of small discrete foci of microscopic necrosis (microcysts) that typically measure less than a millimeter.30 Due to their relatively small size, microcysts are occult lesions that are typically not detected by MRI. Although microcysts have been observed in ∼35% of autopsy cases, they comprised only ∼1-5% of total lesion burden.31 Moreover, the overall burden of human necrotic WMI (cystic and microcystic) was decreased by ∼10-fold in contemporary cohorts relative to retrospective cases from earlier decades.31 Essentially complete myelination failure occurs in these relatively uncommon but clinically significant necrotic lesions as a consequence of the degeneration of all cellular elements.
Figure 1.
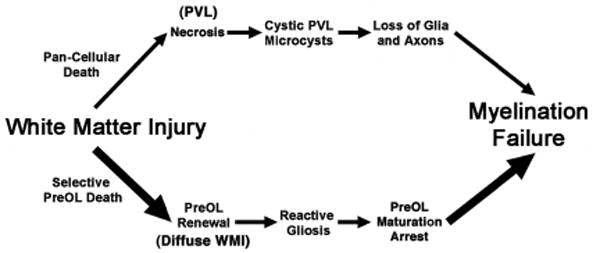
Pathogenesis of myelination failure arising from white matter injury (WMI) related to pan-cellular death and necrosis (periventricular leukomalacia (PVL); upper pathway) or selective preOL death and diffuse WMI (lower pathway). Note that the lower pathway is the dominant one in most contemporary preterm survivors, whereas the minor upper pathway reflects the declining burden of white matter necrosis that has accompanied advances in neonatal intensive care. Severe necrosis results in macrocystic lesions (cystic PVL), whereas milder necrosis results in microcysts. Milder WMI selectively triggers early preOL death. PreOLs renewal arises from the rapid regeneration of preOLs from a pool of early OL progenitors that are much more resistant to cell death in WMI. Chronic lesions are enriched in reactive astrocytes that generate inhibitory signals that block the maturation of preOLs to mature myelinating oligodendrocytes.
Diffuse WMI is currently the most frequently observed form of WMI in premature newborns. Diffuse WMI evolves from early lesions where the oligodendrocyte (OL) lineage is particularly susceptible to oxidative damage32 of a magnitude consistent with hypoxia-ischemia.33,34 Initially, diffuse WMI causes widespread selective degeneration of late OL progenitors (preOLs) in pre-myelinating white matter. Axons are mostly spared in pre-myelinating white matter35 except in necrotic foci.36,37 At the onset of myelination, axons expand in caliber and display increased susceptibility to oxidative stress and hypoxia-ischemia.38-40 Hence, disturbances in myelination are initiated by “selective vulnerability” of preOLs that are enriched in human cerebral white matter during the high-risk period for WMI.41
In about one-third of preterm newborns at 24-32 weeks gestation, diffuse WMI is readily identified on MRI as multi-focal lesions in the first weeks of life.1,42 While MRI-defined focal lesions are associated with increased risk for neurocognitive and motor dysfunction, they likely underestimate the extent of WMI and the burden of neurodevelopmental disability.1,43-46 As premature newborns grow to term age, early multifocal lesions give rise to more widespread abnormalities in microstructural and metabolic brain development.42,47-49 The consequence is adverse long-term neurodevelopmental outcomes, which are associated with disrupted white matter maturation that manifests in childhood as altered brain structure and connectivity. 13,44,50-55
Abnormal Myelination in Diffuse WMI is Mediated by Degeneration and Dysmaturation of PreOLs
PreOLs predominate in human cerebral white matter throughout the high-risk period for WMI and are the primary cell type that degenerates in early WMI in the setting of hypoxia-ischemia or inflammation.32,56,57 Subsequently, abnormal myelination occurs via a process that involves a more complex and potentially reversible process linked to arrested pre-OL maturation. Several studies found, paradoxically, that although pronounced extensive degeneration of preOLs occurs in acute lesions, no significant depletion of OL lineage cells was observed in chronic human or experimental lesions.31,58-61 This is related to the fact that developing white matter is populated by a large reservoir of early OL progenitors that are resistant to hypoxia-ischemia and proliferate robustly in the setting of preOL degeneration.62 Thus, after WMI, developing white matter is capable of engaging repair mechanisms that trigger the rapid regeneration and pronounced expansion of preOLs that derive from early OL progenitors.58,63,64 Although, regeneration of preOLs compensates for preOL death, these newly generated preOLs display persistent arrested differentiation in chronic lesions and fail to myelinate intact axons. Arrested maturation of preOLs was shown to contribute to myelination failure in diffuse WMI in both preterm fetal sheep and human WMI.31,60. Hence, chronic diffuse WMI is characterized by an aberrant response to acute injury, which involves a disrupted regeneration and repair process where preOLs are regenerated but remain dysmature.
PreOL maturation arrest may adversely influence subsequent white matter maturation in several ways. Oligodendrocytes are critical for axon survival,65 raising the possibility that preOL arrest could also adversely affect the functional integrity of axons in chronic lesions. Chronic WMI may also coincide with an expanded developmental window during which preOL maturation-arrest persists and confers an enhanced risk for recurrent and potentially more severe WMI.58
There continues to be a significant proportion of preterm infants for whom WMI derives from poorly defined antenatal or perinatal factors that may not be recognized until after the early injury period. Hence, there is a need for alternative therapies that enhance myelination and promote regeneration and repair of chronic WMI. PreOL maturation arrest appears to be related to a complex array of intrinsic, extrinsic and epigenetic factors that regulate preOL cell cycle exit, OL lineage progression and myelination.66-70 Excitatory and inhibitory synaptic inputs to OL progenitors may influence preOL maturation, although this has not been defined in preterm WMI.71,72 Multiple molecules likely act in concert with other signals in chronic white matter lesions to prevent preOL maturation and normal myelination. A number of these signals appear to be linked to reactive astrogliosis.73,74
In diffuse WMI, the extracellular matrix (ECM) is a rich source of reactive astrocyte-derived inhibitory factors that are potential mediators of myelination failure. One such factor is hyaluronic acid (HA) and one of its receptors, CD44, which are both markedly elevated in chronic human WMI.31 Arrest of preOL maturation is stimulated both in vitro and in vivo by bioactive HA fragments that are generated by a CNS enriched hyaluronidase, PH20 that is persistently expressed in chronic WMI.75 Pharmacological inhibition of PH20 promotes OL maturation in vitro and myelination in vivo, which is accompanied by enhanced nerve conduction.76 Reactive astrocytes also express bone morphogenetic proteins that inhibit OL progenitor differentiation.77 Dysregulation of WNT-beta catenin signaling in OL progenitors promotes preOL arrest, delays normal myelination and disrupts remyelination.78-84 Constitutive expression of the epidermal growth factor receptor (EGFR) in neonatal white matter promotes pronounced proliferation of OL progenitors.85 EGFR activation via an intranasally administered form of EGF reduced OL death from neonatal chronic hypoxia, promoted OL maturation and myelination and lead to functional improvement.86 Other astrocyte-derived growth factors also may contribute to preOL arrest. Insulin-like growth factors promote OL lineage cell survival and myelination and protect against OL progenitor loss in fetal and neonatal models of WMI.87-89 Definition of the mechanisms that link these inhibitory pathways to trigger myelination failure will be critical to develop new regeneration and repair therapies for neonatal WMI and other chronic myelin disorders that include multiple sclerosis and vascular dementia.
II. Pathogenesis of Gray Matter Abnormalities
Preterm Survivors Display Impaired Cerebral Gray Matter Growth and Function
Several large human neuroimaging studies have identified that preterm survivors display significant reductions in the growth of the cerebral cortex and subcortical gray matter structures that include the basal ganglia, thalamus, hippocampus and cerebellum.50,90-93 Reduced cortical growth further manifests as varying degrees of abnormal cortical folding and reduced gyral complexity.94 As children and adults, preterm survivors with normal neurocognitive function nevertheless display altered cortical activation and functional connectivity during language and visual processing.13,95-98 In prior decades, these abnormalities were attributed to primary or secondary gray matter injury related to severe WMI. Currently, many premature newborns still acquire extensive gray matter abnormalities, but with a lack of overt gray or white matter injury, as identified by signal abnormalities on structural MRI. On fcMRI, preterm newborns at term display reduced functional connectivity between the cortex and thalamus.99,100 In fact, altered functional connectivity can persist into adolescence and is now recognized as a critical risk factor for adverse neurocognitive outcomes.53,95,96 As has been observed for WMI, such findings thus support the notion that for many patients, the spectrum of gray matter abnormalities has shifted to milder forms that involve primarily developmental disturbances rather than destructive processes.
Factors Related to the Extent of Preterm Gray Matter Abnormalities
Although cerebral neurons in the full term neonate are highly susceptible to hypoxic-ischemic degeneration, the susceptibility of preterm neurons to hypoxia-ischemia is more variable and appears to be related to the severity of the underlying insult and the associated severity of WMI. Neuronal degeneration is particularly prominent in the setting of significant white matter necrosis. In experimental studies, widespread death of preterm neurons was triggered by prolonged hypoxia-ischemia that also caused cystic necrotic WMI.34 Significant neuronal loss has been observed in human cortex, subplate, basal ganglia, thalamus and cerebellum in association with necrotic WMI,30,101-103 but not in autopsy cases where isolated diffuse non-necrotic WMI occurred.30,104 Neuronal degeneration thus may arise directly from primary energy failure or secondary to retrograde axonal degeneration triggered by necrotic WMI.
By contrast, immature neurons in the preterm cerebrum do not appear to significantly degenerate under conditions that primarily generate diffuse non-necrotic WMI (Figure 2). In human autopsy cases with early diffuse WMI (Fig. 2A-D), neither preterm gray matter neurons or white matter axons displayed significant oxidative injury or degeneration.32 Similarly, in preterm fetal sheep with diffuse WMI and preOL degeneration (Fig. 2E-H), neurons and axons rarely degenerated.34 Importantly, quantitative cerebral blood flow studies demonstrated that the magnitude of ischemia in fetal sheep periventricular white matter was very similar to that in superficial cortex and subcortical structures including the caudate nucleus.34,105 Hence, neurons in the preterm gray matter appear to be relatively resistant to hypoxic-ischemic degeneration, which is not prominent unless more severe necrotic WMI occurs.
Figure 2.
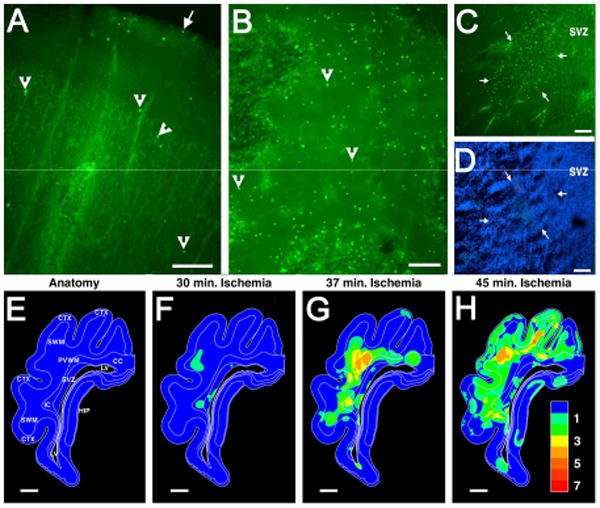
Diffuse WMI is associated with markedly less cerebral gray matter injury in preterm human autopsy cases (A-D) and in a pre-clinical large animal model of WMI in preterm fetal sheep (E-H). From a preterm human autopsy case diagnosed with early diffuse WMI, the relative amounts of cell death are compared in parietal cerebral cortex (A) relative to a diffuse deep cerebral white matter lesion (B). From these images of fluorescently-labeled degenerating cells (arrowheads provide typical examples), the paucity of degenerating cells in the cortex (A) relative to the white matter (B) can be appreciated. The large arrow in A indicates the pial surface. (C) This image shows an early small focal deep white matter lesion (arrows), adjacent to the subventricular zone (SVZ), that was less than a millimeter in diameter. Such lesions typically evolve to microscopic necrosis. (D) This image visualizes all of the cells present in the region shown in C and demonstrates the high density of cells in the SVZ relative to the adjacent deep white matter in the center of the image. (E) Anatomical diagram of the preterm fetal sheep brain at 0.65 gestation, which is approximately equivalent to the preterm human brain at 26-28 weeks gestation. Animals were analyzed for the distribution of degenerating cells that occurred in response to cerebral hypoxia-ischemia of durations of 30 minutes (min.) (F) 37 minutes (G) or 45 minutes (H). The pseudocolor maps in F-H indicate the overlapping distributions of degenerating cells observed in four animals at the level of parietal cortex. The pseudocolor probability scale (1, low; 7, high) indicates the relative frequency with which cell death was observed throughout this level of the brain. Note that WMI in the periventricular white matter (PVWM), superficial white matter (SWM), internal capsule (IC) and corpus callosum (CC) increased as the duration of ischemia was increased from 30 to 45 min. Injury to the cerebral cortex (CTX) and hippocampus (HIP) was rarely observed until the duration of ischemia was very prolonged to 45 min., which also coincided with a pronounced increase in necrotic WMI (H). Panels A-D adapted from Back et al., 2005.32 Panels E-H adapted from Riddle et al., 2006.34 Scale Bars: = 200 μm (A); 100 μm (B-D); 2 mm (E-H).
Gray Matter Injury and Neuronal Dysmaturation
Despite the greater resistance of preterm neurons to hypoxic-ischemic degeneration, cerebral gray matter growth is paradoxically reduced in the setting of diffuse WMI. To study this paradox, we developed a preterm fetal sheep model of global cerebral ischemia that sustains chronic diffuse WMI and reduced growth of the cerebral cortex and caudate nucleus. Reduced cortical and caudate growth was not related to axonal degeneration in the white matter.37 Neither was it related to degeneration of migrating neuronal progenitors in the white matter. Degeneration of GABAergic interneurons has been proposed to occur during their migration through human white matter during periods of WMI.106 However, reduced growth of the caudate was not explained by loss of GABAergic interneurons or projection neurons. Reduced growth of the cerebral cortex and the caudate was also not related to loss of cortical pyramidal neurons107 or medium spiny projection neurons in the caudate,108 which comprise the majority of neurons in each region.
A detailed analysis of the maturation of the dendritic arbor of cortical107 and caudate108 projection neurons demonstrated that reduced growth of both regions was accompanied by a significant reduction in the complexity of the dendritic arbor; consistent with the notion that neuronal maturation was disrupted in the setting of cerebral ischemia. Compared to controls, the ischemic animals displayed neuronal dysmaturation that was reflected in a reduction in the total dendritic length as well as the number of branches, branch endings and branch points. Notably, the dendritic arbor was most simplified closer to the cell body where synaptic integration occurs. Hence, widespread disturbances in maturation of cortical and caudate projection neurons occurred in association with non-destructive diffuse WMI.
Disturbances in Synaptic Activity Accompany Neuronal Dysmaturation
Since disturbances in neuronal maturation occur during a critical window in the establishment of neuronal connections, we determined whether neuronal dysmaturation also disrupts the development of normal CNS circuitry at the level of dendritic spines, the key sites for synaptic activity. In response to ischemia, reduced numbers of spines were observed on the dysmature dendrites of projection neurons in both the cortex107 and caudate.108 Electrophysiological studies in the caudate nucleus employed patch clamp recordings on medium spiny projection neurons, which identified significant abnormalities in excitatory synaptic activity mediated by both NMDA and AMPA glutamate receptors in fetal sheep that were studied one month after preterm hypoxia-ischemia.108 Collectively, these findings begin to provide an explanation for the global disturbances in gray matter growth in preterm survivors that may occur via reduced dendritic complexity and widespread disturbances in neuronal connectivity and synaptic activity. Abnormalities in synaptic activity in the caudate, for example, may contribute to a wide variety of neurobehavioral disabilities in preterm survivors that include motor dyspraxias and transient dystonia, as well as disturbances in learning, memory, attention and executive function.
Neuronal Dysmaturation gives rise to MRI-defined Abnormalities in Cortical Fractional Anisotropy (FA)
MRI-defined changes in fractional anisotropy (FA) have been extensively studied as a non-invasive means to define the progression of normal cortical maturation. A progressive decline in FA accompanies cortical maturation in human109 and non-human primates.110-112 Two recent human studies found that the normal progressive loss of cortical FA was delayed in human preterm survivors with impaired postnatal growth93 or reduced cortical growth.113 Similarly, in response to hypoxia-ischemia, we found that preterm fetal sheep displayed impaired cortical growth that was accompanied by significantly higher cortical FA.107 To explain this observation, we analyzed whether disrupted maturation of cortical projection neurons was related to these cortical FA disturbances. We employed a mathematical model that was developed to estimate FA based upon the predicted patterns of water diffusion along the dendritic arbor of cortical projection neurons defined by Golgi stain.107 This approach confirmed that the FA values derived from MRI and neuronal morphology were very similar.107 Moreover, the cortical FA values derived from neuronal morphology were higher for the ischemic neurons relative to control neurons, consistent with less random water diffusion along the processes of the more immature ischemic neurons. Interestingly, one factor associated with abnormal cortical FA in human preterm neonates was impaired somatic growth (weight, length, and head circumference); even after accounting for WMI and systemic illness (e.g., infection).93 In addition to abnormal nutritional status, impaired cortical growth and function was also recently related to stressful procedures sustained by preterm neonates during intensive care.98,114,115 Hence, a number of additional factors other than cerebral ischemia may also potentially contribute to the pathogenesis of neuronal dysmaturation in preterm survivors and the persistence of widespread disturbances in neuronal connectivity.
Conclusions
Advances in neonatal care have markedly reduced the overall severity of cerebral injury in infants born prematurely. At the more severe end of the spectrum are preterm survivors with major motor and cognitive disabilities related to destructive lesions where focal or more diffuse loss of neurons and glia occurs. For the majority of more mildly affected children, the burden of chronic disabilities may be related to a primary diffuse cerebral dysmaturation disorder that may respond to interventions directed at promoting brain maturation and improved neurological outcome. The activation of dysmaturation processes is emerging as a key factor that disrupts regeneration and repair in the preterm brain after injury. Glial progenitors respond to WMI by mounting an aberrant repair process in which the preOL pool regenerates and expands but fails to mature. The maturation block may involve both intrinsic and extrinsic factors that prevent preOL maturation and myelination. In response to hypoxia-ischemia, major populations of immature cortical and caudate projection neurons do not degenerate, but fail to normally mature. In contrast to mature neurons in the full term neonate that degenerate in response to hypoxia-ischemia,116 immature neurons in the preterm brain are markedly more resistant to similar injury. However, after hypoxia-ischemia, they still respond aberrantly and survive with a simplified dendritic arbor that contributes to a reduction in cerebral growth and abnormal synaptic activity. Thus, cellular dysmaturation in gray and white matter may disrupt a critical developmental window that coincides with rapid brain growth and enhanced neuronal connectivity related to elaboration of the dendritic arbor, synaptogenesis and myelination.
The timing and nature of neuronal and glia dysmaturation suggests that each may respond to distinct interventions to potentially enhance cerebral gray or white matter growth by promoting regeneration and repair of cerebral injury. Factors such as improved infant nutrition, reduction in postnatal infections, a lower burden of painful procedures and earlier behavioral interventions may all potentially enhance cerebral gray matter growth and maturation. Pharmacological interventions aimed at blocking the inhibitory pathways that sustain preOL maturation arrest may reverse or prevent myelination failure with the potential for enhanced connectivity of CNS pathways. Hence, new insights into the pathogenesis of preterm cerebral injury have redirected our focus to a variety of promising new strategies to promote enhanced brain maturation and growth that were not previously feasible when impaired brain development was largely attributable to severe tissue destruction.
Acknowledgments
This work was supported by the NIH (National Institutes of Neurological Disorders and Stroke: 1R01NS054044, and R37NS045737-06S1/06S2, the National Institute on Aging: 1R01AG03189, the American Heart Association (#11GRNT7510072) and the March of Dimes Birth Defects Foundation (#6-FY11-323).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005 Nov;147(5):609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Vohr BR, Allan WC, Westerveld M, et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003 Apr;111(4 Pt 1):e340–346. doi: 10.1542/peds.111.4.e340. [DOI] [PubMed] [Google Scholar]
- 3.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005 Jan 6;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 4.Bodeau-Livinec F, Marlow N, Ancel PY, Kurinczuk JJ, Costeloe K, Kaminski M. Impact of intensive care practices on short-term and long-term outcomes for extremely preterm infants: comparison between the British Isles and France. Pediatrics. 2008 Nov;122(5):e1014–1021. doi: 10.1542/peds.2007-2976. [DOI] [PubMed] [Google Scholar]
- 5.Roberts G, Anderson PJ, Doyle LW. Neurosensory disabilities at school age in geographic cohorts of extremely low birth weight children born between the 1970s and the 1990s. J Pediatr. 2009 Jun;154(6):829–834 e821. doi: 10.1016/j.jpeds.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Walsh MC, Hibbs AM, Martin CR, et al. Two-year neurodevelopmental outcomes of ventilated preterm infants treated with inhaled nitric oxide. J Pediatr. 2010 Apr;156(4):556–561 e551. doi: 10.1016/j.jpeds.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts G, Anderson PJ, Doyle LW. The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Arch Dis Child. 2010 Jun 16;95(10):786–790. doi: 10.1136/adc.2009.160283. [DOI] [PubMed] [Google Scholar]
- 8.Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (< or =800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004 Dec;114(6):e725–732. doi: 10.1542/peds.2004-0932. [DOI] [PubMed] [Google Scholar]
- 9.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002 Jan 17;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 10.Lindstrom K, Winbladh B, Haglund B, Hjern A. Preterm infants as young adults: a Swedish national cohort study. Pediatrics. 2007 Jul;120(1):70–77. doi: 10.1542/peds.2006-3260. [DOI] [PubMed] [Google Scholar]
- 11.Saigal S, den Ouden L, Wolke D, et al. School-age outcomes in children who were extremely low birth weight from four international population-based cohorts. Pediatrics. 2003 Oct;112(4):943–950. doi: 10.1542/peds.112.4.943. [DOI] [PubMed] [Google Scholar]
- 12.Synnes AR, Anson S, Arkesteijn A, et al. School Entry Age Outcomes for Infants with Birth Weight </=800 Grams. J Pediatr. 2010 Jul 31;157(6):989–994. doi: 10.1016/j.jpeds.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Doesburg SM, Ribary U, Herdman AT, et al. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage. 2011 Feb 1;54(3):2330–2339. doi: 10.1016/j.neuroimage.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007 Nov 30;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004 Jul;114(1):50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Grunau RV, Kearney SM, Whitfield MF. Language development at 3 years in pre-term children of birth weight below 1000 g. Br J Disord Commun. 1990 Aug;25(2):173–182. doi: 10.3109/13682829009011972. [DOI] [PubMed] [Google Scholar]
- 17.Whitfield MF, Grunau RV, Holsti L. Extremely premature (< or = 800 g) schoolchildren: multiple areas of hidden disability. Arch Dis Child Fetal Neonatal Ed. 1997 Sep;77(2):F85–90. doi: 10.1136/fn.77.2.f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Arch Pediatr Adolesc Med. 2002 Jun;156(6):615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- 19.Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004 Mar;10(2):149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- 20.Saavalainen P, Luoma L, Bowler D, et al. Spatial span in very prematurely born adolescents. Dev Neuropsychol. 2007;32(3):769–785. doi: 10.1080/87565640701539535. [DOI] [PubMed] [Google Scholar]
- 21.Luu TM, Vohr BR, Schneider KC, et al. Trajectories of receptive language development from 3 to 12 years of age for very preterm children. Pediatrics. 2009 Jul;124(1):333–341. doi: 10.1542/peds.2008-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM. Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc. 2007 Jul;13(4):571–581. doi: 10.1017/S1355617707070725. [DOI] [PubMed] [Google Scholar]
- 23.Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain. 2002 Jul;125(Pt 7):1646–1659. doi: 10.1093/brain/awf159. [DOI] [PubMed] [Google Scholar]
- 24.Hamrick S, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145(5):593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 25.Counsell S, Allsop J, Harrison M, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112(1):176–180. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonagraphy findings. AJNR Am J Neuroradiol. 2003;24:1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 27.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003 May;24(5):805–809. [PMC free article] [PubMed] [Google Scholar]
- 28.Maalouf E, Duggan P, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–727. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 29.Groenendaal F, Termote JU, van der Heide-Jalving M, van Haastert IC, de Vries LS. Complications affecting preterm neonates from 1991 to 2006: what have we gained? Acta Paediatr. 2010 Mar;99(3):354–358. doi: 10.1111/j.1651-2227.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- 30.Pierson CR, Folkerth RD, Billiards SS, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007 Dec;114(6):619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buser J, Maire J, Riddle A, et al. Arrested pre-oligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71(1):93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 33.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22(2):455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddle A, Luo N, Manese M, et al. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci. 2006;26:3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alix JJ, Zammit C, Riddle A, et al. Central axons preparing to myelinate are highly sensitivity to ischemic injury. Ann Neurol. 2012 Dec;72(6):936–951. doi: 10.1002/ana.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008 Jun;63(6):656–661. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddle A, Maire J, Gong X, et al. Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke. 2012;43(1):178–184. doi: 10.1161/STROKEAHA.111.632265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarran W, Goldberg M. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury Is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 2009 Nov;66(5):682–693. doi: 10.1002/ana.21767. [DOI] [PubMed] [Google Scholar]
- 40.Tekkok SB, Goldberg MP. AMPA/Kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21(12):4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001 Feb 15;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009 Aug;66(2):155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 43.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006 Aug 17;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 44.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008 Dec;131(Pt 12):3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007 Sep;120(3):e604–609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 46.Chau V, Synnes A, Grunau R, Poskitt K, Brant R, Miller S. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81(24):2082–20889. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. Journal of magnetic resonance imaging : JMRI. 2002 Dec;16(6):621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 48.Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr. 2010 Jun;156(6):882–888. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bassi L, Chew A, Merchant N, et al. Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res. 2011 Jun;69(6):561–566. doi: 10.1203/PDR.0b013e3182182836. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007 Apr;119(4):759–765. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- 51.Ment LR, Kesler S, Vohr B, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009 Feb;123(2):503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008 Apr;152(4):513–520. 520 e511. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mullen KM, Vohr BR, Katz KH, et al. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011 Feb 14;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalpakidou AK, Allin MP, Walshe M, et al. Neonatal brain injury and neuroanatomy of memory processing following very preterm birth in adulthood: an fMRI study. PloS one. 2012;7(4):e34858. doi: 10.1371/journal.pone.0034858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandit AS, Robinson E, Aljabar P, et al. Whole-Brain Mapping of Structural Connectivity in Infants Reveals Altered Connection Strength Associated with Growth and Preterm Birth. Cereb Cortex. 2013 Mar 31; doi: 10.1093/cercor/bht086. [DOI] [PubMed] [Google Scholar]
- 56.Back S. Mechanisms of Acute and Chronic Brain Injury in the Preterm Infant. In: Miller S, Shevell M, editors. Acquired Brain Injury in the Fetus and Newborn. London: Mac Keith Press; 2012. pp. 29–52. [Google Scholar]
- 57.Favrais G, van de Looij Y, Fleiss B, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011 May 20;70(4):550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 58.Segovia K, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63(4):517–526. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Billiards S, Haynes R, Folkerth R, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathology. 2008;18(2):153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riddle A, Dean J, Buser JR, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011 Sep;70(3):493–507. doi: 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verney C, Pogledic I, Biran V, Adle-Biassette H, Fallet-Bianco C, Gressens P. Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J Neuropathol Exp Neurol. 2012 Mar;71(3):251–264. doi: 10.1097/NEN.0b013e3182496429. [DOI] [PubMed] [Google Scholar]
- 62.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002 Jan 15;22(2):455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhiheng H, Liu J, Cheung PY, Chen C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemia brain injury. Brain Res. 2009;1301:100–109. doi: 10.1016/j.brainres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Wright J, Zhang G, Yu TS, Kernie S. Age-related changes in the oligodendrocyte progenitor pool influence brain remodeling after injury. Dev Neurosci. 2010;32(5-6):499–509. doi: 10.1159/000322081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012 Jul 26;487(7408):443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barres B, Schmid R, Sendnte M, Raff M. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 67.Emery B, Agalliu D, Cahoy JD, et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009 Jul 10;138(1):172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sizonenko SV, Bednarek N, Gressens P. Growth factors and plasticity. Semin Fetal Neonatal Med. 2007 Aug;12(4):241–249. doi: 10.1016/j.siny.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Silbereis J, Huang E, Back S, Rowitch D. Toward improved animal models of neonatal white matter injury associate with cerebral palsy. Disease models & mechanisms. 2010;3(11-12):678–688. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fancy SP, Kotter MR, Harrington EP, et al. Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp Neurol. 2010 Sep;225(1):18–23. doi: 10.1016/j.expneurol.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 71.Bergles DE, Jabs R, Steinhauser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010 May;63(1-2):130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;16:3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherman L, Back S. A GAG reflex prevents repair of the damaged CNS. Trends in neurosciences. 2008;31(1):44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014 Jul 16;83(2):283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagen MW, Riddle A, McClendon E, et al. Role of recurrent hypoxia-ischemia in preterm white matter injury severity. PloS one. 2014;9(11):e112800. doi: 10.1371/journal.pone.0112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preston M, Gong X, Su W, et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013 Feb;73(2):266–280. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Cheng X, He Q, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fancy SP, Harrington EP, Baranzini SE, et al. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat Neurosci. 2014 Apr;17(4):506–512. doi: 10.1038/nn.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009 Nov;42(3):255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Fancy SP, Baranzini SE, Zhao C, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes & development. 2009 Jul 1;23(13):1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fancy S, Harrington E, Yuen T, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14(8):1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tawk M, Makoukji J, Belle M, et al. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011 Mar 9;31(10):3729–3742. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McClain CR, Sim FJ, Goldman SA. Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. J Neurosci. 2012 Oct 24;32(43):15066–15075. doi: 10.1523/JNEUROSCI.1320-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye F, Chen Y, Hoang T, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009 Jul;12(7):829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008 Jan 23;28(4):914–922. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scafidi J, Hammond TR, Scafidi S, et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014 Feb 13;506(7487):230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guan J, Bennet L, George S, et al. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. Journal of Cerebral Blood Flow and Metabolism. 2001 May;21(5):493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Pang Y, Zheng B, Campbell LR, Fan LW, Cai Z, Rhodes PG. IGF-1 can either protect against or increase LPS-induced damage in the developing rat brain. Pediatr Res. 2010 Jun;67(6):579–584. doi: 10.1203/PDR.0b013e3181dc240f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wood TL, Loladze V, Altieri S, et al. Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev Neurosci. 2007;29(4-5):302–310. doi: 10.1159/000105471. [DOI] [PubMed] [Google Scholar]
- 90.Keunen K, Kersbergen KJ, Groenendaal F, Isgum I, de Vries LS, Benders MJ. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J Matern Fetal Neonatal Med. 2012 Apr;25(Suppl 1):89–100. doi: 10.3109/14767058.2012.664343. [DOI] [PubMed] [Google Scholar]
- 91.Tam EW, Ferriero DM, Xu D, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009 Jul;66(1):102–106. doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nossin-Manor R, Chung AD, Whyte HE, Shroff MM, Taylor MJ, Sled JG. Deep gray matter maturation in very preterm neonates: regional variations and pathology-related age-dependent changes in magnetization transfer ratio. Radiology. 2012 May;263(2):510–517. doi: 10.1148/radiol.12110367. [DOI] [PubMed] [Google Scholar]
- 93.Vinall J, Grunau RE, Brant R, et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med. 2013 Jan 16;5(168):168ra168. doi: 10.1126/scitranslmed.3004666. [DOI] [PubMed] [Google Scholar]
- 94.Engelhardt E, Inder TE, Alexopoulos D, et al. Regional impairments of cortical folding in premature infants. Ann Neurol. 2015 Jan;77(1):154–162. doi: 10.1002/ana.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gozzo Y, Vohr B, Lacadie C, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009 Nov 1;48(2):458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schafer RJ, Lacadie C, Vohr B, et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009 Mar;132(Pt 3):661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Narberhaus A, Lawrence E, Allin MP, et al. Neural substrates of visual paired associates in young adults with a history of very preterm birth: alterations in fronto-parieto-occipital networks and caudate nucleus. Neuroimage. 2009 Oct 1;47(4):1884–1893. doi: 10.1016/j.neuroimage.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 98.Doesburg SM, Chau CM, Cheung TP, et al. Neonatal pain-related stress, functional cortical activity and visual-perceptual abilities in school-age children born at extremely low gestational age. Pain. 2013 Apr 8;154:1946–1952. doi: 10.1016/j.pain.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb Cortex. 2010 Mar 17;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex. 2014 Oct 20; doi: 10.1093/cercor/bhu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andiman SE, Haynes RL, Trachtenberg FL, et al. The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 2010 Jul;20(4):803–814. doi: 10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagasunder AC, Kinney HC, Bluml S, et al. Abnormal microstructure of the atrophic thalamus in preterm survivors with periventricular leukomalacia. AJNR Am J Neuroradiol. 2011 Jan;32(1):185–191. doi: 10.3174/ajnr.A2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kinney H, Haynes R, Xu G, et al. Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann Neurol. 2012;71(3):397–406. doi: 10.1002/ana.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pogledic I, Kostovic I, Fallet-Bianco C, Adle-Biassette H, Gressen P, Verney C. Involvement of the subplate zone in preterm infants with periventricular white matter injury. Brain Pathol. 2014 Mar;24(2):128–141. doi: 10.1111/bpa.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McClure M, Riddle A, Manese M, et al. Cerebral blood flow heterogeneity in preterm sheep: lack of physiological support for vascular boundary zones in fetal cerebral white matter. J Cereb Blood Flow Metab. 2008;28(5):995–1008. doi: 10.1038/sj.jcbfm.9600597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu G, Broadbelt KG, Haynes RL, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol. 2011 Oct;70(10):841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dean J, McClendon E, Hansen K, et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5(166-170):101–111. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McClendon E, Chen K, Gong X, et al. Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann Neurol. 2014 Jan 3; doi: 10.1002/ana.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKinstry R, Mathur A, Miller J, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12(2):1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 110.Kroenke CD, Bretthorst GL, Inder TE, Neil JJ. Modeling water diffusion anisotropy within fixed prenatal primate brain using Bayesian probability theory. Magn Reson Med. 2006;55:187–197. doi: 10.1002/mrm.20728. [DOI] [PubMed] [Google Scholar]
- 111.Kroenke C, Van Essen D, Inder T, Rees S, Bretthorst G, Neil J. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 2007;14(27):12506–12515. doi: 10.1523/JNEUROSCI.3063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cereb Cortex. 2009 Dec;19(12):2916–2929. doi: 10.1093/cercor/bhp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ball G, Srinivasan L, Aljabar P, et al. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012 Mar;71(3):385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011 Oct;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004 Nov 4;351(19):1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]


