Vector exposure showed a direct association with Chagas cardiomyopathy, but an inverse relationship with maternal parasitemia and congenital transmission. We hypothesize that repeated antigen exposure maintains an inflammatory response, increasing cardiomyopathy, but this upregulation improves control of parasitemia during pregnancy.
Keywords: Chagas disease, Trypanosoma cruzi, infectious disease transmission; vertical, cardiomyopathy
Abstract
Background. We studied women and their infants to evaluate risk factors for congenital transmission and cardiomyopathy in Trypanosoma cruzi–infected women.
Methods. Women provided data and blood for serology and quantitative polymerase chain reaction (PCR). Infants of infected women had blood tested at 0 and 1 month by microscopy, PCR and immunoblot, and serology at 6 and 9 months. Women underwent electrocardiography (ECG).
Results. Of 1696 women, 456 (26.9%) were infected; 31 (6.8%) transmitted T. cruzi to their infants. Women who transmitted had higher parasite loads than those who did not (median, 62.0 [interquartile range {IQR}, 25.8–204.8] vs 0.05 [IQR, 0–29.6]; P < .0001). Transmission was higher in twin than in singleton births (27.3% vs 6.4%; P = .04). Women who had not lived in infested houses transmitted more frequently (9.7% vs 4.6%; P = .04), were more likely to have positive results by PCR (65.5% vs 33.9%; P < .001), and had higher parasite loads than those who had lived in infested houses (median, 25.8 [IQR, 0–64.1] vs 0 [IQR, 0–12.3]; P < .001). Of 302 infected women, 28 (9.3%) had ECG abnormalities consistent with Chagas cardiomyopathy; risk was higher for older women (odds ratio [OR], 1.06 [95% confidence interval {CI}, 1.01–1.12] per year) and those with vector exposure (OR, 3.7 [95% CI, 1.4–10.2]). We observed a strong dose-response relationship between ECG abnormalities and reported years of living in an infested house.
Conclusions. We hypothesize that repeated vector-borne infection sustains antigen exposure and the consequent inflammatory response at a higher chronic level, increasing cardiac morbidity, but possibly enabling exposed women to control parasitemia in the face of pregnancy-induced Th2 polarization.
In the Americas, Trypanosoma cruzi causes the highest disease burden of any parasite, accounting for 7 times more disability-adjusted life years lost than malaria [1]. An estimated 6 million people are infected, of whom 20%–30% will develop potentially life-threatening heart disease [2]. Chagas cardiomyopathy is characterized by a chronic inflammatory process [3]. The earliest signs are usually conduction system abnormalities, followed by atrioventricular blocks, ventricular arrhythmias, sinus node dysfunction, and progressive dilated cardiomyopathy [4, 5]. The determinants of progression to cardiomyopathy are not well understood, but may include repeated T. cruzi superinfection, parasite virulence and tissue tropism, and host immune response [3].
Although vector exposure remains the most frequent infection route, T. cruzi can also be transmitted by transfusion or transplantation, and from mother to child [6]. The Southern Cone Initiative has made major advances in blood screening and control of the principal vector, Triatoma infestans [7]. With the decrease in other routes, congenital transmission has become proportionately more important, accounting for an estimated 22% of new infections in 2010 [2]. In meta-analyses, the median transmission rate from T. cruzi–infected women is estimated at 5% [8], but rates vary widely, from 1% to >15% across study populations [9–12].
From 2010 to 2013, we conducted a study of women and their infants in 2 sites in Santa Cruz Department, Bolivia. The ability to study congenital T. cruzi transmission and cardiac status in the same women enabled us to examine the epidemiology of these 2 phenomena simultaneously. Our aims were to evaluate the incidence of vertical transmission and prevalence of early cardiomyopathy in T. cruzi–infected women, and to assess risk factors for both of these outcomes.
MATERIALS AND METHODS
Ethics Statement
The protocol was approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health, Hospital Universitario Japonés, Universidad Católica Boliviana, Universidad Peruana Cayetano Heredia, Asociación Benéfica Proyectos en Informatica, Salud, Medicina y Agricultura, and the Centers for Disease Control and Prevention. Each woman provided written informed consent for herself and her infant.
Study Population and Data and Specimen Collection
The study was conducted in Hospital Universitario Japonés in urban Santa Cruz (city population 1.7 million) and Hospital Municipal Camiri in Camiri (city population 30 191) (www.ine.gob.bo) [13, 14]. Both hospitals are referral centers with a large proportion of cesarean deliveries. Although vector-borne T. cruzi transmission is absent in urbanized areas, both cities receive migrants from rural areas with intense transmission. Camiri is the capital of Cordillera province (population 111 171) in the Bolivian Chaco and located a few miles from villages where 50%–100% of houses are heavily infested, with rapid reinfestation after insecticide application [14, 15]. The estimated national T. cruzi prevalence is 6% [2], but in rural areas of the Chaco, the all-age prevalence is 50%, and adult infection prevalence reaches ≥80% [14, 16].
Trained study nurses enrolled women presenting for delivery and collected data, including a record of all houses lived in throughout the woman's life, duration of residence, construction materials, and observed vector infestation. The study instrument was developed and validated in earlier studies in Santa Cruz [13, 17]; 2 nurses involved in the earlier validation supervised the interviews. Blood was collected, centrifuged, and immediately screened by 2 rapid tests, Trypanosoma Detect (InBios, Seattle, Washington) and Polychaco indirect hemagglutination assay (IHA) at a single dilution of 1:16 (Lemos Laboratories, Santiago del Estero, Argentina). Sera were later tested in Santa Cruz by IHA with multiple dilutions and Chagatest lysate enzyme-linked immunosorbent assay (ELISA), with Recombinante 3.0 ELISA as a tie-breaker (both from Wiener Laboratories, Rosario, Argentina). A confirmed infection required positive results by ≥2 conventional tests [18]. For infected women, a standard 12-lead electrocardiogram (ECG) was performed during a follow-up visit.
Management of Congenital T. cruzi Infection
A study nurse attended the delivery of each rapid test–positive woman to collect cord blood. Infants of infected women had follow-up blood collection at 1, 6, and 9 months of age. Cord blood and 1-month specimens were examined by micromethod, the standard technique to diagnose congenital T. cruzi infection in the first months of life in most Latin American healthcare facilities [19]. Blood is collected in 4–6 heparinized microhematocrit tubes, sealed, and processed within 24 hours by centrifugation (12 000 rpm for 7 minutes) followed by microscopy. Six- and 9-month specimens were centrifuged, and serum samples were tested by immunoglobulin G assays as described above for maternal testing.
Western blots were performed in our Lima laboratory to detect immunoglobulin M (IgM) to trypomastigote excreted-secreted antigens (IgM TESA-blot) in infant specimens following published methods [20]. Blots with clear bands at the expected molecular weights were considered positive; blots with weak or ambiguous bands were repeated. Ladder-like bands at 130–200 kDa on IgM TESA-blot demonstrate antibodies to shed acute-phase antigens, indicating acute or congenital infection [20]. In our earlier analysis, the TESA-blot showed 67% sensitivity and 100% specificity [13].
Quantitative polymerase chain reaction (PCR) was performed on maternal and infant specimens in Lima. For the first 598 women and the first 1000 infants in the study, phenol-chloroform extractions were performed from blood clot [13]. DNA was amplified using the primer set Cruzi1/Cruzi2 following published methods [13, 21]. Parasite loads were calculated based on the standard curve included in each run; the threshold of detection was estimated at 1 parasite equivalent/mL. For later specimens, extractions were conducted using the automated Qiacube system with Qiagen kits (Qiagen N.V., Hilden, Germany). The assay was restandardized following this change, but analysis showed that the amount of DNA extracted was much lower, and sensitivity, especially for maternal specimens whose parasite loads were relatively low, dropped significantly. For this reason, analyses related to maternal parasite load are limited to the specimens extracted by phenol-chloroform. Parasite loads were much higher in infant specimens, and the diagnosis of congenital infection was based on multiple samples and assays (Supplementary Table 1). We are therefore confident that the infections included in this analysis are true infections, and believe that few, if any, congenital infections were missed based on the methodological change.
A neonatologist (M. C. A.) managed infected infants following Bolivian Control Program guidelines, which require positive results by micromethod or serology at 6–9 months [22]. For this analysis, we considered an infant to have congenital infection based on positive results by >1 assay or positive serology at 6–9 months confirmed by IHA and ELISA. Infants with borderline serological results at 6 months were asked to return for repeat testing at 9 months.
Electrocardiographic Readings
Two cardiologists blinded to each other's reading evaluated each ECG and recorded abnormalities following standardized methods [23]. We considered the following to be consistent with Chagas cardiomyopathy: complete right or left bundle branch block, left anterior or posterior fascicular block, complex or multiform ventricular premature beats, atrioventricular blocks in absence of drugs slowing AV conduction, sinus bradycardia <45 beats/minute or sinus pauses >3.0, atrial fibrillation, junctional rhythm, or complex ventricular arrhythmias (multiform, couplets, or nonsustained ventricular tachycardia). Incomplete right bundle branch block was not considered to meet the criteria, because this finding can be a normal variant in young individuals [24].
Data Analysis
Variables related to vector exposure were derived from the listing of residences, construction material, and infestation. Statistical analyses were performed using SAS software for Windows 9.0. Categorical variables were compared by χ2 or Fisher exact test as appropriate. Continuous variables were analyzed using the Wilcoxon rank-sum test. Multivariable models were constructed using forward stepwise logistical regression, testing variables with P < .10 in univariate analyses. Model fit was assessed by the Akaike information criterion.
RESULTS
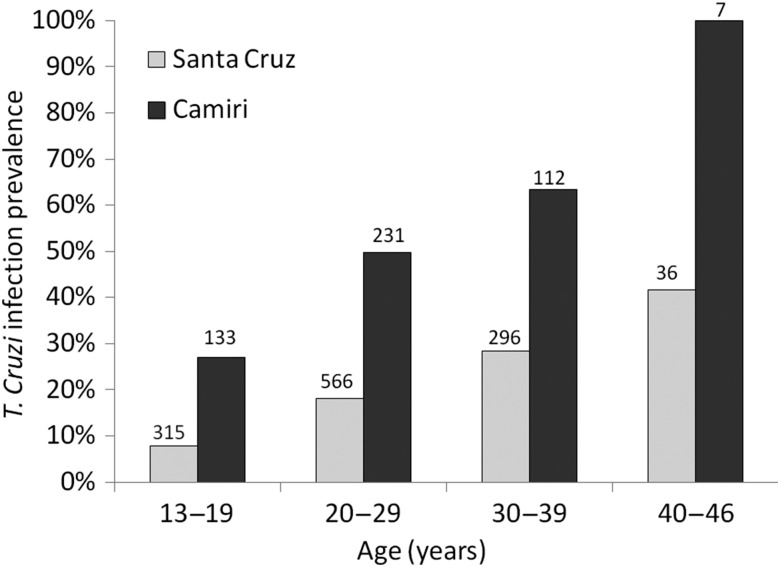
A total of 1696 women were screened for Chagas disease, 1213 in Santa Cruz between 1 June 2010 and 31 May 2013, and 483 in Camiri between 8 October 2012 and 5 March 2013. Women who delivered in Camiri were more likely to have T. cruzi infection than those in Santa Cruz (47.4% vs 18.7%, respectively; P < .0001; Table 1). The difference in prevalence was significant within every maternal age group (P < .001 for 3 younger age groups; P < .01 for women 40–46 years old) (Figure 1). Women delivering in Camiri were slightly younger than those in Santa Cruz, and more likely to report having lived in infested houses and in houses with mud walls and earth floors, materials associated with vector infestation.
Table 1.
Characteristics of Women Presenting for Delivery in Hospital Municipal Camiri, Camiri, and Hospital Japonés, Santa Cruz, Bolivia
| Maternal Characteristics | Camiri (n = 483), No. (%) | Santa Cruz (n = 1213), No. (%) | Total (N = 1696), No. (%) | P Value |
|---|---|---|---|---|
| Trypanosoma cruzi infection | 229 (47.4) | 227 (18.7) | 456 (26.9) | <.0001 |
| Cesarean delivery | 233 (48.5) | 565 (46.6) | 798 (47.1) | .47 |
| Twin births | 4 (0.8) | 19 (1.6) | 23 (1.4) | .35 |
| Congenital transmissiona | 8 (1.7) | 23 (1.9) | 31 (1.8) | .74 |
| Ever lived in rural areab | 246 (51.0) | 606 (52.2) | 852 (51.9) | .66 |
| Lives in rural area now | 160 (33.1) | 311 (25.6) | 471 (27.8) | .002 |
| Ever lived in an infested housec | 165 (34.2) | 293 (24.2) | 458 (27.1) | <.0001 |
| Lives in infested house now | 107 (22.2) | 74 (6.1) | 181 (10.7) | <.0001 |
| Ever lived in mud-wall house | 210 (43.5) | 410 (33.8) | 620 (36.0) | .0002 |
| Lives in mud-wall house now | 139 (28.8) | 51 (4.2) | 190 (11.2) | <.0001 |
| Ever lived in house with earth floor | 212 (43.9) | 405 (33.4) | 617 (36.4) | <.0001 |
| Lives in house with earth floor now | 139 (28.8) | 58 (4.8) | 197 (11.6) | <.0001 |
| Completed secondary school | 225 (46.6) | 426 (35.1) | 651 (38.4) | <.0001 |
| Lives in house with electricity | 404 (83.6) | 1194 (98.4) | 1598 (94.2) | <.0001 |
| Owns televisiond | 369 (76.4) | 1156 (95.4) | 1525 (90.0) | <.0001 |
| Owns refrigeratore | 253 (52.4) | 814 (67.2) | 1067 (37.0) | <.0001 |
| Owns motor vehiclee | 76 (15.7) | 164 (13.5) | 240 (14.2) | .25 |
| Owns computere | 85 (17.6) | 76 (6.3) | 161 (9.5) | <.0001 |
a Data excluded for 3 women from Camiri and 4 women from Santa Cruz because infant infection status was unresolved (see text).
b Data missing for 1 woman from Camiri and 53 women from Santa Cruz.
c Data missing for 3 women from Santa Cruz.
d Data missing for 1 woman from Santa Cruz.
e Data missing for 2 women from Santa Cruz.
Figure 1.
Trypanosoma cruzi infection prevalence by age among pregnant women in Santa Cruz and Camiri, Bolivia. The numbers above each bar show the denominator for that age group. The difference in prevalence between sites was significant within every maternal age group (P < .001 for the 3 younger age groups; P < .01 for women aged 40–46 years).
Thirty-four infants from 31 women were diagnosed with congenital T. cruzi infection (Supplementary Table 1). Congenital transmission occurred to 3 sets of twins; all 6 babies were infected. Three of 7 dichorionic twin births had transmission compared with 0 of 4 monochorionic twin births (P = .24). Infection status was inconclusive for 7 infants, 2 with borderline 6-month serology who were treated by the national program before results could be repeated, and 5 with a single positive result by PCR with threshold cycle close to the cutoff who failed to return for subsequent testing. The mothers of these 7 infants were excluded from further analyses.
Women who transmitted to their infants were significantly more likely to have positive results by PCR and had higher parasite loads compared to those without transmission (Table 2). Other significant predictors included younger maternal age and twin births. Mothers of infected infants were significantly less likely to report residence in an infested house, with an inverse relationship between residence duration and transmission risk. Variables associated with domestic infestation such as mud walls showed similar associations.
Table 2.
Factors Associated With Congenital Transmission Among Trypanosoma cruzi–Infected Women Presenting for Delivery in Camiri Municipal Hospital, Camiri, and Hospital Japonés, Santa Cruz, Bolivia
| Factor | Transmitted T. cruzi (n = 31) | Did Not Transmit T. cruzi (n = 418) | P Valuea |
|---|---|---|---|
| Positive results by PCRb | 13 (100) | 44 (41.9) | <.0001 |
| Cesarean section | 19 (61.3) | 204 (48.8) | .18 |
| Twin birth | 3 (9.7) | 8 (1.9) | .04 |
| Primiparous | 6 (19.4) | 86 (20.6) | .87 |
| Parity >3 | 19 (61.3) | 237 (56.7) | .62 |
| Ever lived in rural area | 24 (77.4) | 263 (64.6)c | .15 |
| Lives in rural area now | 15 (48.4) | 142 (34.0) | .11 |
| Ever lived in an infested house | 11 (35.5) | 230 (55.2)d | .04 |
| Lives in infested house now | 4 (12.9) | 98 (23.4) | .26 |
| Ever lived in house with mud walls | 12 (38.7) | 243 (58.1) | .04 |
| Lives in house with mud walls now | 4 (12.9) | 100 (23.9) | .19 |
| Ever lived in house with earth floor | 13 (41.9) | 242 (57.9) | .09 |
| Lives in house with earth floor now | 5 (16.1) | 100 (23.9) | .39 |
| Completed secondary school | 6 (19.4) | 141 (33.7) | .11 |
| Lives in house with electricity | 30 (96.8) | 363 (86.8) | .16 |
| Owns television | 30 (96.8) | 334 (80.1)d | .02 |
| Owns refrigerator | 17 (54.8) | 218 (52.3)d | .78 |
| Owns motor vehicle | 2 (6.5) | 48 (11.5) | .56 |
| Owns computer | 0 (0) | 42 (10.1) | .10 |
| Age, y, median (IQR) | 24.0 (20.1–30.2) | 26.8 (22.0–34.0) | .04e |
| Parasite equivalents/mL by quantitative PCRb, median (IQR) | 62.0 (25.8–204.8) | 0.05 (0–29.6) | <.0001e |
| Years living in rural area, median (IQR) | 15.0 (0–22.0) | 12.0 (0–21) | .30e |
| Years living in infested house, median (IQR) | 0 (0–15) | 6.5 (0–20) | .02e |
| Years living in house with mud walls, median (IQR) | 0 (0–15) | 8 (0–20) | .02e |
| Years living in house with earth floor, median (IQR) | 0 (0–17) | 8 (0–20) | .07e |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
a By χ2 or Fisher exact test.
b Data available for 13 women who transmitted and 105 women who did not transmit.
c Data missing for 11 women.
d Data missing for 1 woman.
e By Wilcoxon rank-sum test for continuous variables.
Women without reported residence in an infested house had a higher transmission rate than those with this reported exposure (20/207 [9.7%] vs 11/241 [4.6%]; P = .04). Women with no history of living in an infested house were more likely to have positive results by PCR (36/55 [65.5%] vs 21/62 [33.9%]; P < .001) and had higher parasite loads than those with a history of residence in an infested house (median, 25.8 [interquartile range {IQR}, 0–64.1] vs 0 [IQR, 0–12.3]; P < .001). The multivariable model failed to converge because all women who transmitted had positive PCR results.
Of 302 T. cruzi–infected women with ECG data, 28 (9.3%) showed at least 1 abnormality consistent with Chagas cardiomyopathy (Supplementary Table 2). The most common abnormalities were bundle branch block and bradycardia. One woman had right bundle branch block, intermittent complete atrioventricular block, bradycardia, and junctional escape rhythm; she developed hypotension requiring vasopressors and received a pacemaker shortly after delivery [25].
Compared to women with normal ECGs, women with abnormal ECGs were older and more likely to report living in an infested house at any time and at the time of study (Table 3). The median duration of residence in an infested house was significantly longer for women with abnormal ECGs than those with normal ECGs (17.5 vs 1.0 years; P = .001). Other variables associated with house infestation showed similar associations. Women with ECG abnormalities were less likely than those with normal ECGs to transmit to their infants, but this association was not statistically significant (1/28 [3.6%] vs 22/274 [8.0%]; P = .40). There was no significant difference in parasite load for those with and without ECG abnormalities. In a multivariable model, increasing maternal age and residence in an infested house were associated with 5% and 4% increased risk, respectively, of ECG abnormalities per year of exposure (Table 4). A second multivariable model estimated a 3.7-fold increased risk of ECG abnormalities among women who ever lived in an infested house vs those who had not.
Table 3.
Factors Associated With Electrocardiographic Abnormalities Consistent With Chagas Cardiomyopathy Among 302 Trypanosoma cruzi–Infected Women
| Maternal Characteristic | Abnormal ECG (n = 28) | Normal ECG (n = 274) | P Valuea |
|---|---|---|---|
| Positive results by PCRb | 3 (30.0) | 33 (45.2) | .50 |
| Ever lived in rural area | 18 (64.3) | 175 (65.8)c | .87 |
| Lives in rural area now | 12 (42.9) | 85 (31.0) | .20 |
| Ever lived in an infested house | 23 (82.1) | 142 (51.8) | .002 |
| Lives in infested house now | 10 (35.7) | 49 (17.9) | .03 |
| Ever lived in house with mud walls | 21 (75.0) | 152 (55.5) | .05 |
| Lives in house with mud walls now | 11 (39.3) | 52 (19.0) | .03 |
| Ever lived in house with earth floor | 21 (75.0) | 151 (55.1) | .04 |
| Lives in house with earth floor now | 11 (39.3) | 50 (18.3) | .008 |
| Completed secondary school | 11 (39.3) | 98 (35.8) | .71 |
| Lives in house with electricity | 24 (85.7) | 243 (88.7) | .55 |
| Owns television | 22 (78.6) | 230 (84.3)d | .42 |
| Owns refrigerator | 16 (57.1) | 152 (55.7)d | .88 |
| Owns motor vehicle | 1 (3.6) | 37 (13.5) | .23 |
| Owns computer | 5 (17.9) | 26 (9.5) | .19 |
| Age, y, median (IQR) | 32 (24–39) | 27 (22–34) | .03e |
| Parasite equivalents/mL by quantitative PCRb, median (IQR) | 0 (0–27) | 0.3 (0–33.7) | .33e |
| Years lived in rural area, median (IQR) | 14 (0–28) | 10 (0–20)c | .39e |
| Years lived in infested house, median (IQR) | 17.5 (8–28) | 1 (0–18) | .001e |
| Years lived in house with mud walls, median (IQR) | 17.5 (1.5–27) | 5 (0–18) | .01e |
| Years lived in house with earthen floor, median (IQR) | 18.5 (1.5–28) | 5 (0–18) | .01e |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: ECG, electrocardiogram; IQR, interquartile range; PCR, polymerase chain reaction.
a By χ2 or Fisher exact test.
b PCR data available for 10 women with abnormal ECG and 73 women with normal ECG.
c Data missing for 9 women.
d Data missing for 1 woman.
e By Wilcoxon rank-sum test.
Table 4.
Logistic Regression Model of Factors Associated With Electrocardigraphic Abnormalities Consistent With Chagas Cardiomyopathy Among 302 Trypanosoma cruzi–Infected Women
| Factor | Univariable Analyses |
Multivariable Model 1 |
Multivariable Model 2 |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | Adjusted ORa (95% CI) | P Value | |
| Per year of age | 1.07 (1.02–1.13) | .012 | 1.06 (1.01–1.12) | .04 | 1.05 (.99–1.12) | .10 |
| Ever lived in infested house | 4.3 (1.6–11.6) | .004 | 3.7 (1.4–10.2) | .01 | … | |
| Lives in infested house now | 2.6 (1.1–5.9) | .03 | … | … | ||
| Per year of living in infested house | 1.05 (1.02–1.08) | .002 | … | 1.04 (1.01–1.07) | .02 | |
| Ever lived in house with mud walls | 2.4 (.99–5.9) | .05 | … | … | ||
| Lives in house with mud walls now | 2.8 (1.2–6.3) | .02 | … | … | ||
| Per year in house with mud walls | 1.04 (1.01–1.08) | .008 | … | … | ||
| Ever lived in house with earth floor | 2.4 (1.0–5.9) | .05 | … | … | ||
| Lives in house with earth floor now | 2.9 (1.3–6.6) | .01 | … | … | ||
| Per year in house with earth floor | 1.04 (1.01–1.07) | .008 | … | … | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
a OR adjusted for the other variables listed in the column.
The presence of ECG abnormalities demonstrated a strong dose-response relationship with increasing years of living in an infested house, whereas congenital transmission showed a strong dose-response relationship for maternal parasite load (Table 5). Congenital transmission risk was significantly lower for women in older age strata and those with longer duration of residence in an infested house.
Table 5.
Dose-Response Analyses for Congenital Trypanosoma cruzi Transmission and Electrocardiographic Abnormalities Consistent With Chagas Cardiomyopathy
| Characteristic | Congenital Transmission, no./No.(%) | Stratum OR | χ2 for Trend, P Value | Abnormal ECG no./No. (%) | Stratum OR | χ2 for Trend, P Value |
|---|---|---|---|---|---|---|
| Age group | ||||||
| 13–19 | 7/60 (11.7) | Referent | 3/35 (8.6) | Referent | ||
| 20–29 | 16/215 (7.4) | 0.61 | 8/138 (5.8) | 0.66 | ||
| 30–46 | 8/174 (4.6) | 0.36 | 4.22, P = .04 | 18/133 (13.5) | 1.67 | 2.3, P = .13 |
| Years in infested house | ||||||
| None | 20/207 (9.7) | Referent | 5/138 (3.6) | Referent | ||
| 1–19 | 9/134 (6.7) | 0.67 | 11/96 (11.5) | 3.44 | ||
| ≥20 | 2/107 (1.9) | 0.18 | 7.12, P = .008 | 13/72 (18.1) | 5.86 | 11.2, P = .0008 |
| Parasite loada | ||||||
| 0 | 0/61 (0)b | Referent | 7/47 (14.9)c | Referent | ||
| 1–34 | 4/24 (14.3) | … | 2/19 (10.5) | 0.67 | ||
| ≥35 | 9/29 (31.0) | … | 18.0, P < .0001 | 1/17 (5.9) | 0.36 | 1.5, P = .23 |
Increasing parasite load shows a direct dose-response relationship, whereas increasing maternal age and duration of vector exposure show an inverse relationship with congenital T. cruzi transmission risk. Increasing duration of living in an infested house shows a direct dose-response relationship with the presence of ECG abnormalities.
Abbreviations: ECG, electrocardiogram; OR, odds ratio.
a Parasite equivalents/mL by quantitative polymerase chain reaction (PCR).
b Data available for 13 women who transmitted and 105 who did not transmit. ORs undetermined because of 0 cell in referent stratum.
c PCR data available for 10 women with abnormal ECG and 73 women with normal ECG.
DISCUSSION
Bolivia has the highest T. cruzi infection prevalence in the world [18]. More than 70% of cases of congestive heart failure in some Santa Cruz hospitals are attributed to Chagas disease, and an estimated 1 in 175 Bolivian infants is born with T. cruzi [17, 18]. In the Bolivian Chaco, people continue to live in infested houses, and in some villages, 80%–90% of adults have Chagas disease [14, 16]. In this setting, we examined factors associated with early Chagas cardiomyopathy and congenital transmission among women presenting for delivery. We observed a higher prevalence of ECG abnormalities in women with longer residence times in infested houses, confirming the impression of longtime Chagas disease researchers [26, 27]. Remarkably, vector exposure variables showed an inverse relationship with maternal parasitemia and mother-to-child transmission. Our data thus suggest that sustained vector exposure increases the risk of Chagas cardiomyopathy, but may be associated with some protection against congenital transmission.
We hypothesize that both pathogenic and protective effects may result from frequent exposure to infected vectors and repeated superinfection by the parasite [26]. In the acute phase of Chagas disease, a robust inflammatory response involving innate immune cells and macrophages activated by interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) brings the parasitemia under control [28]. Acute symptoms resolve spontaneously as patent parasitemia disappears, but without treatment, total parasite clearance virtually never occurs [18]. During chronic infection, T-cell–mediated immunity keeps the parasite in check [29]. However, failure to downregulate the inflammatory response, triggered by parasite persistence and modulated by both host and parasite factors, is thought to play a key role in the pathogenesis of Chagas cardiomyopathy [3, 28]. Mice superinfected with the same or a different T. cruzi strain had more frequent, severe ECG changes [30]. Repeated vector-borne infection is thought to sustain antigen exposure and the consequent Th1-mediated inflammatory response at a higher chronic level, increasing cardiac morbidity [3, 26].
In our data, reported time living in an infested house was inversely related to maternal parasitemia, which in turn is the strongest immediate predictor of congenital transmission [13, 31, 32]. Repeated antigen exposure may reinforce the Th1 response that controls the parasite in the face of the maternal Th2 polarization necessary to maintain the pregnancy [33]. Only 2 previous studies have examined vector exposure as a factor in congenital transmission [34, 35]. An Argentine study reported findings similar to ours; women from areas with high infestation rates had the lowest risk of transmission compared with those from areas under vector control (who had intermediate risk) and those living in a city that had never had vector-borne transmission (the highest risk group) [34]. A Bolivian study reported lower morbidity in infected infants of mothers in areas currently under vector control, but no difference in the transmission rate [35]. In our data, duration of residence showed a much stronger association than current house infestation, suggesting that the effect may require prolonged, repeated exposure to develop.
Parasite load was by far the strongest predictor of transmission. Vector exposure is likely one of several factors that influence risk. Other determinants may include T. cruzi genotype and tropism, and maternal immunogenetics [36]. The predominant genotype detected in congenital infections in Bolivia and Argentina is T. cruzi V, followed by II and VI; no differences have been found in transmission rates by genotype [32, 37]. Studies show lower levels of TNF-α in mothers who transmit than those without transmission [38]. Similarly, lower levels of T-cell and macrophage activation markers and T. cruzi–induced IFN-γ release were found in mothers who transmitted compared with infected mothers of uninfected infants [39]. The association of twin births with increased transmission may be related to the intensified downregulation in multiple pregnancies [40].
Our study had a number of limitations. Our measures of vector exposure were retrospective and self-reported. Because T. cruzi infection is chronic and usually acquired in childhood, studies of adults necessarily assess exposures that occurred years before data collection. However, T. infestans is a memorable vector: Adults are >1 inch long, have a pungent odor, and leave visible fecal trails on the walls of infested houses. Light infestations are more likely to have been missed than heavy ones. Some women without reported residence in an infested house were likely infected congenitally themselves, whereas others undoubtedly had more transient or less intense vector exposure not captured in our data. Intensity (number of infective bites per unit time) is impossible to assess retrospectively, but likely plays a role in the effects we observed. We had technical difficulties when we changed extraction methodology midway through the study and were only able to include PCR data from a subset of women. This impeded our ability to analyze epidemiologic risk factors together with parasite load in multivariable models. Our cardiac analysis would have been strengthened by the addition of an uninfected control group, but this was not feasible in the current study. However, we used rigorous criteria to define ECG abnormalities, and all analyses were comparisons within the group of infected mothers. In a meta-analysis of population-based studies, the prevalence of bundle branch blocks in women aged <45 years was 0.2% [41], compared with 6.6% in study women, suggesting that the observed abnormalities were due to Chagas disease. Our study enrolled nearly 1700 women; an even larger sample size would have increased our statistical power but would have strained our resources. Even in this high-prevalence population, we screened an average of 54 women and conducted follow-up on 15 mother–infant pairs to yield a single transmission event.
Chagas disease researchers have long postulated that repeated T. cruzi superinfection is a major determinant of cardiomyopathy risk [26, 27]. In areas under effective vector control, there is a widespread impression that cardiomyopathy severity is lower, and onset and mortality are delayed until later in life [42, 43]. Our maternal ECG data provide epidemiologic support for this hypothesis, and underscore the importance of the vector control effort in the Bolivian Chaco. Nevertheless, congenital transmission risk will remain as long as there are infected women in the reproductive age group. Recent data give strong support to the contention that antitrypanosomal treatment of women prior to pregnancy markedly decreases congenital transmission risk [44]. Sustained vector control efforts, especially in the most affected communities, and programs to safely treat infected individuals are essential to decrease morbidity and prevent continued congenital transmission in the future. Treatment of children and young adults should be given high priority in Latin America and other regions with infected residents [44, 45].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Members of the Working Group on Chagas Disease in Bolivia and Peru include Leny Sanchez, Lisbeth Ferrufino, Edith Malaga, Sara Quispe, Edith Hinojosa, Margot Ramirez, Eliana Saenza, Jorge Luis Flores-Franco, Janet Acosta, Gerardo Sanchez, Maribel Suxo, Hilsen Roncales, Fernando Ramirez, Nazaret Bozo Escalera, Celia Espinoza, and Janet Vizcarra. We are grateful to the nurses and physicians of the obstetric services of Hospital Japonés and Hospital Municipal Camiri for their collaboration and their dedication to the welfare of the women and infants of Santa Cruz Department.
Disclaimer. The funding sources had no role in the study design, collection, analysis and interpretation of the data, preparation of the manuscript, or the decision to submit for publication.
Financial support. This work was supported by the National Institutes of Health (grant number R01-AI087776, Global Research Training grant number D43 TW006581) and by discretionary funds awarded to R. H. G. from Asociacion Benefica PRISMA (www.prisma.org.pe).
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Working Group on Chagas Disease in Bolivia and Peru, Leny Sanchez, Lisbeth Ferrufino, Edith Malaga, Sara Quispe, Edith Hinojosa, Margot Ramirez, Eliana Saenza, Jorge Luis Flores-Franco, Janet Acosta, Gerardo Sanchez, Maribel Suxo, Hilsen Roncales, Fernando Ramirez, Nazaret Bozo Escalera, Celia Espinoza, and Janet Vizcarra
References
- 1.World Health Organization. Global burden of disease estimates for 2000–2012. Available at: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html Accessed 18 June 2015.
- 2.World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 2015; 90:33–44. [PubMed] [Google Scholar]
- 3.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation 2007; 115:1109–23. [DOI] [PubMed] [Google Scholar]
- 4.Rassi A Jr, Rassi A, Little WC. Chagas’ heart disease. Clin Cardiol 2000; 23:883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire JH, Hoff R, Sherlock I et al. . Cardiac morbidity and mortality due to Chagas’ disease: prospective electrocardiographic study of a Brazilian community. Circulation 1987; 75:1140–5. [DOI] [PubMed] [Google Scholar]
- 6.Maguire JH. Trypanosoma. In: Gorbach S, Bartlett J, Blacklow N, eds. Infectious diseases. 2nd ed Philadelphia: Lippincott, Williams & Wilkins, 2004:2327–34. [Google Scholar]
- 7.Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz 2002; 97:603–12. [DOI] [PubMed] [Google Scholar]
- 8.Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG 2014; 121:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azogue E, Darras C. Prospective study of Chagas disease in newborn children with placental infection caused by Trypanosoma cruzi (Santa Cruz-Bolivia) [in Portugese]. Rev Soc Bras Med Trop 1991; 24:105–9. [DOI] [PubMed] [Google Scholar]
- 10.Basombrio MA, Nasser J, Segura MA et al. . The transmission de Chagas disease in Salta and the detection of congenital cases [in Spanish]. Medicina (B Aires) 1999; 59(suppl 2):143–6. [PubMed] [Google Scholar]
- 11.Schenone H, Contreras MC, Borgono JM et al. . Overview of the epidemiology of Chagas’ disease in Chile [in Spanish]. Bol Chil Parasitol 1991; 46:19–30. [PubMed] [Google Scholar]
- 12.Torrico F, Alonso-Vega C, Suarez E et al. . Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg 2004; 70:201–9. [PubMed] [Google Scholar]
- 13.Bern C, Verastegui M, Gilman RH et al. . Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clin Infect Dis 2009; 49:1667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuels AM, Clark EH, Galdos-Cardenas G et al. . Epidemiology of and impact of insecticide spraying on Chagas disease in communities in the Bolivian Chaco. PLoS Negl Trop Dis 2013; 7:e2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lardeux F, Depickere S, Aliaga C, Chavez T, Zambrana L. Experimental control of Triatoma infestans in poor rural villages of Bolivia through community participation. Trans R Soc Trop Med Hyg 2015; 109:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chippaux JP, Postigo JR, Santalla JA, Schneider D, Brutus L. Epidemiological evaluation of Chagas disease in a rural area of southern Bolivia. Trans R Soc Trop Med Hyg 2008; 102:578–84. [DOI] [PubMed] [Google Scholar]
- 17.Hidron A, Gilman R, Justiniano J et al. . Chagas cardiomyopathy in the context of the chronic disease transition. PLoS Negl Trop Dis 2010; 4:e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet 2010; 375:1388–402. [DOI] [PubMed] [Google Scholar]
- 19.Freilij H, Muller L, Gonzalez Cappa SM. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J Clin Microbiol 1983; 18:327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umezawa ES, Nascimento MS, Kesper N Jr et al. . Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J Clin Microbiol 1996; 34:2143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piron M, Fisa R, Casamitjana N et al. . Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop 2007; 103:195–200. [DOI] [PubMed] [Google Scholar]
- 22.Ministerio de Salud y Deportes. Manual de normas tecnicas y operativas para el tamizaje, diagnóstico y tratamiento de la enfermedad de chagas cronica reciente infantil. 2nd ed La Paz, Bolivia: Ministerio de Salud y Deportes, 2007. [Google Scholar]
- 23.Lazzari JO, Pereira M, Antunes CM et al. . Diagnostic electrocardiography in epidemiological studies of Chagas’ disease: multicenter evaluation of a standardized method. Rev Panam Salud Publica 1998; 4:317–30. [DOI] [PubMed] [Google Scholar]
- 24.Le VV, Wheeler MT, Mandic S et al. . Addition of the electrocardiogram to the preparticipation examination of college athletes. Clin J Sport Med 2010; 20:98–105. [DOI] [PubMed] [Google Scholar]
- 25.Clark EH, Sherbuk J, Okamoto E et al. . Hyperendemic Chagas disease and the unmet need for pacemakers in the Bolivian Chaco. PLoS Negl Trop Dis 2014; 8:e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias JCP. Chagas disease control and the natural history of human Chagas disease: a possible interaction? Mem Inst Oswaldo Cruz 2000; 95(suppl II):14–22. [Google Scholar]
- 27.Dias E. Os efeitos da superinfecção sobre a evolução da cardiopatia crônica chagásica. Rev Goiana Med 1962; 9(suppl):233–9. [Google Scholar]
- 28.Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis 2008; 21:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacal F, Silva CP, Pires PV et al. . Transplantation for Chagas’ disease: an overview of immunosuppression and reactivation in the last two decades. Clin Transplant 2010; 24:E29–34. [DOI] [PubMed] [Google Scholar]
- 30.Bustamante JM, Rivarola HW, Fernandez AR et al. . Trypanosoma cruzi reinfections in mice determine the severity of cardiac damage. Int J Parasitol 2002; 32:889–96. [DOI] [PubMed] [Google Scholar]
- 31.Rendell VR, Gilman RH, Valencia E et al. . Trypanosoma cruzi-infected pregnant women without vector exposure have higher parasitemia levels: implications for congenital transmission risk. PLoS One 2015; 10:e0119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virreira M, Truyens C, Alonso-Vega C et al. . Comparison of Trypanosoma cruzi lineages and levels of parasitic DNA in infected mothers and their newborns. Am J Trop Med Hyg 2007; 77:102–6. [PubMed] [Google Scholar]
- 33.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993; 14:353–6. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez Negrette O, Mora MC, Basombrio MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics 2005; 115:e668–72. [DOI] [PubMed] [Google Scholar]
- 35.Torrico F, Vega CA, Suarez E et al. . Are maternal re-infections with Trypanosoma cruzi associated with higher morbidity and mortality of congenital Chagas disease? Trop Med Int Health 2006; 11:628–35. [DOI] [PubMed] [Google Scholar]
- 36.Carlier Y, Truyens C. Maternal-fetal transmission of Trypanosoma cruzi. In: Telleria J, Tibayrenc M, eds. American trypanosomiasis-Chagas disease: one hundred years of research. New York, NY: Elsevier, 2010:539–81. [Google Scholar]
- 37.Ortiz S, Zulantay I, Solari A et al. . Presence of Trypanosoma cruzi in pregnant women and typing of lineages in congenital cases. Acta Trop 2012; 124:243–6. [DOI] [PubMed] [Google Scholar]
- 38.Cardoni RL, Garcia MM, De Rissio AM. Proinflammatory and anti-inflammatory cytokines in pregnant women chronically infected with Trypanosoma cruzi. Acta Trop 2004; 90:65–72. [DOI] [PubMed] [Google Scholar]
- 39.Hermann E, Truyens C, Alonso-Vega C et al. . Congenital transmission of Trypanosoma cruzi is associated with maternal enhanced parasitemia and decreased production of interferon-gamma in response to parasite antigens. J Infect Dis 2004; 189:1274–81. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki S, Okudaira S. Maternal peripheral T helper 1-type and T helper 2-type immunity in women during the first trimester of twin pregnancy. Arch Gynecol Obstet 2004; 270:260–2. [DOI] [PubMed] [Google Scholar]
- 41.De Bacquer D, De Backer G, Kornitzer M. Prevalences of ECG findings in large population based samples of men and women. Heart 2000; 84:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acquatella H, Catalioti F, Gomez-Mancebo JR, Davalos V, Villalobos L. Long-term control of Chagas disease in Venezuela: effects on serologic findings, electrocardiographic abnormalities, and clinical outcome. Circulation 1987; 76:556–62. [DOI] [PubMed] [Google Scholar]
- 43.Lima-Costa MF, Barreto SM, Guerra HL. Chagas’ disease among older adults: branches or mainstream of the present burden of Trypanosoma cruzi infection? Int J Epidemiol 2002; 31:688–9. [DOI] [PubMed] [Google Scholar]
- 44.Fabbro DL, Danesi E, Olivera V et al. . Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis 2014; 8:e3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murcia L, Carrilero B, Munoz-Davila MJ, Thomas MC, Lopez MC, Segovia M. Risk factors and primary prevention of congenital Chagas disease in a nonendemic country. Clin Infect Dis 2013; 56:496–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.