Unlike human immunodeficiency virus (HIV)-exposed but uninfected children, many perinatally HIV-infected children lack serologic evidence of immunity to measles, mumps, and rubella despite past immunization and current antiretroviral therapy (ART). Effective ART before immunization is a strong predictor of current seroimmunity.
Keywords: perinatal HIV, measles, mumps, rubella, immunity
Abstract
Background. Children with perinatal human immunodeficiency virus (HIV) infection (PHIV) may not be protected against measles, mumps, and rubella (MMR) because of impaired initial vaccine response or waning immunity. Our objectives were to estimate seroimmunity in PHIV-infected and perinatally HIV-exposed but uninfected (HEU) children and identify predictors of immunity in the PHIV cohort.
Methods. PHIV and HEU children were enrolled in the Pediatric HIV/AIDS Cohort Study (PHACS) at ages 7–15 years from 2007 to 2009. At annual visits, demographic, laboratory, immunization, and clinical data were abstracted and serologic specimens collected. Most recent serologic specimen was used to determine measles seroprotection by plaque reduction neutralization assay and rubella seroprotection and mumps seropositivity by enzyme immunoassay. Sustained combination antiretroviral therapy (cART) was defined as taking cART for at least 3 months.
Results. Among 428 PHIV and 221 HEU PHACS participants, the prevalence was significantly lower in PHIV children for measles seroprotection (57% [95% confidence interval {CI}, 52%–62%] vs 99% [95% CI, 96%–100%]), rubella seroprotection (65% [95% CI, 60%–70%] vs 98% [95% CI, 95%–100%]), and mumps seropositivity (59% [95% CI, 55%–64%] vs 97% [95% CI, 94%–99%]). On multivariable analysis, greater number of vaccine doses while receiving sustained cART and higher nadir CD4 percentage between last vaccine dose and serologic testing independently improved the cumulative prediction of measles seroprotection in PHIV. Predictors of rubella seroprotection and mumps seropositivity were similar.
Conclusions. High proportions of PHIV-infected children, but not HEU children, lack serologic evidence of immunity to MMR, despite documented immunization and current cART. Effective cART before immunization is a strong predictor of current seroimmunity.
Routine administration of combination measles, mumps, and rubella (MMR) vaccine at ages 12–15 months and 4–6 years has resulted in elimination of measles and rubella and dramatic reductions in mumps in the United States [1–3]. However, as recent outbreaks of measles and mumps have demonstrated, children in the United States continue to be exposed to these viruses [3–5].
The risk of serious disease and complications, especially for measles, is high for children with human immunodeficiency virus (HIV) infection [6–8]. Live-attenuated MMR vaccine is contraindicated for HIV-infected children with severe immunosuppression, in whom it can cause serious illness [9]. Fortunately, the widespread use of combination antiretroviral therapy (cART) has resulted in improved immunologic status for most HIV-infected children in the United States today [10]. However, HIV-infected children may be susceptible to vaccine-preventable illnesses despite immunization because past or persistent immunologic abnormalities may have led to lower response rates and shorter duration of protective responses following immunization [11]. Unrecognized susceptibility puts this subpopulation at risk of contracting these infections and could contribute to augmenting outbreaks. The goals of the present study were to estimate and compare the prevalences of MMR immunity in perinatally HIV-infected (PHIV) children and in perinatally HIV-exposed but uninfected (HEU) comparison children in a large US cohort and to evaluate independent predictors of current immunity in PHIV children.
METHODS
Pediatric HIV AIDS Cohort Study
The Adolescent Master Protocol (AMP) of the Pediatric HIV AIDS Cohort Study (PHACS) is a prospective cohort study designed to evaluate the effect of HIV infection and antiretroviral therapy (ART) among PHIV youth [9]. Between March 2007 and November 2009, the study enrolled 451 PHIV and 227 HEU children aged 7–15 years, from 15 sites across the United States, including Puerto Rico. Participants were required to have complete lifetime information regarding ART use, plasma HIV RNA concentrations (viral load), and CD4+ T-lymphocyte (CD4) measurements. The protocol was approved by the institutional review board (IRB) at each site and at the Harvard School of Public Health. Written informed consent was obtained from the parent or legal guardian and assent was obtained from child participants, per local IRB guidelines.
Data Collection
Demographic and clinical characteristics of interest included age, sex, race/ethnicity, body mass index (BMI), vaccination history, viral load, CD4 percentage (CD4%), Centers for Disease Control and Prevention (CDC) clinical classification for HIV disease [12, 13], and prescribed ART. Characteristics were collected through self-report (eg, race/ethnicity), medical chart abstraction (eg, vaccination, immunologic, virologic, and ART characteristics), or physical exam (eg, height and weight). BMI, included as a measure of nutritional status, was calculated as weight in kilograms divided by height in meters squared and expressed as z scores for age and sex [14]. cART was defined as a regimen consisting of at least 3 antiretroviral drugs from at least 2 different drug classes. Sustained cART was defined a priori as receiving cART for at least 3 consecutive months in an effort to exclude participants who may have initiated cART but discontinued due to intolerance or unsuccessful adherence.
Serologic Testing
Repository sera were obtained yearly in the AMP study. Specimens were frozen at ≤ − 70°C in 0.5 mL aliquots. The most recently available serum specimen as of 10 October 2011 was selected for this cross-sectional seroprevalence study, and all serologic testing was performed by the Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC.
Rubella and Mumps Testing
Serum specimens were analyzed in duplicate for rubella and mumps immunoglobulin G (IgG) antibodies using commercially available, US Food and Drug Administration–cleared, indirect enzyme-linked IgG immunoassays (Wampole Laboratories, Inc, Princeton, New Jersey). An index standard ratio of at least 1.10 on both runs was considered evidence of seroprotection for rubella and seropositivity for mumps, as no serologic correlate of protection has been defined for mumps.
Measles Plaque Reduction Neutralization Assay
Seroprotection against measles was assessed using a plaque reduction neutralization (PRN) assay, as this method has been clinically validated [15].
In brief, Vero cell monolayers were infected with a low-passage Edmonston measles virus strain and incubated with serially diluted serum specimens in duplicate. The 50% endpoint titers were interpolated using the Karber method [16]. Measles seroprotection was defined as a PRN titer of >120 milli–international units (mIU) of neutralizing antibody per milliliter of serum relative to World Health Organization II reference serum 66/202 (supplied by National Institute for Biological Standards and Control, South Mimms, United Kingdom) [16].
Statistical Analysis
The prevalences of measles seroprotection, rubella seroprotection, and mumps seropositivity were compared between PHIV and HEU participants, with 95% exact binomial confidence intervals (CIs) and Fisher exact test. Demographic and clinical characteristics assessed at the times of first and last MMR dose and date of serologic specimen were compared between PHIV and HEU participants using Wilcoxon rank-sum and Fisher exact tests as appropriate. Among PHIV participants, HIV severity measures and ART use were also compared by MMR antibody status. The prevalences of measles seroprotection, rubella seroprotection, and mumps seropositivity were compared by the number of vaccine doses received while on sustained cART with Fisher exact test. Further analyses assessed whether 1 or more (compared to zero) vaccine doses prior to participant exposure to sustained cART modified the relationship between seroimmunity and the number of vaccine doses received while on sustained cART. To identify key sets of covariates that would be most predictive of seroimmunity and to discriminate between children who have seroimmunity and those who may need to be reimmunized, multivariable models for immunity to each virus were built by initially including the number of doses while on sustained cART and subsequently adding covariates 1 at a time by descending order of their univariable c-statistic, which is analogous to the area under the curve in a receiver-operator curve (ie, a measure of discrimination). To obtain an efficient set of independent clinical predictors, covariates were retained if they were significant at α = .05 and did not nullify the significance of any already included predictors. All analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
As of 10 October 2011, 428 PHIV and 221 HEU participants had serum specimens available for serologic testing. A serologic specimen from 1 PHIV participant did not contain a sufficient quantity of serum to be tested in the measles PRN assay; this participant's data was included only in analyses of mumps seropositivity and rubella seroprotection. The 428 PHIV children, compared with the 221 HEU children, were more likely born before 1996 (when cART became available) and had a different racial/ethnic composition (Table 1). At the time the serologic specimen was obtained, the PHIV children were older (14.6 vs 12.2 years, P < .001) and had a lower BMI z score (0.30 vs 0.85, P < .001). In both groups, 87% had received 2 doses of MMR, but the distribution of MMR doses was different between the groups (P < .001), as PHIV children were more likely to have received >2 MMR doses (8% vs 2%) and less likely to have received 0–1 MMR doses (4% vs 11%). The median interval from last MMR dose to serologic specimen was longer for the PHIV group (9.8 vs 8.0 years, P < .001).
Table 1.
Descriptive Characteristics by HIV Status
| Characteristics | Total (N = 649) | Cohort |
P Value | |
|---|---|---|---|---|
| HIV-Infected (n = 428) | HIV-Uninfected (n = 221) | |||
| Birth year ≥1996 | 402 (62) | 218 (51) | 184 (83) | <.001a |
| Male sex | 313 (48) | 198 (46) | 115 (52) | .185a |
| Race/ethnicity | .005a | |||
| White non-Hispanic/other | 50 (8) | 30 (7) | 20 (9) | |
| Black non-Hispanic | 408 (63) | 288 (67) | 120 (54) | |
| Hispanic (regardless of race) | 186 (29) | 107 (25) | 79 (36) | |
| Missing | 5 (1) | 3 (1) | 2 (1) | |
| MMR doses receivedb | <.001 | |||
| 0 | 12 (2) | 6 (1) | 6 (3) | |
| 1 | 32 (5) | 14 (3) | 18 (8) | |
| 2 | 564 (87) | 372 (87) | 192 (87) | |
| 3 or 4 | 41 (6) | 36 (8) | 5 (2) | |
| Years from last MMR dose to date of specimenb | ||||
| Median (Q1, Q3) | 9.2 (6.6, 11.7) | 9.8 (6.9, 12.1) | 8.0 (6.0, 10.3) | <.001c |
| Missing | 12 (2) | 6 (1) | 6 (3) | |
| Age, in years, at first MMR doseb | ||||
| Median (Q1, Q3) | 1.13 (1.02, 1.37) | 1.17 (1.03, 1.46) | 1.07 (1.02, 1.21) | <.001c |
| Missing | 12 (2) | 6 (1) | 6 (3) | |
| BMI z score at first MMR doseb | ||||
| Median (Q1, Q3) | −0.30 (−0.78, 0.84) | −0.00 (−0.78, 1.20) | −0.58 (−0.92, 0.09) | .640c |
| Missing | 627 (97) | 410 (96) | 217 (98) | |
| Age, in years, at last MMR doseb | ||||
| Median (Q1, Q3) | 4.32 (4.04, 5.03) | 4.44 (4.08, 5.34) | 4.14 (4.02, 4.66) | <.001c |
| Missing | 12 (2) | 6 (1) | 6 (3) | |
| BMI z score at last MMR doseb | ||||
| Median (Q1, Q3) | 0.27 (−0.35, 0.95) | 0.32 (−0.24, 0.89) | 0.15 (−0.58, 1.83) | .922c |
| Missing | 355 (55) | 222 (52) | 133 (60) | |
| Age, in years, at specimen, median (Q1, Q3) | 13.9 (11.5, 16.2) | 14.6 (12.3, 16.7) | 12.2 (10.4, 14.3) | <.001c |
| BMI z score at specimen date | ||||
| Median (Q1, Q3) | 0.51 (−0.39, 1.45) | 0.30 (−0.44, 1.20) | 0.85 (−0.16, 1.91) | <.001c |
| Missing | 1 (0) | 0 (0) | 1 (0) | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; MMR, trivalent measles, mumps, and rubella vaccine.
a Fisher exact test.
b Only MMR doses considered when producing table statistics; monovalent doses excluded.
c Wilcoxon test.
Among the PHIV children, 96% had ever received sustained cART, but only 81% were taking sustained cART at the time serum was obtained; median age of first cART initiation was 3.1 years (interquartile range, 1.1–5.8 years). At the time of serologic specimen, the median CD4% was 34%; 29 (7%) had a CD4% <15%, 278 (65%) had HIV RNA <400 copies/mL, and 108 (25%) were (lifetime) CDC clinical stage C.
The prevalence of measles seroprotection in PHIV children (57% [95% CI, 52%–62%]) was significantly lower (P < .001) than that in HEU children (99% [95% CI, 96%–100%]) (Figure 1). The prevalence of rubella seroprotection in PHIV children (65% [95% CI, 60%–70%]) was also significantly lower (P < .001) than that in HEU children (98% [95% CI, 95%–100%]). Similarly, the prevalence of mumps seropositivity in PHIV children (59% [95% CI, 55%–64%]) was significantly lower (P < .001) than that in HEU children (97% [95% CI, 94%–99%]). When adjusted for age at serologic testing, all 3 comparisons remained highly significant (P < .001) (data not shown).
Figure 1.

Prevalence of measles and rubella seroprotection and mumps seropositivity with 95% confidence intervals, for perinatally human immunodeficiency virus (HIV)-infected children (PHIV) and HIV-exposed but uninfected children (HEU).
Among the subset of PHIV children (n = 253) who were receiving sustained cART and had virologic suppression (viral load <400 copies/mL) and no severe immunosuppression (CD4% ≥15%), 164 (65% [95% CI, 59%–71%]) had measles seroprotection, 178 (70% [95% CI, 64%–76%]) had rubella seroprotection, and 161 (64% [95% CI, 57%–70%]) were seropositive for mumps.
Among the 427 PHIV children with measles PRN results, those with measles seroprotection were more likely to have a more recent birth year, earlier age at first initiation of sustained cART, and higher lifetime nadir CD4% (Supplementary Table 1). Although there was no difference in the overall number of measles-containing vaccine doses between PHIV children with and without measles seroprotection (P = .677), the number of vaccine doses administered after receiving sustained cART was significantly greater in PHIV children with measles seroprotection compared to those lacking seroprotection (P < .001). At the time of first vaccine dose, time of most recent vaccine dose, and time of the serologic specimen, CD4% was consistently higher, log viral load consistently lower, and prevalence of sustained cART higher in PHIV children with seroprotection compared to those without seroprotection. Whereas nadir CD4% was significantly higher in the PHIV children with measles seroprotection at the time of the serologic specimen, this association was not present at the time of first or most recent vaccine dose. Those with seroprotection were younger (13.8 vs 15.8 years, P < .001) and had a shorter interval from last vaccine dose (8.7 vs 11.0 years, P < .001) but not BMI z score or CDC stage C, at time of serologic specimen.
Most of the univariable results observed for measles seroprotection were similar for rubella seroprotection and mumps seropositivity with the following differences: CD4% at first or last vaccine dose and CD4% nadir and sustained cART at time of serologic specimen collection did not differ between those with and without rubella seroprotection, whereas the distributions of race/ethnicity, number of vaccine doses overall, and BMI did differ between those with and without rubella seroprotection. Sustained cART and log viral load at time of serologic specimen collection did not differ between those with and without mumps seropositivity, but the distributions of race/ethnicity and less advanced CDC category did.
Figure 2 shows the prevalence of measles seroprotection by 0, 1, and ≥2 doses of vaccine while on sustained cART. Measles seroprotection was present in 43% (95% CI, 35%–50%), 53% (95% CI, 45%–62%), and 85% (95% CI, 77%–91%), respectively. The same significant dose-response relationship was observed for rubella seroprotection and for mumps seropositivity in relation to the number of vaccine doses received after sustained cART.
Figure 2.
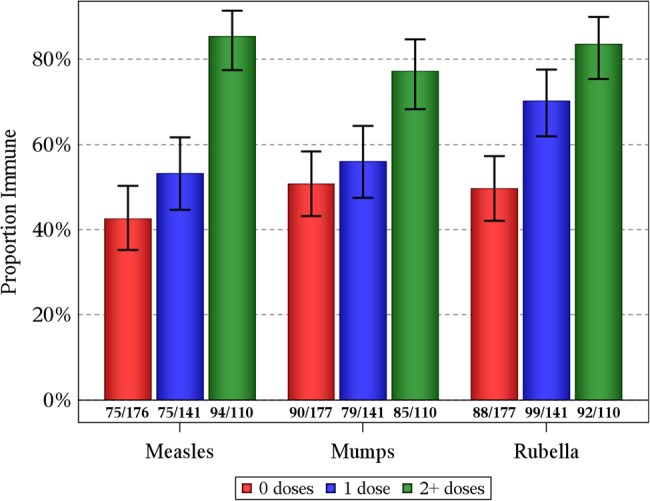
Prevalence of measles and rubella seroprotection and mumps seropositivity in children with perinatal human immunodeficiency virus infection by number of vaccine doses administered while receiving sustained combination antiretroviral therapy (cART), defined as receiving cART for at least 3 months.
To help assess if the number of vaccine doses while receiving sustained cART was a more important determinant of seroimmunity than younger age at first sustained cART initiation, we stratified the number of vaccine doses received while on sustained cART by number of vaccine doses prior to sustained cART (Table 2). The proportions with seroimmunity were higher in those who received more MMR doses after sustained cART regardless of MMR doses received prior to sustained cART. For instance, among those with at least 2 MMR doses after sustained cART, measles seroprotection was present in 85% (82/96) of those with no pre-cART MMR doses and 86% (12/14) of those with at least 1 pre-cART MMR dose.
Table 2.
Prevalence of Measles Seroprotection, Mumps Seropositivity, and Rubella Seroprotection in Children With Perinatal Human Immunodeficiency Virus Infection by Number of Vaccine Doses Received While on Sustained Combination Antiretroviral Therapy (cART), Stratified by ≥1 Versus No Vaccine Doses Prior to Sustained cART
| No. Vaccine Doses on Sustained cARTa | ≥1 Dose Prior to Sustained cART |
Zero Doses Prior to Sustained cART |
||
|---|---|---|---|---|
| Positive Antibody Response |
Positive Antibody Response |
|||
| Yes | No | Yes | No | |
| Measles | ||||
| 0 | 73 (43) | 97 (57) | 2 (33) | 4 (67) |
| 1 | 66 (52) | 61 (48) | 9 (64) | 5 (36) |
| ≥2 | 12 (86) | 2 (14) | 82 (85) | 14 (15) |
| Mumps | ||||
| 0 | 87 (51) | 84 (49) | 3 (50) | 3 (50) |
| 1 | 68 (54) | 59 (46) | 11 (79) | 3 (21) |
| ≥ 2 | 8 (67) | 4 (33) | 77 (79) | 21 (21) |
| Rubella | ||||
| 0 | 85 (50) | 86 (50) | 3 (50) | 3 (50) |
| 1 | 89 (70) | 38 (30) | 10 (71) | 4 (29) |
| ≥2 | 10 (83) | 2 (17) | 82 (84) | 16 (16) |
Data are presented as No. (row %).
Abbreviations: cART, combination antiretroviral therapy; MMR, trivalent measles, mumps, and rubella vaccine.
a Sustained cART is defined as at least 3 months of consecutive cART. Vaccine doses include MMR and disease-specific monovalent vaccinations. Subjects who were never on at least 3 consecutive months of cART had their vaccine doses (if any) classified as having happened prior to sustained cART.
In multivariable analyses, greater number of vaccine doses while receiving sustained cART and higher nadir CD4% between last vaccine dose and serologic testing were independent predictors of measles seroprotection, with a cumulative c-statistic of 0.742 (Table 3). Greater number of vaccine doses while receiving sustained cART, higher nadir CD4% between last vaccine dose and serologic testing, and a shorter interval between last vaccine dose and serologic assessment were independent predictors of mumps seropositivity, with a cumulative c-statistic of 0.675. Similar to mumps, rubella seroprotection was also independently predicted by greater number of vaccine doses while receiving sustained cART, shorter interval between last vaccine dose and serologic assessment, and race/ethnicity (c = 0.734).
Table 3.
Multivariable Model Results for Seroprotection (Measles and Rubella) or Seropositivity (Mumps)
| Covariates | Odds Ratio(95% CI)a | P Valuea | Cumulative c-Statisticb |
|---|---|---|---|
| Measles | |||
| No. of MMR doses while receiving sustained cARTc | <.001 | .682 | |
| 0 | Ref | ||
| 1 | 1.17 (.72–1.91) | ||
| ≥2 | 5.40 (2.83–10.30) | ||
| Lowest CD4% between last MMR dose and serologic specimen collection) (per 1% increase) | 1.05 (1.03–1.08) | <.001 | .742 |
| Mumps | |||
| No. of MMR doses while on sustained cARTc | .041 | .621 | |
| 0 | Ref | ||
| 1 | 0.94 (.56–1.57) | ||
| ≥2 | 1.97 (1.02–3.80) | ||
| Years from last MMR dose to date of specimen draw (per year increase) | 0.92 (.85–1.00) | .048 | .670 |
| Nadir CD4% (last MMR dose to immunity specimen draw) (per 1% increase) | 1.03 (1.00–1.05) | .024 | .675 |
| Rubella | |||
| No. of MMR doses while on sustained cARTc | .030 | .673 | |
| 0 | Ref | ||
| 1 | 1.64 (.96–2.80) | ||
| ≥2 | 2.56 (1.24–5.28) | ||
| Years from last MMR dose to date of specimen draw (per year increase) | 0.84 (.76–.92) | <.001 | .715 |
| Race/ethnicity | .005 | .734 | |
| Black non-Hispanic | Ref | ||
| White non-Hispanic/other | 0.93 (.39–2.23) | ||
| Hispanic (regardless of race) | 0.43 (.26–.71) | ||
Four hundred subjects were analyzed for measles, whereas 401 were analyzed for mumps and rubella. Subjects were lost due to missing information on age at first instance of 3 consecutive months of cART, race/ethnicity, and years from last MMR dose to date of specimen draw.
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; MMR, trivalent measles, mumps, and rubella vaccine; Ref, reference.
a Based on type 3 analysis of effects (controlling for other covariates in the multivariable logistic regression model).
b The cumulative c-statistic for overall model predictability was generated at each step during forward selection of covariates into the multivariable models.
c Sustained cART is defined as at least 3 months of consecutive cART.
DISCUSSION
In this large cohort of PHIV children, the levels of measles and rubella seroprotection and mumps seropositivity were significantly lower than levels in a simultaneously enrolled comparison group of HEU children and markedly lower than population-based studies of healthy US children [17, 18]. Based on this study, one-third to nearly one-half of PHIV children in the United States may be unprotected against these vaccine-preventable diseases. These findings extend and corroborate smaller published studies of reduced levels of immunity to vaccine-preventable diseases in PHIV populations and support currently recommended criteria for revaccination [1, 19]. Of note, nearly all PHIV children in this study had received the full 2-dose series of MMR vaccines, in contrast to other studies in which perinatal HIV infection has been a risk factor for failure to receive recommended immunizations [20–22]. Despite concerns that HEU children may have subtle immunologic abnormalities that could impair response to vaccines [23, 24], the rates of seroprotection and seropositivity in the HEU children in this study were extremely high and reassuringly comparable to those in US children in general [17, 18].
Many studies have demonstrated that PHIV children are at higher risk of lack of response, reduced magnitude of response, and shorter duration of response to MMR vaccines [25–29]. More advanced immunosuppression, incomplete plasma HIV suppression, and lack of cART have all been associated with increased risk of poor response to vaccines [19, 30–32]. In this study, it is not possible to separate contributions of poor initial vaccine response from antibody loss after an adequate vaccine response, because serologic specimens were obtained many years after most vaccine doses were administered. Nonetheless, this group of older children and youths represents the population of PHIV people in the United States [33], and it is concerning that such high proportions of these youths—including those currently achieving virologic suppression and good immune status on cART—may not be protected against MMR.
Determining the factors that help predict which PHIV children and youths are likely not protected against measles, mumps, or rubella can guide approaches to identifying children who are at highest risk. Receipt of cART for at least 3 months before receiving MMR vaccine doses and initiation of cART at an earlier age were important and interrelated predictors of seroprotection. Prior studies have demonstrated that immune responses to vaccines in PHIV children are most similar to responses in healthy children if cART precedes vaccination [34]. In addition, starting cART in infancy permits the normal development and maintenance of the memory B cells, whereas later cART initiation results in impaired number and function of memory B cells [35].
Early cART initiation should allow for robust response to MMR and other vaccines in the small number of new infant HIV infections that occur in the United States. But for many older children and young adults with perinatal HIV infection, cART was not available when they were young and so MMR vaccine was commonly administered before cART. Overall, about half of children who received no vaccine after sustained cART lacked serologic evidence of immunity to MMR. In contrast, high proportions of children who received at least 2 doses of MMR vaccine after they were on sustained cART had seroprotection to rubella and measles (less so to mumps), although the rates of seroprotection still fall short of protection following 2 doses of MMR in healthy children. Furthermore, seroprotection after 2 MMR doses during sustained cART was not improved by having also received MMR vaccine doses before cART or the total number of MMR doses, suggesting that seroprotection is most dependent on the MMR doses administered during sustained cART. In predictive modeling, a higher number of MMR vaccine doses while on sustained cART was a consistent and robust predictor of immunity. This finding is consistent with a recent trial that demonstrated relatively high levels of response to MMR revaccination in PHIV children on cART who had received MMR in the past but who lacked seroprotection to measles [19]. These data also support the recently revised recommendations that PHIV children who start cART before their first MMR dose at age 12–15 months can receive the standard 2-dose MMR schedule recommended generally for children, and that PHIV children who started cART at an older age should be assessed for the need for additional MMR vaccination to ensure receipt of 2 doses of MMR after stable cART has been established (unless they have other evidence of immunity) [1].
This study has important limitations and strengths. Participants were not prospectively assigned to experimental vaccine and cART schedules for comparison. Evaluation of immunity was limited to serologic measures and did not address the role of cell-mediated immunity. Some vaccine doses may have been missed, but the very small number of participants—especially those with HIV infection—with <2 MMR vaccine doses suggests that most doses were captured. The limited size of our study cohort precluded us from developing a separate dataset for validation of our predictive model; we hope that other PHIV cohorts can be used to validate our results. The study is strengthened by the large size of the cohort, lack of selection of participants for a study of vaccine-related immunity, inclusion of a relevant control group, and use of the gold-standard PRN methodology for assessing measles seroprotection. The finding of lower odds of rubella immunity in Hispanics compared with black PHIV children merits further study but is consistent with reports of higher rubella antibody titers following rubella vaccine in black compared with Hispanic healthy children [36].
The control of MMR depends upon ensuring high levels of population immunity. Older children with perinatal HIV infection may contribute to community risk of outbreaks and may be at higher risk of severe disease if they become infected. Prevention of infant HIV infection, early cART for newly HIV-infected infants followed by the standard schedule for MMR immunization, and repeating MMR vaccine doses given to PHIV children before they were receiving sustained cART can prevent these vaccine-preventable infections in this vulnerable population. These data support current CDC recommendations [1] and can inform global policy recommendations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the children and families for their participation in Pediatric HIV AIDS Cohort Study (PHACS), and the individuals and institutions involved in the conduct of PHACS. Data management services were provided by Frontier Science and Technology Research Foundation (Principal Investigator [PI]: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). The following institutions, clinical site investigators, and staff participated in conducting PHACS Adolescent Master Protocol in 2013 (in alphabetical order): Ann & Robert H. Lurie Children's Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia Garvie, James Blood; Children's Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; Rutgers–New Jersey Medical School: Arry Dieudonne, Linda Bettica, Susan Adubato; St Christopher's Hospital for Children: Janet Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Alisa Katai, Jennifer Dunn, Suzanne Paul; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
Author contributions. K. P. has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: G. K. S., K. P., W. J. B., M. U. P., S. K. B., W. A. M., R. B. V. D. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: G. K. S., K. P., B. K. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: K. P., B. K. Administrative, technical, or material support: All authors. Study supervision: G. K. S., K. P., W. J. B., M. U. P., S. K. B., W. A. M., R. B. V. D.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services. Federal government employees and academic investigators participated together in all aspects of study design and supervision of the conduct of the study; interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Data collection, management, and analysis were performed by academic investigators.
Financial support. The PHACS was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (U01 HD052102; PI: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (U01 HD052104; PI: Russell Van Dyke; Co-PI: Kenneth Rich; Project Director: Patrick Davis). Serologic assays were performed in a US federal government (CDC) laboratory.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Pediatric HIV AIDS Cohort Study (PHACS), Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter, William Shearer, Mary Paul, Norma Cooper, Lynnette Harris, Murli Purswani, Mahboobullah Baig, Anna Cintron, Ana Puga, Sandra Navarro, Patricia Garvie, James Blood, Sandra Burchett, Nancy Karthas, Betsy Kammerer, Andrew Wiznia, Marlene Burey, Molly Nozyce, Arry Dieudonne, Linda Bettica, Susan Adubato, Janet Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant, Katherine Knapp, Kim Allison, Megan Wilkins, Midnela Acevedo-Flores, Heida Rios, Vivian Olivera, Margarita Silio, Medea Jones, Patricia Sirois, Stephen Spector, Kim Norris, Sharon Nichols, Elizabeth McFarland, Alisa Katai, Jennifer Dunn, Suzanne Paul, Gwendolyn Scott, Patricia Bryan, and Elizabeth Willen
References
- 1.Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013. summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2013; 62(No. RR-4):1–34. [PubMed] [Google Scholar]
- 2.Papania MJ, Wallace GS, Rota PA et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatr 2014; 168:148–55. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med 2014; 371:1661–3. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Measles cases and outbreaks. Available at: http://www.cdc.gov/measles/cases-outbreaks.html Accessed 3 November 2014.
- 5.Centers for Disease Control and Prevention. Mumps cases and outbreaks. Available at: http://www.cdc.gov/mumps/outbreaks.html Accessed 3 November 2014.
- 6.Albertyn C, van der Plas H, Hardie D et al. Silent casualties from the measles outbreak in South Africa. S Afr Med J 2011; 101:313–4, 316–7. [DOI] [PubMed] [Google Scholar]
- 7.le Roux DM, le Roux SM, Nuttall JJ, Eley BS. South African measles outbreak 2009–2010 as experienced by a paediatric hospital. S Afr Med J 2012; 102:760–4. [DOI] [PubMed] [Google Scholar]
- 8.Moss WJ, Fisher C, Scott S et al. HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin Infect Dis 2008; 46:523–7. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Measles pneumonitis following measles-mumps-rubella vaccination of a patient with HIV infection. MMWR Morb Mortal Wkly Rep 1996; 45:603–6. [PubMed] [Google Scholar]
- 10.Van Dyke RB, Patel K, Siberry GK et al. Pediatric HIV/AIDS Cohort Study. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes . J Acquir Immune Defic Syndr 2011; 57:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis 2010; 10:630–42. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41(RR-17):1–19. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Recomm Rep 1994; 43(RR-12):1–10. [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM et al. CDC growth charts: United States. Adv Data 2000; 314:1–27. [PubMed] [Google Scholar]
- 15.Chen RT, Markowitz LE, Albrecht P et al. Measles antibody: reevaluation of protective titers. J Infect Dis 1990; 162:1036–42. [DOI] [PubMed] [Google Scholar]
- 16.Cohen BJ, Audet S, Andrews N, Beeler J; WHO working group on measles plaque reduction neutralization test. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 2007; 26:59–66. [DOI] [PubMed] [Google Scholar]
- 17.McQuillan GM, Kruszon-Moran D, Hyde TB, Forghani B, Bellini W, Dayan GH. Seroprevalence of measles antibody in the US population, 1999–2004. J Infect Dis 2007; 196:1459–64. [DOI] [PubMed] [Google Scholar]
- 18.Hyde TB, Kruszon-Moran D, McQuillan GM, Cossen C, Forghani B, Reef SE. Rubella immunity levels in the United States population: has the threshold of viral elimination been reached? Clin Infect Dis 2006; 43(suppl 3):S146–50. [DOI] [PubMed] [Google Scholar]
- 19.Abzug MJ, Qin M, Levin MJ et al. International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1024 and P1061s Protocol Teams. Immunogenicity, immunologic memory, and safety following measles revaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis 2012; 206:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinna SS, Bamford A, Cunnington A et al. Immunization status of children with HIV: failure to protect a vulnerable population. HIV Med 2011; 12:447–8. [DOI] [PubMed] [Google Scholar]
- 21.Schulte JM, Burkham S, Squires JE et al. Immunization status of children born to human immunodeficiency virus (HIV)-infected mothers in two Texas cities. South Med J 2000; 93:48–52. [PubMed] [Google Scholar]
- 22.Succi RC, Krauss MR, Harris DR et al. NISDI Pediatric Study Group 2012. Undervaccination of perinatally HIV-infected and HIV-exposed uninfected children in Latin America and the Caribbean. Pediatr Infect Dis J 2013; 32:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidzeru EB, Hesseling AC, Passmore JA et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 2014; 28:1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abramczuk BM, Mazzola TN, Moreno YM et al. Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clin Vaccine Immunol 2011; 18:1406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkelhamer S, Borock E, Elsen C, Englund J, Johnson D. Effect of highly active antiretroviral therapy on the serological response to additional measles vaccinations in human immunodeficiency virus-infected children . Clin Infect Dis 2001; 32:1090–4. [DOI] [PubMed] [Google Scholar]
- 26.Nair N, Moss WJ, Scott S et al. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J Infect Dis 2009; 200:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farquhar C, Wamalwa D, Selig S et al. Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre- and post-highly active antiretroviral therapy and revaccination . Pediatr Infect Dis J 2009; 28:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima M, De Menezes Succi RC, Nunes Dos Santos AM, Weckx LY, De Moraes-Pinto MI. Rubella immunization in human immunodeficiency virus type 1-infected children: cause for concern in vaccination strategies. Pediatr Infect Dis J 2004; 23:604–7. [DOI] [PubMed] [Google Scholar]
- 29.Aurpibul L, Puthanakit T, Siriaksorn S, Sirisanthana T, Sirisanthana V. Prevalence of protective antibody against measles in HIV-infected children with immune recovery after highly active antiretroviral therapy. HIV Med 2006; 7:467–70. [DOI] [PubMed] [Google Scholar]
- 30.Crisinel PA, Posfay-Barbe KM, Aebi C et al. Swiss Mother and Child HIV Cohort Study of Switzerland. Determinants of hepatitis A vaccine immunity in a cohort of human immunodeficiency virus-infected children living in Switzerland. Clin Vaccine Immunol 2012; 19:1751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siberry GK, Williams PL, Lujan-Zilbermann J et al. IMPAACT P1065 Protocol Team. Phase I/II, open-label trial of safety and immunogenicity of meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine in human immunodeficiency virus-infected adolescents . Pediatr Infect Dis J 2010; 29:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhi SA, Adrian P, Cotton MF et al. Comprehensive International Program of Research on AIDS 4 Study Team. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants . J Infect Dis 2010; 202:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. HIV surveillance report, 2011; 23:52–53. [PubMed]
- 34.Simani OE, Izu A, Violari A et al. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS 2014; 28:531–41. [DOI] [PubMed] [Google Scholar]
- 35.Pensieroso S, Cagigi A, Palma P et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci U S A. 2009; 106:7939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haralambieva IH, Salk HM, Lambert ND et al. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine 2014; 32:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


