Abstract
We previously suggested a relationship between ocular immunoglobulin (Ig)G4-related disease (IgG4-RD) and marginal zone lymphomas (MZLs). However, the cytokine background associated with these disorders and whether it differs between ocular adnexal MZLs with (IgG4-associated MZL) and without (IgG4-negative MZL) numerous IgG4+ plasma cells are unknown. In this study, we identified the mRNA expression pattern of Th2 and regulatory T-cell (Treg) cytokines in IgG4-RD and in IgG4-associated MZL and IgG4-negative MZL using real-time polymerase chain reaction analysis. Ocular IgG4-RD and IgG4-associated MZL exhibited significantly higher expression ratios of interleukin (IL)-4/β-actin, IL-10/β-actin, IL-13/β-actin, transforming growth factor (TGF) β1/β-actin, and FOXP3/β-actin than did IgG4-negative MZL (p < 0.05). This finding further supports our prior observations that a significant subset of ocular MZLs arises in the setting of IgG4-RD. Furthermore, the presence of a different inflammatory background in IgG4-negative MZLs suggests that IgG4-associated MZLs may have a different pathogenesis.
Immunoglobulin (Ig)G4-related disease (IgG4-RD) is a systemic syndrome characterized by dense lymphoplasmacytic infiltrates that are rich in IgG4+ plasma cells, fibrosis in the involved organ, and elevated serum IgG4 levels. IgG4-RD can affect almost any organ, including the pancreas, hepatobiliary duct, lacrimal and salivary glands, lung, kidney, retroperitoneum, aorta, and lymph nodes1,2,3,4,5,6,7,8,9,10,11.
Recent reports have described upregulation of T-helper-2 cells (Th2) and regulatory T-cell (Treg) cytokines in tissues with IgG4-RD, suggesting that the immune reaction mediated by these cytokines is responsible for the lesions12,13. This is in contrast to most extranodal marginal zone lymphomas (MZLs) that have a Th1 type inflammatory background, but similar to the Th2 background seen in a large cutaneous MZL subset that is also often IgG4-positive14,15,16.
Previously, we reported cases of ocular adnexal MZLs with numerous IgG4+ plasma cells that fulfilled the histological diagnostic criteria for IgG4-RD; therefore, we suggested that MZLs can arise in a background of IgG4-RD1. However, the expression pattern of cytokines in MZL lesions with IgG4+ plasma cells has not been clarified.
In this study of ocular IgG4-RD and MZLs with (IgG4-associated MZL) and without (IgG4-negative MZL) numerous IgG4+ plasma cells, we aimed to identify the mRNA expression patterns of Th2 and Treg cytokines and to determine the inflammatory background associated with benign and neoplastic ocular lymphoplasmacytic proliferations with numerous IgG4+ plasma cells that is distinct from that associated with ocular IgG4-negative MZL.
Material and Methods
Samples and clinical review
Formalin-fixed excisional biopsies from the ocular adnexal region of patients were selected, including 11 patients with IgG4-RD, 11 with IgG4-negative MZL, and 6 with IgG4-associated MZL (Table 1). All MZL lesions were primary tumors, and there was no other organ involvement. None of the patients were treated prior to the biopsy. Clinical data including serum IgG4 and IgG levels were obtained when available. The IgG4 and IgG levels were measured by routine laboratory blood tests. Informed consent for the use of their samples in research was obtained from all patients.
Table 1. Histological and serological findings.
| Case No. | Diagnosis | Age | Sex | Anatomical location | IgG4+ cells (/HPF) | IgG+ cells (/HPF) | IgG4+/IgG+ cell ratio | Serum IgG4 (mg/dL) [4.8–105] | Serum IgG (mg/dL) [870–1,700] | Serum IgG4/IgG ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IgG4-RD | 47 | F | lacrimal gland | 113 | 135 | 0.837 | n.e. | n.e. | |
| 2 | IgG4-RD | 60 | M | orbit | 178 | 309 | 0.575 | n.e. | n.e. | |
| 3 | IgG4-RD | 66 | F | lacrimal gland | 260 | 365 | 0.713 | 106 | 1400 | 0.076 |
| 4 | IgG4-RD | 73 | M | lacrimal gland | 137 | 198 | 0.690 | 136 | 1437 | 0.095 |
| 5 | IgG4-RD | 56 | F | lacrimal gland | 177 | 275 | 0.642 | n.e. | n.e. | |
| 6 | IgG4-RD | 61 | M | orbit | 298 | 440 | 0.678 | 3320 | 4168 | 0.797 |
| 7 | IgG4-RD | 66 | M | lacrimal gland | 365 | 409 | 0.892 | 969 | 2454 | 0.395 |
| 8 | IgG4-RD | 60 | F | orbit | 184 | 223 | 0.825 | 120 | 1499 | 0.080 |
| 9 | IgG4-RD | 58 | F | orbit | 207 | 248 | 0.836 | 376 | 1351 | 0.278 |
| 10 | IgG4-RD | 36 | F | orbit | 160 | 174 | 0.918 | 561 | 1958 | 0.287 |
| 11 | IgG4-RD | 72 | M | orbit | 311 | 474 | 0.656 | n.e. | n.e. | |
| 12 | IgG4− MZL | 74 | M | conjunctiva | 0 | 9 | 0.000 | n.e. | n.e. | |
| 13 | IgG4− MZL | 56 | F | conjunctiva | 16 | 108 | 0.146 | n.e. | n.e. | |
| 14 | IgG4− MZL | 69 | M | lacrimal gland | 44 | 259 | 0.171 | n.e. | n.e. | |
| 15 | IgG4− MZL | 72 | M | conjunctiva | 49 | 164 | 0.297 | n.e. | n.e. | |
| 16 | IgG4− MZL | 69 | M | orbit | 47 | 669 | 0.071 | n.e. | n.e. | |
| 17 | IgG4− MZL | 66 | F | lacrimal gland | 0 | 15 | 0.000 | 19.3 | 962 | 0.020 |
| 18 | IgG4− MZL | 71 | F | lacrimal gland | 25 | 605 | 0.041 | n.e. | n.e. | |
| 19 | IgG4− MZL | 61 | M | orbit | 0 | 4 | 0.000 | n.e. | n.e. | |
| 20 | IgG4− MZL | 82 | M | orbit | 0 | 26 | 0.000 | 166 | 1520 | 0.109 |
| 21 | IgG4− MZL | 39 | M | conjunctiva | 2 | 17 | 0.100 | n.e. | n.e. | |
| 22 | IgG4− MZL | 74 | F | conjunctiva | 1 | 12 | 0.111 | n.e. | n.e. | |
| 23 | IgG4+ MZL | 57 | F | lacrimal gland | 128 | 183 | 0.699 | n.e. | n.e. | |
| 24 | IgG4+ MZL | 72 | M | orbit | 128 | 152 | 0.838 | 760 | 2709 | 0.281 |
| 25 | IgG4+ MZL | 65 | F | lacrimal gland | 143 | 300 | 0.475 | 116 | 1450 | 0.080 |
| 26 | IgG4+ MZL | 56 | M | orbit | 189 | 219 | 0.863 | 475 | 1536 | 0.309 |
| 27 | IgG4+ MZL | 42 | F | lacrimal gland | 123 | 152 | 0.807 | n.e. | n.e. | |
| 28 | IgG4+ MZL | 71 | F | lacrimal gland | 147 | 450 | 1844 | 0.244 |
IgG4-RD, IgG4-related disease; IgG4− MZL, IgG4-negative marginal zone lymphoma; IgG4+ MZL, IgG4-associated marginal zone lymphoma; n.e., not examined. Normal ranges of serum IgG and IgG4 are shown in square brackets. In case no. 28, number of IgG+ cells is not shown because tumor cells produce IgG.
Real-time quantitative polymerase chain reaction (PCR)
The following evaluations were carried out in accordance with the approved guidelines. All experimental protocols were approved by the Institutional Review Board at Okayama University. Total RNA was extracted from the paraffin-embedded sections of all samples using an miRNeasy FFPE Kit (QIAGEN, Valencia, CA, USA). cDNA was prepared using a SuperScript VILO MasterMix kit (Invitrogen, Carlsbad, CA, USA). Multiplex real-time PCR was performed for quantitative analysis, according to a standard protocol, using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA), a Step One Plus Real-Time PCR System (Applied Biosystems), and specific primers and probes for FOXP3 (Hs01085834_m1), transforming growth factor (TGF)β1 (Hs00998133_m1), interleukin (IL)-4 (Hs00174122_m1), IL-5 (Hs00174200_m1), IL-10 (Hs00961622_m1), IL-13 (Hs00174379_m1), and β-actin (Hs99999903_m1) (Applied Biosystems). The PCR cycling conditions were as follows: 20 s at 95 °C, 50 cycles of 1 s at 95 °C, and 20 s at 60 °C. The expression of each target was normalized to that of β-actin, which was used as an endogenous control.
Histological examination, immunohistochemistry, and in situ hybridization
Serial sections (4 μm) were cut from the block of paraffin-embedded tissue, stained with hematoxylin and eosin, and used for the following immunohistochemical stains: CD20 (L26 [1:400]; DAKO, Glostrup, Denmark), CD3 (LN10 [1:200]; Novocastra, Newcastle, UK), CD5 (4c7 [1:50]; Novocastra), CD10 (56C6 [100:1]; Novocastra), CyclinD1 (SP4 [1:50]; Nichirei, Tokyo, Japan), Ki-67 (MIB-1 [1:2500]; Novocastra), IgG (polyclonal [1:20,000]; DAKO), and IgG4 (HP6025 [1:10000]; The Binding Site, Birmingham, UK). Following immunostaining using an automated Bond Max stainer (Leica Biosystems, Melbourne, Germany), the numbers of IgG4+ and IgG+ cells were estimated in areas with the highest density of IgG4+ cells. In accordance with the consensus statement on the pathological features of IgG4-RD17, three different high-power fields (HPFs) (total magnification, ×400) were examined to calculate the average number of IgG4+ cells per HPF and the IgG4+/IgG+ cell ratio. In situ hybridization was also performed for κ and λ-light chains (Leica Biosystems) using a Bond Max stainer.
Molecular genetic analysis
PCR molecular genetic analysis for immunoglobulin heavy chain gene rearrangements was performed as previously described18,19,20. The primers used in this study were: 5′-TGG[A/G]TCCG[C/A]CAG[G/C]C[T/C][T/C]C[A/C/G/T]GG-3′ as an upstream consensus V-region primer; 5′-TGAGGAGACGGTGACC-3′ as a consensus J-region primer, and 5′-GTGACCAGGGT[A/C/G/T]CCTTGGCCCCAG-3′ as a consensus J-region primer18,19,20.
Statistical analysis
All statistical analyses were performed using the Mann–Whitney U-test with SPSS software (version 14.0; SPSS, Chicago, IL, USA).
Results
Histological/phenotypic examinations
IgG4-related disease
Biopsies from the 11 patients with IgG4-RD showed marked lymphoplasmacytic infiltration with or without dense fibrosis, scattered eosinophils, and interspersed reactive lymphoid follicles. No light-chain restrictions were noted. All cases demonstrated >100 IgG4+ cells/HPF, and the IgG4+/IgG+ cell ratio was >40% (Fig. 1).
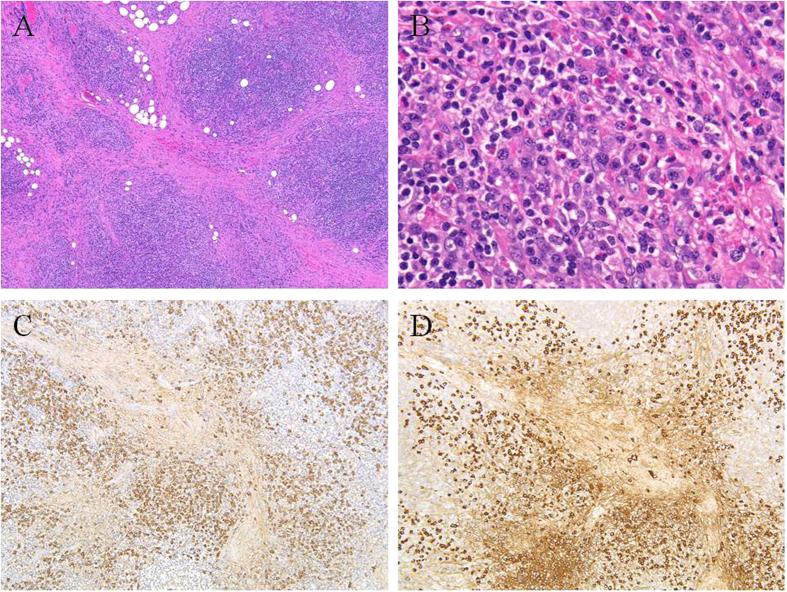
Figure 1. IgG4-related disease (case 6).
Marked lymphoplasmacytic infiltration with dense fibrosis, scattered eosinophils, and interspersed reactive lymphoid follicles are shown (A,B) (hematoxylin & eosin). Numerous IgG+ (C) and IgG4+ (D) cells are present with an IgG4+/IgG+ cell ratio >40%.
IgG4-negative marginal zone lymphoma
The 11 IgG4-negative MZLs showed dense infiltration by small- to medium-sized CD20+, CD3−, CD5−, and CD10− lymphoid cells with a Ki-67 labeling index <10% (Fig. 2). Fibrosis and eosinophil infiltration were rarely present. In 3 specimens (cases 15, 16, and 18), in situ hybridization was performed, and polytypic plasma cells were detected. PCR revealed clonal immunoglobulin heavy chain rearrangement in 1 of 2 cases tested (case 22 but not case 15). All cases revealed few, if any, IgG4+ cells, and the IgG4+/IgG+ cell ratio was <40%.
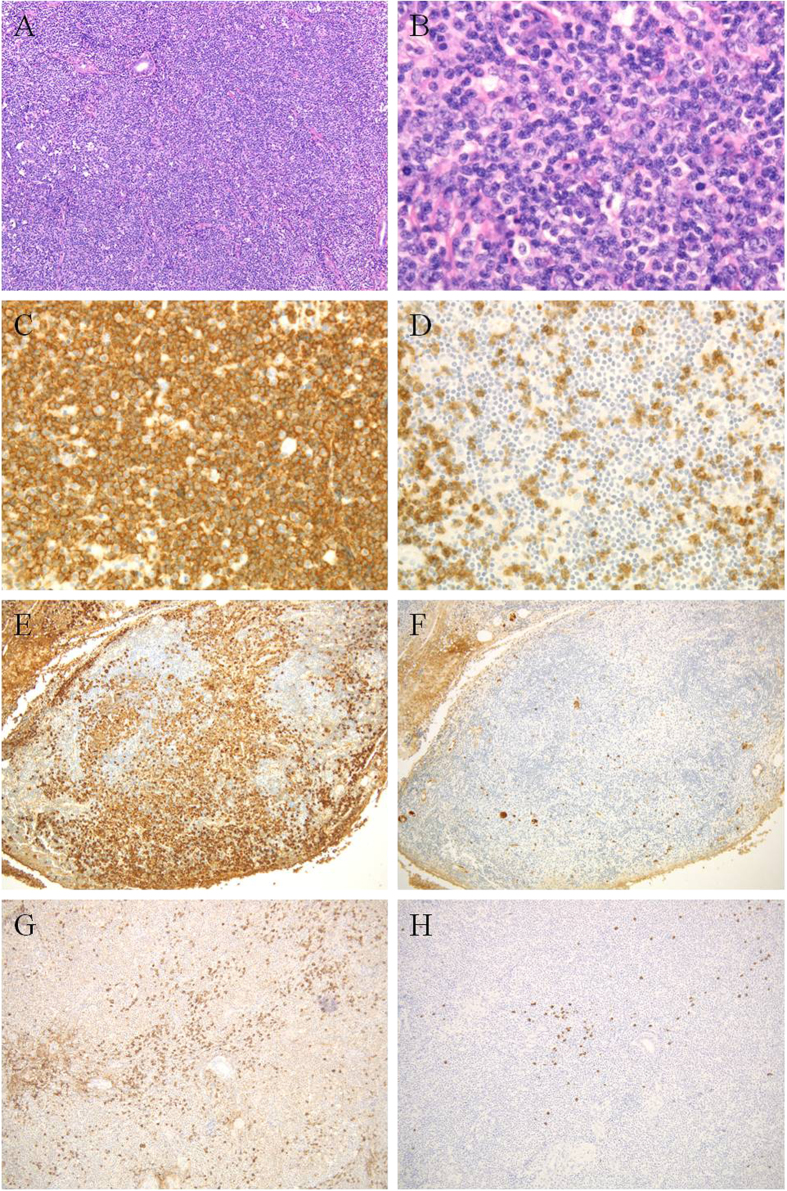
Figure 2. IgG4-negative marginal zone lymphoma (case 16).
There is a diffuse proliferation of small- to medium-sized lymphoid cells (A,B) that are CD20+ (C) and CD3− (D). Numerous Igκ+ plasma cells are present (E), but only very few Igλ+ plasma cells (F). Some IgG+ (G) and IgG4+ (H) cells are present but the IgG4+/IgG+ cell ratio is <40%.
IgG4-associated marginal zone lymphoma
The 6 IgG4-associated MZLs were histologically and phenotypically similar to the IgG4-negative MZLs except that there were numerous IgG4+ cells, and fibrosis was present in 3 cases (cases 26, 27, and 28) (Table 1, Fig. 3). All cases had either clonal immunoglobulin heavy chain rearrangement (cases 23, 25, and 26) and/or were monotypic based on in situ hybridization for κ and λ (cases 24, 25, 27, and 28). Based on the distribution of the IgG4+ cells and non-dominant light chain staining, the IgG4+ cells appeared to be polytypic. In all 6 cases, >100 IgG4+ cells/HPF were detected; therefore, these cases fulfilled the histological diagnostic criteria for IgG4-RD. Furthermore, in 5 cases, the IgG4+/IgG+ cell ratio was >40%. In case 28, the IgG4+/IgG+ cell ratio was not estimated because the lymphoma cells present were IgG positive, and the much fewer IgG4+ cells appeared to reflect a polytypic IgG+ population that could not be quantitated (Fig. 4).
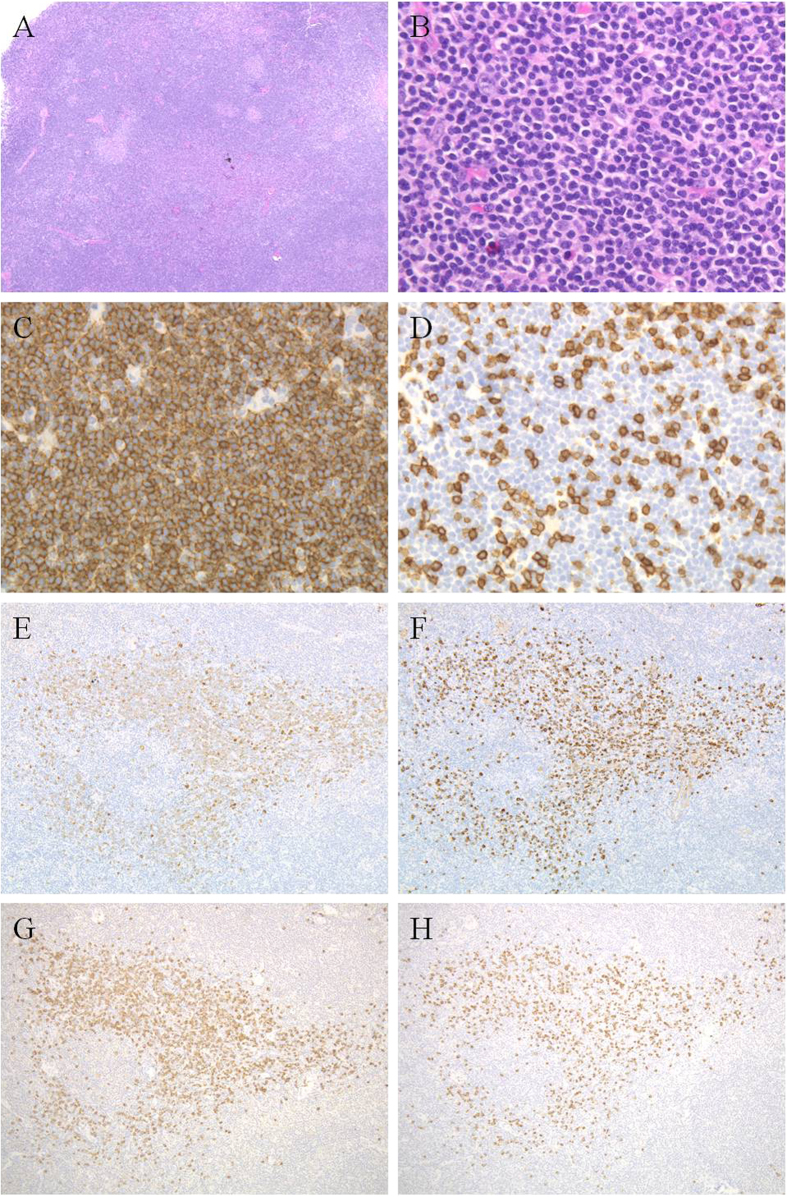
Figure 3. IgG4-associated marginal zone lymphoma.
The diffusely proliferating small- to medium-sized lymphoid cells [(A,B) case 26] are CD20+ (C) and CD3− (D). Infiltration of many IgG+ (E) and IgG4+ (F) cells are observed, but these cells are polytypic (G) (Igκ) and (H) (Igλ). The IgG4+/IgG+ cell ratio is >40%.
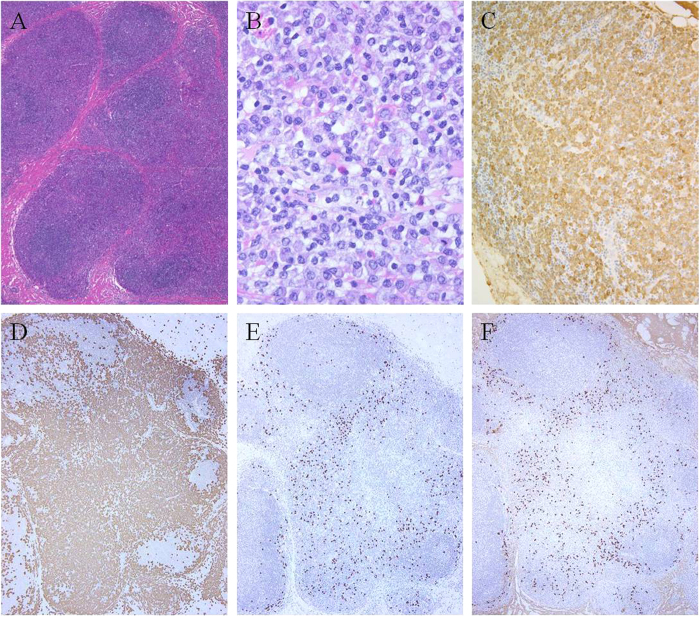
Figure 4. IgG4-associated marginal zone lymphoma (case 28).
Numerous plasmacytic cells are present (A,B) that are IgG+ (C). The lymphoma cells are κ light chain-restricted. (D) Igκ-in situ hybridization and (E) Igλ-in situ hybridization. Admixed IgG4+ plasma cells that appear to be polytypic are present (F).
Serological analysis
Serum IgG4 and IgG levels were determined in 7 patients with ocular IgG4-RD, in 2 patients with IgG4-negative MZL, and in 4 patients with IgG4-associated MZL (Table 1). Serum IgG4 levels were >135 mg/dL (reference range, 4.8–105) in 5 of 7 tested patients with IgG4-RD, 1 of 2 patients with IgG4-negative MZL, and 3 of 4 patients with IgG4-associated MZL.
Expression pattern of Th2 and Treg cytokines
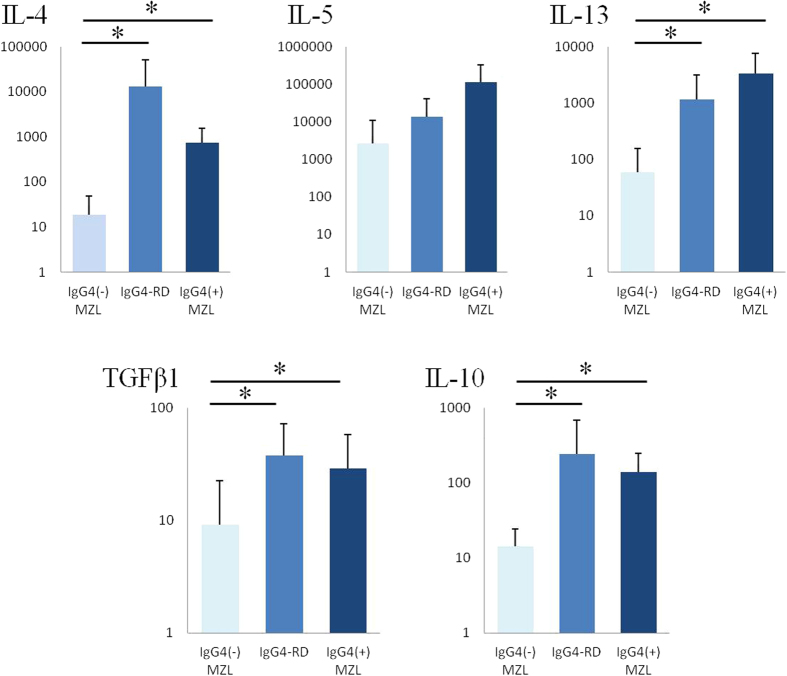
The expressions of IL-4, IL-5, IL-10, IL-13, TGFβ1, and β-actin in the samples were quantitatively analyzed using real-time PCR. Ocular IgG4-RD and the IgG4-associated MZL samples exhibited significantly higher expression ratios of IL-4/β-actin, IL-10/β-actin, IL-13/β-actin, and TGFβ1/β-actin than did the IgG4-negative MZL samples (p < 0.05) (Fig. 5). No significant differences were found for IL-5/β-actin expression.
Figure 5. Cytokine levels were measured by relative quantification of mRNA.
mRNA expression of Th2 cytokines (IL-4, IL-13) and regulatory cytokines (TGFβ1, IL-10) are significantly higher in the ocular adnexal regions from biopsies with IgG4-related disease and IgG4-associated marginal zone lymphoma than in the ocular adnexal regions from IgG4-negative marginal zone lymphomas. The expression levels of IL-5 are not significantly different. Data represent mean ± standard deviation (SD) values. Significant differences between groups were determined using the Mann-Whitney U test. (*p < 0.05). IgG4− MZL, IgG4-negative marginal zone lymphoma; IgG4-RD, IgG4-related disease; IgG4+ MZL, IgG4-associated marginal zone lymphoma.
Expression of FOXP3
The ocular IgG4-RD and IgG4-associated MZL biopsies exhibited significantly higher expression ratios of FOXP3/β-actin than did the IgG4-negative MZL specimens (p < 0.05) (Fig. 6).
Figure 6. FOXP3 expression levels were measured by relative quantification of mRNA.
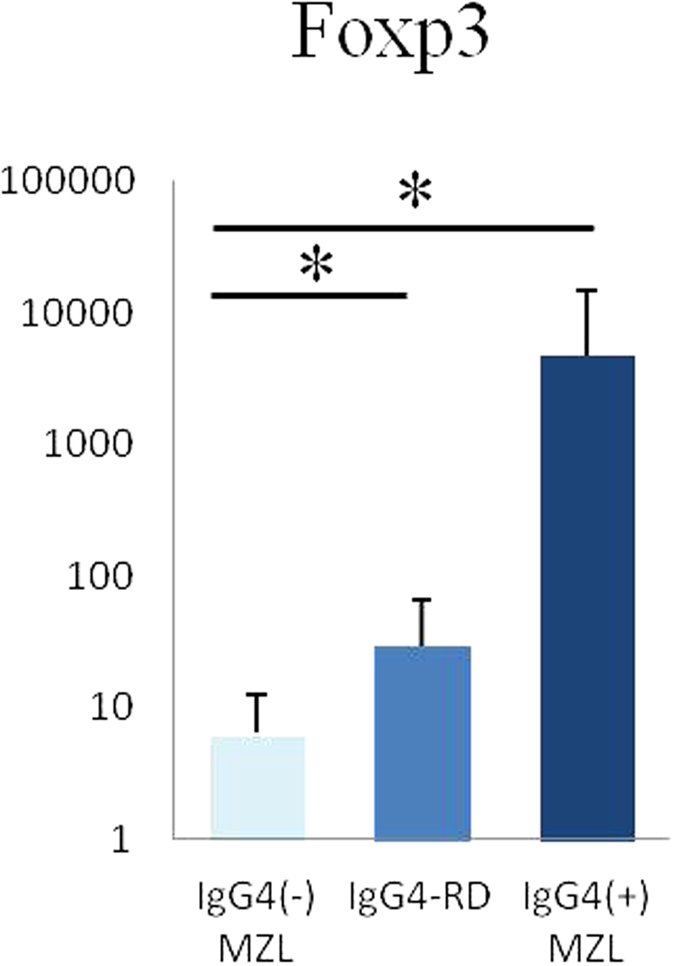
mRNA expressions of FOXP3 are significantly higher in the ocular adnexal regions from biopsies with IgG4-related disease and IgG4-associated marginal zone lymphoma than in the ocular adnexal resions from IgG4-negative marginal zone lymphomas. (*p < 0.05). IgG4− MZL, IgG4-negative marginal zone lymphoma; IgG4-RD, IgG4-related disease; IgG4+ MZL, IgG4-associated marginal zone lymphoma.
Discussion
The Th1/Th2 balance is considered important for healthy immune responses, and a Th1/Th2 imbalance can cause immune-mediated disease. Th1-dominant immune responses are noted in patients with rheumatoid arthritis, type 1 diabetes, and multiple sclerosis, whereas Th2-dominant immune responses are observed in patients with type 1 hypersensitivity disorders, such as allergies or asthma21. Extranodal MZLs are considered to arise in a background of chronic inflammation. High levels of Th1-type cytokines (interferon [IFN]-γ and IL-2) are typically found in the tumor environment of many mucosa associated lymphoid tissue (MALT) lymphomas22,23. Moreover, most extranodal MZLs express high levels of CXCR3, which is the receptor for IFN-γ-induced chemokines24,25,26. In contrast, a major subset of cutaneous MZLs typically shows heavy chain class switching and has a Th2 background14,15. In addition, 39% of cutaneous MZLs with plasmacytic differentiation are reported to be IgG4+, whereas an ocular MZL was the only non-cutaneous IgG4+ MZL found in the same study16.
Recently, high levels of Th2 cytokines (IL-4 and IL-13) and Treg cytokines (TGFβ1 and IL-10) have been detected in tissues with IgG4-RD12. Costimulation with IL-4 and IL-10 is suggested to cause class switching to IgG427. Furthermore, upregulation of IL-13 induces eosinophil infiltration, and TGFβ1 is considered to cause fibrosis in IgG4-RD lesions. In this study, we found higher levels of Th2 and Treg cytokines (including FOXP3) in lesions from patients with either IgG4-RD or ocular IgG4-associated MZL than in samples from patients with ocular adnexal IgG4-negative MZL. However, the expression levels of IL-5, which is a Th2 cytokine that promotes eosinophil infiltration, were not significantly different between sample types. These results indicate that IgG4-associated MZL is characterized by the upregulation of Th2 and Treg cytokines, similar to IgG4-RD. Despite sharing a similar inflammatory background with class-switched cutaneous MZLs, and very much unlike most other MALT lymphomas, the ocular cases, with one exception, had IgG4+ plasma cells that were polytypic and not part of the neoplasm. An explanation for this difference remains to be established, and the heavy chain expressed by the ocular lymphomas is uncertain.
The mechanism for the development of ocular adnexal MZL is not clear. However, recent reports indicated that Chlamydia psittaci infection is associated with the development of ocular adnexal MZL28,29,30. We previously suggested that ocular adnexal MZLs may arise in the setting of ocular IgG4-RD1, and others have reported cases of ocular adnexal lymphoma with IgG4+ cells, suggesting that they arose from IgG4-RD31. Moreover, a case involving two types of mass lesions in the same ophthalmic region has also been reported32. One of the lesions was typical of IgG4-RD, and the other was an MZL admixed with IgG4+ plasma cells. Because the two lesions were adjacent to each other, the MZL may have arisen in the setting of the IgG4-RD lesion. These reports and the results of the present study suggest that a subset of ocular MZLs may arise in the setting of IgG4-RD.
Patients with IgG4-RD are considered to be at high risk of MZLs as well as other malignancies, with malignancy considered a possible complication of IgG4-RD. Lung and colon cancers, as well as malignant lymphomas, are reported to occur in 10.4% of patients with IgG4-RD, an incidence that is approximately 3.5 times higher than that in the general population33. Although the mechanism through which neoplasms arise in a background of IgG4-RD remains unknown, a recent report suggests that IgG4 subclass antibodies impair antitumor immunity against melanomas34. The authors reported that IgG4 antibodies did not induce antitumor immunity, unlike IgG1 antibodies; rather, they inhibited the antitumor activity of the IgG1 antibodies. Therefore, although IgG4 subclass antibodies do not specifically induce neoplasms, they may create an environment where induced malignant neoplasms are more likely to grow.
In the current study, upregulation of FOXP3 was detected in cases of IgG4-RD and IgG4-associated MZL lesions. This is consistent with an earlier report describing the presence of numerous FOXP3+ cells in IgG4-RD12. FOXP3 is a marker of Tregs and is a central control element in their development and function. Because Tregs have been reported to suppress inflammation, including tumor immunity35, FOXP3+ Tregs may infiltrate lesions associated with IgG4-RD and further suppress tumor immunity, also promoting the growth of neoplasms.
Although steroid therapy is effective in IgG4-RD, the most effective treatment for MZLs arising in the setting of IgG4-RD is uncertain. Although evaluation of additional cases of IgG4-associated MZLs is needed to determine the treatment and prognosis, radiation or chemotherapy may be necessary. Therefore, when IgG4-RD is diagnosed, the lesion should be assessed to determine if it includes an MZL component. The possible tumor-promoting environment of IgG4-RD supports treatment for these patients before an MZL arises, in addition to ongoing follow-up.
Conclusion
IgG4-associated MZL is characterized by the upregulation of Th2 and regulatory cytokines, as is the case in IgG4-RD, but unlike the cytokine background of IgG4-negative MZLs. The current results further suggest that a subset of MZLs may arise in an IgG4-RD setting and that this subset of MZLs may have a different pathogenesis than IgG4-negative MZLs.
Additional Information
How to cite this article: Ohno, K. et al. A subset of ocular adnexal marginal zone lymphomas may arise in association with IgG4-related disease. Sci. Rep. 5, 13539; doi: 10.1038/srep13539 (2015).
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (no. 24591447) from the Japan Society for the Promotion of Science and ‘Research on Measures for Intractable Disease’ Project, a matching fund subsidy from the Ministry of Health Labour and Welfare, Japan.
Footnotes
Author Contributions Conceived and designed the experiments: Y.S. Performed the experiments: K.O. and Y.S. Analyzed the data: Y.S., K.O., K.T., M.T., T.M.T., Y.G., T.T., Y.O., T.I., S.H.S. and T.Y. Contributed materials: K.O. Wrote the paper: K.O., Y.S., S.H.S. and T.Y. All authors read and approved the final manuscript.
References
- Sato Y. et al. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol. Int. 58, 465–70 (2008). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. Ocular adnexal IgG4-producing mucosa-associated lymphoid tissue lymphoma mimicking IgG4-related disease. J. Clin. Exp. Hematop. 52, 51–5 (2012). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. IgG4-related disease: historical overview and pathology of hematological disorders. Pathol. Int. 60, 247–58 (2010). [DOI] [PubMed] [Google Scholar]
- Masaki Y., Kurose N. & Umehara H. IgG4-related disease: a novel lymphoproliferative disorder discovered and established in Japan in the 21st century. J. Clin. Exp. Hematop. 51, 13–20 (2011). [DOI] [PubMed] [Google Scholar]
- Stone J. H. et al. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis Rheum. 60, 3139–45 (2009). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman’s disease. Mod. Pathol. 22, 589–99 (2009). [DOI] [PubMed] [Google Scholar]
- Umehara H. et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod. Rheumatol. 22, 1–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y. et al. Association between IgG4-related disease and progressively transformed germinal centers of lymph nodes. Mod. Pathol. 25, 956–67 (2012). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. Clinicopathologic analysis of IgG4-related skin disease. Mod. Pathol. 26, 523–32 (2013). [DOI] [PubMed] [Google Scholar]
- Andrew N. H., Sladden N., Kearney D. J. & Selva D. An analysis of IgG4-related disease (IgG4-RD) among idiopathic orbital inflammations and benign lymphoid hyperplasias using two consensus-based diagnostic criteria for IgG4-RD. Br. J. Ophthalmol. 99, 376–81 (2015). [DOI] [PubMed] [Google Scholar]
- Hardy T. G., McNab A. A. & Rose G. E. Enlargement of the infraorbital nerve: an important sign associated with orbital reactive lymphoid hyperplasia or immunoglobulin g4-related disease. Ophthalmology 121, 1297–303 (2014). [DOI] [PubMed] [Google Scholar]
- Zen Y. et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 45, 1538–46 (2007). [DOI] [PubMed] [Google Scholar]
- Tanaka A. et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 64, 254–63 (2012). [DOI] [PubMed] [Google Scholar]
- Van Maldegem F. et al. The majority of cutaneous marginal zone B-cell lymphomas expresses class-switched immunoglobulins and develops in a T-helper type 2 inflammatory environment. Blood 112, 3355–61 (2008). [DOI] [PubMed] [Google Scholar]
- Edinger J. T., Kant J. A. & Swerdlow S. H. Cutaneous marginal zone lymphomas have distinctive features and include 2 subsets. Am. J. Surg. Pathol. 34, 1830–41 (2010). [DOI] [PubMed] [Google Scholar]
- Brenner I. et al. Primary cutaneous marginal zone lymphomas with plasmacytic differentiation show frequent IgG4 expression. Mod. Pathol. 26, 1568–76 (2013). [DOI] [PubMed] [Google Scholar]
- Deshpande V. et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 25, 1181–92 (2012). [DOI] [PubMed] [Google Scholar]
- Mannami T. et al. Clinical, histopathological, and immunogenetic analysis of ocular adnexal lymphoproliferative disorders: characterization of malt lymphoma and reactive lymphoid hyperplasia. Mod. Pathol. 14, 641–9 (2001). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. Deviated VH4 immunoglobulin gene usage is found among thyroid mucosa-associated lymphoid tissue lymphomas, similar to the usage at other sites, but is not found in thyroid diffuse large B-cell lymphomas. Mod. Pathol. 19, 1578–84 (2006). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. Duodenal follicular lymphomas share common characteristics with mucosa-associated lymphoid tissue lymphomas. J. Clin. Pathol. 61, 377–81 (2008). [DOI] [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 8, 223–46 (2003). [PubMed] [Google Scholar]
- Riedel S. et al. CD4+ Th1-cells predominate in low-grade B-cell lymphoma of gastric mucosa-associated lymphoid tissue (MALT type). Scand. J. Gastroenterol. 36, 1198–203 (2001). [DOI] [PubMed] [Google Scholar]
- Vyth-Dreese F. A. et al. Localization in situ of costimulatory molecules and cytokines in B-cell non-Hodgkin’s lymphoma. Immunology 94, 580–6 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bende R. J. et al. Among B cell non-Hodgkin’s lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 201, 1229–41 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Benjamin R. J., Shahsafaei A. & Dorfman D. M. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood 95, 627–32 (2000). [PubMed] [Google Scholar]
- Suefuji H. et al. CXCR3-positive B cells found at elevated frequency in the peripheral blood of patients with MALT lymphoma are attracted by MIG and belong to the lymphoma clone. Int. J. Cancer 114, 896–901 (2005). [DOI] [PubMed] [Google Scholar]
- Meiler F., Klunker S., Zimmermann M., Akdis C. A. & Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy 63, 1455–63 (2008). [DOI] [PubMed] [Google Scholar]
- Chanudet E. et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J. Pathol. 209, 344–51 (2006). [DOI] [PubMed] [Google Scholar]
- Aigelsreiter A. et al. Chlamydia psittaci Infection in nongastrointestinal extranodal MALT lymphomas and their precursor lesions. Am. J. Clin. Pathol. 135, 70–5 (2011). [DOI] [PubMed] [Google Scholar]
- Collina F. et al. Chlamydia psittaci in ocular adnexa MALT lymphoma: a possible role in lymphomagenesis and a different geographical distribution. Infect. Agent. Cancer 7, 8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk W. et al. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am. J. Surg. Pathol. 32, 1159–67 (2008). [DOI] [PubMed] [Google Scholar]
- Kase S. et al. IgG4-related inflammation of the orbit simulating malignant lymphoma. Anticancer Res. 33, 2779–83 (2013). [PubMed] [Google Scholar]
- Yamamoto M. et al. Risk of malignancies in IgG4-related disease. Mod. Rheumatol. 22, 414–8 (2012). [DOI] [PubMed] [Google Scholar]
- Karagiannis P. et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J. Clin. Invest. 123, 1457–74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M. W. L. et al. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 70, 7800–9 (2010). [DOI] [PubMed] [Google Scholar]