Abstract
A 78-year-old woman with metastatic low-grade serous ovarian cancer presented with rapidly progressive exertional dyspnoea and hypoxia, and was found to have new-onset severe pulmonary hypertension (PH) by right heart catheterisation. A diagnosis of pulmonary tumour thrombotic microangiopathy (PTTM) was made at autopsy. PTTM is a rare complication of advanced cancer that often presents as rapidly progressive PH or acute hypoxic respiratory failure. Widespread tumour cell emboli in the pulmonary arteries and arterioles are hypothesised to induce fibrocellular subintimal proliferation and microthrombi, leading to increased pulmonary vascular resistance and PH. PTTM arising from serous ovarian cancer is exceedingly rare, with only two previously reported cases. A discussion of the pathophysiology, diagnosis and management of PTTM is presented.
Background
Pulmonary tumour thrombotic microangiopathy (PTTM) is a rare but increasingly recognised complication of advanced cancer that often presents as rapidly progressive pulmonary hypertension (PH) or acute hypoxic respiratory failure. PTTM is marked by an aggressive course, presents a diagnostic challenge and has few therapeutic options. PTTM arising from serous ovarian cancer is exceedingly rare.
Case presentation
A 78-year-old woman with recurrent, stage IV, low-grade serous ovarian carcinoma, presented with 2 weeks of shortness of breath and a rapid decline in exercise tolerance. At the time of her cancer diagnosis in 2006, she underwent a right salpingo-oophorectomy, debulking omentectomy and pelvic lymphadenectomy. She was initially treated with anastrozole for 5 years, and then transitioned to carboplatin and bevacizumab when malignant ascites developed. Her last cycle of chemotherapy had been 3 weeks prior to this presentation. Her history was also significant for atrial fibrillation on anticoagulation, hypertension and prior hysterectomy and left salpingo-oophorectomy.
On presentation, she was afebrile with a blood pressure of 106/68 mm Hg, heart rate of 79 bpm and respiratory rate of 22 breaths/min. Her oxygenation saturation was 98% on room air. Her examination was notable for jugular venous distension, an irregularly irregular heart rhythm with a pronounced P2 heart sound, II/VI holosystolic murmur at the left lower sternal border and a right ventricular heave. Her abdomen was distended with a fluid wave. She notably had clear lungs and no peripheral oedema.
Investigations
A staging CT of the chest, abdomen and pelvis performed 1 week prior to admission showed peritoneal nodularity with moderate ascites, cardiomegaly and no pulmonary embolism.
Initial admission laboratory investigations were significant for serum sodium of 128 mmol/L, serum creatinine 0.9 mg/dL, white cell count 5.7 K/µL, haemoglobin 12.2 g/dL, platelet count 227/mm3, brain natriuretic peptide 335 pg/mL, activated partial thromboplastin time (PTT) 30.0 s and international normalised ratio 1.4. A peripheral smear did not reveal schistocytes.
An ECG on admission showed atrial fibrillation but was otherwise normal. Routine chest X-ray showed increased interstitial lung markings and small right pleural effusion (figure 1).
Figure 1.
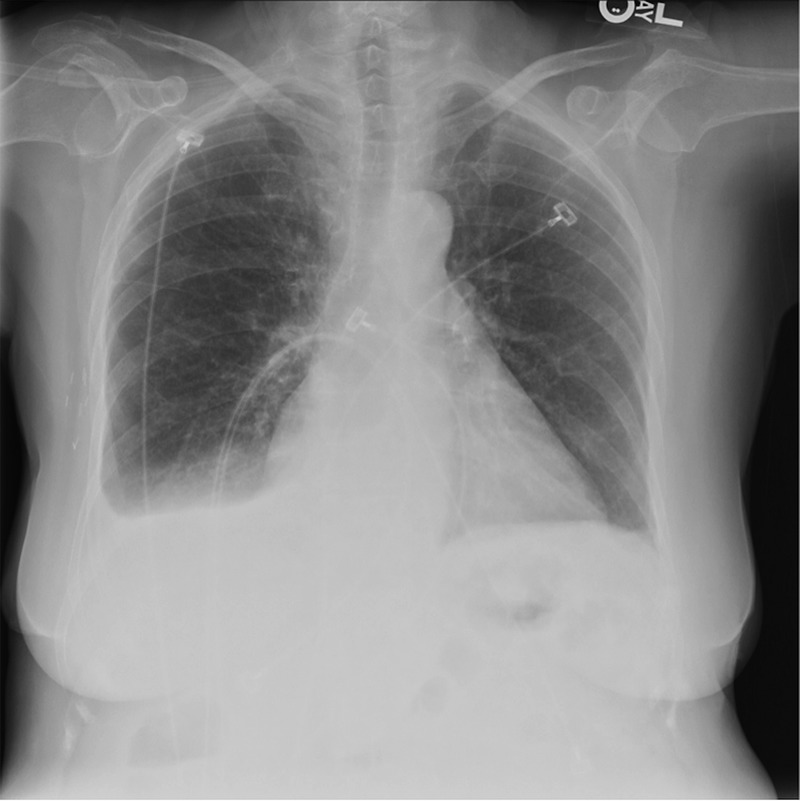
Posteroanterior chest X-ray on admission showing increased interstitial lung markings and a small right pleural effusion.
A transthoracic echocardiogram demonstrated normal left ventricular function (left ventricular ejection fraction 55%), severe biatrial enlargement, moderate mitral regurgitation, new severe PH (estimated pulmonary artery systolic pressure 85 mm Hg), new severe tricuspid regurgitation and newly reduced right ventricular function.
A right heart catheterisation (RHC) performed on admission revealed severe PH, with minimal response to inhaled nitric oxide (iNO; table 1). After clinical decompensation, a repeat RHC on hospital day 24, while the patient was on dobutamine, dopamine, vasopressin and iNO, showed unchanged haemodynamics (table 1).
Table 1.
Right heart catheterisation on admission and hospital day 24
| Admission, baseline | Admission, iNO 40 ppm | Hospital day 24 | |
|---|---|---|---|
| Cuff blood pressure | 109/64 | 107/49 | 108/46 |
| Arterial oxygen saturation (%) | 99 | 99 | 92 |
| Right atrial pressure (mean) | 2 | – | 8 |
| Right ventricular pressure (S/D) | 62/1 | – | |
| Pulmonary arterial pressure (S/D/M) | 64/22/37 | 58/23/37 | 70/20/38 |
| Pulmonary arterial oxygen saturation (%) | 58 | 60 | 49 |
| Pulmonary capillary wedge pressure (mean) | 7 | 8 | 18 |
| Cardiac output/cardiac index (Fick) | 3.1/1.7 | 3.2/1.8 | 3.5/2.0 |
| Pulmonary vascular resistance (wu) | 9.8 | 9.1 | 5.7 |
iNo, inhaled nitric oxide; S/D/M, systolic/diastolic/mean; wu, Wood unit
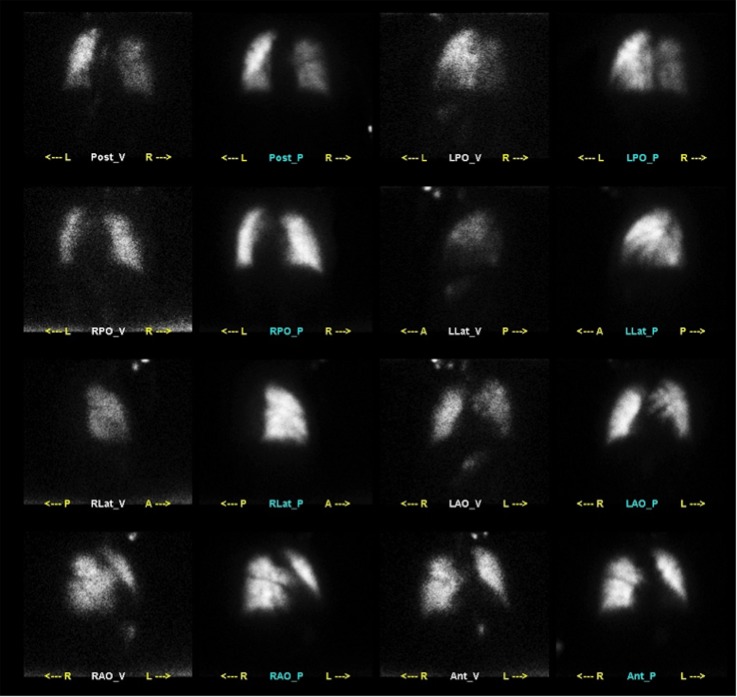
A nuclear ventilation-perfusion scan was interpreted as intermediate probability of pulmonary embolism with possible subsegmental thromboembolic disease (figure 2).
Figure 2.
Ventilation-perfusion scintigraphy obtained at day 4 of admission showing peripheral small-to-moderate perfusion-ventilation mismatched defects, interpreted as intermediate probability for pulmonary emboli with possible subsegmental thromboembolic disease.
Chest CT scan was performed on hospital day 12 and hospital day 22, and showed progressive bilateral tree-in-bud pattern with patchy ground-glass opacities, with associated bilateral pleural effusions (figure 3A, B).
Figure 3.
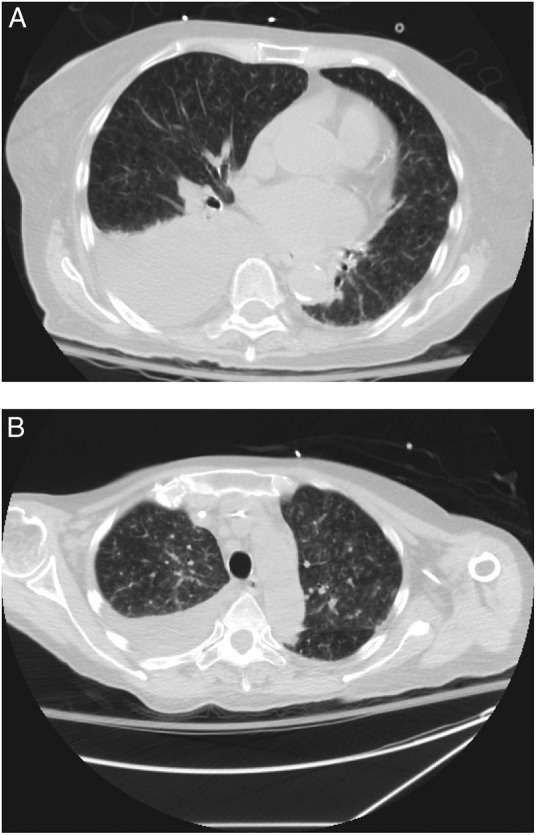
(A) Axial image from CT scan obtained at day 12 of admission. CT scan demonstrating tree-in-bud pattern and patchy ground-glass opacities throughout both lungs, as well as a moderate right pleural effusion. (B) Axial images from CT scan on day 22 of admission. Follow-up CT scan demonstrating marked increase in tree-in-bud pattern and ground-glass opacities bilaterally, and stable right pleural effusion.
Differential diagnosis
In view of new-onset PH, the diagnoses of idiopathic PH, chronic thromboembolic PH (CTEPH), acute pulmonary embolism and PTTM were considered. Lymphangitic carcinomatosis was also considered. On the basis of the RHC haemodynamics, WHO Group II PH was thought to be less likely.
Treatment
The patient was initially treated with oral sildenafil and ambrisentan. During the second week of her hospitalisation, she developed acute hypoxic respiratory failure, hypotension and acute renal failure. Treatment with dobutamine, dopamine, vasopressin and iNO was initiated. A right-sided diagnostic and therapeutic thoracentesis was performed on hospital day 23. Fluid analysis was consistent with a malignant pleural effusion. After a second decompensation, intravenous treprostinil was started.
Outcome and follow-up
After minimal improvement was noted with uptitration of intravenous treprostinil, the patient expressed wishes to be transitioned to comfort measures; she died on hospital day 29. Consent for autopsy was obtained. The principal findings were metastatic ovarian serous carcinoma with peritoneal tumour implants, and pleural tumour implants with bilateral pleural effusions. Notably, there was pulmonary lymphatic involvement by tumour and widely disseminated tumour emboli in the pulmonary arterial tree, with associated subintimal and luminal fibrocellular proliferation, and acute fibrin thrombi (figure 4A, B). The cause of death was attributed to PH secondary to metastatic ovarian serous carcinoma with pulmonary arterial tumour emboli.
Figure 4.
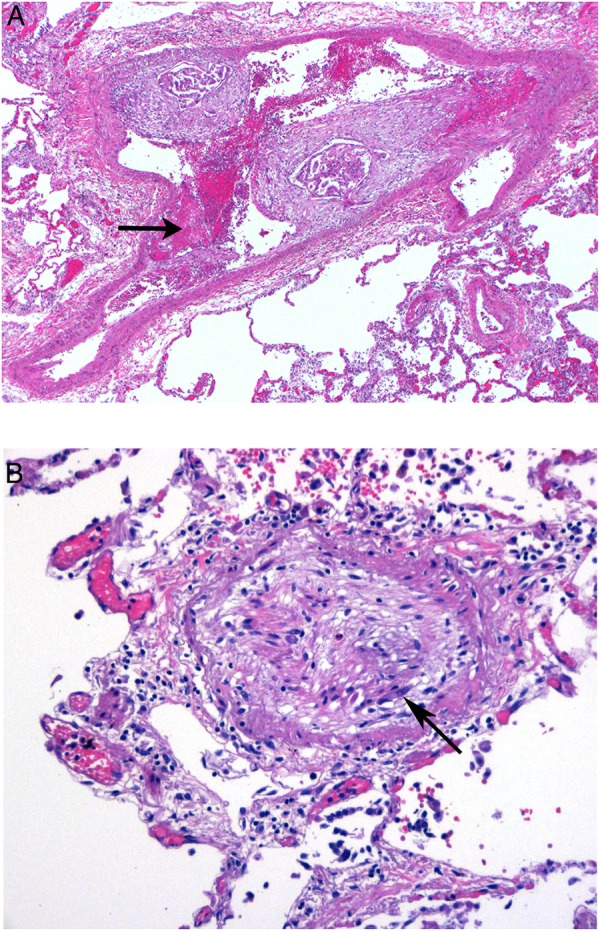
(A) Carcinoma in pulmonary artery associated with fibrointimal proliferation and acute luminal thrombus (arrow), magnification ×4, H&E stain. (B) Intimal proliferation and fibrous obliteration of a pulmonary arteriole with residual tumour cells (arrow), magnification ×20, H&E stain.
Discussion
PTTM is a rare clinicopathological entity seen in patients with metastatic cancer; it is characterised by widespread tumour cell emboli in the small arteries and arterioles of the lung, often associated with thrombus formation.1 2 The associated clinical syndrome is often one of rapidly progressive shortness of breath and hypoxia, secondary to pulmonary hypertension, as was seen in this patient. PTTM can rarely present as an acute thrombotic microangiopathy (TMA).3
PTTM has been most frequently described in patients with poorly differentiated signet-ring gastric cancer, with a postmortem incidence of 16–26%, although often asymptomatic.1 4 To a lesser degree, adenocarcinomas of the oesophagus, prostate, bladder, lung, breast, colon and gallbladder have been associated with PTTM.5 6 PTTM arising from a serous ovarian cancer is exceedingly rare with only two cases previously reported, both presenting as acute respiratory failure.7 It is thought that tumour cell adherence to the vascular endothelium in PTTM induces fibrocellular subintimal proliferation, and deposition of platelet and fibrin microthrombi, leading to increased pulmonary vascular resistance and progressive PH.4 8 9 The role of cancer cell expression of angiogenic factors, such as platelet-derived growth factor A (PDGF-A) and vascular endothelial growth factor, in inducing intimal proliferation, is increasingly being recognised in the pathogenesis of PTTM.10
PTTM poses a difficult diagnostic problem. Lung biopsy is optimal for diagnosis, but rarely an option given illness severity.5 A high-resolution chest CT scan with an ‘ultrafine granular appearance’, and a ventilation-perfusion scan with a ‘mottled appearance’, can be helpful, but are insensitive.11 12 Pulmonary angiography is useful only in ruling out CTEPH and acute pulmonary embolism.13 Cytological analysis on pulmonary microvascular samples drawn through a wedged PA catheter has been reported to have a sensitivity of 80–88% and specificity of 82–94% for the diagnosis of lymphangitic carcinomatosis, and may have a role in the diagnosis of PTTM.5 14 The role of fluorodeoxyglucose (FDG)-positron emission tomography/CT is still undefined, but it has the potential to differentiate CTEPH from PTTM given FDG avidity only in the latter.15 Laboratory investigations are often non-specific, and may reveal elevated lactate dehydrogenase in over half of patients, as well as schistocytes and low-grade disseminated intravascular coagulation (DIC), which was not suggested in this case.16 Although such presentation is not specific for PTTM, it may prompt clinicians to search for metastatic disease. Fulminant TMA with significant microangiopathic haemolysis, thrombocytopenia, advanced DIC and acute renal failure, is seen in a much smaller minority of cases.3 16
Unfortunately, PTTM is marked by an aggressive course with few therapeutic options. Advanced treatments for primary PH, such as endothelin receptor antagonists, iNO and prostacyclin analogues, can acutely reduce pulmonary pressures, but lack long-term efficacy.5 Anticoagulation has not been shown to change prognosis.6 Early administration of systemic chemotherapy has the potential to reduce the burden of malignant cells in the pulmonary circulation, but such cells constitute only a small component of increased pulmonary vascular resistance in PTTM.11 Recent evidence suggests the PDGF-receptor inhibitor, imatinib, may be effective in select cases of PTTM with tumour overexpression of PDGF, as in some gastric malignancies.17 18 The potential benefits of imatinib must be weighed against the increased risk of bleeding when this agent is combined with coumadin. Further investigation into effective therapies for PTTM is certainly needed.
Patient's perspective.
Prior to her illness, my mother enjoyed an active life—walking several days a week, and tending to her yard and gardens. She loved raising dogs; she loved us very much. When she was first referred to Sloan Kettering towards the end of her illness, her local doctor told her how rare her case was to him. He said it was an enigma. The autopsy amplified this, but of course did not comfort us in any way. She was a strong willed woman who was liked and respected by everyone she met. Her sickness and death has been so hard for us to accept.
Learning points.
Pulmonary tumour thrombotic microangiopathy is a rare clinicopathological entity seen in patients with metastatic cancer.
Clinical presentation is often one of rapidly progressive pulmonary hypertension (PH) or acute hypoxic respiratory failure.
PH is thought to arise from both direct tumour cell adherence to pulmonary arterioles, as well as angiogenic factor-induced fibrointimal proliferation.
High-resolution chest CT scan, ventilation-perfusion imaging, fluorodeoxyglucose-positron emission tomography/CT, pulmonary capillary wedge specimen sampling and lung biopsy may be helpful in diagnosis, however, most cases are diagnosed antemortem.
Treatment options are limited, but may include pulmonary vasodilators, systemic chemotherapy and tyrosine-kinase inhibitors.
Acknowledgments
The authors would like to acknowledge David J Pisapia, Irina Sobol, David M Hyman, Meredith L Turetz and Jana Ivanidze, for contributions in the care of this patient and the manuscript production.
Footnotes
Contributors: SLP was involved in design of the manuscript, literature review, obtaining consent and drafting the article. NN, EMH and MGK were involved in care of the patient, critical revision of the article and final approval of the version to be published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.von Herbay A, Illes A, Waldherr R et al. . Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990;66:587–92. [DOI] [PubMed] [Google Scholar]
- 2.Chinen K, Fujino T, Horita A et al. . Pulmonary tumor thrombotic microangiopathy caused by an ovarian cancer expressing tissue factor and vascular endothelial growth factor. Pathol Res Pract 2010;206:682–9. 10.1016/j.prp.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Gainza E, Fernandez S, Martinez D et al. . Pulmonary tumor thrombotic microangiopathy: report of 3 cases and review of the literature. Medicine (Baltimore) 2014;93:359–63. 10.1097/MD.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinen K, Tokuda Y, Fujiwara M et al. . Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: an analysis of 6 autopsy cases and review of the literature. Pathol Res Pract 2009;205:63–8. 10.1016/j.prp.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Keenan NG, Nicholson AG, Oldershaw PJ. Fatal acute pulmonary hypertension caused by pulmonary tumour thrombotic microangiopathy. Int J Cardiol 2008;124:e11–13. 10.1016/j.ijcard.2006.11.162 [DOI] [PubMed] [Google Scholar]
- 6.Kitamura A, Nishimura A, Jinta T et al. . A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep Pulmonol 2013;2013:259080 10.1155/2013/259080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gru AA, Pai RK, Roma AA. Pulmonary tumor thrombotic microangiopathy in patients with low-grade ovarian serous neoplasm: a clinicopathologic review of 2 cases of a previously unknown association. Int J Gynecol Pathol 2012;31:438–42. 10.1097/PGP.0b013e318249287d [DOI] [PubMed] [Google Scholar]
- 8.Pinkcard JK, Wick MR. Tumor-related thrombotic pulmonary microangiopathy: review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol 2000;4:154–7. 10.1016/S1092-9134(00)90038-8 [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Marutsuka K, Asada Y et al. . Pulmonary tumor thrombotic microangiopathy. Pathol Int 1995:45:436–40. 10.1111/j.1440-1827.1995.tb03481.x [DOI] [PubMed] [Google Scholar]
- 10.Abe H, Hino R, Fukayama M. Platelet-derived growth factor-A and vascular endothelial growth factor-C contribute to the development of pulmonary tumor thrombotic microangiopathy in gastric cancer. Virchows Arch 2013;462:523–31. 10.1007/s00428-013-1403-7 [DOI] [PubMed] [Google Scholar]
- 11.Kayatani H, Matsuo K, Ueda Y et al. . Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern Med 2012;51:2767–70. 10.2169/internalmedicine.51.7682 [DOI] [PubMed] [Google Scholar]
- 12.Bourdreau RJ, Lisbona R, Sheldon H. Ventilation-perfusion mismatch in tumor embolism. Clin Nucl Med 1982;7:320–2. 10.1097/00003072-198207000-00005 [DOI] [PubMed] [Google Scholar]
- 13.Schriner RW, Ryo JH, Edwards WD. Microscopic pulmonary tumor embolism causing subacute cor pulmonale: a difficult antemortem diagnosis. Mayo Clin Proc 1991;66:143–8. 10.1016/S0025-6196(12)60485-6 [DOI] [PubMed] [Google Scholar]
- 14.Abati A, Landucci D, Danner RL et al. . Diagnosis of pulmonary microvascular metstases by cytologic evaluation of pulmonary artery catheter-derived blood specimens. Hum Pathol 1994;25:257–62. 10.1016/0046-8177(94)90197-X [DOI] [PubMed] [Google Scholar]
- 15.Tashima Y, Abe K, Matsuo Y et al. . Pulmonary tumor thrombotic microangiopathy: FDG-PET/CT findings. Clin Nucl Med 2009;34:175–7. 10.1097/RLU.0b013e3181966f5c [DOI] [PubMed] [Google Scholar]
- 16.Goldhaber SZ, Dricker E, Buring JE et al. . Clinical suspicion of autopsy-proven thrombotic and tumor pulmonary embolism in cancer patients. Am Heart J 1987;114:1432–5. 10.1016/0002-8703(87)90548-5 [DOI] [PubMed] [Google Scholar]
- 17.Minatsuki S, Miura I, Yao A et al. . Platelet-derived growth factor receptor-tyrosine kinase inhibitor, Imatinib, is effective for treating pulmonary hypertension induced by pulmonary tumor thrombotic microangiopathy. Int Jeart J 2015;56:245–8. 10.1536/ihj.14-220 [DOI] [PubMed] [Google Scholar]
- 18.Ogawa A, Yamadori I, Matsubara O et al. . Pulmonary tumor thrombotic microangiopathy with circulatory failure treated with imatinib. Intern Med 2013;52:1927–30. 10.2169/internalmedicine.52.0718 [DOI] [PubMed] [Google Scholar]