Treatment with daptomycin for vancomycin-resistant enterococcal bloodstream infection resulted in significantly lower treatment failure, 30-day mortality, microbiologic failure, 7-day mortality, and duration of bacteremia in comparison to linezolid.
Keywords: bloodstream infection, Enterococcus, vancomycin-resistant Enterococcus, daptomycin, linezolid
Abstract
Background. Vancomycin-resistant Enterococcus bloodstream infections (VRE-BSIs) are becoming increasingly common. Linezolid and daptomycin are the primary treatment options for VRE-BSI, but optimal treatment is unclear.
Methods. This was a national retrospective cohort study comparing linezolid and daptomycin for the treatment of VRE-BSI among Veterans Affairs Medical Center patients admitted during 2004–2013. The primary outcome was treatment failure, defined as a composite of (1) 30-day all-cause mortality; (2) microbiologic failure; and (3) 60-day VRE-BSI recurrence. Poisson regression was conducted to determine if antimicrobial treatment was independently associated with clinical outcomes.
Results. A total of 644 patients were included (linezolid, n = 319; daptomycin, n = 325). Overall, treatment failure was 60.9% (n = 392/644), and 30-day all-cause mortality was 38.2% (n = 246/644). Linezolid was associated with a significantly higher risk of treatment failure compared with daptomycin (risk ratio [RR], 1.37; 95% confidence interval [CI], 1.13–1.67; P = .001). After adjusting for confounding factors in Poisson regression, the relationship between linezolid use and treatment failure persisted (adjusted RR, 1.15; 95% CI, 1.02–1.30; P = .026). Linezolid was also associated with higher 30-day mortality (42.9% vs 33.5%; RR, 1.17; 95% CI, 1.04–1.32; P = .014) and microbiologic failure rates (RR, 1.10; 95% CI, 1.02–1.18; P = .011). No difference in 60-day VRE-BSI recurrence was observed between treatment groups.
Conclusions. Treatment with linezolid for VRE-BSI resulted in significantly higher treatment failure in comparison to daptomycin. Linezolid treatment was also associated with greater 30-day all-cause mortality and microbiologic failure in this cohort.
(See the Editorial Commentary by McKinnell and Arias on pages 879–82.)
Vancomycin-resistant Enterococcus (VRE) is a significant healthcare-associated pathogen with increasing impact in recent years [1–4]. As many as 28% of all enterococcal bloodstream isolates are now resistant to vancomycin, including a majority of Enterococcus faecium strains [1]. Multiple studies have shown vancomycin resistance to be independently associated with mortality in VRE bloodstream infections (VRE-BSIs), with mortality rates 2–3 times that of vancomycin-susceptible infections [5–7]. Among critically ill and neutropenic patients with VRE-BSI, mortality may exceed 60% [6].
Despite the prevalence and severity of these infections, optimal treatment for VRE-BSI remains unclear. Most VRE strains are resistant to ampicillin, and current recommendations suggest linezolid or daptomycin as first-line treatment options [8, 9]. Linezolid is US Food and Drug Administration (FDA) approved for the treatment of VRE infections, including bacteremia [10]. Despite its efficacy, concerns for myelosuppression and serotonergic toxicity limit linezolid use in patients with underlying hematologic disturbances or those using concomitant serotonergic agents [10]. The bacteriostatic activity of linezolid may also limit its effectiveness in patients with endocarditis or immunosuppression [6, 11]. In these cases, an agent such as daptomycin, which exhibits concentration-dependent bactericidal activity against VRE, may be an attractive alternative [6, 12–14]. Daptomycin is recommended at a dose of 6 mg/kg/day for gram-positive BSIs, but in vitro and clinical data suggest that outcomes may be improved with higher doses [15–18]. Although daptomycin lacks an FDA indication for VRE-BSI, it is commonly used in this setting in clinical practice [19].
Multiple clinical studies have compared daptomycin and linezolid for the treatment of VRE-BSI [20–23]. A recent meta-analysis pooled data from these investigations and noted an apparent superiority of linezolid over daptomycin in overall mortality [24]. However, the heterogeneity among inclusion and exclusion criteria and outcome definitions in the pooled studies make it difficult to properly adjust for confounding factors. Nearly all the included studies noted a trend toward daptomycin treatment among patients with neutropenia, thrombocytopenia, and endocarditis [24]. Another meta-analysis noted similar treatment selection bias [25]. Previous studies comparing the 2 agents have failed to find a difference in outcomes due to inadequate statistical power [20–22]. Due to the high mortality associated with these infections, optimal treatment is essential. Therefore, the objective of this study was to compare the safety and effectiveness of linezolid vs daptomycin for treatment of VRE-BSI in a population not vulnerable to some of the limitations of previous studies.
METHODS
Study Population
This was a national retrospective cohort study of hospitalized patients admitted to any Veterans Affairs Medical Center (VAMC) between 1 January 2004 and 1 January 2013. All adult patients with at least 1 blood culture positive for VRE were included. Exclusion criteria were (1) treatment with another anti-VRE agent; (2) treatment with linezolid and daptomycin combination therapy (including sequential treatment); and (3) treatment with daptomycin or linezolid for <48 hours. In recurrent VRE-BSI, only the first case encountered in the study period was analyzed. This study was approved by the Kansas City VAMC institutional review board.
Data Sources
National clinical databases comprised of inpatient, outpatient, and administrative data from all VAMCs were queried to identify patients meeting study criteria. Data were abstracted from these databases and included patient demographics, laboratory and microbiologic data, vital signs, antimicrobial treatment data, comorbidities, admissions records, and dates of death. Additionally, retrospective review of the electronic medical record was conducted to collect data that were not available in these databases at the time of this study, including negative culture results, VRE-BSI source, and source control as documented by a treating physician. Susceptibilities to antimicrobial agents were determined during routine clinical care.
Outcome Measures
The primary outcome was treatment failure, defined as a composite of (1) 30-day all-cause mortality; (2) microbiologic failure (lack of microbiologic clearance among those with at least 1 follow-up blood culture); and (3) recurrence of VRE-BSI within 60 days of therapy completion. Secondary outcomes were 30-day all-cause mortality, early (7-day) mortality, hospital length of stay (LOS), and duration of bacteremia. The starting time for 30-day and 7-day mortality determinations was designated as the time of first positive VRE blood culture. Hospital LOS was defined as the number of days from the beginning of linezolid or daptomycin treatment until discharge. Duration of bacteremia was defined as the number of days between the first positive VRE blood culture and the first negative blood culture.
Adverse Events
Platelet and creatine phosphokinase (CPK) data were collected for each patient at the beginning of treatment until 3 days after the end of therapy, when available. CPK elevation was determined according to previously defined criteria [26]. Thrombocytopenia was defined as platelets <50 000 cells/µL.
Statistical Analysis
Baseline categorical variables were compared by χ2 or 2-tailed Fisher exact test, when appropriate. Continuous variables were compared by t test or Mann–Whitney U test. Variables that were associated with treatment group or treatment failure (P < .2) were manually entered into a backward stepwise Poisson regression model with robust variance estimates. Variables that confounded the relationship between treatment and the primary outcome, resulting in a ≥10% change in the associated risk ratio, were retained in the final multivariable model. Time-to-event analyses were conducted for 30-day all-cause mortality and microbiologic failure using the Kaplan–Meier method, with differences in survival distributions for treatment groups compared using the log-rank test. Additionally, Cox proportional hazards models were fitted with covariates selected using a backward stepwise approach. For time-dependent analyses, cases that did not experience the outcome of interest were right-censored at the end of the treatment period to control for potential differences in duration of therapy. Analyses were conducted to compare 30-day mortality stratified for VRE-BSI species (E. faecium vs Enterococcus faecalis) and source of infection (line vs nonline) using the Mantel–Haenszel procedure. An analysis of the effect of concomitant treatment with at least 1 dose of a β-lactam or aminoglycoside agent was conducted among daptomycin-treated subjects. A sensitivity analysis excluding cases in which the causative VRE species was not defined in the final microbiology report was conducted. Propensity score–matched analyses were also performed (Supplementary Appendix). Proportions of adverse events were compared by logistic regression. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina), with a 2-tailed P value <.05 considered statistically significant.
RESULTS
A total of 1109 cases of VRE-BSI met inclusion criteria during the study period. Of those cases, patients were excluded due to treatment with another anti-VRE agent (n = 25), treatment with both linezolid and daptomycin (n = 140), treatment <48 hours (n = 119), and recurrent infection (n = 181). There were 644 patients included in the final analysis, with 319 (49.5%) treated with linezolid and 325 (50.5%) treated with daptomycin. These individuals were treated at 47 distinct VAMCs across 29 states and Puerto Rico. All isolates were resistant to ampicillin. Among those treated with linezolid, susceptibility data was reported for 141 (44.2%) of VRE isolates, all of which were susceptible. Daptomycin susceptibility testing was not routinely reported and was only available in 33 (10.1%) cases, all of which were daptomycin susceptible (1 µg/mL, 7/33 [21.2%]; 2 µg/mL, 15/33 [45.5%]; 4 µg/mL, 11/33 [33.3%]).
The median daptomycin dose was 5.93 mg/kg (interquartile range [IQR], 5.33–6.10 mg/kg). Nearly all (99.4%) patients treated with linezolid were given 600-mg doses twice daily. Polymicrobial bacteremia occurred in 7.6% of cases overall, and the microbiology of these infections are reported in Supplementary Table 1. Baseline characteristics were compared according to treatment group in Table 1. As can be interpreted, there were many significant differences between linezolid- and daptomycin-treated subjects with regard to these characteristics.
Table 1.
Baseline Patient Characteristics by Antimicrobial Treatment for Vancomycin-Resistant Enterococcus Bloodstream Infection
| Characteristic | Linezolid (n = 319) | Daptomycin (n = 325) | P Value |
|---|---|---|---|
| Age, y, median (IQR) | 67 (59–76) | 64 (58–74) | .192 |
| Age ≥65 y | 175 (54.9) | 150 (46.2) | .027 |
| Male sex | 309 (96.9) | 321 (98.8) | .098 |
| Body mass index, kg/m2, median (IQR) | 25.5 (21.8–31.1) | 26.1 (22.6–31.0) | .336 |
| Enterococcus faecium | 276 (86.5) | 263 (80.9) | .055 |
| Enterococcus faecalis | 24 (7.5) | 25 (7.7) | .936 |
| Other VRE species or unspecified | 19 (6.0) | 37 (11.4) | .015 |
| Polymicrobial bacteremiaa | 19 (6.0) | 30 (9.2) | .117 |
| Concomitant broad-spectrum antipseudomonal β-lactam treatmentb | 200 (62.7) | 192 (59.1) | .347 |
| Infection source | |||
| Line | 109 (34.2) | 134 (41.2) | .065 |
| Genitourinary | 35 (11.0) | 27 (8.3) | .252 |
| Abdominal | 39 (12.2) | 44 (13.5) | .619 |
| Gastrointestinal | 43 (13.5) | 17 (5.2) | <.001 |
| Endocarditis/cardiac device | 10 (3.1) | 29 (8.9) | .002 |
| Wound/bone | 22 (6.9) | 15 (4.6) | .214 |
| Unknown | 61 (19.1) | 59 (18.2) | .752 |
| Source controlc | |||
| Yes | 188 (72.9) | 207 (77.8) | .188 |
| No | 16 (6.2) | 14 (5.3) | .644 |
| Undocumented | 54 (20.9) | 45 (16.9) | .241 |
| Facility complexity leveld | |||
| 1a | 138 (43.3) | 193 (59.1) | <.001 |
| 1b | 77 (24.1) | 104 (32.0) | .026 |
| 1c | 97 (30.4) | 26 (8.0) | <.001 |
| 2 | 4 (1.3) | 3 (0.9) | .723 |
| 3 | 3 (0.9) | 0 (0.0) | .121 |
| Solid organ transplant | 18 (5.6) | 32 (9.8) | .046 |
| Kidney | 11 (3.4) | 14 (4.3) | .572 |
| Liver | 5 (1.6) | 17 (5.2) | .015 |
| Heart-lung | 2 (0.6) | 1 (0.3) | .621 |
| Time to VRE-BSIe, d, median (IQR) | 5 (2–14) | 5 (1–15) | .970 |
| Time to treatmentf, h, median (IQR) | 83 (55–107) | 72 (38–102) | .124 |
| >1 d of VRE-positive blood cultures prior to treatment | 46 (14.4) | 46 (14.2) | .923 |
| Intensive care unit admission | 267 (83.7) | 229 (70.5) | <.001 |
| No. of follow-up blood cultures, mean ± SD | 1.45 ± 0.89 | 1.39 ± 1.02 | .551 |
| Sepsis | 208 (65.2) | 210 (64.6) | .768 |
| Charlson comorbidity index, median (IQR) | 9 (7–11) | 9 (7–11) | .448 |
| Past myocardial infarction | 76 (23.8) | 69 (21.2) | .431 |
| Congestive heart failure | 112 (35.1) | 125 (38.5) | .378 |
| Peripheral vascular disease | 109 (34.2) | 77 (23.7) | .003 |
| Cerebrovascular disease | 86 (27.0) | 89 (27.4) | .903 |
| Dementia | 35 (11.0) | 37 (11.4) | .868 |
| Chronic obstructive pulmonary disease | 150 (47.0) | 143 (44.0) | .441 |
| Rheumatoid arthritis | 12 (3.8) | 10 (3.1) | .632 |
| Mild liver disease | 38 (11.9) | 42 (12.9) | .697 |
| Diabetes, uncomplicated | 62 (19.4) | 82 (25.2) | .078 |
| Diabetes, with end-organ damage | 61 (19.1) | 80 (24.6) | .092 |
| Hemiplegia | 31 (9.7) | 26 (8.0) | .443 |
| Moderate or severe renal disease | 214 (67.1) | 235 (72.3) | .149 |
| Any malignancy | 122 (38.2) | 135 (41.5) | .393 |
| Severe liver disease | 28 (8.8) | 50 (15.4) | .010 |
| Metastatic solid tumor | 29 (9.1) | 27 (8.3) | .724 |
| HIV infected | 5 (1.6) | 3 (0.9) | .460 |
| Peptic ulcer disease | 31 (9.7) | 33 (10.2) | .853 |
| Hematologic malignancy | 38 (11.9) | 63 (19.4) | .009 |
| Neutropenia | 39 (12.2) | 62 (19.1) | .017 |
| Acute kidney injury | 143 (44.8) | 154 (47.4) | .515 |
| Mechanical ventilation | 70 (21.9) | 37 (11.4) | <.001 |
| Thrombocytopenia | 34 (10.7) | 41 (12.6) | .439 |
| APACHE II score, median (IQR) | 16 (12–21) | 14 (10–20) | .005 |
Data are presented as No. (%) unless otherwise specified. Categorical variables compared by χ2 or Fisher exact test. Continuous variables compared by Mann–Whitney U test or t test.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; BSI, bloodstream infection; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; VRE, vancomycin-resistant Enterococcus.
a ± 72 hours of index VRE blood culture.
b At least 1 dose of cefepime, ticarcillin-clavulanate, piperacillin-tazobactam, meropenem, doripenem, or imipenem-cilastatin following positive VRE blood culture.
c Comparison among those with a known source of infection (linezolid, n = 258; daptomycin, n = 266).
d Facility complexity designation at the time of index VRE blood culture. Facility complexity levels are based on patient population, complexity of clinical services, and education/research, with level 1a designated as the most complex.
e Time from beginning of hospitalization to first positive VRE blood culture.
f Time from index VRE blood culture to first dose of linezolid or daptomycin.
Overall, treatment failure was 60.9%, 30-day all-cause mortality was 38.2%, 7-day mortality was 9.9%, median hospital LOS was 13 days (IQR, 6–25 days), and median duration of bacteremia was 3 days (IQR, 2–6 days). The association between VRE-BSI treatment and clinical outcomes is displayed in Table 2. Factors associated with treatment failure are also reported (Supplementary Table 2). In univariable analysis, treatment failure was significantly higher in the linezolid-treated group compared with the daptomycin-treated group (67.1% vs 54.8%; risk ratio [RR], 1.37; 95% confidence interval [CI], 1.13–1.67; P = .001). This association was driven primarily by differences between treatment groups with regard to 30-day all-cause mortality (42.9% vs 33.5%; RR, 1.17; 95% CI, 1.04–1.32; P = .014) and microbiologic failure (14.6% vs 6.4%; RR, 1.10; 95% CI, 1.02–1.18; P = .011). Treatment with linezolid also resulted in a significantly higher frequency of early mortality (12.9% vs 7.1%; RR, 1.07; 95% CI, 1.01–1.12; P = .016). No difference in median hospital LOS between treatment groups was observed (14 days vs 12 days; P = .228). Median duration of bacteremia was significantly higher among patients treated with linezolid vs daptomycin (4 days vs 3 days; P = .033). Excluding cases in which the causative VRE species was not defined, treatment failure remained higher among those treated with linezolid (67.0% [n = 201/300] vs 56.9% [n = 164/288]; P = .012).
Table 2.
Clinical Outcomes by Antimicrobial Treatment for Vancomycin-Resistant Enterococcus Bloodstream Infection
| Outcome | Linezolid (n = 319) | Daptomycin (n = 325) | Risk Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Treatment failure | 214 (67.1) | 178 (54.8) | 1.37 (1.13–1.67) | .001 |
| 30-day all-cause mortality | 137 (42.9) | 109 (33.5) | 1.17 (1.04–1.32) | .014 |
| Microbiologic failurea | 23 (14.6) | 15 (6.4) | 1.10 (1.02–1.18) | .011 |
| 60-day VRE-BSI recurrence | 80 (25.1) | 72 (22.2) | 1.04 (.96–1.14) | .347 |
| Early (7-day) mortality | 41 (12.9) | 23 (7.1) | 1.07 (1.01–1.12) | .016 |
| Hospital length of stay, d, median (IQR) | 14 (7–25) | 12 (6–25) | … | .228 |
| Duration of bacteremia, d, median (IQR) | 4 (2–7) | 3 (2–5) | … | .033 |
Data are presented as No. (%) unless otherwise specified. Reference group: linezolid treatment.
Abbreviations: CI, confidence interval; IQR, interquartile range; VRE-BSI, vancomycin-resistant Enterococcus bloodstream infection.
a Percentages among those with ≥1 follow-up blood culture drawn during treatment period (linezolid, n = 157; daptomycin, n = 233).
Variables that were selected in the backward stepwise Poisson regression model for treatment failure included linezolid treatment, intensive care unit admission, severe liver disease, and Acute Physiology and Chronic Health Evaluation II (APACHE II) score. The relationship between increased failure among those treated with linezolid remained after adjusting for these factors in Poisson regression (adjusted RR, 1.15; 95% CI, 1.02–1.30; P = .026; Table 3). In this model, every 1-unit increase in APACHE II score was associated with a 2.5% greater risk of treatment failure. All other variables, including time to VRE-BSI, time to treatment, and solid organ transplant did not confound the relationship between treatment group and composite treatment failure and therefore were not retained in the final parsimonious model.
Table 3.
Poisson Regression of Factors Associated With Treatment Failure Among Patients With Vancomycin-Resistant Enterococcus Bloodstream Infection
| Factor (N = 644) | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Linezolid treatment | 1.37 (1.13–1.67) | .001 | 1.15 (1.02–1.30) | .026 |
| Intensive care unit admission | 1.67 (1.34–1.96) | <.001 | 1.31 (1.08–1.60) | .007 |
| Severe liver disease | 1.60 (1.08–2.36) | .009 | 1.19 (1.03–1.37) | .016 |
| APACHE II score | 1.04 (1.03–1.05) | <.001 | 1.03 (1.02–1.03) | <.001 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval.
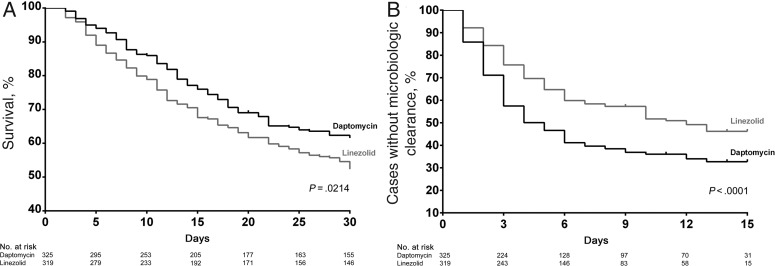
Kaplan–Meier curves for 30-day all-cause mortality and microbiologic failure are depicted in Figure 1. Compared to linezolid, daptomycin treatment demonstrated significantly improved survival (log-rank P = .021) and microbiologic clearance (log-rank P < .001). Unadjusted and adjusted Cox proportional hazard ratios were also derived (Table 4). Significant differences in duration of therapy were noted between groups, with a median duration of linezolid therapy of 7 days (IQR, 4–12 days) compared with a median duration of daptomycin therapy of 11 days (IQR, 5–14; P < .001). However, even after controlling for duration of therapy and other factors, linezolid treatment remained significantly associated with mortality (Table 4). Factors associated with 30-day mortality are reported in Supplementary Table 3.
Figure 1.
Kaplan–Meier curves for outcomes (A) 30-day mortality and (B) microbiologic failure.
Table 4.
Cox Proportional Hazards Model of Factors Associated With 30-Day Mortality Among Patients With Vancomycin-Resistant Enterococcus Bloodstream Infection
| Factor (N = 644) | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Linezolid treatment | 1.36 (1.05–1.76) | .021 |
| Age ≥65 y | 1.27 (.97–1.67) | .088 |
| Intensive care unit admission | 1.90 (1.29–2.80) | .001 |
| Severe liver disease | 1.83 (1.26–2.66) | .002 |
| Hematologic malignancy | 1.57 (1.11–2.22) | .011 |
| Thrombocytopenia | 1.52 (1.07–2.16) | .019 |
| Unknown infection source | 1.69 (1.25–2.28) | <.001 |
| APACHE II score | 1.03 (1.01–1.05) | <.001 |
Cases right-censored at end of treatment period.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval.
The treatment groups were statistically balanced with regard to baseline characteristics following propensity score matching (Supplementary Table 4). In this analysis, treatment failure remained significantly more common in the linezolid-treated group (54.5% vs 45.5%; P = .019), which was driven primarily by differences in mortality and microbiologic failure (Supplementary Table 5). Overall, outcomes in the propensity score–matched cohort were consistent with other analyses.
The association between linezolid treatment and 30-day all-cause mortality persisted after stratifying by VRE-BSI species (E. faecium vs E. faecalis; Mantel–Haenszel common RR, 1.16; 95% CI, 1.01–1.32; P = .027). Among those with VRE-BSI caused by E. faecium, the RR for 30-day all-cause mortality was 1.14 (95% CI, 1.00–1.31) compared with 1.28 (95% CI, .89–1.85) among infections caused by E. faecalis. Linezolid treatment was also associated with 30-day all-cause mortality stratified by source of infection (line vs nonline; Mantel–Haenszel common RR, 1.16; 95% CI, 1.03–1.31; P = .016). Among those with a line source of VRE-BSI, the RR for 30-day all-cause mortality was 1.14 (95% CI, .94–1.39), and among those with a nonline source of infection, the RR for 30-day all-cause mortality was 1.17 (95% CI, 1.01–1.37).
We conducted an analysis of daptomycin-treated subjects with or without concomitant β-lactam or aminoglycoside treatment. Only agents that have been shown to be synergistic against some VRE strains in previous in vitro experiments were included [27]. These agents included ampicillin or ampicillin-sulbactam (n = 14), aztreonam (n = 13), cefazolin (n = 10), cefepime (n = 62), cefotaxime (n = 4), ceftazidime (n = 18), ceftriaxone (n = 35), imipenem-cilastatin (n = 49), doripenem (n = 8), ertapenem (n = 17), meropenem (n = 33), piperacillin-tazobactam (n = 96), ticarcillin-clavulanate (n = 10), amikacin (n = 10), tobramycin (n = 10), and gentamicin (n = 27). Unexpectedly, patients treated with a concomitant β-lactam agent appeared to have a higher proportion of treatment failure (56.3% [n = 135/240] vs 50.6% [n = 43/85]); however, this association was not statistically significant (P = .367). This relationship was also true for concomitant aminoglycoside therapy and treatment failure (57.8% [n = 26/45] vs 54.3% [n = 152/280]; P = .662).
Adverse Events
The frequency of adverse events compared by antimicrobial treatment group is displayed in Table 5. Of the 569 patients with platelet measurements available during the treatment period (linezolid, n = 285; daptomycin, n = 284), thrombocytopenia occurred more frequently among those treated with linezolid (6.3% vs 4.9%). However, no statistically significant association between VRE-BSI treatment and development of thrombocytopenia was observed (odds ratio [OR], 1.30; 95% CI, .60–2.87; P = .593). Among the 275 patients with CPK measurements available during the time period evaluated, CPK elevation was observed in 6 of 211 daptomycin-treated patients (2.8%) and 1 of 64 linezolid-treated patients (1.6%; OR, 0.54; 95% CI, .01–4.61; P = .974).
Table 5.
Adverse Events by Antimicrobial Treatment Group for Vancomycin-Resistant Enterococcus Bloodstream Infection
| Outcome | Linezolid | Daptomycin | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Thrombocytopenia, No. (%) | 18/285 (6.3) | 14/284 (4.9) | 1.30 (.60–2.87) | .593 |
| Creatine phosphokinase elevation, No. (%) | 1/64 (1.6) | 6/211 (2.8) | 0.54 (.01–4.61) | .974 |
Reference group: linezolid treatment. Adverse events compared by logistic regression.
Abbreviation: CI, confidence interval.
DISCUSSION
The purpose of this study was to compare the safety and effectiveness of linezolid and daptomycin for the treatment of VRE-BSI. To our knowledge, this is the first nationwide cohort study comparing these agents and the largest single investigation to date. Treatment with daptomycin resulted in significantly less treatment failure, 30-day mortality, microbiologic failure, 7-day mortality, and duration of bacteremia. The mortality rates we observed were consistent with previous studies of VRE-BSI [25]. However, the present study is the first to demonstrate improved clinical outcomes associated with daptomycin treatment.
Consistent with previous studies, microbiologic clearance was common and occurred in 90.0% of cases overall [25]. Microbiologic clearance is especially important in critically ill and neutropenic patients, and a shorter duration of bacteremia corresponds with better survival in this population [6]. Previous researchers hypothesized that treatment with a bactericidal agent such as daptomycin may lead to improved clinical outcomes, but this had not been demonstrated prior to the present study [9, 25]. As can be interpreted from the associated Kaplan–Meier curves (Figure 1), the effect of daptomycin treatment on microbiologic clearance appeared to be greatest within the first 3–7 days of treatment. This finding corresponds with the improved early mortality benefit with daptomycin treatment we observed, which may be a result of the rapid bactericidal activity of daptomycin in comparison to linezolid.
Multiple retrospective studies have aimed to compare clinical outcomes between linezolid and daptomycin for VRE-BSI, but have failed due to inadequate sample sizes [20–23]. Recently, researchers have pooled data from these investigations [24, 25]. In direct contrast to the present study, one of these meta-analyses reported improved survival measured by overall mortality, defined as a composite of 7-day, 30-day, hospital, and infection-related mortality, among those treated with linezolid [24]. Significant limitations associated with this meta-analysis may have resulted in misinformed conclusions. Most important, there were profound differences in inclusion and exclusion criteria across studies that would lead to a heterogeneous study population.
Previous data suggest that VRE-BSI recurrence may be higher among daptomycin-treated subjects [22]. This finding has been attributed to the increased use of daptomycin among immunosuppressed patients [22, 25]. Although more patients with hematologic malignancy and neutropenia were treated with daptomycin in the present study, we did not observe an increase in VRE-BSI recurrence. The reason for this is unclear, but may be related to more accurate patient follow-up within the integrated Veterans Affairs healthcare system.
In the present analysis, daptomycin consistently performed better than linezolid in all the clinical outcomes evaluated, whereas no differences in adverse events were observed. Of note, we only collected objective laboratory data during the treatment period as part of our safety evaluation. Side effects such as myalgias may manifest in the absence of CPK elevation and may differ between treatment groups [28]. However, the frequency of CPK elevation we observed was consistent with previous analyses [26].
Synergy with daptomycin has been demonstrated with multiple β-lactam and aminoglycoside agents against VRE, although this effect is not observed with all strains [27]. In this cohort, addition of a β-lactam agent or aminoglycoside to daptomycin did not appear to significantly reduce treatment failure. Because we were unable to analyze the synergistic activity of these combinations, it is impossible to conclude if this observation was due to nonsuperiority of combination therapy or some other factor. It is important to note that this study was not designed to evaluate the effect of daptomycin combinations on clinical outcomes.
A number of considerations should be taken into account to appropriately interpret the data from the present study. First, this was a retrospective observational study and suffers from the limitations of this design. Second, microbiologic failure and duration of bacteremia are largely dependent on the timing of follow-up cultures, which may vary based on individual patient condition and between practitioners. Third, susceptibility data were not reported for the majority of cases. However, due to rarity of linezolid resistance and daptomycin nonsusceptibility among VRE isolates, we do not anticipate that this would have a significant impact on our findings [29, 30]. Fourth, standard 6 mg/kg doses of daptomycin were used in the majority of patients, despite newer evidence that higher doses may lead to better outcomes in patients with VRE-BSI [15–17]. High-dose daptomycin (>8 mg/kg) was only used in 4.3% of subjects, preventing an analysis of the effectiveness of these doses. However, our findings likely underestimate the treatment difference between daptomycin and linezolid because of the lower daptomycin doses utilized. Additionally, data on VRE colonization, infectious diseases specialist consultation, and time to positivity were not available at the time of this study and could not be considered in analyses. Although data on hematologic malignancy and neutropenia were collected, hematopoietic stem cell transplant status was not assessed. Last, we were likely underpowered to evaluate differences in the observed proportions of 60-day VRE-BSI recurrence and adverse events in respective subsets.
In summary, treatment with linezolid rather than daptomycin for VRE-BSI resulted in significantly greater treatment failure. The association between treatment failure and linezolid treatment persisted even after adjusting for confounding factors in Poisson regression and propensity score matching. In a Cox proportional hazards model, treatment with linezolid was also associated with lower 30-day survival compared with daptomycin. Overall, daptomycin treatment for VRE-BSI appeared to result in better clinical outcomes than linezolid.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Mary Oehlert and Allen Faler for their expertise and assistance in study coordination and database management.
Disclaimer. The contents of this report do not represent the views of the Department of Veterans Affairs or the US government.
Financial support. This work was supported by the National Institutes of Health (grant number TL1 TR000120-03 to N. S. B.). This material is the result of work supported with resources and the use of facilities at the Dwight D. Eisenhower VAMC, Leavenworth, Kansas.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 2007; 58:163–70. [DOI] [PubMed] [Google Scholar]
- 3.Reik R, Tenover FC, Klein E, McDonald LC. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn Microbiol Infect Dis 2008; 62:81–5. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey AM, Zilberberg MD. Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States, 2000–2006. Infect Control Hosp Epidemiol 2009; 30:184–6. [DOI] [PubMed] [Google Scholar]
- 5.Salgado CD, Farr BM. Outcomes associated with vancomycin-resistant enterococci: a meta-analysis. Infect Control Hosp Epidemiol 2003; 24:690–8. [DOI] [PubMed] [Google Scholar]
- 6.DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis 2005; 191:588–95. [DOI] [PubMed] [Google Scholar]
- 7.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis 2005; 41:327–33. [DOI] [PubMed] [Google Scholar]
- 8.Mermel LA, Allon M, Bouza E et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect 2010; 16:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zyvox (linezolid for injection) [package insert]. New York: Pharmacia & Upjohn Company, Division of Pfizer, Inc, 2010. [Google Scholar]
- 11.Talbot GH, Bradley J, Edwards JE Jr et al. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 2006; 42:657–68. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher JC, Perez ME, Marino EA, LoCastro LG, Abrardo LA, MacDougall C. Daptomycin therapy for vancomycin-resistant enterococcal bacteremia: a retrospective case series of 30 patients. Pharmacotherapy 2009; 29:792–9. [DOI] [PubMed] [Google Scholar]
- 13.Poutsiaka DD, Skiffington S, Miller KB, Hadley S, Snydman DR. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J Infect 2007; 54:567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak MJ, Hershberger E, Moldovan T, Grucz RG. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin- intermediate and -resistant strains. Antimicrob Agents Chemother 2000; 44:1062–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall AD, Steed ME, Arias CA, Murray BE, Rybak MJ. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 2012; 56:3174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullar R, Davis SL, Levine DP et al. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy 2011; 31:527–36. [DOI] [PubMed] [Google Scholar]
- 17.King EA, McCoy D, Desai S, Nyirenda T, Bicking K. Vancomycin-resistant enterococcal bacteraemia and daptomycin: are higher doses necessary? J Antimicrob Chemother 2011; 66:2112–8. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Abraham T, Rapp J, Vastey F, Saad N, Balmir E. Daptomycin: evaluation of a high-dose treatment strategy. Int J Antimicrob Agents 2011; 38:192–6. [DOI] [PubMed] [Google Scholar]
- 19.Canton R, Ruiz-Garbajosa P, Chaves RL, Johnson AP. A potential role for daptomycin in enterococcal infections: what is the evidence? J Antimicrob Chemother 2010; 65:1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mave V, Garcia-Diaz J, Islam T, Hasbun R. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? J Antimicrob Chemother 2009; 64:175–80. [DOI] [PubMed] [Google Scholar]
- 21.Crank CW, Scheetz MH, Brielmaier B et al. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin Ther 2010; 32:1713–9. [DOI] [PubMed] [Google Scholar]
- 22.Twilla JD, Finch CK, Usery JB, Gelfand MS, Hudson JQ, Broyles JE. Vancomycin-resistant Enterococcus bacteremia: an evaluation of treatment with linezolid or daptomycin. J Hosp Med 2012; 7:243–8. [DOI] [PubMed] [Google Scholar]
- 23.McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol Infect 2011; 139:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balli EP, Venetis CA, Miyakis S. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother 2014; 58:734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whang DW, Miller LG, Partain NM, McKinnell JA. Systematic review and meta-analysis of linezolid and daptomycin for treatment of vancomycin-resistant enterococcal bloodstream infections. Antimicrob Agents Chemother 2013; 57:5013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin Infect Dis 2010; 50:1568–74. [DOI] [PubMed] [Google Scholar]
- 27.Steenbergen JN, Mohr JF, Thorne GM. Effects of daptomycin in combination with other antimicrobial agents: a review of in vitro and animal model studies. J Antimicrob Chemother 2009; 64:1130–8. [DOI] [PubMed] [Google Scholar]
- 28.Cubicin (daptomycin for injection) [package insert]. Lexington, MA: Cubist Pharmaceuticals Inc, 2012. [Google Scholar]
- 29.Kelesidis T, Humphries R, Uslan DZ, Pegues DA. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin Infect Dis 2011; 52:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayakawa K, Marchaim D, Pogue JM et al. Predictors and outcomes of linezolid-resistant vancomycin-resistant Enterococcus: a case-case-control study. Am J Infect Control 2012; 40:e261–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.