Abstract
Purpose:
The purpose was to study choroidal thickness and its profile based on location in healthy Indian children using enhanced depth spectral-domain-optical coherence tomography (SD-OCT).
Methods:
In this cross-sectional observational study 255 eyes of 136 children with no retinal or choroidal disease were consecutively scanned using enhanced depth SD-OCT. Eyes with any ocular disease or axial length (AXL) >25 mm or < 20 mm were excluded. A single observer measured choroidal thickness from the posterior edge of the retinal pigment epithelium to the choroid/sclera junction at 500-microns intervals up to 2500 microns temporal and nasal to the fovea. Generalized estimating equations were used to evaluate the correlation between choroidal thickness at various locations and age, AXL, gender and spherical equivalent (SEq).
Results:
Mean age of the subjects was 11.9 ± 3.4 years (range: 5–18 years). There were 62 Females and 74 males. The mean AXL was 23.55 ± 0.74 mm. Mean subfoveal choroidal thickness was 312.1 ± 45.40 μm. Choroid was found to be thickest subfoveally, then temporally. Age, AXL and SEq showed a significant correlation with choroidal thickness, whereas gender did not affect choroidal thickness.
Conclusion:
Our study provides a valid normative database of choroidal thickness in healthy Indian children. This database could be useful for further studies evaluating choroidal changes in various chorioretinal disorders. Age and AXL are critical factors, which negatively correlated with choroidal thickness.
Keywords: Children, choroid, choroidal imaging, enhanced-depth imaging, pediatric, spectral-domain-optical coherence tomography
The choroid is the most vascular tissue in the eye. It provides a blood supply to the outer retinal structures and plays a vital role in the pathophysiology of many diseases affecting the retina.[1,2,3,4,5] Several reports in the past have highlighted choroidal abnormalities such as thinning, vascular hyperpermeability and loss which are critical to the onset and progression of chorioretinal diseases such as central serous chorioretinopathy,[2] Vogt–Koyanagi–Harada disease,[3] high myopia-related chorioretinal atrophy,[6] age-related macular degeneration,[7] and polypoidal choroidal vasculopathy.[5]
Both ultrasonography and optical coherence tomography (OCT) have been used for measuring the choroidal thickness, however, the ultrasonography suffers from the limitations of low resolution (except in the peripapillary area) and high test-retest variability. On the other hand, with the development of high-resolution spectral-domain-OCT (SD-OCT) and enhanced depth imaging (EDI), in vivo assessment of choroid is possible which allows accurate and highly reproducible quantitative assessment of the choroid,[8,9,10] making this a noninvasive noncontact modality to image choroid in normal and pathological states. This information could, therefore, be useful in decision making for the management and monitoring of disease progression.
Recently, several factors such as age, gender, axial length (AXL), and refractive error have been shown to affect choroidal thickness.[11,12] However, there is scant literature regarding the choroidal thickness measurements in children. Measuring choroidal thickness may provide an insight into the influence of normal eyeball development on choroidal structure in children belonging to different age groups. Moreover, there is no literature available on normative choroidal thickness profile in Indian children, the previous reports being mainly from the western world.[13,14,15,16,17]
This prospective observational study aims to report normative database of choroidal thickness in healthy Indian children.
Methods
This was a cross-sectional observational study. We evaluated the choroidal thickness of healthy children ≤18 years of age through EDI SD-OCT. This prospective study was performed at our institute, from June 2012 to August 2013. Prior approval from the Institutional Review Board of the institute was taken, and informed consent was obtained from the parents or guardians of each participant. This study was conducted in accordance with the tenets of the Declaration of Helsinki. Only children aged 5 years or older were included because younger children were considered unable to cooperate for SD-OCT examination. Exclusion criteria included high myopia (>−6 D) or hyperopia (>+4 D); eyes with any ocular disease or AXL >25 mm or <20 mm; any retinal or retinal pigment epithelium (RPE) abnormality detectable on OCT scan or vitreoretinal disorders; poor image quality because of unstable fixation; evidence of amblyopia or strabismus; history of systemic disease or systemic medications with known ocular effects; history of any ocular surgery/injury, strabismus, amblyopia, congenital cataract. Subjects who were unable to cooperate for SD-OCT examination were also excluded. Both eyes of the patient were included in the study.
All participants underwent a comprehensive ophthalmic examination including visual acuity testing using, slit-lamp biomicroscopy, intraocular pressure measurement using Goldmann applanation tonometer, dilated fundoscopic examination, and cycloplegic refraction with cyclopentolate hydrochloride 1% eye drops. AXL measurement was performed using ocular biometry (IOL Master; Carl Zeiss Meditec, Jena, Germany).
Choroidal imaging
The SD-OCT scans were obtained using Cirrus high-definition (HD)-OCT (Carl Zeiss Meditec, Inc., Dublin, CA. Software Version 6.0) with dilated pupils. The scan used for imaging in this study is HD 5-line raster. Scan 3 of the 5, which passes through the fovea and was used for all the measurements. Only scans with a signal strength of more than or equal to 6 were used for analysis.
Using the Cirrus linear measurement tool, single observer measured choroidal thickness, which was defined as the vertical distance from the hyperreflective line of the RPE along the direction of the scan, to the hyperreflective line of the inner surface of the sclera, at 500 microns intervals temporal and nasal from the fovea, up to 2500 microns as published in the literature.[18] Intraclass correlation coefficient for intra-observer reproducibility was 0.97.
Statistical analysis
Descriptive statistics included mean and standard deviation for continuous variables. As both eyes of most subjects were included for analysis, the correlation between the two eyes of the same subject was adjusted using generalized estimating equations (GEE) during the calculation of summary descriptive parameters. Multivariate models adjusted using GEE methods were fit to assess the effects of age, gender, AXL, and spherical equivalent (SEq) on the choroidal thickness measurements. Statistical analyses were performed using commercial software (Stata version 12.1; StataCorp, College Station, TX). The alpha level (type I error) was set at 0.05.
Results
We included two fifty-five eyes of 136 healthy Indian children. Mean age (mean ± standard deviation) of the subjects was 11.9 ± 3.4 years (range: 5–18 years). There were 62 females and 74 males. The mean AXL was 23.55 ± 0.74 mm (range: 21.12–24.98 mm) and mean SEq was − 0.5 ± 1.09 D. All patients were phakic.
Mean subfoveal choroidal thickness was 311.2 ± 45.19 μm (range: 185–396 μm). Mean macular thickness was 194.4 ± 30.71 μm. When compared between two eyes of one patient, there was no significant difference in choroidal thickness at all locations.
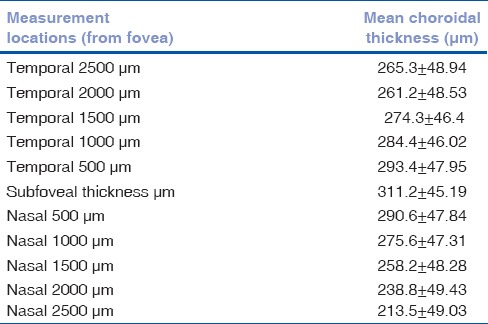
Table 1 shows the distribution of the choroidal thickness across the various points. Maximum choroidal thickness was noted subfoveally and gradually decreased as the distance from the fovea increased (P < 0.00001). Nasal choroidal was found to be thinner than the temporal choroid (P < 0.00001). Hence, the choroid was noted to be thinnest near optic nerve head.
Table 1.
Mean choroidal thickness at various locations from fovea
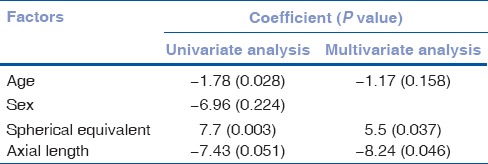
In this study, age and AXL showed significant negative correlation with choroidal thickness on univariate analysis whereas on multivariate analysis only AXL showed a significant negative correlation. SEq had a significant positive correlation with choroidal thickness [Fig. 1]. There was no significant difference in choroidal thickness between the males and females [Table 2].
Figure 1.
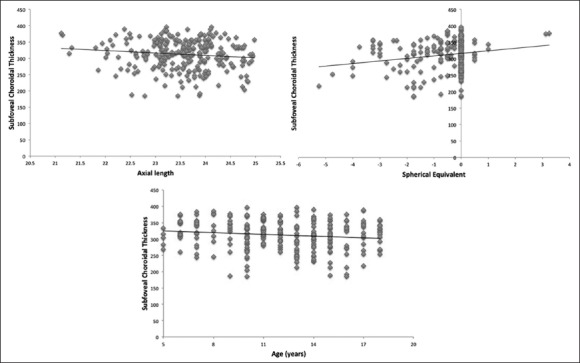
Correlation between subfoveal choroidal thickness and age, spherical equivalent and axial length
Table 2.
Relationship between various factors on the subfoveal choroidal thickness
Discussion
The development of normative choroidal thickness profile is necessary to make the diagnosis of the choroidal abnormalities. To the best of authors’ knowledge, this is the first study, which reports normative choroidal thickness profile in healthy Indian children and studies effect of various factors affecting choroidal thickness using SD-OCT [Table 3]. We found the thickest choroid at the subfoveal area, and temporal choroid was thicker than nasal choroid. Similar findings were reported in children[14,17] as well as adults.[19,20]
Table 3.
Comparison of present study with previously published literature on choroidal thickness in healthy children
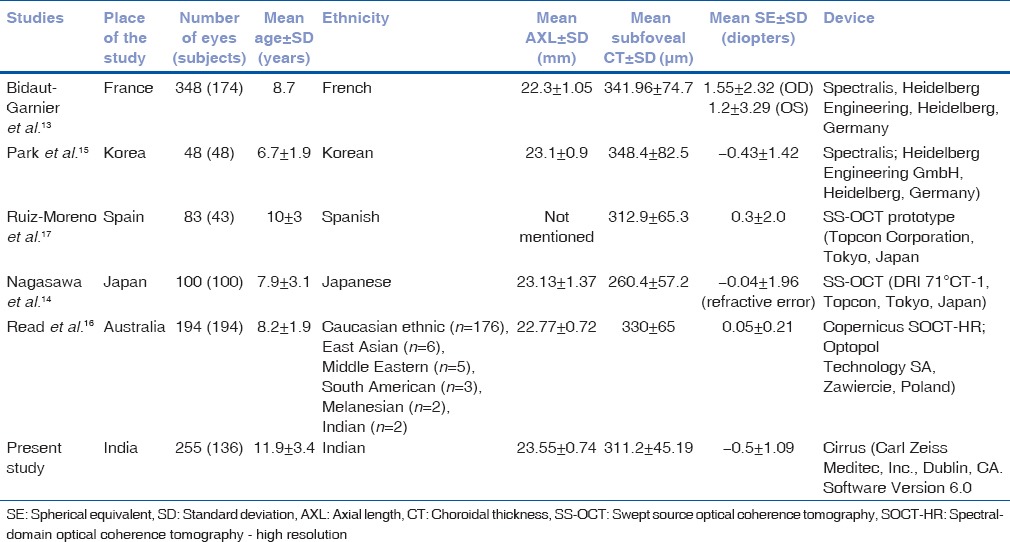
We report the negative correlation of age with choroidal thickness in Indian children. Our unpublished data revealed that the mean subfoveal choroidal thickness in 3rd decade was 294.8 ± 46.5 microns and that of in 8th decade was 249.6 ± 36.0 microns. Our present study reports that the mean choroidal thickness of children (5–18 years) of 311.2 ± 45.19 microns was significantly more (≤0.001) than adult age group. This supports the progressive decrease in choroidal thickness with age. This is consistent with previously published reports on children as well as in adults.[11,14,15,16,19,21,22] However, Ruiz-Moreno et al.[17] reported no difference in choroidal thickness in children compared to adults. The reason for this discrepancy could be manual measurements of choroidal thickness.
Significant negative correlation has been seen between AXL and choroidal thickness in the previous studies done in adults[19,21,23,24] as well as in the pediatric population, similar to the present study.[13,14,15,16] In contrary, Park et al. found no correlation between AXL and choroidal thickness.[15] This could be due to less number of eyes in their study. Ruiz-Moreno et al.[17] did not include AXL in their analysis, which is one of the very important factors to affect choroidal thickness.
In our study, we did not find the difference in choroidal thickness between males and females, similar to previous reports.[15,16] However, Mapelli et al.[22] recently reported a thicker choroid in females with slight significance (P = 0.056). In contrary, adult men have been reported to have thicker choroid than adult females.[11] Reason for this discrepancy in reports and the biological significance of choroidal thickness with regards to gender remains unclear.
We observed that healthy Indian children have slightly thinner choroid compared to French[13] and Korean[15] children, but thicker than Japanese[14] children, which could be due to ethnic differences or different devices used for choroidal scans. Similarly, differences in retinal parameters in various ethnic groups have also been reported.[25] However, there are no studies comparing choroidal parameters in various ethnic groups.
In our study, choroidal thickness measurements were not performed at the same time of the day in all children, therefore, our results are affected by the diurnal variation as reported previously.[12] We did not perform a topographic profile of choroidal thickness on superior and inferior quadrants. Furthermore, our study does not provide data about children below 5 years of age due to technical difficulties in obtaining scans in younger children.
Conclusion
Our study provides a valid normative database of choroidal thickness in healthy Indian children. This database could be useful for future studies evaluating choroidal changes in various chorioretinal disorders. Future studies including longitudinal follow-up of children since birth using hand-held SD-OCT instruments will give more information regarding the trend in choroid thickness with normal development of children.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Maruko I, Iida T, Sugano Y, Oyamada H, Sekiryu T, Fujiwara T, et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina. 2011;31:510–7. doi: 10.1097/IAE.0b013e3181eef053. [DOI] [PubMed] [Google Scholar]
- 2.Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22:166–73. doi: 10.1097/ICU.0b013e3283459826. [DOI] [PubMed] [Google Scholar]
- 3.Read RW, Rao NA, Cunningham ET. Vogt-Koyanagi-Harada disease. Curr Opin Ophthalmol. 2000;11:437–42. doi: 10.1097/00055735-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Guyer DR, Puliafito CA, Monés JM, Friedman E, Chang W, Verdooner SR. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992;99:287–91. doi: 10.1016/s0161-6420(92)31981-5. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- 6.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–5. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119:119–23. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chhablani J, Barteselli G, Wang H, El-Emam S, Kozak I, Doede AL, et al. Repeatability and reproducibility of manual choroidal volume measurements using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:2274–80. doi: 10.1167/iovs.12-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:2267–71. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- 11.Barteselli G, Chhablani J, El-Emam S, Wang H, Chuang J, Kozak I, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: A three-dimensional analysis. Ophthalmology. 2012;119:2572–8. doi: 10.1016/j.ophtha.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–6. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 13.Bidaut-Garnier M, Schwartz C, Puyraveau M, Montard M, Delbosc B, Saleh M. Choroidal thickness measurement in children using optical coherence tomography. Retina. 2014;34:768–74. doi: 10.1097/IAE.0b013e3182a487a4. [DOI] [PubMed] [Google Scholar]
- 14.Nagasawa T, Mitamura Y, Katome T, Shinomiya K, Naito T, Nagasato D, et al. Macular choroidal thickness and volume in healthy pediatric individuals measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:7068–74. doi: 10.1167/iovs.13-12350. [DOI] [PubMed] [Google Scholar]
- 15.Park KA, Oh SY. Choroidal thickness in healthy children. Retina. 2013;33:1971–6. doi: 10.1097/IAE.0b013e3182923477. [DOI] [PubMed] [Google Scholar]
- 16.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in childhood. Invest Ophthalmol Vis Sci. 2013;54:3586–93. doi: 10.1167/iovs.13-11732. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Moreno JM, Flores-Moreno I, Lugo F, Ruiz-Medrano J, Montero JA, Akiba M. Macular choroidal thickness in normal pediatric population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:353–9. doi: 10.1167/iovs.12-10863. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita T, Yamashita T, Shirasawa M, Arimura N, Terasaki H, Sakamoto T. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012;53:1102–7. doi: 10.1167/iovs.11-8836. [DOI] [PubMed] [Google Scholar]
- 19.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–6. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 20.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Li J, Zeng J, Ma W, Liu R, Li T, et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52:9555–60. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 22.Mapelli C, Dell’Arti L, Barteselli G, Osnaghi S, Tabacchi E, Clerici M, et al. Choroidal volume variations during childhood. Invest Ophthalmol Vis Sci. 2013;54:6841–5. doi: 10.1167/iovs.13-12761. [DOI] [PubMed] [Google Scholar]
- 23.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–9.e1. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata M, Tsujikawa A, Matsumoto A, Hangai M, Ooto S, Yamashiro K, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:4971–8. doi: 10.1167/iovs.11-7729. [DOI] [PubMed] [Google Scholar]
- 25.Tariq YM, Samarawickrama C, Pai A, Burlutsky G, Mitchell P. Impact of ethnicity on the correlation of retinal parameters with axial length. Invest Ophthalmol Vis Sci. 2010;51:4977–82. doi: 10.1167/iovs.10-5226. [DOI] [PubMed] [Google Scholar]


