Abstract
Background and Aims:
Traditional clinical methods like auscultation or inspection have been found to be inaccurate in confirmation of double-lumen tube (DLT) position. Lung ultrasonography (USG) reliably identifies the tidal movement (lung sliding) and the collapse of the lung (lung pulse). We intended to check whether the accuracy of clinical methods can be improved by the addition of USG in confirmation of left DLT (LDLT) position.
Methods:
A single centred, prospective, comparative study was conducted involving 70 patients undergoing thoracic surgeries requiring the use of LDLT. The patients were assigned to Group A - where LDLT position was assessed by using clinical methods alone, and Group B - where LDLT position was assessed by USG and clinical methods. The correct position was predicted when USG demonstrated the absence of lung sliding and the presence of lung pulse on the operative side, the presence of lung sliding on non-operative side, along with normal airway pressures and oxygenation. The final verification of LDLT position was done by direct observation of lung isolation by one surgeon who was blinded to the method of confirmation. Contingency tables were drawn to calculate sensitivity, specificity, positive predictive value, negative predictive value and accuracy of each method.
Results:
Compared to clinical methods alone, addition of lung USG improved sensitivity (75% vs. 88%), specificity (18% vs. 75%) and accuracy (57% vs. 85%) for correct prediction of LDLT position.
Conclusion:
USG is a useful addition to the armamentarium of anaesthesiologist for the confirmation of LDLT position.
Keywords: Double lumen tube, lung sliding, ultrasonography
INTRODUCTION
Double-lumen tubes (DLTs) are frequently used to establish one lung ventilation (OLV). Correct placement of a DLT is evaluated by means of clinical methods which include inspection of chest wall movements and auscultation of breath sounds, and instrumental methods like fibre optic bronchoscope (FOB), before starting surgery. However, clinical evaluation does not always recognise malposition of DLT. In fact, if placement of the DLT is checked only by clinical methods, intra-operative problems with either deflation of the non-dependent lung or ventilation of the dependent lung have been observed in up to 25% of cases.[1] Smith et al. using FOB, revealed that 48% of blindly placed DLTs were malpositioned.[2] Hence, it is recommended that the position of DLT should be confirmed using FOB.[3] However, one must also remember that FOB confirms location of DLT, and hence, anatomical separation of lungs, but actual deflation of lung on desired side needs to be assessed using methods like underwater seal technique for air leak, auscultation or lung ultrasonography (USG).
Lung USG has been recently added as a tool to confirm the position of the endotracheal tube.[4] It is a non-invasive, bedside technique that identifies tidal movement of the lung, as well as the collapse of the lung. Hence, adequate lung isolation can be ensured if lung collapse is seen on one side and adequate ventilation is confirmed on other side. The purpose of this study was to check whether the accuracy of clinical methods can be improved by the addition of USG to confirm left DLT (LDLT) position.
METHODS
This was a single centred, prospective and comparative study. After receiving Institutional Review and Ethics Board approval, 70 adult (age >18 years) patients, with American Society of Anesthesiologists physical status I or II, posted for elective thoracic surgery requiring OLV with LDLT were enrolled, during May 2011–August 2012, following their informed consent. The patients were assigned alternately to the clinical assessment group (Group A: 35 patients) and to the clinical and ultrasound assessment group (Group B: 35 patients). Patients with anticipated difficult intubation or with tracheostomy tube were excluded. Furthermore, patients with pneumothorax, pleural effusion, empyema or past history of pleurodesis or with deranged pulmonary function tests (anyone out of forced expiratory volume in 1 s, total lung capacity, forced vital capacity <50% of the predicted values) were excluded.
All patients were intubated by an experienced anaesthesiologist in operating room (OR) with standard polyvinyl chloride (35F/37F/39F) LDLTs (Smiths Medical International Ltd., UK) after routine anaesthesia induction and muscle relaxation. Electrocardiogram, blood pressure and oxygen saturation were monitored during induction. Intra-tracheal placement of DLT was confirmed by end-tidal carbon dioxide (ETCO2) monitor. During bilateral lung ventilation (BLV), all patients received a tidal volume of 8 ml/kg with oxygen air mixture (50:50) and isoflurane. During OLV, all patients were ventilated with tidal volume of 6 ml/kg with oxygen air mixture (50:50) and isoflurane with positive end-expiratory pressure (PEEP) of 5 cm H2O to ventilated lung. Respiratory rate was adjusted to maintain ETCO2 between 30 and 35 mm Hg.
The prediction of DLT position was based on three parameters-lung isolation, airway pressure and oxygenation status. We made an assumption that if lung isolation is adequate along with normal airway pressures and oxygenation, then the position of DLT would be correct. Normal airway pressure was defined as peak airway pressure <35 cm H2O with above-mentioned ventilator settings during OLV. Adequate oxygenation was defined as no need for intervention like continuous positive airway pressure (CPAP) or increments in fraction of inspired oxygen (FiO2) more than 0.5 or PEEP more than 5 cm H2O, to maintain oxygen saturation more than 93%. Three steps were carried out for assessment of lung isolation. In the first step, the tracheal cuff was inflated to seal leak at glottis, and both lungs were ventilated. In the second step, the bronchial cuff was inflated to seal air entry on the right side when the left lung was ventilated through the bronchial lumen. In the third step, ventilation of both lungs was confirmed again. The initial assessment was performed with the patient in supine position, immediately after intubation. Clinical assessment was performed by OR consultant anaesthesiologist and findings were noted in case record form (CRF). For Group B, same three steps were repeated. In addition, in Group B, lung USG was performed by principal investigator, who was blinded for the results of clinical assessment, using SonoSite™ (Fujifilm SonoSite Inc.) scanner with linear transducer (5–10 MHz) held between 2nd and 4th ribs in anterior axillary line, bilaterally. In case of prediction of tube malposition, the tube was re-adjusted to the satisfaction of OR anaesthesiologist. Re-assessments were performed after changing position of the tube or position of the patient (supine to lateral), in both groups and final findings noted in CRF were considered for analysis. In Group B, in case of any discrepancy between findings of clinical assessment and USG, sonography findings about lung isolation were considered as final.
In Group A, correct position of LDLT was predicted when, during OLV, clinical methods revealed correct lung isolation (no air entry or chest inflation on the side of surgery, and adequate air entry and chest expansion on opposite side) and normal airway pressure and oxygenation. Similarly, in Group B, correct position of LDLT was predicted when, during OLV, lung USG revealed correct lung isolation (absence of ‘lung sliding’ and presence of ‘lung pulse’ seen on the side of surgery and ‘lung sliding’ sign seen on opposite side) and normal airway pressure and oxygenation. The absence of any of above-mentioned parameters was predicted the incorrect position of LDLT. Intra-operatively, lung isolation was confirmed by direct observation of lung collapse on operative side by a thoracic surgeon, who was not present in OR at the time of assessment of DLT. Correct position of LDLT was confirmed if there is correct lung isolation (lung to be operated/on the side of surgery-deflated and other lung-ventilated) as observed by operating surgeon, and normal airway pressure and oxygenation. A clinical situation that deviates from any of above-mentioned parameters confirmed the incorrect position of LDLT.
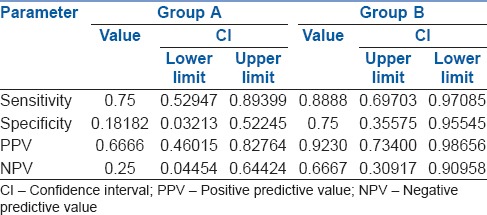
Prior data indicate that the average accuracy rate of clinical methods to determine correct DLT position is 0.60.[5,6,7,8] If the true accuracy for experimental subjects is 0.9, we needed 35 experimental and 35 control subjects to reject the null hypothesis that failure rates for experimental and control subjects are equal with probability (power) 0.8 with type I error being 0.05. Analysis of contingency table [Table 1] was done to calculate sensitivity, specificity, positive predictive value (PPV), negative predictive value and accuracy, for each group. Improvement in the parameter in Group B was considered as significant if the value lies beyond upper limit of the confidence interval for the same parameter in Group A. Receiver operating characteristic (ROC) curve was plotted to find the area under curve for each group.
Table 1.
Contingency table

The primary endpoint of this study was to assess the accuracy rate of clinical methods and USG in confirmation of the position of LDLT upon an intra-operative assessment of lung isolation, airway pressures and oxygenation parameters. Secondary endpoints in this study were the incidence of intra-operative complications associated with lung isolation and need for conversion of OLV to BLV intra-operatively.
RESULTS
Seventy adult patients undergoing elective thoracic surgeries were included in the study. Forty-five patients (Group A: 20, Group B: 25) underwent thoracotomy whereas 25 patients (Group A: 15, Group B: 10) underwent video-assisted thoracoscopic surgeries. The patients in both the groups did not differ with regard to age, sex and height [Table 2]. The size of LDLT was selected by OR consultant anaesthesiologist, according to the clinical judgement (35 F: 18 patients, 37 F: 38 patients and 39 F: 14 patients).
Table 2.
Patient characteristics

In Group A, sensitivity and specificity of clinical methods in confirmation of LDLT were 75% and 18%, respectively. In Group B, sensitivity and specificity of the combination of clinical methods and lung USG were 88% and 75%, respectively, [Tables 3 and 4]. In Group A, final confirmation identified the incorrect position of LDLT in 11 patients (6: Poor lung isolation, 2: Raised airway pressures and 3: Both poor lung isolation and raised airway pressure). In Group B, LDLT was in incorrect position in 8 patients (2: Poor lung isolation, 2: Raised airway pressure and 4: Both). Patients with raised airway pressures during OLV required LDLT adjustments intra-operatively. Among patients who had poor lung isolation, 8 patients had non-deflation of the lung, whereas seven patients had ventilation of lung on the operative side. All these patients required intraoperative interventions like withdrawal or deeper insertion of LDLT, repeated suctioning, manual deflation of lung by a surgeon or use of low tidal volumes. Oxygenation parameters were within normal limits in all patients. None of the patients required the application of CPAP or increments in FiO2 >0.5 or PEEP >5 cm H2O. None of the patients required resumption of BLV due to unstable parameters.
Table 3.
Contingency table for group A and B
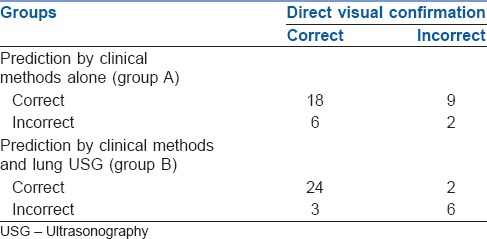
Table 4.
Analysis of Group A and B
In Group B, findings of clinical methods and USG were correlating in 28 patients whereas they differed in 7 patients. Subgroup analysis of these 7 patients showed that USG findings had 100% correlation with findings of direct visual confirmation. Overall, the accuracy of clinical methods in confirmation of LDLT position was 57% whereas addition of lung USG increased it to 85%. The analysis of ROC curves showed that area under the curve for Group A is 0.53 whereas that for Group B is 0.82. It indicates that addition of lung USG to clinical methods improved the accuracy for confirmation of DLT [Figures 1 and 2].
Figure 1.
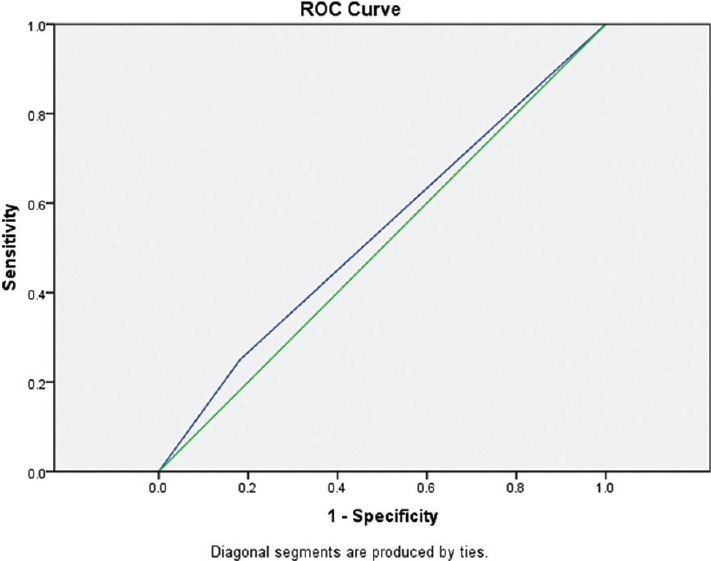
Receiver operating characteristic curve for Group A
Figure 2.
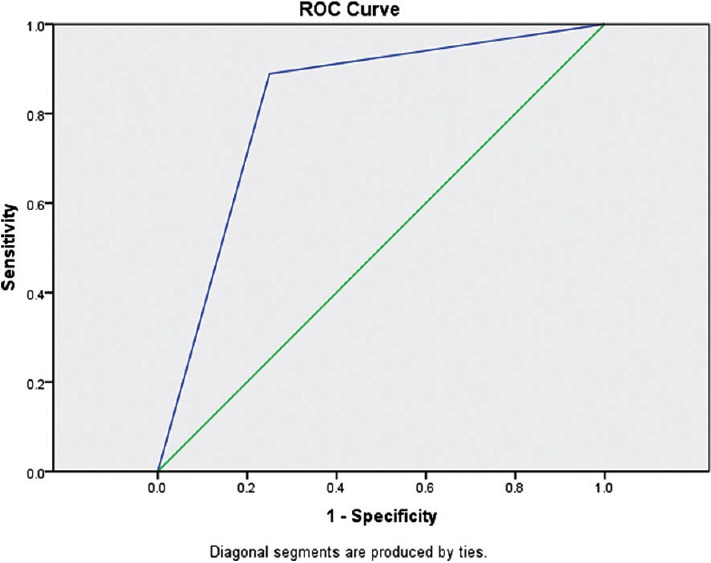
Receiver operating characteristic curve for Group B
DISCUSSION
Reliable and rapid identification of the correct distribution of ventilation is fundamental in surgeries involving OLV. Traditional clinical methods such as inspection of chest wall movements and auscultation of breath sounds have been less accurate.[5,6,7,8] Our results are in congruence with these findings. Auscultation is often subjective and depends upon tidal volume, consistency of underlying lung tissue, the thickness of chest wall, the sensitivity of stethoscope and hearing acuity of the individual. Auscultation of one side of the chest is also confounded by the conductance of sound from other side of chest. Hence, alternative techniques have been proposed to verify correct position of DLT like chest X-ray,[9] selective ETCO2 monitoring,[10,11] analysis of pressure-volume loops,[12] intraoperative palpation of the DLT by surgeon[13] and acoustic monitoring.[14] However, FOB remains the method of choice.[3] Nevertheless, FOB has got some limitations. Besides availability and cost of maintenance, paediatric FOB confirms tube position and adequacy of bronchial cuff inflation and thus, anatomical separation of the lung. However, it does not show that the lung on the non-ventilated side is deflated. This confirmation of lung deflation on non-ventilated side or ‘functional isolation’ can be done by lung USG. An audit of DLT intubation by Seymour et al., showed that use of FOB did not alter the incidence of intra-operative problems like desaturation or collapse of upper lobe.[7] In our study, we did not use FOB as it was not available at our centre during the study interval.
In recent times, lung USG has been identified as a useful tool for the confirmation of the correct position of the endotracheal tube in critical care settings.[4,15,16] With the inter-costal approach, an interface between soft tissues of the chest wall and aerated lung is seen as a hyperechoic line called as ‘pleural line’. In ventilated lung, there is to-and-fro movement at the pleural line which corresponds with tidal movement of the lung (’lung sliding sign’). In the non-ventilated lung, there is the absence of lung sliding whereas in collapsed lung, pleural line moves with heart beats in a pulsatile manner (’lung pulse sign’). ‘Lung pulse’ is 93% sensitive and 100% specific for identification of lung collapse.[17] Thus, if USG demonstrates ‘lung sliding’ on one side and ‘lung pulse’ on other side, then adequate ‘functional lung isolation’ can be predicted. However, adequate lung isolation does not rule out the advancement of the bronchial lumen beyond secondary carina. Such advancement would result in increased airway pressures or poor oxygenation or both. Hence, in addition to adequate functional lung isolation, if airway pressure and oxygenation status (SpO2) of the patient are within normal limits during OLV, then one can safely comment that the DLT position is satisfactory. Brodsky and Lemmens described the position of DLT as ‘satisfactory’ if DLT is inappropriate bronchus, with effective and safe isolation and no de-oxygenation due to malposition.[18,19] Lung USG helps to identify functional lung isolation in a better way than auscultation does. Few other studies further support this finding. Šustić et al. evaluated the role of a brief ultrasound examination in detecting correct position of LDLT in a prospective, randomised study and found that addition of lung USG to clinical methods amplified the specificity, accuracy and PPV for detection of correct LDLT position.[20] Álvarez-Dνaz et al. (2015) compared transthoracic lung ultrasound and clinical methods to confirm the position of DLT in 105 patients and concluded that lung ultrasound was superior to clinical methods in confirming the adequate position of DLT.[21] Our study had similar results as well. Saporito et al. found that thoracic ultrasound done by a trained nurse anaesthetist can be as specific and sensitive as FOB in confirming DLT position. In addition, lung USG was more rapid and cost-effective than FOB.[22] Thus, transthoracic lung ultrasound is a quick, non-invasive method which is superior to clinical methods for confirmation of the position of DLT. Hence, lung USG is a better complimentary method to FOB than clinical methods. However, it may not be an alternative to FOB as there are some distinct advantages of FOB over lung ultrasound. For example, FOB can guide placement of right DLT to align lumen against right upper lobe orifice. Advancement of DLT beyond secondary carina is easily diagnosed by FOB. Furthermore, intra-operative adjustment of DLT is best done with FOB.
Finally, it's worth noting disadvantages of lung USG. Besides availability and cost of the machine, USG imaging is always subjective and the possibility of inter-individual variation exists. This holds true for anaesthesiologist who may not be an expert at performing lung USG. The possibility of DLT advancing beyond secondary carina or lobar atelectasis cannot be ruled out by USG. Lung USG may not be reliable in cases where air tight seal is mandatory, like in bronchoalveolar lavage.
There are few limitations to this study. First, patients were not randomised in two groups but were included on an alternate basis. Second, lung USG does not detect selective lobar atelectasis. Hence, patients with right-sided DLT or with bronchial blocker were not included in this study. Clinical methods alone need to be compared with USG methods alone. Furthermore, final confirmation of LDLT position could not be done with FOB.
CONCLUSION
This study reconfirms that clinical methods alone have low accuracy for confirmation of DLT position. The addition of lung USG to clinical methods significantly improves the accuracy for the confirmation of functional lung isolation and is a useful method in the case of unavailability of FOB. Thus, transthoracic lung ultrasound is a valuable addition to the armamentarium of anaesthesiologist for the confirmation of LDLT position.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wilson WC, Benumof JL. Anesthesia for thoracic surgery. In: Miller RD, Fleisher LA, Savarese JJ, Wiener-Kronish JP, Young WL, editors. Miller's Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 1847–939. [Google Scholar]
- 2.Smith GB, Hirsch NP, Ehrenwerth J. Placement of double-lumen endobronchial tubes. Correlation between clinical impressions and bronchoscopic findings. Br J Anaesth. 1986;58:1317–20. doi: 10.1093/bja/58.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Cohen E. Double-lumen tube position should be confirmed by fiberoptic bronchoscopy. Curr Opin Anaesthesiol. 2004;17:1–6. doi: 10.1097/00001503-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chun R, Kirkpatrick AW, Sirois M, Sargasyn AE, Melton S, Hamilton DR, et al. Where's the tube? Evaluation of hand-held ultrasound in confirming endotracheal tube placement. Prehosp Disaster Med. 2004;19:366–9. doi: 10.1017/s1049023x00002004. [DOI] [PubMed] [Google Scholar]
- 5.Klein U, Karzai W, Bloos F, Wohlfarth M, Gottschall R, Fritz H, et al. Role of fiberoptic bronchoscopy in conjunction with the use of double-lumen tubes for thoracic anesthesia: A prospective study. Anesthesiology. 1998;88:346–50. doi: 10.1097/00000542-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Alliaume B, Coddens J, Deloof T. Reliability of auscultation in positioning of double-lumen endobronchial tubes. Can J Anaesth. 1992;39:687–90. doi: 10.1007/BF03008231. [DOI] [PubMed] [Google Scholar]
- 7.Seymour AH, Prasad B, McKenzie RJ. Audit of double-lumen endobronchial intubation. Br J Anaesth. 2004;93:525–7. doi: 10.1093/bja/aeh229. [DOI] [PubMed] [Google Scholar]
- 8.Moloney JT, Fowler SJ, Chang W. Anesthetic management of thoracic trauma. Curr Opin Anaesthesiol. 2008;21:41–6. doi: 10.1097/ACO.0b013e3282f2aadc. [DOI] [PubMed] [Google Scholar]
- 9.Merli G, Guarino A, Della Rocca G, Frova G, Petrini F, Sorbello M, et al. Recommendations for airway control and difficult airway management in thoracic anesthesia and lung separation procedures. Minerva Anestesiol. 2009;75:59. [PubMed] [Google Scholar]
- 10.Shafieha MJ, Sit J, Kartha R, Sabnis L, Hajianpour B, Pappas AL, et al. End-tidal CO2 analyzers in proper positioning of the double-lumen tubes. Anesthesiology. 1986;64:844–5. doi: 10.1097/00000542-198606000-00052. [DOI] [PubMed] [Google Scholar]
- 11.Shankar KB, Moseley HS, Kumar AY. Dual end-tidal CO2 monitoring and double-lumen tubes. Can J Anaesth. 1992;39:100. doi: 10.1007/BF03008694. [DOI] [PubMed] [Google Scholar]
- 12.Simon BA, Hurford WE, Alfille PH, Haspel K, Behringer EC. An aid in the diagnosis of malpositioned double-lumen tubes. Anesthesiology. 1992;76:862–3. doi: 10.1097/00000542-199205000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Cohen E, Kirschner PA, Goldofsky S. Intraoperative manipulation for positioning of double-lumen tubes. Anesthesiology. 1988;68:170. doi: 10.1097/00000542-198801000-00040. [DOI] [PubMed] [Google Scholar]
- 14.Tejman-Yarden S, Lederman D, Eilig I, Zlotnik A, Weksler N, Cohen A, et al. Acoustic monitoring of double-lumen ventilated lungs for the detection of selective unilateral lung ventilation. Anesth Analg. 2006;103:1489–93. doi: 10.1213/01.ane.0000240909.48774.49. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh KS, Lee CL, Lin CC, Huang TC, Weng KP, Lu WH. Secondary confirmation of endotracheal tube position by ultrasound image. Crit Care Med. 2004;32(9 Suppl):S374–7. doi: 10.1097/01.ccm.0000134354.20449.b2. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108:1345–8. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein DA, Lascols N, Prin S, Mezière G. The “lung pulse”: An early ultrasound sign of complete atelectasis. Intensive Care Med. 2003;29:2187–92. doi: 10.1007/s00134-003-1930-9. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky JB, Lemmens HJ. Left double-lumen tubes: Clinical experience with 1,170 patients. J Cardiothorac Vasc Anesth. 2003;17:289–98. doi: 10.1016/s1053-0770(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky JB. Fiberoptic bronchoscopy need not be a routine part of double-lumen tube placement. Curr Opin Anaesthesiol. 2004;17:7–11. doi: 10.1097/00001503-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Sustic A, Protic A, Cicvaric T, Zupan Z. The addition of a brief ultrasound examination to clinical assessment increases the ability to confirm placement of double-lumen endotracheal tubes. J Clin Anesth. 2010;22:246–9. doi: 10.1016/j.jclinane.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Álvarez-Díaz N, Amador-García I, Fuentes-Hernández M, Dorta-Guerra R. Comparison between transthoracic lung ultrasound and a clinical method in confirming the position of double-lumen tube in thoracic anaesthesia. A pilot study. Rev Esp Anestesiol Reanim. 2015;62:305–12. doi: 10.1016/j.redar.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Saporito A, Lo Piccolo A, Franceschini D, Tomasetti R, Anselmi L. Thoracic ultrasound confirmation of correct lung exclusion before one-lung ventilation during thoracic surgery. J Ultrasound. 2013;16:195–9. doi: 10.1007/s40477-013-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


