INTRODUCTION
Pre-operative anxiety has been described as a subjective feeling of apprehension, fear, and nervousness.[1] Many pharmacological and non-pharmacological methods have been used to allay anxiety in the pre-operative period. Anticonvulsants such as gabapentin and pregabalin are used effectively to attenuate pre-operative anxiety. Levetiracetam that is a novel antiepileptic drug has also shown anxiolysis property in various studies.[2,3] To date, no clinical study has evaluated the efficacy of levetiracetam in reducing pre-operative anxiety. We conducted this study to assess the effects of levetiracetam on attenuating pre-operative anxiety.
METHODS
This prospective, randomized, double-blind, and placebo-controlled study was carried out after taking approval by Local Ethical Committee and written informed consent from the patients. The study protocol was registered prospectively at Clinical Trial Registry of India (CTRI/2014/01/004323). A total 102 patients, aged 18–70 years, of American Society of Anesthesiologists physical status I and II, who were scheduled to undergo laparoscopic cholecystectomy, were included in the study. Exclusion criteria were: Body mass index <19 and >35 kg/m2 known sensitivity to study drugs, inability to tolerate oral medication and understand visual analogue scale score (VAS) significant respiratory, cardiac, renal or hepatic dysfunction, patients on chronic pain medication, anticonvulsant or antidepressant drugs.
A resident doctor who was not participating in the trial prepared the randomization sequence using ‘research randomizer’ software (http://www.randomizer.org/) before starting the study.
Pre-operative anxiety was assessed using VAS score on which 0 indicated ‘no anxiety’ and 100 represented ‘worst imaginable anxiety’.
The patients were randomly assigned into two groups. Group 1 (control) received placebo (iron) tablet that was looking similar to levetiracetam tablet, Group 2 (levetiracetam) received tablet levetiracetam 500 mg. (Leveroxa®, Ranbaxy). After assessing base line anxiety in the ward, one nurse who was not part of the study gave one table to each patient in sequentially numbered, sealed, opaque envelops. Patients, investigators, and outcome assessor were not aware of the type of medication. Anxiety was assessed again in the pre-operative holding area and in the operation theatre just before induction of anaesthesia by a nurse who did not participate in the study.
Other anxiolytic pre-medications were omitted. All patients received same standardized general anaesthesia. The primary outcome measure of the study was the level of pre-operative anxiety in term of VAS score, and incidence of any side effects as secondary outcomes. Adverse effects that is, headache, nausea, blurred vision, vomiting, respiratory depression, etc., were noted.
The superiority sample size calculation was done on the basis of VAS score for anxiety assessment as reported in previous published literature.[4] Assuming that levetiracetam would decrease VAS score by 30%, at significance level of α = 0.05 and β = 0.10,46 patients patients were needed to be enrolled in each group. We enrolled 51 patients in each group to minimize the effect of any missing data.
The distribution of data was determined by Kolmogorov–Smirnov analysis and Bartlellet's test. The groups were compared with unpaired Student's t-test, unpaired t-test with Welch correction, and Mann–Whitney U-test. Z-test was done to detect a significant difference among proportion. Data were presented as mean ± SD, median ± interquartile range, numbers (n), and percentages (%). The package SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad instate were used for statistical analysis. P < 0.05 was considered significant.
RESULTS
Total 113 patients were assessed for eligibility, out of which 102 patients were randomized either to get study drug or placebo. Patients in both the groups were comparable in regards to demographic variables and baseline anxiety. There was no significant difference in the level of pre-operative anxiety between the groups [Figure 1]. Two patients in the control group had pre-mature ventricular contractions which subsided within a minute without any medications. One patient in Levetiracetam group developed bronchospasm which was treated with 400 μg of inhaled salbutamol.
Figure 1.
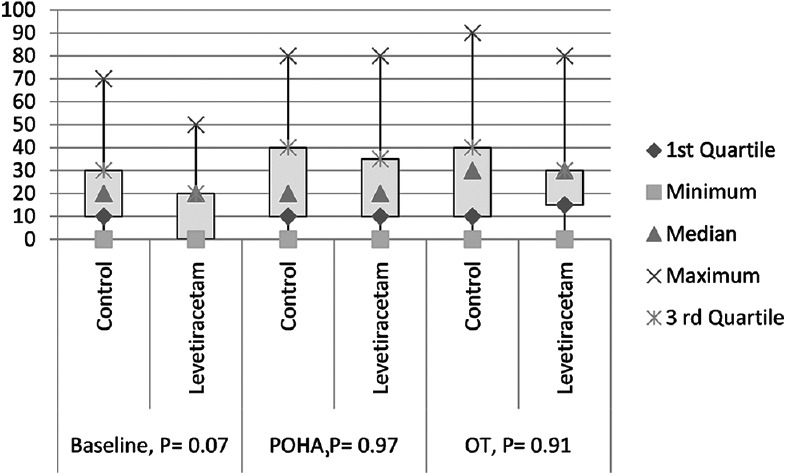
Level of pre-operative anxiety (VAS) X axis = VAS score in 0–100. Y = level of anxiety in the different time interval. Base line = visual analogue scale score before giving the study drugs, pre-operative holding area = visual analogue scale score at pre-operative holding area, operating theatre = visual analogue scale score at operation table before induction, P = P value
DISCUSSION
The precise mechanism of anxiolysis by levetiracetam is yet to be elucidated. Unlike other anticonvulsants, levetiracetam does not bind with gamma-aminobutyric acid (GABA) receptors. But it can facilitate the action of GABA by altering its metabolism or transportation.[5] It may also cause anxiolysis by inhibiting excitatory neurotransmitters such as glutamine and glutamate.[6] After oral ingestion, levetiracetam is rapidly absorbed, with peak concentration attained after 1½ h, and its bioavailability is more than 95%.[7]
One study on refractory anxiety disorder found that levetiracetam at mean (SD) dose of 1969 ± 819 mg/day was an effective anxiolytic agent.[2] This finding was supported by a study of social anxiety disorder where levetiracetam was flexibly titrated to 3000 mg/day.[3] Our study failed to show anxiolysis efficacy of levetiracetam in the setting of pre-operative anxiety. Similar to our study, one multi centric study found that levetiracetam was not effective in social anxiety disorder; the lower doses may be responsible.[8]
Present study is the first clinical trial that examined the pre-operative anxiolysis efficacy of levetiracetam. The study has some limitations. One may criticize that we used VAS score to assess anxiety which is not the gold standard. Inadequate dose of levetiracetam was a major limitation of our study. Other studies that evaluated anxiolysis property of levetiracetam started with smaller dose that is, 250 mg, then it was gradually increased over weeks to a higher dose in an attempt to avoid any untoward effect and increase tolerability of medication. We avoided the use of higher dose that may possibly increase the incidence of side effects.
CONCLUSION
A single dose of levetiracetam at 500 mg dose was not useful in decreasing pre-operative anxiety.
Acknowledgments
We express our sincere gratitude to an administrator, all the doctors, and staff nurse of Department of Surgery for their kind support without which the study would never be completed.
Financial support and sponsorship
The necessary fund was provided by the institute.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238–45. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 2.Kinrys G, Worthington JJ, Wygant L, Nery F, Reese H, Pollack MH. Levetiracetam as adjunctive therapy for refractory anxiety disorders. J Clin Psychiatry. 2007;68:1010–3. doi: 10.4088/jcp.v68n0705. [DOI] [PubMed] [Google Scholar]
- 3.Simon NM, Worthington JJ, Doyle AC, Hoge EA, Kinrys G, Fischmann D, et al. An open-label study of levetiracetam for the treatment of social anxiety disorder. J Clin Psychiatry. 2004;65:1219–22. doi: 10.4088/jcp.v65n0909. [DOI] [PubMed] [Google Scholar]
- 4.Jawaid M, Mushtaq A, Mukhtar S, Khan Z. Preoperative anxiety before elective surgery. Neurosciences (Riyadh) 2007;12:145–8. [PubMed] [Google Scholar]
- 5.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–6. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löscher W, Hönack D, Bloms-Funke P. The novel antiepileptic drug levetiracetam (ucb L059) induces alterations in GABA metabolism and turnover in discrete areas of rat brain and reduces neuronal activity in substantia nigra pars reticulata. Brain Res. 1996;735:208–16. doi: 10.1016/0006-8993(96)00587-2. [DOI] [PubMed] [Google Scholar]
- 7.Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43:707–24. doi: 10.2165/00003088-200443110-00002. [DOI] [PubMed] [Google Scholar]
- 8.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: Response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:739–43. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]


