Abstract
Background:
Endothelial progenitor cells (EPCs) are present in circulation and contribute to vasculogenesis in adults. The aim of the present study was to determine the number of circulating EPCs in patients with optic neuritis (ON).
Materials and Methods:
Fifty patients with ON were diagnosed by expert neurologist and optometrist at the Feiz Hospital, Isfahan, Iran (2012–2013). Blood samples were collected from ON patients in the first attack. The number of EPCs was measured by flow cytometry through the assessment of CD34+ and CD309+ in patients and healthy individuals.
Results:
With using flow cytometry, CD34+ and CD309+ cells detected in peripheral blood cells of patients (n = 50) with ON, and healthy individuals (n = 30). Patients with ON had (mean = 66.71 ± 17.82) CD34+ and CD309+ cells compared with healthy controls (mean = 28.72 ± 22.46). In addition, there was no significant difference in CD309+ cells in both groups.
Conclusion:
This study showed elevated CD34+ and CD309+ cells in the early stage of the disease. Regarded to EPC increment in neural repair, it expected the EPC level be increased in these patients, but no detectable differences were observed among both markers in healthy and patient with first attack.
Keywords: CD309+, CD34+, endothelial progenitor cells, optic neuritis
INTRODUCTION
Optic neuritis (ON) is an inflammation of the optic nerve.[1] Classically there is a triad of clinical features-reduced vision, eye pain, and impaired color vision.[1,2] Where multiple sclerosis (MS) is common, acute demyelization of the optic nerve is a common cause of ON in some parts. However, there are many other possible causes which must not be overlooked, as they may require different and urgent management.[3,4]
Endothelial cells have a critical role in development and maintenance of blood vessels after injury. Low endothelial progenitor cells (EPCs) number has also been reported in patients with cerebrovascular disease.[5] Astrocytes interaction with endothelial cells maintain the blood-brain barrier (BBB), which normally restricts the entrance of immune system effectors unless localized or distant events disturb the BBB, thus allowing access of cellular or soluble immune effectors.[5] Since optic nerve, as an area of the central nervous system (CNS), is more permissive, as evidenced by immunostaining for markers of intact BBB,[5,6] tissues of the optic nerve might be more sensitive to aquaporin-4 (AQP4) dysfunction mediated by anti-AQP4 antibodies.[5] While ON can be caused following other disease development, MS is the most likely reason in a young, otherwise healthy individual.[6,7]
The rate of circulating EPC have not been studied commonly in patients with ON, and the results were strife. Functional changes in cerebral endothelial cells (CECs) cause BBB disruption and correlate with a vascular system and MS risk.[8,9,10] In vitro studies show that, at least in the earlier stages of MS, strong interactions have been shown between the CECs, CD8+ T-lymphocytes, macrophages, chemokines, activated CD4+ T-lymphocytes, and cytokines. The impairment in CECs function allows entrance of inflammatory cells to BBB, which arouse constant of cascade of Th1 cytokine toward CNS environment.[9,10]
The circulating EPCs which release from bone marrow may possibly sustain the major increase of neoangiogenesis in the bone marrow.[11,12,13] The aim of the study was to evaluate the number of circulating EPCs in patients with first ON attack compare to healthy individuals, if any; the relationship could be among number of EPCs and severity of the ON disease.
MATERIALS AND METHODS
Fifty patients were included with ON, diagnosed by expert neurologist and optometrist, referred to the Feiz Hospital, Isfahan, Iran (July 2012–June 2013) The protocol for this study was approved by the Institutional Review Board of the Feiz Hospital. Study design, data collection, analysis, and reporting were performed independently by a study committee. Blood samples were collected from ON patients with the first attack, MS patients with a history of ON attack and healthy individuals (mean ages: 26 ± 5). Together with the collection of demographic data, a sample of venous blood was drawn to assess a complete blood count with differential; circulating CD34+ as a marker for hematopoietic stem cells (HSCs) and circulating EPC marker or CD309 cells count. The white blood cell (WBC) count was corrected for the number of circulating erythroblasts.
Flow cytometry analysis
Circulating EPCs were measured by phenotypic analysis in unselected peripheral blood cells. Blood samples were processed (2 h) after they were drawn. 50 μl of ethylenediamine tetraacetic acid-anticoagulated blood incubated (30 min, at 4°C) with 20 μl of fluorescein isothiocyanate (FITC)-conjugated anti-CD34 and 10 μl of phycoerythrin (PE)-conjugated anti-CD309. Appropriate isotype controls were used for each staining procedure. A volume of 1 ml of lysis solution was added (5 min, at room temperature). Then, samples were centrifuged, and pellets were resuspended (in 300 μl of phosphate buffered saline). Cells (2 × 105) were acquired by a fluorescence-activated cell sorting (FACS) calibur flow cytometer, and analyzed. Results were expressed as percentage of CD34 + cells that co-expressed CD309. On the basis of the peripheral blood nucleated cell count, we also calculated the absolute number of CD34+ CD309+ positive cells.
The expression of cell surface antigens was determined by 2-color immunofluorescence staining. Briefly, 2 × 106 cells were incubated in buffer containing 2% bovine serum albumin with 20 μ Fc-blocking agent (10 min, at 25°C). Then, cells were incubated (30 min, at 4°C) with 20 μl of CD34-FITC and CD309-PE (total volume of 200 μl). The cells were washed twice before re-suspension in 400 μl stain buffer. FACS analysis was performed in triplicate for each sample.
MATERIALS
The following were purchased from the sources indicated; antibodies, Calibur flow cytometer (Becton Dickinson, San Jose, CA); Lysis solution (Dako, Glostrup, Denmark); Fetal calf serum (HyClone, Logan, UT); Cell Quest software, Stain buffer (BD Biosciences, San Diego, CA); Fc-blocking agent (Miltenyi Biotech); CD34-FITC, CD309-PE (Miltenyi Biotech).
Data analysis
Data are expressed as mean ± standard deviation. Results were analyzed statistically, using the Independent sample t-test. Values of P < 0.001 were taken as significant.
RESULTS
The hematologic and clinical characteristics of total 50 patients with ON were studied. The median absolute percentage of circulating CD34+ as a marker for HSCs in the overall population of ON patients was 18 ± 9.96. Against in the healthy was 26.31 ± 14.33% (P < 0.001). Additionally, WBC count was noticeably high in patients with ON in compare with control groups. Moreover, level of circulating EPC marker or CD309 (18.70 ± 36.47%) was not substantially higher in patients with first attack of ON than in control subjects (28.72 ± 15.53). Although, a level of circulating CD309+ EPCs (17.8 ± 9.5%) was observed in MS patients with history of ON attack but there was no significant difference in patients comparison to the healthy [Figure 1 compared these markers in both groups].
Figure 1.
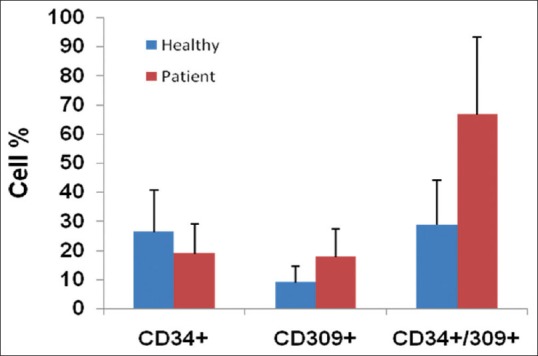
Cells analysis from the healthy controls and optic neuritis patients. The number of CD34+ CD309+ cells/ml compared in both groups. Results are expressed as mean ± standard deviation; *P ≤ 0.05
DISCUSSION
At sites of endothelial cell damage, recruitment and integration of EPCs which derived bone marrow has been shown to re modulate endothelialization.[13,14] Regard to rare number of EPC in the peripheral blood of healthy individuals, decrement or increment in these can be used in neovasculogenesis and vascular injury.[15,16] Quantitative lesion numbers may be served up as substitute markers of ON severity.
To assess the number of circulating EPCs, the percentage of CD309+ cells was evaluated. We found increasing EPC counts in our patients with first ON attach compared with control group, but there was not significant compared with healthy individuals. Based on previous literature,[17,18] which stated high level of circulating EPC in some neurological disorder may improve neurorepair,[19] in our study, EPC increment in ON patient was not significant, and it is unclear if elevated EPCs have good outcome for these patients.
In this study in patients with the first event of ON, as a result of the level of circulating EPCs, several clinically striking implications were observed. In these patients, levels of circulating EPCs was significantly higher in patients with onset of ON in compare with control subjects and MS patients with a history of ON attack but was not significant.
Recent studies have shown which rapidly increasing level of migratory circulation of EPC following acute ischemic stroke, acute coronary syndrome, and traumatic vascular injury are seen.[17,18,19,20,21,22] Similarity our results demonstrated that level of circulating EPCs increased rapidly in the onset of ON. Previous studies claim that mobilization of EPCs from bone marrow to circulation is a rapid response to BBB and tissue injury.[23,24,25] On the other hand, the findings from recent observational studies[26] demonstrated that the number of circulating EPCs was significantly lower with cerebrovascular disease than in control subjects, but it was not significantly lower with acute stroke disease. In addition, serial changes of circulating level of EPCs were demonstrated in patients after acute Ischemic Stroke and coronary artery disease.[27,28,29]
It suggested with regard to difference in the time interval for blood sampling between the present and the recent studies in acute response of EPC mobilization from bone marrow to tissue injury into consideration. In addition, the different number and migratory activity of circulating EPCs has been reported to depend on with risk factors for, ischemic attack (IA), coronary artery disease[30,31,32,33] and ON attack, as well. Another study is reported which circulating EPC number to be somewhat lower in coronary artery diseases. Increased circulating EPCs have been reported in diverse conditions associated with vascular injury, including vascular trauma, cardiovascular risk, exercise-induced ischemia, aortic aneurysm repair, and myocardial infarction. This is the first study that demonstrates evidence of a high number of circulating EPCs in the peripheral blood of patients with first ON attack.
CONCLUSION
These finding indicated that EPCs circulate in peripheral blood of ON patients at an early phase of disease was increased numbers. In literature studies have been shown increase in circulating EPC after ischemic attack be associated with a positive result.[34] So it seems to increase of EPC in MS as vascular disease[8] relates to a good outcome. The data showed in spite of various risk factors that could affect EPC levels in the neurodegenerative disease and also in ON patients. This study proffers a number of new standpoints in the pathogenesis of the disease. Yet, it is not clear if circulating EPCs have a role in neoangiogenesis and/or whether neoangiogenesis influence the disease progression.
ACKNOWLEDGMENTS
This study was supported by grant from CinnaGen Company. We appreciated Dr. Shiva Salami who coordinates financial process of the present project. The authors would like to thanks all patients who participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Morrow MJ, Wingerchuk D. Neuromyelitis optica. J Neuroophthalmol. 2012;32:154–66. doi: 10.1097/WNO.0b013e31825662f1. [DOI] [PubMed] [Google Scholar]
- 2.Lidster K, Baker D. Optical coherence tomography detection of neurodegeneration in multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11:518–27. doi: 10.2174/187152712801661185. [DOI] [PubMed] [Google Scholar]
- 3.Etemadifar M, Abtahi SH, Razmjoo H, Abtahi MA, Dehghani A, Salari M, et al. 25-hydroxyvitamin D concentrations in patients with optic neuritis as a clinically isolated syndrome and healthy controls. Int J Prev Med. 2012;3:313–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Dehghani L, Meamar R, Etemadifar M, Sheshde ZD, Shaygannejad V, Sharifkhah M, et al. Can vitamin d suppress endothelial cells apoptosis in multiple sclerosis patients? Int J Prev Med. 2013;4:S211–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–3. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 6.Lund H, Krakauer M, Skimminge A, Sellebjerg F, Garde E, Siebner HR, et al. Blood-brain barrier permeability of normal appearing white matter in relapsing-remitting multiple sclerosis. PLoS One. 2013;8:e56375. doi: 10.1371/journal.pone.0056375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minagar A, Maghzi AH, McGee JC, Alexander JS. Emerging roles of endothelial cells in multiple sclerosis pathophysiology and therapy. Neurol Res. 2012;34:738–45. doi: 10.1179/1743132812Y.0000000072. [DOI] [PubMed] [Google Scholar]
- 8.Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res. 2006;28:230–5. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 9.Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–41. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JS, Zivadinov R, Maghzi AH, Ganta VC, Harris MK, Minagar A. Multiple sclerosis and cerebral endothelial dysfunction: Mechanisms. Pathophysiology. 2011;18:3–12. doi: 10.1016/j.pathophys.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Goldschmidt-Clermont PJ. Endothelial progenitor cells: A promising therapeutic alternative for cardiovascular disease. J Interv Cardiol. 2007;20:93–9. doi: 10.1111/j.1540-8183.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 13.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: A novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 14.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108:1917–23. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 16.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 19.Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–64. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 20.Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, et al. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110:2039–46. doi: 10.1161/01.CIR.0000143161.01901.BD. [DOI] [PubMed] [Google Scholar]
- 21.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell-based vascular therapy. Circulation. 2003;108:2710–5. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 22.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–9. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 23.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribó M, Alvarez-Sabín J, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80:317–23. doi: 10.1016/j.mvr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Assmus B, Iwasaki M, Schächinger V, Roexe T, Koyanagi M, Iekushi K, et al. Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J. 2012;33:1911–9. doi: 10.1093/eurheartj/ehr388. [DOI] [PubMed] [Google Scholar]
- 25.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 26.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 27.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 28.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 29.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 31.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 32.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 33.Tao J, Wang Y, Yang Z, Tu C, Xu MG, Wang JM. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J Hum Hypertens. 2006;20:490–5. doi: 10.1038/sj.jhh.1001996. [DOI] [PubMed] [Google Scholar]
- 34.Bogoslovsky T, Chaudhry A, Latour L, Maric D, Luby M, Spatz M, et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology. 2010;75:2059–62. doi: 10.1212/WNL.0b013e318200d741. [DOI] [PMC free article] [PubMed] [Google Scholar]


