Abstract
Objectives
To develop a candidate definition for central line-associated blood stream infection (CLABSI) in neonates with presumed mucosal barrier injury due to gastrointestinal (MBI-GI) conditions; to evaluate epidemiology and microbiology of MBI-GI CLABSI in infants.
Design
Multicenter retrospective cohort study
Setting
Neonatal intensive care units (NICU) from 14 U.S. children’s hospitals and pediatric facilities
Methods
A multidisciplinary focus group developed a candidate MBI-GI CLABSI definition based on presence of a MBI-GI condition, parenteral nutrition (PN) exposure, and an eligible enteric organism. CLABSI surveillance data from participating hospitals were supplemented by chart review to identify MBI-GI conditions and PN exposure.
Results
During 2009–12, 410 CLABSI occurred in 376 infants. MBI-GI conditions and PN exposure occurred in 149 (40%) and 324 (86%) of these 376 neonates, respectively. The distribution of pathogens was similar among neonates with versus without MBI-GI conditions and PN exposure. Fifty-nine (16%) of the 376 initial CLABSI episodes met the candidate MBI-GI CLABSI definition. Subsequent versus initial CLABSI were more likely to be caused by an enteric organism (22 of 34, 65% vs. 151 of 376, 40%; p = 0.009) and to meet the candidate MBI-GI CLABSI definition (19 of 34, 56% vs. 59 of 376, 16%; p < 0.01).
Conclusions
While MBI-GI conditions and PN exposure were common, only 16% of initial CLABSI met the candidate definition of MBI-GI CLABSI. The high proportion of MBI-GI CLABSI among subsequent infections suggests infants with MBI-GI CLABSI should be a population targeted for further surveillance and interventional research.
Keywords: central line associated bloodstream infection, infant, neonate, surveillance
INTRODUCTION
Healthcare-associated infections (HAI) are responsible for increased morbidity, mortality, and utilization of health care resources. Bloodstream infections (BSI) are the most frequent type of HAI in pediatric patients.1 Recent efforts to prevent central line-associated BSI (CLABSI) have yielded significant reductions in many pediatric inpatient settings and institutions.2–5 However, as public reporting requirements expand and pay-for-performance strategies are implemented, clinicians and members of the hospital epidemiology community have expressed concern that not all CLABSI can be prevented by strict adherence to current CLABSI prevention bundles.6–9 Much of this concern has focused upon patients who are presumed to have disrupted integrity of mucosal barriers, particularly of the gastrointestinal (GI) tract. Many have hypothesized that translocation of colonizing bacteria across a damaged mucosal barrier, rather than introduction of bacteria along a percutaneous central venous catheter, may be a common mechanism of CLABSI in specific patient populations.10,11
In January 2013, a modified definition for laboratory-confirmed bloodstream infection was introduced by the National Healthcare Safety Network’s (NHSN).12 This new definition provides a mechanism to designate a BSI as related to mucosal barrier injury (MBI), provided both patient- and organism-specific criteria are met.10,11 Although there are scant empirical data to define the pathogenesis of these infections, this novel definition provides an opportunity to better understand CLABSI events in a specific, vulnerable patient population.
Other patient populations with impaired intestinal mucosal barrier may also be at risk of BSI due to bacterial translocation. In pediatrics, patients with chronic intestinal dysfunction due to structural or functional abnormalities of the GI tract, such as congenital malformations or complications of prematurity, often have a prolonged need for parenteral nutrition (PN) and thus prolonged need for central venous catheterization. Anecdotal reports suggest that these patients may contribute disproportionately to the current CLABSI rates in institutions that have achieved marked success in CLABSI prevention among other patient populations.11 a We conducted this multicenter, retrospective cohort study to explore the epidemiology and microbiology of CLABSI in neonatal patients with and without chronic GI dysfunction.
METHODS
Participating Hospitals and Locations
Thirty-three children’s hospitals and pediatric facilities, which comprise the Pediatric Prevention EpiCenters Consortium, were invited to participate in this study. Of those, 14 sites elected to participate, including 11 free-standing children’s hospitals. The study hospitals had a median of 288 beds and 3 infection preventionists. All participating sites performed surveillance for laboratory-confirmed BSI in neonatal intensive care unit (NICU) patients with central lines as part of their ongoing surveillance plans. Thirteen hospitals were in states that required public reporting of NICU CLABSI and at least 7 had undergone an external review of their CLABSI surveillance data. Each site obtained approval from their local Institutional Review Board.
Data Collection
Each site submitted 1–3 years of retrospective data (ranging from January 2009 through June 2012) on all episodes of laboratory-confirmed BSI in NICU patients with a central line at the time of infection onset. Only infections determined by the site to be a CLABSI by NHSN criteria13 were included in this analysis. Data collected for each event included patient age, month and year of infection, and organism(s) isolated. In addition, sites were asked whether the event was the patient’s initial or a subsequent event during the reporting period. For each event, study participants conducted chart review to determine the presence of any underlying GI condition(s) at the time of event, and whether PN and/or lipids had been administered during the 7 days prior to infection.
For each month during the data-reporting period, sites submitted the monthly number of NICU central line days.
Development of a Candidate Definition of a BSI related to MBI associated with a chronic GI condition
A focus group of neonatologists and pediatric gastroenterologists was convened to discuss the underlying conditions and clinical features of patients with GI conditions (other than those secondary to neutropenia or graft vs. host disease) that might be associated with an increased risk of bacteremia due to translocation. The following characteristics were identified as being associated with an increased risk of intestinal translocation: presence of ≥ 1 specific GI conditions associated with MBI (MBI-GI conditions, Table 1) AND receipt of PN ≤ 7 days prior to BSI. This timeframe was selected in recognition that some patients may receive lipid supplementation on a weekly basis and others may have had PN held due to temporary lack of venous access. We used organisms identified in the new NHSN MBI-laboratory confirmed bloodstream infection definition12 as being eligible organisms for an MBI-GI event although viridans group streptococci were not included because they are considered oral commensal organisms (Table 1). Thus, a MBI-GI BSI was defined as 1) laboratory-confirmed bloodstream infection unrelated to another NHSN-defined infection/process,13 2) in a patient with an eligible GI condition and PN exposure, and 3) caused by an eligible organism.
Table 1.
Criteria for Candidate Definition of Mucosal-barrier Injury associated Gastrointestinal Insufficiency Event
| Patient Factors | Underlying Intestinal Condition | Autoimmune enteropathy Bowel resection for trauma, tumor, ischemia, obstruction, gastrointestinal congenital anomaly^ Gastroschisis or Omphalocele Necrotizing enterocolitis Inflammatory bowel disease Intestinal aganglionosis Intestinal atresia Intestinal pseudo-obstruction Intestinal transplantation Microvillous inclusion disease Midgut volvulus Motility disorder Radiation enteritis Tufting enteropathy |
| Evidence of Intestinal Insufficiency | Receipt of PN within 7 days prior to onset of infection | |
| Organism | NHSN-defined MBI Organisms^ |
Bacteroides species Candida species Clostridium species Enterobacteriaceae Enterococcus species Fusobacterium species Peptostreptococcus species Prevotella species Veillonella species |
excludes esophageal anomalies
Classification of GI Conditions
Sites were asked to report all GI conditions that were present at the time of CLABSI onset from a pre-specified list of conditions (Table 1). For conditions not listed, free-text entry of other GI conditions was encouraged. For patients who had multiple GI conditions at the time of CLABSI onset, a hierarchy of conditions was created that assigned a primary GI condition. For patients who had both MBI-GI and non-MBI-GI conditions, the MBI-GI condition was assigned as the primary GI condition. For patients with multiple MBI-GI diagnoses, the condition with the presumed earliest onset was assigned as the primary GI condition. For patients with CLABSI and non-NHSN or non-acute NEC, we created an additional category of “non-NHSN NEC” which included: 1) a clinical syndrome of NEC that did not meet NHSN criteria (“suspected NEC”); 2) a history of NEC without reported complications (“past medical NEC”); and 3) a history of complicated NEC due to perforation or bowel resection (“past surgical NEC”).
Data Analysis
To understand differences between MBI-GI and non-MBI-GI CLABSI, our primary analysis focused on initial CLABSI events. Patient-level clinical and microbiologic data for CLABSI were summarized, with categorical data displayed as frequencies and percentages and continuous data described using medians and interquartile ranges. The pathogen mix of CLABSI from patients with and without MBI-GI conditions was compared using Pearson’s χ2 test and Fisher’s exact test, with a 2-tailed p value of <0.05 indicating statistical significance. Overall and site-specific monthly CLABSI rates (infections per 1000 catheter days) were calculated with and without inclusion of cases of MBI-GI CLABSI in the numerator. Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Event Characteristics
A total of 410 CLABSI were reported from 376 NICU patients. Subsequent (n=34) CLABSI were excluded from the primary analysis of 376 initial CLABSI described below. At the time of initial CLABSI, most patients were < 30 days of age (Table 2).
Table 2.
Patient characteristics at time of initial CLABSI (n = 376)
| Characteristic | Number (%) |
|---|---|
|
| |
| Sex | |
| Male | 217 (58) |
|
| |
| Age | |
| < 30 days | 195 (52) |
| 30–60 days | 80 (21) |
| 61–90 days | 39 (11) |
| 91 days to 6 months | 46 (12) |
| > 6 months | 16 (4) |
|
| |
| PN^ exposure within prior 7 days | 324 (86) |
|
| |
| Gastrointestinal (GI) condition | |
| Single MBI-GI condition | 87 (23) |
| >1 MBI-GI conditions | 62 (17) |
| Other GI condition | 16 (4) |
| None | 211 (56) |
|
| |
| Intestinal Insufficiency* | 139 (37) |
PN, parenteral nutrition
intestinal insufficiency defined as the presence of an MBI-GI qualifying condition and PN exposure within 7 days prior to CLABSI
GI Conditions and PN Exposure
One or more GI conditions were reported from 165 of 376 patients (44%) for a total of 282 reported conditions, of which most (n=236, 84%) were MBI-GI conditions. The most common non-MBI-GI conditions were intestinal perforation (n=19) and esophageal processes (n= 8). A total of 149 patients were found to have ≥1 MBI-GI conditions (Table 3). The most prevalent GI condition among patients with initial CLABSI was non-NHSN NEC, including suspected NEC (n=20), past medical NEC (n=23), and past surgical NEC (n=37). At least 63 patients (17%) had undergone bowel resection. Most patients had recent PN exposure at the time of CLABSI (324 of 376, 86%), although PN was more common in patients with (139 of 149, 93%) than without MBI-GI conditions (185 of 227, 81%, p = 0.0012).
Table 3.
Final categorization of gastrointestinal conditions for 376 NICU patients
| Final GI Categories by Patient | N (%) |
|---|---|
| Non-NHSN NEC* | 80 (21.3) |
| Gastroschisis/omphalocele w/ or w/o resection | 31 (8) |
| Intestinal atresias w/ or w/o resection | 16 (4) |
| Bowel resection | 15 (4) |
| Motility disorder | 6 (2) |
| Inflammatory bowel disease w/bowel resection | 1 (0.3) |
| Extra-intestinal, intra-abdominal | 9 (2.4) |
| Other intestinal | 7 (2) |
| None | 211 (56) |
Non-NHSN NEC (necrotizing enterocolitis) included suspected NEC, past medical NEC and past surgical NEC
Microbiology of CLABSI
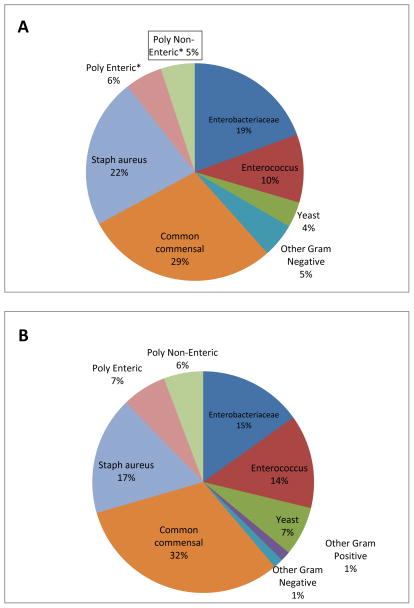
Skin commensal organisms and S. aureus were the most commonly isolated organisms from patients with initial CLABSI. Enteric organisms, including Enterobacteriaceae (67) and Enterococcus spp. (43), were recovered from approximately one-third of patients with initial CLABSI, including 22 patients who had polymicrobial infections with at least one enteric organism. When the microbiology of initial CLABSI from patients with and without an eligible GI condition and PN exposure was compared, no significant differences were noted (Figure 1). NHSN MBI organisms were recovered from 59 of 139 CLABSI (42%) from patients with and 92 of 237 CLABSI (39%) from patients without an MBI-GI condition and PN exposure. Similarly, when the microbiology of CLABSI for patients with any as compared to no GI conditions was examined, no differences were observed (data not shown).
Figure 1.
Microbiology of initial CLABSI reported from 376 NICU patients with (panel A) and without (panel B) an MBI-GI condition and recent PN exposure. *Poly Enteric = polymicrobial infection that included one or more MBI enteric organisms; *Poly Non-Enteric = polymicrobial infection that did not include any MBI enteric organisms.
We examined the microbiology of CLABSI in patients with various specific GI conditions and noted no significant differences associated with specific GI conditions (Supplemental Table).
Classification of CLABSI
We identified 59 of 376 (16%) initial events that met the candidate definition of MBI-GI CLABSI. In contrast, 19 of 34 (56%) subsequent events were classified as MBI-GI CLABSI (p<0.05).
Patients with multiple CLABSI
There were a total of 34 subsequent CLABSI during the reporting period. These included 28 patients with 2 CLABSI, 5 patients with 3 CLABSI and 1 patient with 4 CLABSI. Of the 28 patients with more than 1 CLABSI, 17 (61%) had ≥ 1 MBI-GI condition, most commonly bowel resection (n=9), non-NHSN NEC (n=8), intestinal atresia (n=5) and gastroschisis (n=4). Eight (29%) patients had more than 1 MBI-GI condition, including 6 who had past surgical NEC. Twenty-four (86%) had recent PN exposure and 16 (57%) had both an eligible GI condition and recent PN exposure. NHSN MBI organisms were recovered from 22 of 34 CLABSI (65%). When the microbiology of initial and subsequent CLABSI were compared, enteric organisms were more common in subsequent than initial infections (22 of 34, 65% vs. 151 of 376, 40%; p = 0.009). Subsequent versus initial CLABSI were more likely to meet the candidate MBI-GI CLABSI definition (19 of 34, 56% vs. 59 of 376, 16%; p < 0.01).
Impact of Candidate Definition on NICU CLABSI Rates
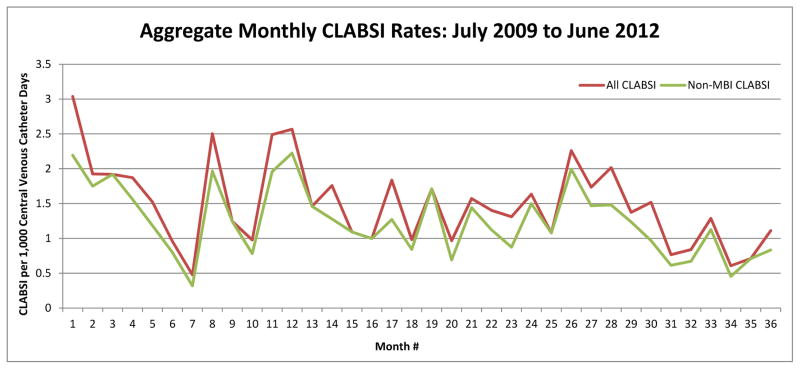
We determined the monthly CLABSI rates across all study sites with MBI-GI CLABSI both included and excluded from the numerator (Figure 2). Of 206 data months contributed by all sites, 140 months (68%) had no difference in monthly rates after removal of MBI-GI. We also examined the potential impact of removal of MBI-GI CLABSI from the monthly CLABSI rates of individual sites. We noted substantial differences across sites in the proportion of CLABSI meeting criteria for MBI-GI CLABSI (see Supplemental Figure).
Figure 2.
Aggregate monthly CLABSI rate with all CLABSI (green) and non-MBI-GI CLABSI (red) included.
DISCUSSION
In this multicenter cohort study of infants hospitalized in NICUs, we found that GI conditions were common and that approximately one-third of CLABSI occurred in infants with an MBI-GI condition and PN exposure. Contrary to our hypothesis, the distribution of organisms causing CLABSI was similar for patients with and without MBI-GI condition(s) and PN exposure. Sixteen percent of all NICU CLABSI met our proposed case definition of MBI-GI CLABSI, although the proportion of infections that fulfilled the criteria for this definition varied markedly by institution.
We found that 40% of infants with initial CLABSI had underlying GI conditions that were hypothesized to increase the risk of bacteremia due to translocation of enteric organisms across a chronically disrupted intestinal epithelium. NEC was the most prevalent GI condition, including past surgical NEC that was presumed to have led to “short bowel syndrome”. While our study design did not allow us to evaluate the relative risk of CLABSI among patients with and without various underlying GI conditions, the high prevalence of these conditions is consistent with prior reports that underlying GI conditions were a risk factor for CLABSI in both neonatal and older pediatric patients.14–16,17, although this finding has not been universally observed.18
Enteric organisms were common causes of infection in our cohort and were isolated in approximately 40% of the reported CLABSI. Similarly, using data reported to the NHSN between 2006–2008, Hocevar and colleagues found that at least one-third of NICU CLABSI were due to an enteric organism.19 Bacteremia due to enteric organisms, particularly in patients with vascular devices, may arise from a variety of mechanisms. Translocation of organisms that colonize the intestinal tract has been hypothesized to be a mechanism of bacteremia. Studies performed in adult surgical patients in the 1990s demonstrated that enteric organisms were isolated from the mesenteric nodes of 14–21% of adult patients undergoing laparotomy20–22 and were associated with an increased risk of post-operative sepsis in one study.20 More recent studies of the intestinal microbiome in stem cell transplant patients suggested that “intestinal domination” by potentially pathogenic organisms was associated with markedly increased risk of bacteremia due to an enteric organism, presumably via translocation.23,24 Alternately, some episodes of bacteremia due to an enteric organism in patients with vascular catheters may arise from mechanisms related to the catheter. Hospitalized neonates can rapidly acquire both intestinal and cutaneous colonization with enteric organisms.25 Additionally, factors such as the presence of an ostomy or diarrhea might increase the likelihood of cutaneous colonization with enteric organisms. In the setting of suboptimal catheter care, these organisms could contaminate the interior aspect of a needleless connector or migrate along the subcutaneous catheter tunnel and give rise to a CLABSI due to an enteric organism.26
Our a priori hypothesis was that CLABSI due to enteric organisms would be more prevalent among patients with an MBI-GI condition and PN exposures as compared to patients who did not have these characteristics. In this retrospective cohort, however, we observed that there was little difference in the pathogens that caused CLABSI in these two patient populations. Several major reasons might explain this observation. First, translocation might not be a common mechanism of bacteremia in patients with chronic GI conditions and PN dependence. In a report of findings from a successful multicenter collaborative to reduce NICU CLABSI, Schulman and colleagues found that despite significant reductions in the rate of CLABSI, the proportion of infections due to enteric organisms remained unchanged: 34% prior to and 32% after implementation of a prevention bundle.5 This finding suggests that the bundle of interventions, all of which focused on insertion or maintenance of the catheter, had a similar impact on both CLABSI caused by skin organisms and CLABSI caused by enteric organisms. Presumably, enhanced care of a vascular catheter would not alter the risk of bacteremia due to translocation of organisms across a damaged intestinal epithelium.
Alternatively, misclassification bias might have masked possible differences in the microbiology of CLABSI between patients with and without chronic GI conditions and PN dependence. Although our candidate definition was derived by experienced clinicians who care for children with short bowel syndrome, it is unknown if this proposed surveillance definition is able to accurately identify infants with impaired integrity of their intestinal epithelial barrier. Our study design did not allow us to capture data on total duration of PN or to assess intestinal permeability, both characteristics of infants with intestinal failure.27,28 Additionally, we were unable to measure other recognized risk factors for CLABSI, such as number and location of central lines, that might confound this observation. Thus, our study was unable to examine potential differences in the microbiology among patients with varying degrees of intestinal dysfunction. Additionally, the retrospective nature of our study might have limited our ability to capture accurately the complex GI conditions of our cohort. Our study was also significantly limited by the use of existing data from infection control departments. For example, misclassification bias may also have been introduced by our need to omit events previously designated as secondary BSI from this analysis because we lacked the resources needed to re-adjudicate these events. Therefore, we were unable to assess whether patients with GI conditions were at increased risk of CLABSI or to determine other risk factors for CLABSI such as catheter type and location. Finally, this study may have limited generalizability because all participating sites were academic level IV NICUs. Even within our study sites, there were may have been significant differences in the populations served (e.g. high proportion of term infants referred for surgical conditions) that led to the observed differences in the proportion of CLABSI that might be designated as MBI-GI CLABSI. Alternatively, there may have been important differences in the way lab-confirmed BSI in patients with chronic GI conditions were classified (ie. as secondary BSI or CLABSI) or important practice differences at some hospitals that were successful in preventing CLABSI in this patient population.
We identified an increased prevalence of enteric organisms in infants with MBI-GI condition(s) and PN exposure who experienced multiple CLABSI, an observation that might be consistent with translocation-related CLABSI. Similarly, a study from a national neonatal network reported that recurrent bloodstream infections were common among very low birth weight infants with intestinal failure, although skin flora were recovered from nearly half of the infections.29 Future initiatives might focus on the development of an “enteric CLABSI prevention bundle” that could be selectively applied to infants with chronic GI conditions and PN dependence who develop an initial CLABSI due to an enteric organism.
In summary, we developed a candidate definition for MBI BSI that may be used to identify a high-risk patient population with chronic underlying GI conditions associated with an increased risk of recurrent CLABSI. We observed that the microbiology of CLABSI among patients with chronic GI conditions and PN dependence who experienced multiple events was distinct from that observed for patients who did not have underlying GI conditions. Larger scale evaluation of this definition is needed to determine its capacity to differentiate BSI more likely to arise from translocation from CLABSI that likely arose related to routine care of a central venous catheter. This definition might be used to evaluate enhanced CLABSI prevention bundles in this high-risk patient population.
Supplementary Material
Acknowledgments
Financial support. This work was supported in part by the CDC Prevention Epicenters Program (U54-CK000163). CD was supported in part by K24HD058795.
The authors would like to thank the infection preventionists at all of our institutions for their commitment to conducting high quality surveillance and to acknowledge the dedicated work of NICU clinicians to prevent infections.
Participants from the Pediatric Prevention EpiCenter Consortium
Kris Bryant, MD (Kosair Children’s Hospital, Louisville, KY); Elaine Cox, MD (Riley Hospital for Children, Indianapolis, IN); Audra Deveikis, MD and David Michalik, DO (Miller Children’s Hospital, Long Beach, CA); Jane Gould, MD (St. Christopher’s Hospital for Children, Philadelphia, PA); Judith Guzman-Cotrill, DO (Doernbecher Children’s Hospital, Portland, OR); Anne Hansen, MD (Boston Children’s Hospital, Boston, MA); Galit Holzmann-Pazgal, MD (Children’s Memorial Hermann Hospital, Houston, TX); Larry Kociolek, MD (Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL); Latania Logan, MD and Angela Rupp, MT, MS, CIC (Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL); Edward Septimus, MD (Hospital Corporation of America, Nashville, TN); Eileen Sherman, MS, CIC (duPont Hospital for Children, Wilmington, DE); Sarah Wittig, CIC (Johns Hopkins Medical Institutions, Baltimore, MD)
Footnotes
The lead author previously presented a portion of these data at the 2013 meeting of the European Society of Paediatric Infectious Diseases (ESPID) in an abstract entitled “Microbiology of Bloodstream Infections in Infants with and without Intestinal Insufficiency”
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
References
- 1.Dudeck MA, Horan T, Peterson KD, et al. American Journal of Infection Control. JIC. 2013;41(4):286–300. doi: 10.1016/j.ajic.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller MR, Griswold M, Harris JM, et al. Decreasing PICU Catheter-Associated Bloodstream Infections: NACHRI’s Quality Transformation Efforts. Pediatrics. 2010;125(2):206–213. doi: 10.1542/peds.2009-1382. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler DS, Giaccone MJ, Hutchinson N, et al. A Hospital-wide Quality-Improvement Collaborative to Reduce Catheter-Associated Bloodstream Infections. Pediatrics. 2011;128(4):e995–e1007. doi: 10.1542/peds.2010-2601. [DOI] [PubMed] [Google Scholar]
- 4.Bizzarro MJ, MD, Sabo B, APRN, Noonan M, RN, Bonfiglio MP, RN, Northrup V, MPH, Diefenbach K., MD A Quality Improvement Initiative to Reduce Central Line–Associated Bloodstream Infections in a Neonatal Intensive Care Unit. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2010;31(3):241–248. doi: 10.1086/650448. [DOI] [PubMed] [Google Scholar]
- 5.Schulman J, Stricof R, Stevens TP, et al. Statewide NICU Central-Line-Associated Bloodstream Infection Rates Decline After Bundles and Checklists. Pediatrics. 2011;127(3):436–444. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg JP, Robichaux C, Tejedor SC, Reyes MD, Jacob JT. Distribution of Pathogens in Central Line–Associated Bloodstream Infections among Patients with and without Neutropenia following Chemotherapy: Evidence for a Proposed Modification to the Current Surveillance Definition. Infection Control and Hospital Epidemiology. 2013;34(2):171–175. doi: 10.1086/669082. [DOI] [PubMed] [Google Scholar]
- 7.Fraser TG, Gordon SM. CLABSI Rates in Immunocompromised Patients: A Valuable Patient Centered Outcome? Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2011;52(12):1446–1450. doi: 10.1093/cid/cir200. [DOI] [PubMed] [Google Scholar]
- 8.Beekmann SE, Diekema DJ, Huskins WC, et al. Diagnosing and Reporting of Central Line–Associated Bloodstream Infections. Infection Control and Hospital Epidemiology. 2012;33(9):875–882. doi: 10.1086/667379. [DOI] [PubMed] [Google Scholar]
- 9.Sexton DJ, MD, Chen LF, MBBS, MP, Anderson DJ., MD, MPH Current Definitions of Central Line–Associated Bloodstream Infection: Is the Emperor Wearing Clothes? Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2010;31(12):1286–1289. doi: 10.1086/657583. [DOI] [PubMed] [Google Scholar]
- 10.Lukenbill J, Rybicki L, Sekeres MA, et al. Defining Incidence, Risk Factors, and Impact on Survival of Central Line-Associated Blood Stream Infections Following Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biology of Blood and Marrow Transplantation. 2013;19(5):720–724. doi: 10.1016/j.bbmt.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 11.DiGiorgio MJ, Fatica C, Oden M, et al. Development of a Modified Surveillance Definition of Central Line–Associated Bloodstream Infections for Patients with Hematologic Malignancies. Infection Control and Hospital Epidemiology. 2012;33(9):865–868. doi: 10.1086/667380. [DOI] [PubMed] [Google Scholar]
- 11a.Cole CR, Frem JC, Schmotzer B, Gewirtz AT, Meddings JB, Gold BD, Zigler TR. Ther rate of bloodstream infection is high in infants with short bowel syndrome. Journal of Pediatrics. 2010;156:941–947. doi: 10.1016/j.jpeds.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.See I, Iwamoto M, Allen-Bridson KT, Horan T, Magill SS, Thompson ND. Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infection: Results from a Field Test of a New National Healthcare Safety Network Definition. Infection Control and Hospital Epidemiology. 2013;34(8):769–776. doi: 10.1086/671281. [DOI] [PubMed] [Google Scholar]
- 13.NHSN. 17 CDC NHSN Surveillance Definitions. 2012. pp. 1–34. [Google Scholar]
- 14.Squires RH, Duggan C, Teitelbaum DH, et al. Natural History of Pediatric Intestinal Failure: Initial Report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161(4):723–728. e2. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham PL, III, Begg MD, Larson E, Della-Latta P, Allen A, Saiman L. Risk Factors for Late Onset Gram-Negative Sepsis in Low Birth Weight Infants Hospitalized in the Neonatal Intensive Care Unit. Pediatr Infect Dis J. 2006;25(2):113–117. doi: 10.1097/01.inf.0000199310.52875.10. [DOI] [PubMed] [Google Scholar]
- 16.Niedner MF, Huskins WC, Colantuoni E, et al. Epidemiology of Central Line–Associated Bloodstream Infections in the Pediatric Intensive Care Unit. Infection Control and Hospital Epidemiology. 2011;32(12):1200–1208. doi: 10.1086/662621. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard AC, Fortin E, Rocher I, et al. Central Line–Associated Bloodstream Infection in Neonatal Intensive Care Units. Infection Control and Hospital Epidemiology. 2013;34(11):1167–1173. doi: 10.1086/673464. [DOI] [PubMed] [Google Scholar]
- 18.Advani S, Reich NG, Sengupta A, Gosey L, Milstone AM. Central Line-Associated Bloodstream Infection in Hospitalized Children with Peripherally Inserted Central Venous Catheters: Extending Risk Analyses Outside the Intensive Care Unit. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2013;52(9):1108–1115. doi: 10.1093/cid/cir145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hocevar SN, Edwards JR, Horan TC, Morrell GOC, Iwamoto M, Lessa FC. Device-Associated Infections among Neonatal Intensive Care Unit Patients: Incidence and Associated Pathogens Reported to the National Healthcare Safety Network, 2006–2008. Infection Control and Hospital Epidemiology. 2012;33(12):1200–1206. doi: 10.1086/668425. [DOI] [PubMed] [Google Scholar]
- 20.MacFie J, Reddy BS, Gatt M, Jain PK, Sowdi R, Mitchell CJ. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2005;93(1):87–93. doi: 10.1002/bjs.5184. [DOI] [PubMed] [Google Scholar]
- 21.MacFie J, O’Boyle C, Mitchel CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Boyle C, MacFie J, Mitchel CJ, Johnstone D, Sagar PM, Sedman AB. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taur Y, Xavier JB, Lipuma L, et al. Intestinal Domination and the Risk of Bacteremia in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America. 2012;55(7):905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milisavljevic V, Garg M, Vuletic I, et al. Prospective assessment of the gastroesophageal microbiome in VLBW neonates. BMC Pediatrics. 2013;13(1):1–1. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowan ME, Frost MR. A comparison between a detergent baby bath additive and baby soap on the skin flora of neonates. Journal of Hospital Infection. 1986;7:91–95. doi: 10.1016/0195-6701(86)90033-2. [DOI] [PubMed] [Google Scholar]
- 26.Polin RA, Denson S, Brady MT THE COMMITTEE ON FETUS AND NEWBORN and COMMITTEE ON INFECTIOUS DISEASES. Strategies for Prevention of Health Care-Associated Infections in the NICU. Pediatrics. 2012;129(4):e1085–e1093. doi: 10.1542/peds.2012-0145. [DOI] [PubMed] [Google Scholar]
- 27.Hull MA, Jones BA, Zurakowski D, et al. Low serum citrulline concentration correlates with catheter-related bloodstream infections in children with intestinal failure. JPEN J Parenter Enteral Nutr. 2011;35(2):181–187. doi: 10.1177/0148607110381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler TR, Luo M, Estívariz CF, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R402–10. doi: 10.1152/ajpregu.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole CR, Hansen NI, Higgins R, et al. Bloodstream Infections in Very Low Birth Weight Infants with Intestinal Failure. J Pediatr. 2012;160(1):54–59. e2. doi: 10.1016/j.jpeds.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.