Abstract
Spontaneous coronary artery dissection (SCAD) is an increasingly recognised cause of acute coronary syndrome, particularly in women. A 36-year-old Caucasian woman presented to our hospital with sudden onset chest pain and was diagnosed with a non-ST elevation myocardial infarction. Coronary angiography revealed mid and distal left anterior descending artery (LAD) dissection with distal LAD occlusion. A short segment of apical LAD filled late with incomplete opacification (Thrombolysis In Myocardial Infarction (TIMI) 1 flow). A decision was made to treat the patient conservatively, with subsequent resolution of dissection over the next 3 months. Our patient made a good clinical recovery with healing of her affected coronary vasculature on subsequent angiogram. The case illustrates that SCAD can be managed conservatively with antiplatelet agents, β-blockers, heparin and statins, if the patient is haemodynamically stable and coronary flow is adequate.
Background
Optimal treatment for spontaneous coronary artery dissection (SCAD) is unknown to date; there is debate over whether to perform percutaneous coronary intervention (PCI) with stenting in an attempt to restore luminal patency or manage it conservatively. The risks of complete periprocedural vessel occlusion and technical failure of PCI should be weighed against the risk of inadequate coronary flow leading to worsening left ventricular function and potentially fatal arrhythmias with medical management. We present a case report of successful medical management of SCAD, with the rationale for choosing medical management versus surgical treatment in this patient and a concise review of the literature, in order to discuss varying views on the available management options.
Case presentation
A 36-year-old obese, non-smoking Caucasian woman with no significant medical or family history presented with sudden onset of severe left-sided chest pain at rest. The pain radiated to the left shoulder and jaw, was exacerbated by deep breathing and relieved by intravenous opioids in the emergency room. Initial vital signs showed a temperature of 97.7°F oral, pulse 78 bpm, blood pressure 155/95 mm Hg, respiratory rate 24 breaths/min and oxygen saturation of 98% on room air. The patient appeared to be in mild distress due to the pain, which was reproducible on palpation of the left side of the chest. Cardiac auscultation revealed that first and second heart sounds were normal without any additional heart sounds or murmurs. The rest of the physical examination was unremarkable. The patient's initial ECG showed T wave inversion in leads V1–V5, III and aVF. The troponin-I peaked at 48 ng/mL, 7 h after the onset of chest pain. D dimer was less than 0.25 μg/mL and chest radiograph showed mild cardiomegaly.
Treatment
The patient was started on aspirin, ticagrelor, carvedilol, atorvastatin and heparin infusion for non-ST elevation myocardial infarction.
She underwent coronary angiography 24 h after the onset of chest pain, demonstrating 90% stenosis of mid left anterior descending artery (LAD) and 100% occlusion of the distal LAD (figure 1). The affected segment of the vessel appeared to be of irregular contour and had severe, diffuse narrowing. These findings were consistent with diagnosis of a type 2 dissection of the mid and distal LAD. A short segment of the apical LAD filled late and had incomplete opacification with Thrombolysis In Myocardial Infarction (TIMI) 1 flow. No other coronary abnormalities were noted during the procedure.
Figure 1.
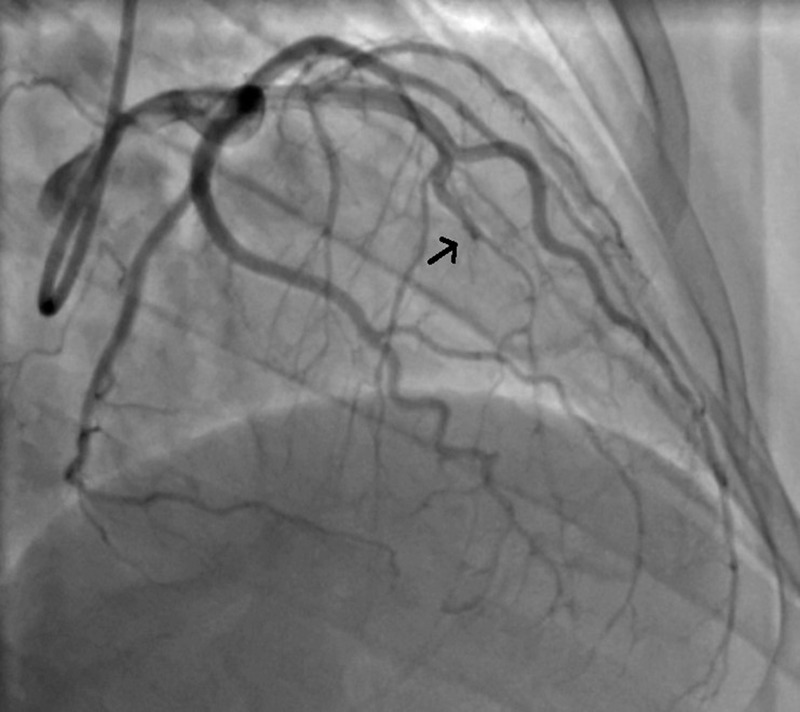
An image from coronary angiogram, 24 h after presentation. The arrow points to the beginning of diffuse narrowing of affected segment of left anterior descending artery (LAD). These findings were consistent with diagnosis of spontaneous dissection of mid and distal LAD.
The decision was made to pursue conservative management given that the patient's chest pain had resolved, and she was haemodynamically and electrically stable. Her repeat ECG showed resolving T wave changes and her troponins had started to decline. Her transthoracic echocardiogram revealed an ejection fraction of 55% with hypokinesis of the anteroseptal and apical walls, which correlated with affected LAD segment. Additionally, risk of postprocedural occlusion of LAD was also recognised. The patient remained clinically stable during the remaining 4 days of her hospital stay and was discharged on aspirin, ticagrelor, atorvastatin and carvedilol.
Outcome and follow-up
A myocardial perfusion imaging study was performed 3 weeks after the patient's discharge, which showed a fixed defect in the distribution of the distal LAD with no areas of ischaemia. A repeat echocardiogram was also performed, which showed an ejection fraction of 60%, and previously documented hypokinesis of the apical and anteroseptal walls. Three months after the initial presentation, the patient underwent repeat coronary angiogram, which demonstrated complete resolution of dissection with restoration of normal coronary flow in LAD (figure 2).
Figure 2.
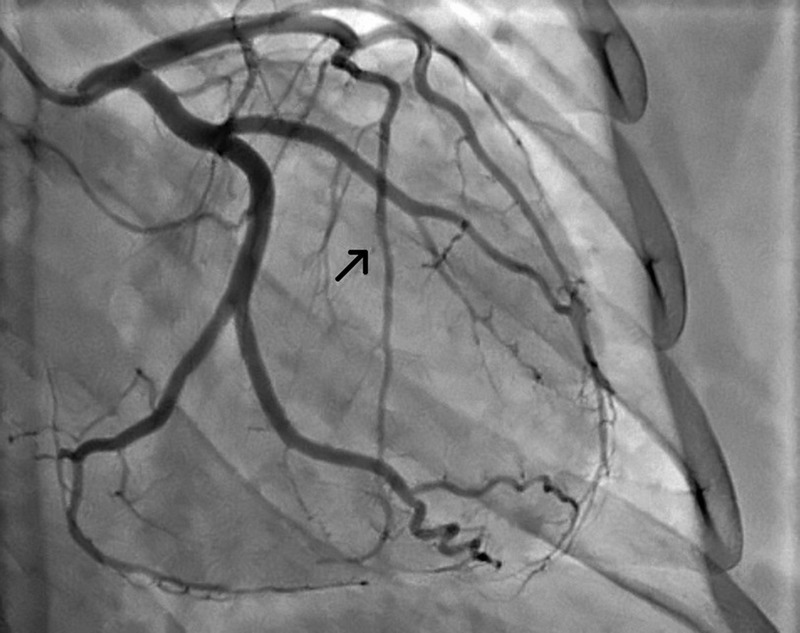
An image from the repeat coronary angiogram, 3 months after initial presentation. The arrow points to the left anterior descending artery, which showed complete resolution of dissection with restoration of normal coronary flow.
Given her young age and lack of risk factors for atherosclerosis, there was a suspicion that she may have underlying fibromuscular dysplasia (FMD) predisposing her to SCAD. A renal angiogram was performed at the time of repeat coronary angiogram, which showed diffuse irregularity of vessel lumen, consistent with the diagnosis of FMD (figure 3). The patient was closely followed in cardiology clinic over the next 4 months and remained clinically stable.
Figure 3.
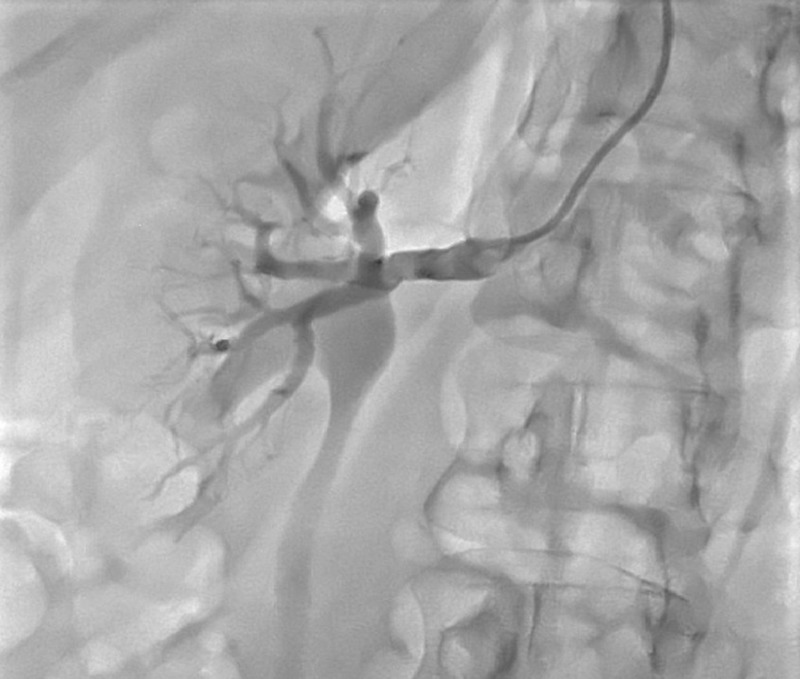
Renal angiogram was evident of diffuse irregularity of vessel lumen, consistent with the diagnosis of fibromuscular dysplasia.
Discussion
SCAD is found in up to 10% of women younger than 50 years of age with acute coronary syndrome and could be present in up to 1.1% of patients undergoing angiography.1 Contrary to the earlier belief that SCAD mostly affected young women (younger than 50 years of age), contemporary studies reveal that the average age of diagnosis is the mid-40s to mid-50s, with Saw et al2 observing one of the largest cohort of patients with non-atherosclerotic SCAD containing 58% women aged 50 years or above. It has been reported that almost 80% of patients diagnosed with SCAD are women, with up to 25% diagnosed during the peripartum period,3 usually presenting within the first 2 weeks after delivery.4
In up to 56.5% of patients, possible predisposing conditions associated with the occurrence of SCAD are present, including FMD (which our patient had), pregnancy, postpartum state, multiparity, hormonal therapy, connective tissue disorders and systemic inflammatory diseases.2 SCAD can be precipitated by vigorous exercise, Valsalva manoeuver, parturition or recreational drugs with hypersympathetic response or extreme emotional stress, none of which were reported by our patient.2 A close association between FMD and SCAD has been described.3 5–7 As many as one-third of young women with non-ST elevation myocardial infarction and positive biomarkers may have FMD/SCAD.5 FMD is an idiopathic, segmental, non-inflammatory angiopathy without atherosclerosis that occurs mainly in young to middle-aged females. It can affect renal, carotid, vertebral and iliac as well as coronary arteries, leading to vascular stenosis and tissue ischaemia.5 Although not all patients with SCAD have FMD, it is important to subsequently evaluate as it can affect vital vascular territories in the body, as described above.5
There are three angiographic types of dissection without differences in prognosis: type 1, with multiple radiolucent lumens and arterial wall staining from contrast dye; type 2 (most common), with diffuse stenosis; and type 3, with resemblance to atherosclerotic lesions.8 Over time, the survival of patients with SCAD has dramatically improved from 50% to approximately 95%, likely owing to better diagnostic and treatment options, since coronary angiography, PCI and coronary artery bypass grafting (CABG) were not widely available before 1990.6 9
There is no consensus on treatment guidelines for SCAD. Conservative medical management is favoured in most cases, particularly if there is no evidence of ongoing cardiac ischaemia or haemodynamic instability. In a study published in 2012 by Alfonso et al, 45 patients with SCAD were included and followed for 6 years. Twenty-nine of the 45 patients (64%) were managed conservatively while 16 underwent revascularisation. The study concluded that conservative management of SCAD had excellent outcomes with favourable prognosis.10 Up to 10% of patients who are managed conservatively may, eventually, require coronary intervention, however, a 5-year revascularisation rate and recurrence of SCAD are not significantly different when compared with patients undergoing early intervention.11 Clinically, significant progression of dissection may occur in some medically managed patients, usually during the first week after the initial event.11 However, cases treated with stent placement can also pose risk of extension of dissection as well as displacement of intramural haematoma leading to worsening myocardial infarction. PCI failure, including total occlusion of the target vessel, may be as high as 53%, thus possibly making early conservative management more favourable.11
Medical management of SCAD is similar to the treatment of acute coronary syndrome, including the use of antiplatelet drugs, intravenous heparin and β-blockers. The medical regimen for conservative management is largely observational as there are no randomised controlled trials comparing different regimens.2 12 Anticoagulation with heparin, ACE inhibitors, β-blockers and dual antiplatelet therapy for up to 1 year is usually undertaken.8 12 Clopidogrel can be discontinued if healing of dissection is confirmed.2 Heparin can be discontinued if the cause of acute coronary syndrome is confirmed to be SCAD.13
The role of new P2Y12 antagonists is uncertain and IIb/IIIa antagonists should generally be avoided due to a potential for extending the dissection.12 13 Calcium channel blockers may be used in cases of coronary spasm,12 however, their use in SCAD has not been studied.2 The role of statins in non-atherosclerotic SCAD is not clear. Some experts recommend that statins should only be used if there is concomitant dyslipidaemia due to a small potential increase in risk of SCAD recurrence with the use of statins, however, this association is still under investigation.2 Thrombolytics are traditionally avoided due to the potential risk of extension of dissection and its accompanying haematoma, though one study did not find worsening outcomes with their use.14
Advocates of early coronary intervention argue that patient survival is improved with an aggressive revascularisation strategy compared with conservative medical management. Shamloo et al concluded that early intervention with either CABG or PCI following the diagnosis of SCAD leads to better outcomes, longer symptom-free intervals and, consequently, less need for future intervention. Their study sample comprised of 440 patients who were treated with early intervention (PCI or CABG) and followed for a period of up to 400 days.9 They reported that 21.2% of the patients originally treated conservatively ultimately required CABG or PCI compared with 2.5% for whom an early revascularisation approach was pursued (p=0.001).9 Of note, the study characteristics were different from that conducted by Alfonso et al,10 with more female study participants (70% vs 58%), greater number of pregnancy-related cases (26% vs 4%), higher number of critically ill patients with at least two-vessel dissection (16.1% vs 11%), higher incidence of ST elevation myocardial infarction (64.9% vs 40%) and left main dissection (6.1% vs 2%).9 It is noteworthy that the Shamloo et al study covered a period from 1931 to 2008, a time when coronary diagnostic and treatment modalities, both invasive and medical therapy, were undergoing tremendous transformation. This broad timeframe may influence the findings of Shamloo et al,9 including the fact that 21.5% of their cases were diagnosed during autopsy examination. To the contrary, other contemporary studies, including Alfonso et al,10 included SCAD cases, and were all diagnosed premortem by coronary angiography or by other newer tomographic imaging techniques (optical coherence tomography, multislice CT, intravascular ultrasound, etc).
In light of current evidence, the management approach should be individualised for each case, based on patient stability, location of dissection (coronary segment involved) and TIMI flow. Many researchers use a Bypass Angioplasty Revascularisation Investigation (BARI) classification to describe the location of dissection. Lettieri et al15 found that distal location of the dissection (based on BARI classification) and TIMI flow 2–3 are more likely to be managed conservatively compared with proximal or mid-vessel location and TIMI flow 0–1, respectively. Furthermore, they also found that the inpatient cardiovascular complication rate was higher for surgical treatment (16.1% vs 3.8%; p 0.028) but long-term outcome was similar for medical and surgical approaches over a mean follow-up of 31 months. Therefore, patients with TIMI flow 0–1 or clinical instability may benefit from early coronary revascularisation and inpatient monitoring for 5–7 days.7 11 PCI and stenting may be preferable in proximal vessel dissection with significant reduction in coronary perfusion whereas conservative management can be chosen in mid-to-distal dissection involving only a single vessel.3 14 The more distal lesions, usually, do not require any intervention.16 Watchful waiting also seems prudent in stable patients, to avoid the risk of PCI guide wire being passed into false lumen thereby extending dissection.17
The majority of medically managed cases tend to result in natural healing of dissection, which should be reassessed with repeat angiogram in 4–6 weeks with the understanding that a minority of these patients may still end up requiring coronary revascularisation. Late angiographic follow-up can be carried out to assess for progression of dissection. Alfonso et al10 conducted late follow-up angiographies with a median of 262 days (range 175–284 days). They found that 2 of the 10 patient managed conservatively who underwent late angiography showed progression of the dissection requiring late revascularisation.
Delayed PCI may allow better visualisation of vessel lumen and thus more accurate stent placement to completely exclude the dissection.3 17 CABG is usually reserved for patients with failed PCI or cases involving left main or multiple vessel dissection, though stenting of spontaneous left main dissection may be appropriate in emergent situations.3 6 14 Further screening for FMD should be considered in all cases of SCAD, especially in women. This usually includes abdominal angiography to evaluate renal and iliac arteries, and CT angiogram of the head to investigate carotid vessels.5
Learning points.
Most cases of spontaneous coronary artery dissection (SCAD) can be managed medically with natural healing of the dissection.
Early surgical management (percutaneous coronary intervention or coronary artery bypass grafting) is recommended in dissection involving left main coronary artery, multiple vessels, ongoing or worsening cardiac ischaemia or haemodynamic instability.
Follow-up coronary angiogram is recommended after 4–6 weeks to document healing of the dissection. Late angiographic follow-up may be undertaken at approximately 6–9 months, as some patients may show late progression of dissection requiring late revascularisation.
Screening for fibromuscular dysplasia should be considered in SCAD with angiogram of the cerebral, renal and iliac vasculature, as it may be associated with up to one-third of the cases of SCAD in young women.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rosengarten JA, Dana A. Recurrent spontaneous coronary artery dissection: acute management and literature review. Eur Heart J Acute Cardiovasc Care 2012;1:53–6. 10.1177/2048872612442404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saw J, Aymong E, Sedlak T et al. . Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–55. 10.1161/CIRCINTERVENTIONS.114.001760 [DOI] [PubMed] [Google Scholar]
- 3.Garcia NA, Khan AN, Boppana RC et al. . Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014;4:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cropp EM, Turner JS, Kreutz RP. Spontaneous coronary artery dissection in a 14-year-old. Am J Emerg Med 2013;31:461.e5–7. 10.1016/j.ajem.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 5.Saw J, Poulter R, Fung A et al. . Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012;5:134–7. 10.1161/CIRCINTERVENTIONS.111.966630 [DOI] [PubMed] [Google Scholar]
- 6.Graidis C, Dimitriadis D, Karasavvidis V et al. . Percutaneous treatment of spontaneous left main coronary artery dissection using drug-eluting stent. BMC Cardiovasc Disord 2014;14:191 10.1186/1471-2261-14-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motreff P, Souteyrand G, Dauphin C et al. . Management of spontaneous coronary artery dissection: review of the literature and discussion based on a series of 12 young women with acute coronary syndrome. Cardiology 2010;115:10–18. 10.1159/000244608 [DOI] [PubMed] [Google Scholar]
- 8.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115–22. 10.1002/ccd.25293 [DOI] [PubMed] [Google Scholar]
- 9.Shamloo BK, Chintala RS, Nasur A et al. . Spontaneous coronary artery dissection: aggressive vs conservative therapy. J Invasive Cardiol 2010;22:222–8. [PubMed] [Google Scholar]
- 10.Alfonso F, Paulo M, Lennie V et al. . Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv 2012;5:1062–70. 10.1016/j.jcin.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 11.Tweet MS, Eleid MF, Best PJ et al. . Spontaneous coronary artery dissection: revascularization vs conservative therapy. Circ Cardiovasc Interv 2014;7:777–86. 10.1161/CIRCINTERVENTIONS.114.001659 [DOI] [PubMed] [Google Scholar]
- 12.Alfonso F, Bastante T, Cuesta J et al. . Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015;5:133–40. 10.3978/j.issn.2223-3652.2015.03.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29:1027–33. 10.1016/j.cjca.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 14.Vanzetto G, Berger-Coz E, Barone-Rochette G et al. . Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250–4. 10.1016/j.ejcts.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 15.Lettieri C, Zavalloni D, Rossini R et al. . Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 2015;116:66–73. 10.1016/j.amjcard.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 16.Vrints CJ. Spontaneous coronary artery dissection. Heart 2010;96:801–8. 10.1136/hrt.2008.162073 [DOI] [PubMed] [Google Scholar]
- 17.Arrivi A, Bazzucchi M, Paolis MD et al. . Spontaneous-idiopathic left anterior descending artery dissection: is watchful waiting better than immediate stenting? Case Rep Vasc Med 2013;2013:639384 10.1155/2013/639384 [DOI] [PMC free article] [PubMed] [Google Scholar]


