Abstract
A patient taking regular flecainide for paroxysmal atrial fibrillation presented with broad complex tachycardia and circulatory compromise. With no history of pacemaker insertion and no pacing spikes visible on the ECG, this was presumed to be ventricular tachycardia and treated with electrical cardioversion, leading to p-wave asystole. An indwelling pacemaker was now recognised and ventricular capture was eventually attained by significantly increasing ventricular lead output. Invasive haemodynamic support was required due to new ventricular systolic dysfunction. Pacing thresholds and ventricular function normalised within 72 h consistent with flecainide toxicity; levels were shown to be toxic. Pacemaker interrogation revealed evidence of an undiagnosed atrial flutter, at presentation this was likely slowed by flecainide toxicity to a rate below the pacemaker mode switch, such that it was tracked in the ventricle at the upper tracking rate (120 bpm). Cardioversion terminated the arrhythmia but raised the capture threshold of the ventricle above the maximum lead output.
Background
The class 1C antiarrhythmic, flecainide, is commonly used to suppress atrial fibrillation (AF).1 By binding sodium channels, the influx of sodium ions that causes cellular depolarisation in phase 0 of the cardiac action potential is slowed, thus prolonging its duration, and lengthening the QRS width and QT interval on the ECG.2 Such properties also mediate a reversible negative inotropic effect on the left ventricle3 and increase myocardial capture thresholds,4 even at therapeutic doses. Flecainide therapy may also widen the ‘excitable gap’, defined as the length of time between the end of the refractory period and the arrival of the next impulse in the re-entrant circuit.5 Such properties promote macro re-entry circuits in the atria, which include typical and atypical atrial flutters.
Case presentation
An 82-year-old man presented in cardiogenic shock with broad complex tachycardia (BCT; figure 1) of rate 120 bpm. Available medical history was of paroxysmal AF, for which the patient had been taking flecainide 100 mg two times per day and warfarin; he was taking ramipril 5 mg daily for hypertension, but no atrioventricular (AV) nodal blocking agents, being previously intolerant of bisoprolol due to fatigue and dizziness. The presenting rhythm was treated as ventricular tachycardia (VT) with electrical cardioversion; this resulted in p-waves, apparent ventricular pacing without capture, and consequent ventricular asystole. Transcutaneous pacing failed to consistently capture the myocardium despite an applied current of 190 mA, mandating periods of cardiopulmonary resuscitation. The chest film from arrival was reviewed revealing that the patient had a dual-chamber pacemaker, not appreciated when the patient was first assessed and in extremis. Interrogation of the device (Boston Scientific ALTRUA 50) found that the V lead threshold had increased substantially with consistent ventricular capture only attained at an output of 6.5 V pulse amplitude and 1 ms pulse duration. Pacemaker insertion was previously warranted for episodes of complete heart block with syncope.
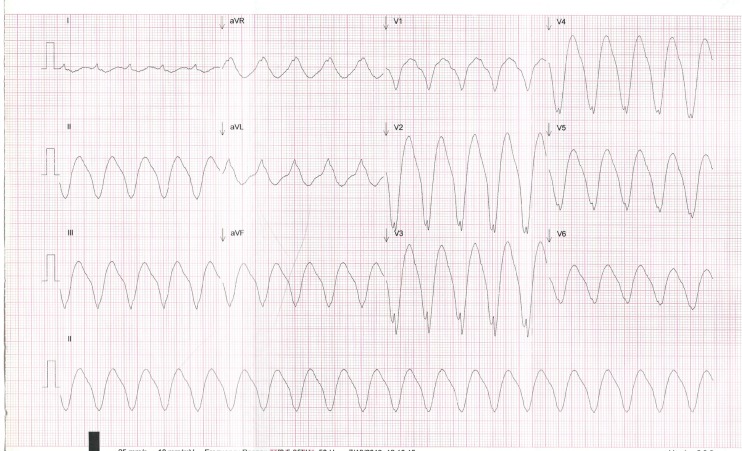
Figure 1.
Presenting ECG demonstrating monomorphic sinusoidal broad complex tachycardia with rate 120 bpm (the programmed maximum tracking rate of the pacemaker) and QRS morphology left bundle branch block. No pacing spikes are seen.
Despite restoration of AV synchrony, mechanical circulatory support with intra-aortic balloon pump (IABP) was needed, with echocardiography demonstrating new severe global left ventricular systolic impairment with an ejection fraction of 20%. Blood results revealed acute renal failure with serum creatinine of 175 µmol/L compared with previously normal values. Flecainide level (therapeutic range 200–700 µg/L) drawn 24 h postadmission was 1400 µg/L, reducing to 760 µg/L 36 h after admission, thus levels were severely toxic at presentation. The clinical picture improved as flecainide was cleared, with V lead threshold falling back to 1 V at 1 m/s; the patient was weaned from IABP. The patient reverted to AF before discharge, which was accepted, flecainide was stopped and the patient was discharged on warfarin, digoxin, ramipril and furosemide. Two months after discharge, his ventricular function had normalised, with V-lead pacing threshold back to a premorbid level of 0.6 V at 0.4 m/s, both consistent with flecainide toxicity.
Device interrogation revealed that ventricular pacing had at times been at the maximum tracking rate (MTR (programmed at 120 bpm)), in response to paroxysms of a previously undiagnosed atrial tachyarrhythmia. On occasion, this new atrial rhythm had exceeded the automatic mode switch (AMS) rate, set at 170 bpm, thus triggering ventricular fall back pacing at 80 bpm. However, more recently the atrial rate during this rhythm was less than the AMS rate, and so was tracked in the ventricle by the pacemaker (programmed DDD) with the pacemaker demonstrating pseudo-Wenckebach (upper rate) behaviour (figure 2). Such behaviour allows AV synchrony to be preserved on conducted p-waves with occasional dropped p-waves ensuring that the upper tracking rate is not exceeded.
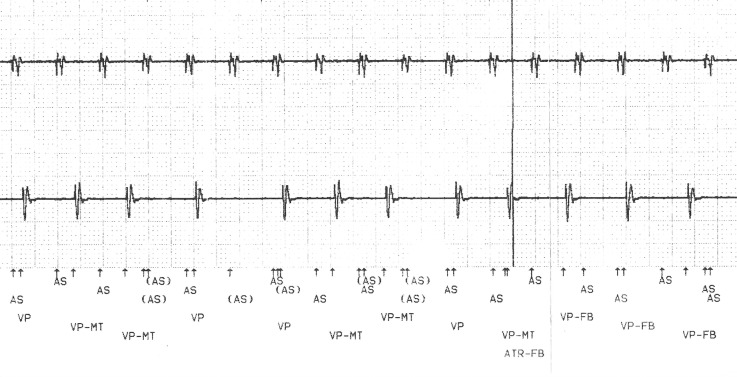
Figure 2.
Stored atrial (top trace) and ventricular (bottom trace) EGM demonstrating an atrial tachycardia sensed appropriately (AS) and traced to the ventricles at the upper tracking rate (VP-MT). Pseudo-Wenckebach behaviour is seen at VP-MT with AS events not conducted to the ventricles, (denoted as (AS)). Towards the end of this trace, the atrial rate is sensed as exceeding the automatic mode switch rate (ATR—FB) triggering fall back ventricular pacing (VP-FB)
The broad ‘toxic’ QRS rhythm seen here likely did not represent VT but instead indicated an atrial flutter, slowed by the significant action potential prolongation effects of flecainide toxicity, to a rate falling below the AMS rate, thus triggering ventricular pacing at the upper rate limit (120 bpm) for an extended period of time. In the emergency room, the atrial rate approximated the MTR so that the Wenckebach cycle had lengthened to such an extent that it was not appreciated during clinical examination, or on the ECG. A magnet placed over the device would have proved diagnostic—this would have induced asynchronous DOO pacing, that is, pacing of both chambers with no sensing. In the context of this tracked atrial flutter, the ventricular rate would now have fallen to the magnet rate, between 80 and 100 bpm, depending on battery life. Cardioversion, itself shown to alter capture thresholds,6 terminated the atrial rhythm but exposed the effect of class 1c drug toxicity with loss of lead capture due to a threshold rise from 0.5 to 6.5 V. Some pacemakers offer automated ‘capture management’ algorithms that seek to continuously ensure pacemaker output exceeds capture threshold; it is unlikely that this would have averted ventricular asystole, however, as this process takes time. Rather inexplicably, pacing spikes were too small to be appreciated at presentation but were apparent (without capture) after cardioversion.
Differential diagnosis
On arrival, the BCT was treated as VT with immediate cardioversion, given the patient was clinically shocked; if it was appreciated at this point that the patient had a pacemaker, a trial of a magnet over the device to induce asynchronous pacing would have been appropriate. After cardioversion, ventricular pacing did not occur. The differential for this scenario includes capture failure due to lead displacement or fracture, metabolic abnormalities, myocardial infarction, or flecainide toxicity. Failure to generate a pacing impulse could also explain the situation due to oversensing.
Discussion
We report a case of flecainide toxicity, likely mediated by acute renal failure, which led to a failure of ventricular pacemaker lead capture after cardioversion of a patient in shock with a BCT. Device interrogation revealed that the presenting rhythm may have represented an atrial tachyarrhythmia slowed by flecainide toxicity, tracked to the right ventricle at the upper tracking rate. With drug clearance, the capture threshold normalised. Such a scenario has not been previously described. At therapeutic levels, flecainide can convert AF to a slow atrial flutter (typically 200 bpm) with the possibility of 1:1 ventricular conduction, given that flecainide does not slow AV nodal conduction, such actions are considered proarrhythmic. AV nodal blocking agents such as β-blockers are prescribed to prevent such conduction, should flutter occur.1 The overwhelming driver of this atrial flutter, which slowed profoundly to below the AMS rate, was flecainide toxicity; AV conduction velocity was irrelevant since the patient was thus pacing the ventricles. Our patient was not taking AV nodal blocking agents, being previously intolerant of β-blockade; such drugs offer additive rhythm control and may, however, have kept the patient in sinus rhythm. Flecainide is considered an important prescription along with β-blockers to those presenting with accessory pathway mediated pre-excited AF, to ensure conduction slowing in the AV node and accessory pathway in equal measure, thus preventing degeneration to ventricular fibrillation.
Experimentally, therapeutic doses of intravenous and oral flecainide have been shown to alter the ventricular pacing capture threshold in healthy subjects, with thresholds rising more than 200% after chronic oral therapy.4 Direct current cardioversion itself has also been shown to acutely increase capture thresholds.6 In our patient, markedly increased capture thresholds were likely driven predominantly by flecainide toxicity, given that thresholds returned to normal within days.
Aside from its negative inotropic effect, consequences of flecainide overdose are manifest: bradyarrhythmia is commonly reported with AV block, and pulseless electrical activity or asystole is reported within 30–120 min of overdose.7 Less commonly, flecainide's proarrhythmic effect mediated by rate-dependent blockage of fast sodium channels with subsequent prolongation of phase 0 of the cardiac action potential may promote re-entry circuit formation in ventricular tissue,8 thus leading to ventricular arrhythmia.7
No treatments have consistently shown benefit in the context of flecainide toxicity, although hypertonic sodium bicarbonate has been shown to reverse ECG changes.9 Negative inotropic effects on myocardial function are fully reversible as flecainide is cleared; our patient was supported with IABP during a period of cardiogenic shock. More invasive circulatory support with extracorporeal membrane oxygenation,10 has been shown to be lifesaving in providing a bridge until flecainide is cleared and cardiac function recovers.
Learning points.
Electrical cardioversion is the treatment of choice in patients presenting with broad complex tachycardia (BCT) and haemodynamic compromise, the diagnosis often being ventricular tachycardia (VT). In patients with cardiac devices, a paced rhythm must also be considered.
Class 1C antiarrhythmic drugs such as flecainide and propafenone, commonly used to suppress atrial fibrillation, are renally cleared. During periods of renal insufficiency, levels may become toxic, mediating a reversible negatively inotropic effect on the left ventricle.
Atrial tachyarrhythmias may be slowed by class1C toxicity, often meaning that in those with an indwelling pacemaker, a mode switch will not occur, and thus a broad ventricular paced rhythm will ensue, which should be considered alongside VT in the differential of BCT.
In such patients, a magnet placed over the device to induce asynchronous pacing (DOO) allows the differentiation between a paced, or ventricular rhythm. Ventricular pacing is also more likely if QRS morphology at presentation is identical to that seen on a previous, paced ECG, if available.
If necessary, electrical cardioversion of unwell patients with cardiac devices and taking class 1C drugs should be undertaken with pacing support, given that transcutaneous pacing, if needed, may prove challenging due to increased capture thresholds caused by drug toxicity.
Footnotes
Contributors: AA wrote the document. MJ provided expert electrophysiology guidance on the case. SF and CPM helped to collect clinical data from the case.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aliot E, Capucci A, Crijns HJ et al. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace 2011;13:161–73. 10.1093/europace/euq382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Fermini B, Nattel S. Mechanism of flecainide's rate-dependent actions on action potential duration in canine atrial tissue. J Pharmacol Exp Ther 1993;267:575–81. [PubMed] [Google Scholar]
- 3.Santinelli V, Arnese M, Oppo I et al. Effects of flecainide and propafenone on systolic performance in subjects with normal cardiac function. Chest 1993;103:1068–73. 10.1378/chest.103.4.1068 [DOI] [PubMed] [Google Scholar]
- 4.Hellestrand KJ, Burnett PJ, Milne JR et al. Effect of the antiarrhythmic agent flecainide acetate on acute and chronic pacing thresholds. Pacing Clin Electrophysiol 1983;6:892–9. 10.1111/j.1540-8159.1983.tb04410.x [DOI] [PubMed] [Google Scholar]
- 5.Fei H, Hanna MS, Frame LH et al. Assessing the excitable gap in reentry by resetting. Implications for tachycardia termination by premature stimuli and antiarrhythmic drugs. Circulation 1996;94:2268–77. 10.1161/01.CIR.94.9.2268 [DOI] [PubMed] [Google Scholar]
- 6.Waller C, Callies F, Langenfeld H. Adverse effects of direct current cardioversion on cardiac pacemakers and electrodes: is external cardioversion contraindicated in patients with permanent pacing systems? Europace 2004;6:165–8. 10.1016/j.eupc.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 7.Winkelmann BR, Leinberger H. Life–threatening flecainide toxicity: a pharmacodynamic approach. Ann Intern Med 1987;106:807–14. 10.7326/0003-4819-106-6-807 [DOI] [PubMed] [Google Scholar]
- 8.Krishnan SC, Antzelevitch C. Flecainide-induced arrhythmia in canine ventricular epicardium. Phase 2 reentry? Circulation 1993;87:562–72. 10.1161/01.CIR.87.2.562 [DOI] [PubMed] [Google Scholar]
- 9.Rognoni A, Bertolazzi M, Peron M et al. Electrocardiographic changes in a rare case of flecainide poisoning: a case report. Cases J 2009;2:9137–41. 10.1186/1757-1626-2-9137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auzinger GM, Scheinkestel CD. Successful extra-corporeal life support in a case of severe flecainide intoxication. Crit Care Med 2001;29:887–90. 10.1097/00003246-200104000-00041 [DOI] [PubMed] [Google Scholar]