Abstract
Background:
Uterine receptivity for the implantation is a complicated process, that ovarian factors (hormonal), endometrium and embryo simultaneously are involved in this phenomenon. A successful implantation needs appropriate development of the endometrium. Furthermore, embryo must be capable of reacting with the endometrium and producing suitable adhesion molecules. This study aimed to examine one of endometrial maturation indices in mice before implantation, i.e., proliferation of stromal cells.
Materials and Methods:
A total of 40 adult female mice were divided into four groups: Control, gonadotropin, gonadotropin + progesterone, and gonadotropin + sildenafil citrate. The three experimental groups were first injected 7.5 IU of human chorionic gonadotropin (HCG) and then 7.5 IU of human menopausal gonadotropin (HMG). Then, every two female mice were placed in a cage with a male mouse for mating. Two groups were injected 1 mg of progesterone and 3 mg/kg of sildenafil citrate at intervals of 24, 48, and 72 h after injection of HMG. After 96 h, all the mice were killed, and their uterine samples subjected to tissue passage and prepared for analysis. Immunohistochemical method, Ki-67, and stromal mitotic cell count were used in this study.
Results:
Our observations in all groups showed changes in the luminal epithelium. ANOVA analysis Ki-67-positive stromal cells among all groups were not statistically significant.
Conclusion:
The results showed that administration of HMG and HCG following that of progesterone and sildenafil citrate could change the indices of endometrial maturation, and they were not involved in the phase immediately before implantation in stromal mitotic index.
Keywords: Endometrium, implantation, sildenafil citrate
INTRODUCTION
A successful implantation requires two factors: The first one comprises endometrial development and maturation, intact embryo in the blastocyst stage, and proper mutual function and effects of these two, and the second one is synchronization of uterine endometrium and blastocyst.[1]
Uterine endometrial maturation immediately before embryo implantation in the blastocyst stage includes the following phases: (1) Zona pellucida hatching, (2) the phase before contact of luminal epithelial cells and embryonic trophoblast cells, (3) apposition, (4) adhesion (attachment) wherein luminal epithelial cells attach to the trophoblast cells, (5) invasion (to penetrate and invade the uterine endometrium), and (6) decidualization and placentation.[2,3]
Morphologic and functional changes under the influence of ovarian steroid hormones are required to prepare the uterus for implantation. Moreover, a large group of intermediate molecules including cytokines, cell adhesion molecules, lipids, growth factors, and many other molecules are involved in implantation under the influence of the earlier mentioned hormones.[4,5]
Progesterone secreted from the ovaries causes fundamental changes in uterus, such as growth of lining epithelial cells and glandular cells of glands, decidual reaction, and transformation of stromal cells into decidual cells.[6]
Studies show tha ART (Assisted Reproductive Technologies) drugs can increase endometrial thickness and the likelihood of pregnancy.[7,8]
In recent years, the focus was on the role of nitric oxide and its moderating effects on uterine blood flow.[9,10]
Nitric oxide relaxes vascular smooth muscles by releasing and moderating cyclic guanosine monophosphate (cGMP), and its isoforms have been also detected in uterus.[11]
Sildenafil citrate was among the very successful drugs that were first used for treatment of erectile dysfunction in men in 1997. This drug is a phosphodiesterase type 5 inhibitor that inhibits degradation of cGMP and causes the nitric oxide to affect the vascular smooth muscles.[12]
Ki-67 antigen is one of the cell proliferation markers and is expressed in the nucleus of the proliferating mammalian cells, with a molecular weight of 320 and 360 kDa after splicing stages. The monoclonal antibody produced against this antigen is called MIB-5(Anti-Rat Ki-67 Antigen. Clone MIB-5. Code No. M7248). Both molecules bind to the chromatin, but their effect is not known.[13,14,15,16] Ki-67 antigen is expressed during G1, S, G2, and M phases. In G1 phase, Ki-67 is often detected around the nucleus. During the next phases, Ki-67 antigen is identified all over the nucleus and is shown throughout the nuclear matrix.[17] Ki-67 antigen binds to the chromosomes during mitosis[18] and surrounds the chromosomes in the form of a network-like structure in metaphase, which is reduced in anaphase and telophase.[19,20] On the contrary, Ki-67 antigen cannot be identified in silent cells and in DNA that is being repaired.[21] the most important feature of this study was that it studied the endometrial conditions only if the blastocyst entered the uterine cavity, while in previous studies, previous studies have been based on this point of time.
It seems that sildenafil citrate is effective in endometrial maturation by increasing perfusion and it consequently causes cell proliferation, thus examining the rate of cell proliferation in stroma can guide the researchers toward the objective of this study.
MATERIALS AND METHODS
Animals and groups
In this study, 40 Syrian adult female mice with a mean weight of 25-30 g and 20 Swiss adult male mice were selected. The female mice were divided to four groups, each containing 10 mice: Control, gonadotropin, gonadotropin + progesterone, and gonadotropin + sildenafil citrate. All the mice were kept in the animal house of the Pharmaceutics Applied Research Center under identical conditions on a 12-h light/dark cycle at 23 ± 1°C. They received municipal water and food (Pars co. Iran). At first, for induction of ovulation, the mice in the experimental groups were injected 7.5 IU of human menopausal gonadotropin (HMG) intraperitoneally (IP) and 48 h later, they were injected 7.5 IU of human chorionic gonadotropin (HCG). Then, every two female mice were placed in a cage with a male mouse for mating. The mice in gonadotropin + progesterone group were injected 1 mg of progesterone at intervals of 24, 48, and 72 h after injection of HMG. The mice in gonadotropin + sildenafil citrate group were also injected 3 mg/kg of sildenafil citrate IP at the same intervals (sildenafil citrate powder, supplied by Rouz Darou Pharmaceutical Company, Tehran, Iran) was dissolved in distilled water and used immediately). After 96 h, all the mice in the control group and experimental groups were killed by dislocating their cervical vertebrae, and their uterus was rinsed with culture medium. Only the uteri of the mice containing blastocyst were sampled. The samples were then buffered in 10% formalin after fixation. Then, they underwent stages of tissue passage with ascending grades of alcohol, cleared with xylol, and finally, molded in paraffin. The samples were stained with periodic acid-Schiff (PAS) and analyzed using an optical microscope. The paraffinic samples were applied for immunohistochemical (IHC) study.
Immunohistochemical staining by Ki-67
The paraffinic block of the formalin-fixed tissue was cut at a thickness of 3 μ and placed on a slide coated with poly-L-lysine. Then, rinsing was done according to the following stages: Transferring the slides into the boiling buffer citrate for 10-15 min, washing the samples in two containers of washing buffer, and putting the slides in hydrogen peroxide solution; incubating the slides in background + antimouse Ki-67 (Dako); incubating in the secondary antibody + wash buffer at room temperature or at 4°C; incubating in HRP/streptavidin + wash buffer at room temperature or at 4°C and more chromogen solution + substrate (DAB + substrate); washing the samples; and staining with hematoxylin for background staining within 1-3 min. The slides were then studied using an optical microscope (Olympus/3H-2) ×40.
Ki-67-positive cells
To count Ki-67-positive cells, the samples stained with Ki-67 kit were photographed using Motic Image software at ×40. Then, among the images, 20 fields were selected randomly in each group and brown Ki-67 positive cells were counted.
Statistical calculations
The counted cells were analyzed by statistically descriptive methods (mean ± standard deviation) and one-way ANOVA using SPSS.13 software. P value less than 0.05 was considered significant in this study.
RESULTS
The analyses with optical microscope showed long cylindrical luminal epithelial cells with numerous PAS-positive granules present mainly on the basal surface of the cells and the area under nucleus in the control group. In the gonadotropin group, long cylindrical luminal epithelial cells with numerous PAS-positive granules were observed on the apical area above the nucleus and on the basal surface of the cells. In the gonadotropin + progesterone and gonadotropin + sildenafil citrate groups, the cells were cylindrical and numerous PAS-positive granules were observed on the apical surface of the cells above the nucleus and also on the basal surface of the cells under the nucleus. The nuclei of the cells of all groups were in center, which showed the luteal phase in the endometrium. The nuclei seemed vacuolated in all groups.
Moreover, the Ki-67-positive cells in endometrial stroma were analyzed using optical microscope [Figures 1 and 2]. Within pre-implantation phase, stromal cells reacted positively with Ki-67, which showed cell proliferation in the endometrial stroma of mice uteri in all groups. Ki-67-positive cell counting in endometrial stroma of all groups and statistical analyses of data did not show a significant difference in terms of Ki-67 positivity [Figure 3].
Figure 1.
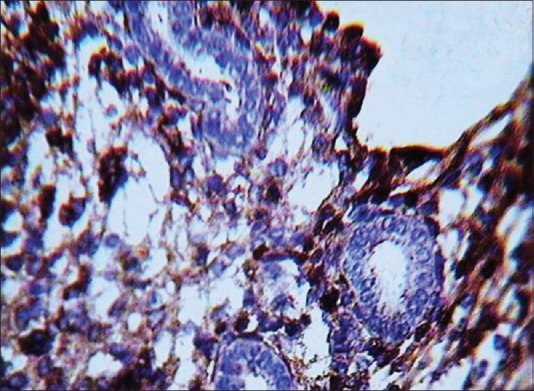
Photomicrograph of uterine endometrium of mice in control group for Ki-67 (the brown cells are Ki-67 positive, M=660)
Figure 2.
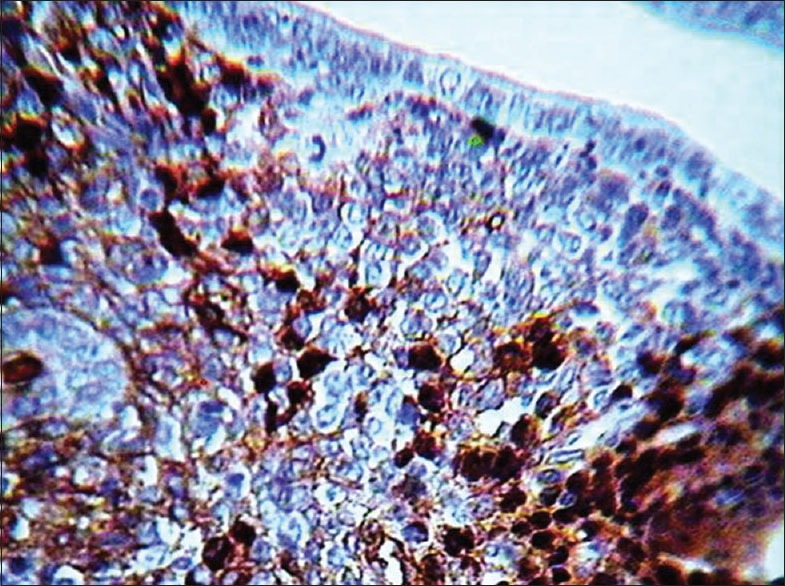
Photomicrograph of uterine endometrium of mice in human menopausal gonadotropin/human chorionic gonadotropin + sildenafil citrate group for Ki-67 (the brown cells are Ki-67 positive, M=660)
Figure 3.
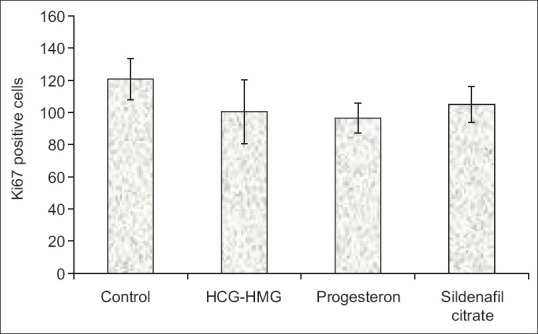
Comparison of the number of Ki-67-positive cells in endometrial stroma of studied groups (P > 0.05)
DISCUSSION
The effects of ovulation-inducing drugs on the process of endometrial maturation in ART cycles have always been considered by gynecologists and infertility specialists; however, comparison of the endometrial samples under treatment protocol with control samples of normal cycle has been almost impossible. The reason is that access to human endometrial samples in pre-implantation conditions is practically impossible. Therefore, in this regard, experimental studies on animal models would be valuable. Although progesterone is used for acceleration of endometrial maturation, the use of other drugs is also taken into account. Sildenafil citrate is primarily used to cure male impotence; however, it was found that this drug can be used for vasodilation[22,23] and relaxation of myometer muscles.[24] Although this drug is in its preliminary and experimental application and its direct use under in vitro condition has harmful effects on sperm,[25] it seems that it facilitates endometrial maturation.
Induction of ovulation using HMG/HCG and then application of progesterone and sildenafil citrate in the three experimental groups did not induce cell proliferation in luminal epithelial. Furthermore, despite cell proliferation in stroma in this phase, application of ovulation-inducing drugs, progesterone, and sildenafil citrate did not cause cell proliferation. Previous reports of the researchers also revealed that the height of luminal cells in the group that received ovulation-inducing HMG–HCG increased compared to that in the control group; however, comparison of the HMG–HCG + progesterone group and HMG–HCG + sildenafil citrate group with the control group showed a reduction in the height of luminal epithelium. Moreover, there was no difference in the height of luminal cells in the HMG–HCG + progesterone group and the HMG–HCG + sildenafil citrate group.[26]
Previous reports of the researchers also showed that the height of endometrial glandular epithelial cells in the group receiving ovulation-inducing HMG–HCG did not increase or decrease compared to that in the control group. Furthermore, comparison of the HMG–HCG + progesterone group and HMG–HCG + sildenafil citrate group and the control group showed no difference in the height of endometrial glandular epithelial cells. Moreover, there was no difference in the height of endometrial glandular epithelial cells in the HMG–HCG + progesterone group and the HMG–HCG + sildenafil citrate group. There were no morphological variations in glandular epithelial cells in terms of the appearance of the nuclei, cytoplasm, and surface mucous.[27] Previous studies have also shown that the use of estrogen alone in adult mice whose ovaries had been removed caused cell proliferation in luminal and glandular epithelium; however, a combination of estrogen and progesterone caused development of endometrial stroma.[28,29] During pre-implantation period, a similar pattern of cell-specific behavior is regulated by ovarian steroids.
On the specificity of staining with Ki-67 antigen for proliferating cells, it must be mentioned that the monoclonal antibody was first selected in 1983 to react with the nuclei of proliferating cells in human tissues.[30] Analysis of cellular cycle has shown that the antigens associated with cell proliferation are actively expressed during G1, S, G2, and mitosis phases, although in G0 phase, resting cells are absent.[31]
There are various methods for detection of cell proliferation in tissue sections. A standard method for detecting cell proliferation under in vivo condition is the application of DNA labeling using a modified analog of pyrimidine and derivative of thymidine named BrdU (5-bromo-2’-deoxyuridine).[32,33] BrdU is used in immunohistochemistry for identification of the S phase of cell proliferation.
Another common method for detection of cell proliferation in tissue sections without using BrdU is identification of proliferating cell nuclear antigen (PCNA) using IHC method, which was first introduced by Miyachi et al.[34] The expression of PCNA increases during G1 phase and reaches its maximum in S phase, whereas it is reduced in G2/M phases. This is the reason that various behaviors of cells can be identified during immunostaining stages in different phases of meiosis.[35]
The monoclonal antibody which is produced against it is called MIB-5 that can detect Ki-67 antigen expressed in the nucleus of the proliferating cells in mammals with a molecular weight of 320 and 360 kDa after splicing stages. Both molecules bind to the chromatin, but their effect is not known.[13,14,15,16]
Salmi et al.'s study on human endometrium using Ki-67 showed that the nuclei of cells in luminal and glandular epithelium and also stroma in follicular phase in endometrial functional layer were positive for Ki-67. This was also true for basal layer of endometrium; however, Ki-67-positive cells were reduced at the beginning of the luteal phase in luminal and glandular epithelium and they disappeared at the end of the phase. Whereas positive nuclei were identified in stroma during the whole luteal phase in functional layer of endometrium, no Ki-67-positive cells were found in the nuclei during the luteal phase in the basal layer of endometrium.[21] Growth regulation and differentiation of glandular and stromal cells in endometrium are associated with sex steroids in ovary.[36]
Another study by Koshiyama et al. (1995)[37] on detection of Ki-67 antigen in endometrium identified Ki-67 positive cells in endometrial glands and stroma during the follicular phase; however, they were seen completely scattered in the basal layer of endometrium. At the beginning of the luteal phase, Ki-67 positive cells were reduced in glandular cells, although they increased in functional stroma. In the middle and late luteal phases, no cell containing Ki-67 was identified; however, functional stroma still contained Ki-67 positive cells. In the basal layer of endometrium, Ki-67 positive cells were completely scattered during the luteal phase.[37] Moreover, the difference between proliferated cells in glands and uterine stroma during the secretion phase in menstrual cycle may represent the difference in receptors of sex hormones. In this respect, the expression of receptors of estrogen and progesterone in glandular cells is regulated negatively during the secretion phase, whereas the expression of progesterone receptors in stromal cells continues positively during the secretion phase.[38,39]
During pre-implantation period in mice, epithelial cells on the 1st and 2nd days, before ovulation, are proliferated under the influence of estrogen secreted from ovary. On the 3rd day, stromal cells are proliferated under the influence of progesterone secreted from a forming corpus luteum. On the 4th day, higher induction occurs by estrogen in pre-implantation and the combination of estrogen and progesterone induces differentiation in epithelial cells followed by inhibition of cell proliferation. At the beginning of implantation, stromal cells around the implantation site are transformed into decidual cells under severe influence and differentiation.[29] In terms of topography, endometrium is divided into two layers of basalis and functionalis, and immunoactivity of Ki-67 in glandular and stromal cells in basalis layer is much less than that in functionalis layer.[37] Furthermore, other researchers have shown that stromal and glandular cells in uterine functional layer in the proliferation phase actively entered the cellular cycle (mitosis), whereas in the secretion phase, only the proliferating and dividing stromal cells remain.[40,41]
CONCLUSION
It can be argued that the absence of Ki-67-positive cells in luminal epithelium, during luteal phase and immediately before implantation, was not unexpected in this study. As shown by the analysis using one-way ANOVA, the difference in mean number of Ki-67-positive cells in endometrial stroma was not significant among the studied groups. Therefore, it can be concluded that sildenafil citrate along with gonadotropin may influence endometrial maturation regarding luminal epithelial cells, although this influence does not intervene with the proliferation process in mice endometrial stroma.
ACKNOWLEDGMENT
This study was supported by funding from the Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. Authors acknowledge for master this center.
Footnotes
Source of Support: Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Conflict of Interest: The authors do not have conflict of interest.
REFERENCES
- 1.Diedrich K, Fauser BC, Devroey P, Griesinger G Evian Annual Reproduction (EVAR) Workshop Group. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13:365–77. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- 2.Guillomot M. Cellular interactions during implantation in domestic ruminants. J Reprod Fertil Suppl. 1995;49:39–51. [PubMed] [Google Scholar]
- 3.Blankenship TN, Enders AC, King BF. Trophoblastic invasion and the development of uteroplacental arteries in the macaque: Immunohistochemical localization of cytokeratins, desmin, type IV collagen, laminin, and fibronectin. Cell Tissue Res. 1993;272:227–36. doi: 10.1007/BF00302728. [DOI] [PubMed] [Google Scholar]
- 4.Lessey BA, Castelbaum AJ. Integrins and implantation in the human. Rev Endocr Metab Disord. 2002;3:107–17. doi: 10.1023/a:1015450727580. [DOI] [PubMed] [Google Scholar]
- 5.Lessey BA. Endometrial responsiveness to steroid hormones: A moving target. J Soc Gynecol Investig. 2004;11:61–2. doi: 10.1016/j.jsgi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Moyer DL, de Lignieres B, Driguez P, Pez JP. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: Influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992–7. doi: 10.1016/s0015-0282(16)55916-0. [DOI] [PubMed] [Google Scholar]
- 7.Gonen Y, Casper RF. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF) t J In Vitro Fert Embryo Transf. 1990;7:146–52. doi: 10.1007/BF01135678. [DOI] [PubMed] [Google Scholar]
- 8.Sher G, Herbert C, Maassarani G, Jacobs MH. Assessment of the late proliferative phase endometrium by ultrasonography in patients undergoing in-vitro fertilization and embryo transfer (IVF/ET) Hum Reprod. 1991;6:232–7. doi: 10.1093/oxfordjournals.humrep.a137312. [DOI] [PubMed] [Google Scholar]
- 9.Amit A, Thaler I, Paz Y, Itskovitz-Eldor J. The effect of a nitric oxide donor on Doppler flow velocity waveforms in the uterine artery during the first trimester of pregnancy. Ultrasound Obstet Gynecol. 1998;11:94–8. doi: 10.1046/j.1469-0705.1998.11020094.x. [DOI] [PubMed] [Google Scholar]
- 10.Cameron IT, Campbell S. Nitric oxide in the endometrium. Hum Reprod Update. 1998;4:565–9. doi: 10.1093/humupd/4.5.565. [DOI] [PubMed] [Google Scholar]
- 11.Sher G, Fisch JD. Vaginal sildenafil (Viagra): A preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15:806–9. doi: 10.1093/humrep/15.4.806. [DOI] [PubMed] [Google Scholar]
- 12.Boolell M, Gepi-Attee S, Gingell JC, Allen MJ. Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br J Urol. 1996;78:257–61. doi: 10.1046/j.1464-410x.1996.10220.x. [DOI] [PubMed] [Google Scholar]
- 13.Birner P, Ritzi M, Musahl C, Knippers R, Gerdes J, Voigtländer T, et al. Immunohistochemical detection of cell growth fraction in formalin-fixed and paraffin-embedded murine tissue. Am J Pathol. 2001;158:1991–6. doi: 10.1016/S0002-9440(10)64670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Mitui H, Udaka N, Hayashi H, Okudela K, Kanisawa M, et al. Ki-67 (MIB 5) immunostaining of mouse lung tumors induced by 4-nitroquinoline 1-oxide. Histochem Cell Biol. 1998;110:589–93. doi: 10.1007/s004180050321. [DOI] [PubMed] [Google Scholar]
- 15.Gerlach C, Golding M, Larue L, Alison MR, Gerdes J. Ki-67 immunoexpression is a robust marker of proliferative cells in the rat. Lab Invest. 1997;77:697–8. [PubMed] [Google Scholar]
- 16.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Verheijen R, Kuijpers HJ, Schlingemann RO, Boehmer AL, van Driel R, Brakenhoff GJ, et al. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. I. Intracellular localization during interphase. J Cell Sci. 1989;92:123–30. doi: 10.1242/jcs.92.1.123. [DOI] [PubMed] [Google Scholar]
- 18.Verheijen R, Kuijpers HJ, van Driel R, Beck JL, van Dierendonck JH, Brakenhoff GJ, et al. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J Cell Sci. 1989;92:531–40. doi: 10.1242/jcs.92.4.531. [DOI] [PubMed] [Google Scholar]
- 19.Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992;25:31–40. doi: 10.1111/j.1365-2184.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 20.Hall PA, McKee PH, Menage HD, Dover R, Lane DP. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993;8:203–7. [PubMed] [Google Scholar]
- 21.Salmi A, Heikkilä P, Lintula S, Rutanen EM. Cellular localization of c-jun messenger ribonucleic acid and protein and their relation to the proliferation marker Ki-67 in the human endometrium. J Clin Endocrinol Metab. 1998;83:1788–96. doi: 10.1210/jcem.83.5.4792. [DOI] [PubMed] [Google Scholar]
- 22.Johns Hopkins University: Medical News Today cardiology news; 2005. Johns Hopkins Medical Institutions David March: Viagra (Sildenafil) effectively treats enlarged hearts, mouse study show; p. 24. [Google Scholar]
- 23.Wareing M, Myers JE, O’Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab. 2005;90:2550–5. doi: 10.1210/jc.2004-1831. [DOI] [PubMed] [Google Scholar]
- 24.Khan RN, Hamoud H, Warren A, Wong LF, Arulkumaran S. Relaxant action of sildenafil citrate (Viagra) on human myometrium of pregnancy. Am J Obstet Gynecol. 2004;191:315–21. doi: 10.1016/j.ajog.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Paulus WE, Strehler E, Zhang M, Jelinkova L, El-Danasouri I, Sterzik K. Benefit of vaginal sildenafil citrate in assisted reproduction therapy. Fertil Steril. 2002;77:846–7. doi: 10.1016/s0015-0282(01)03272-1. [DOI] [PubMed] [Google Scholar]
- 26.Rashidi B, Roshangar L, Soleimani Rad J, Khaki AA, Mohammadnejad D, Azami I. Comparison of morphology and morphometry of preimplantation mouse uterine endometrium in natural cycle with those received superovulatory drugs, progesterone and sildenafil citrate (Viagra) Pharm Sci Autumn. 2008;3:33–39. [Google Scholar]
- 27.Rashidi B, Rad JS, Roshangar L. Comparison of morphological and morphometrical characteristics in the glandular epithelium of mouse endometrium in preimplantation period after administration HMG-HCG, progesterone and sildenafil citrate. J Isfahan Med School. 2010;28:112. [Google Scholar]
- 28.Finn CA, Martin L. The role of the oestrogen secreted before oestrus in the preparation of the uterus for implantation in the mouse. J Endocrinol. 1970;47:431–8. doi: 10.1677/joe.0.0470431. [DOI] [PubMed] [Google Scholar]
- 29.Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: Modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–90. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 31.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 32.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–5. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 33.Alison MR. Assessing cellular proliferation: What's worth measuring? Hum Exp Toxicol. 1995;14:935–44. doi: 10.1177/096032719501401201. [DOI] [PubMed] [Google Scholar]
- 34.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228–34. [PubMed] [Google Scholar]
- 35.Foley J, Ton T, Maronpot R, Butterworth B, Goldsworthy TL. Comparison of proliferating cell nuclear antigen to tritiated thymidine as a marker of proliferating hepatocytes in rats. Environ Health Perspect. 1993;101:199–205. doi: 10.1289/ehp.93101s5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss JF, Gurpide E. The endometrium. In: Yen SS, Jaffe RB, editors. Reproductive Endocrinology. 3rd ed. Philadelphia: W B Saunders; 1991. pp. 309–56. [Google Scholar]
- 37.Koshiyama M, Konishi I, Nanbu K, Nanbu Y, Mandai M, Komatsu T, et al. Immunohistochemical localization of heat shock proteins HSP70 and HSP90 in the human endometrium: Correlation with sex steroid receptors and Ki-67 antigen expression. J Clin Endocrinol Metab. 1995;80:1106–12. doi: 10.1210/jcem.80.4.7714077. [DOI] [PubMed] [Google Scholar]
- 38.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–40. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- 39.Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Schaison G, et al. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab. 1988;67:80–7. doi: 10.1210/jcem-67-1-80. [DOI] [PubMed] [Google Scholar]
- 40.Li SF, Nakayama K, Masuzawa H, Fujii S. The number of proliferating cell nuclear antigen positive cells in endometriotic lesions differs from that in the endometrium. Analysis of PCNA positive cells during the menstrual cycle and in post-menopause. Virchows Arch A Pathol Anat Histopathol. 1993;423:257–63. doi: 10.1007/BF01606888. [DOI] [PubMed] [Google Scholar]
- 41.Tabibzadeh S. Proliferative activity of lymphoid cells in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:437–43. doi: 10.1210/jcem-70-2-437. [DOI] [PubMed] [Google Scholar]


