Abstract
Background and Aims Some carnivorous plants trap not only small animals but also algae and pollen grains. However, it remains unclear if these trapped particles are useless bycatch or whether they provide nutrients for the plant. The present study examines this question in Utricularia, which forms the largest and most widely spread genus of carnivorous plants, and which captures prey by means of sophisticated suction traps.
Methods Utricularia plants of three different species (U. australis, U. vulgaris and U. minor) were collected in eight different water bodies including peat bogs, lakes and artificial ponds in three regions of Austria. The prey spectrum of each population was analysed qualitatively and quantitatively, and correlated with data on growth and propagation, C/N ratio and δ15N.
Key Results More than 50 % of the prey of the Utricularia populations investigated consisted of algae and pollen, and U. vulgaris in particular was found to capture large amounts of gymnosperm pollen. The capture of algae and pollen grains was strongly correlated with most growth parameters, including weight, length, budding and elongation of internodes. The C/N ratio, however, was less well correlated. Other prey, such as moss leaflets, fungal hyphae and mineral particles, were negatively correlated with most growth parameters. δ15N was positively correlated with prey capture, but in situations where algae were the main prey objects it was found that the standard formula for calculation of prey-derived N was no longer applicable.
Conclusions The mass capture of immotile particles confirms the ecological importance of autonomous firing of the traps. Although the C/N ratio was little influenced by algae, they clearly provide other nutrients, possibly including phosphorus and trace elements. By contrast, mosses, fungi and mineral particles appear to be useless bycatch. Correlations with chemical parameters indicate that Utricularia benefits from nutrient-rich waters by uptake of inorganic nutrients from the water, by the production of more traps per unit of shoot length, and by the capture of more prey particles per trap, as nutrient-rich waters harbour more prey organisms.
Keywords: Algae, aquatic plant, bladderwort, carnivorous plant, δ15N, nutrient uptake, prey spectrum, suction trap, Utricularia australis, U. minor, U. vulgaris.
INTRODUCTION
Carnivorous plants trap, digest and utilize small animals. Nitrogen, phosphorus and other elements absorbed from the prey lead to increased growth (Büsgen, 1883; Chandler and Anderson, 1976; Thum, 1988), increased formation of flowers, fruits and offshoots (Thum, 1988; Krafft and Handel, 1991) or increased mineral concentrations in the tissue (Oudman, 1935; Christensen, 1976). At least 724 species of vascular plants use prey capture to improve their mineral nutrition; with about 228 species, Utricularia (bladderworts, Lentibulariaceae) forms the largest and most widely spread genus of carnivorous plants (McPherson, 2010). In Utricularia, prey is captured by means of sophisticated suction traps (Lloyd, 1942; Sasago and Sibaoka, 1985a, b; Adamec, 2011a; Vincent and Marmottant, 2011; Vincent et al., 2011a, b).
In spite of their ability to trap animals, carnivorous plants such as Utricularia are by no means independent of the inorganic nutrient supply from their environment. All carnivorous plants are able to absorb at least limited amounts of nutrients via their roots or, in aquatic species, their shoots (Adamec, 1992, 2002; Adlassnig et al., 2005). Nitrogen absorbed from the prey was originally derived from the non-carnivorous plants on which prey animals had been feeding. Thus, carnivorous plants use their prey animals to accumulate nitrogen. Nutrient-rich conditions increase the abundance of prey animals and therefore the benefit gained from prey (Ulanowicz, 1995). In aquatic Utricularia, epiphytic algae attract prey animals towards the traps; a good supply of inorganic nutrients may lead to enhanced growth of algae and therefore to increased prey attraction (Ulanowicz, 1995).
Despite these benefits, most carnivorous plants are restricted to oligotrophic habitats. Leaves transformed to traps contribute only little to CO2 assimilation (Adamec, 2006; Sirová et al., 2010). The investment into traps leads to reduced photosynthesis and growth compared with non-carnivorous plants (Kostytschew, 1923; Ellison and Gotelli, 2001). Carnivorous plants therefore require full sunlight, especially for trap formation, as shown in Utricularia vulgaris (Maier, 1973; Englund and Harms, 2003) or Pinguicula vallisneriifolia (Zamora et al., 1998). A general model of carnivorous plant ecology, developed by Benzing (1987) and Givnish (1989), predicts a net benefit for plant carnivory only for a combination of high irradiation, sufficient water supply and oligotrophic substrate. Indeed, this model is able to give a satisfying explanation for the distribution and niching of carnivorous plants. Some additional effects limit the growth of carnivorous plants under nutrient-rich conditions as well. In U. vulgaris, extremely high prey concentrations cannot be utilized (Englund and Harms, 2001). Furthermore, dense growth of epiphytic algae inhibits plant growth by shadowing, although it may attract additional prey animals (Ulanowicz, 1995).
Although these basic correlations are roughly understood, the problem becomes more complex by the capture of other objects than animals. In Utricularia, algae may form the most abundant group of prey objects in some water bodies (Garbini, 1898; Schumacher, 1960; Gordon and Pacheco, 2007; Peroutka et al., 2008), although the benefit for the plant is not yet clear (Jobson et al., 2000). It is not clear whether algae are digested in the traps or if they survive and even propagate (Peroutka et al., 2008; Alkhalaf et al., 2011; Płachno et al., 2012). Pinguicula and other terrestrial plants frequently trap pollen grains and seem to gain benefit (Harder and Zemlin, 1968). The capture of protozoa seems to be crucial in Genlisea (Barthlott et al., 1998) and in part of Utricularia (Hegner, 1926; Seine et al., 2002). Other objects such as fungal spores and hyphae, moss leaflets or mineral particles are trapped at least occasionally by various species (our own observations).
This study compares eight populations of aquatic Utricularia with regard to the chemistry of their home water, various growth parameters, δ15N, and their trapping success in order to answer the following questions:
Are prey-derived nutrients able to compensate for a lack of minerals from the water? Is the mineral content and size of Utricularia therefore the same in eutrophic and oligotrophic habitats?
Is the investment into carnivory – i.e. the number of traps – proportional to the benefit gained from the prey? In oligotrophic waters, the plant desperately needs additional mineral supply. However, in nutrient-poor waters prey animals are less frequent as well; therefore, additional trap formation might gain little net benefit.
Is there evidence for a beneficial effect of prey objects other than animals, such as algae or pollen?
Are δ15N measurements a useful tool to study prey benefit in aquatic Utricularia?
MATERIAL AND METHODS
Sampling and measuring of plants
Utricularia plants of three different species (U. australis, U. vulgaris and U. minor) were collected in eight different water bodies including peat bogs, lakes and artificial ponds in three regions of Austria, i.e. the Lungau/Salzburg, Waldviertel/Lower Austria and Vienna. Details on the sampling sites are given in Supplementary Data Table 1. Sampling took place in late spring (May–June) 2009.
For determination of prey-derived nitrogen in carnivorous plants, samples of prey animals and non-carnivorous water plants were required (Schulze et al., 1991). Only plant species floating such as Utricularia were used as control plants. Due to the low biodiversity in all habitats, only one suitable species was available in each habitat; the collected species are shown in Supplementary Data Table 2. Prey animals were collected by sieving of the water as well as by collecting small animals manually. For both control plants and animals, all samples from each water body were pooled, dried at 70 °C and homogenized before analysis.
In Utricularia plants, fresh weight, shoot length, the number of buds and the numbers of traps and leaves within the first 10 cm of the main shoot were determined. In each plant individual, the prey was identified and quantified microscopically in 20 fully grown traps that were randomly selected from the first 10 cm of the main shoot. Older traps were excluded as strong evidence exists that the content of the traps differs significantly between young and old, inactive traps, especially with regard to algae (Płachno et al., 2012). The size of the traps was determined microscopically, although it has been shown to be irrelevant for the capture of algae (Alkhalaf et al., 2011). The rest of the plants were dried at 70 °C, and dry weight was determined. The dried material was used for chemical and isotope measurements.
pH and water conductivity were determined at the natural habitat, using a Voltcraft PH-100ATC for pH and a Lutron Pure Water Tester WA300 for conductivity. Water hardness was measured in the laboratory, using Aquamerck Total Hardness Test no. 1·08048·0001 and Aquamerck Carbonate Hardness Test no. 1·0804·0001. The concentrations of macronutrients were determined by photometry. (Detection limits, as stated by the manufacturer, are given in parentheses.) Spectroquant Cell Test no. 1·14739·0001 was used for ammonium (0·01 mg L−1), no. 1·14542·0001 for nitrate (2·2 mg L−1), no. 1·14543·0001 for phosphate (0·05 mg L−1) and no. 1·14562·0001 for potassium (5·0 mg L−1). Due to the very low concentrations of inorganic nutrients next to the limit of detection, these data were not included into statistical evaluation. All measurements were repeated twice.
To clarify the fate of algae within the traps, U. australis colleted at Postteich was added to growing cultures of the colonial green alga Scenedesmus sp. (Scenedesmaceae). After 1 week, the vitality of algae inside and outside of the traps was checked microscopically.
Element analysis and isotope measurements
The dry plants were homogenized; carbon and nitrogen content were determined using an elemental analyser (EA 1110 by CE Instruments, Milan, Italy) interfaced via a ConFlo III device (Thermo Finnigan, Bremen, Germany) to a continuous-flow stable isotope ratio mass spectrometer (DeltaPLUS, Thermo Finnigan).
For determination of prey-derived nitrogen, δ15N was used. Since Shearer and Kohl (1988), the phenomenon of 15N discrimination has been used in ecological research: autotrophic plants exhibit a low δ15N, whereas herbivorous animals a higher δ15N. In predators and top predators, the highest δ15N values are achieved. Therefore, if the δ15N of an organism is known, its position within the food chain can be determined. The same method can be applied to carnivorous plants, which get part of their nitrogen from prey and part from their substrate. Thus, they exhibit a higher δ15N than non-carnivorous plants. If the δ15N values of the accompanying, non-carnivorous flora and of typical prey animals are known, the percentage of prey-derived nitrogen can be estimated (Schulze et al., 1991). The percentage of animal-derived nitrogen (NPrey) in Utricularia was calculated according to the equation
| (1) |
where NPrey is the percentage of prey-derived nitrogen, δ15NRP is the 15N in the reference plant, δ15NCP is the 15N in the carnivorous plant and δ15NZ is the 15N in the zooplankton.
Statistical analysis
PASW Statistics 18·0 was used for statistical analysis. As there was little evidence for a normal distribution of the data, the Mann–Whitney and the Kruskal–Wallis tests were used to test for differences, and the Pearson rank correlation test for correlations. In some tests, partial correlation was used as well. Correlations are shown as R2, with plus or minus signs indicating positive or negative correlations. For factor analysis, Varimax rotation and normalization to −1 to +1 were used.
Statistical tests were carried out twice: once for all samples, i.e. after pooling the data for all three species of Utricularia, and once for U. australis, the most widespread of the investigated species.
RESULTS
The investigated water bodies and their Utricularia species
All investigated water bodies proved to be rather poor in nutrients (highly oligotrophic to mesotrophic). The most oligotrophic conditions were found in the undisturbed mire lake Seetaler Lake and the two investigated ponds. Higher nutrient concentrations were measured in the three disturbed peat bogs, Schrems, Brand and Hochstand, as well as in the lagg of the Seetal mire. pH was very low in the fens of Waldviertel but circumneutral in the other habitats. Conductivity and hardness were low in the fen habitats but high in the calcareous Postteich. Details on water chemistry are given in Supplementary Data Table 2.
Both U. minor and U. australis showed differences in most growth parameters between the various water bodies (Mann–Whitney Test): U. minor from Seetaler Lake had a larger fresh weight compared with U. minor from the Lagg of the same fen (P < 0·01), together with fewer buds (P < 0·01), and a lower leaf and a higher trap density (P < 0·01). The C/N ratio was lower in Seetaler Lake (P < 0·01). Similarly, differences between the five investigated populations of U. australis were found (Kruskal–Wallis test) regarding fresh weight, dry weight, shoot length, number of buds, leaf and trap density, traps per leaf and C/N ratio (all P < 0·01)). Within each population, the variation was much higher for shoot length and dry weight than for C/N ratio or leaf and trap density, which were rather uniform. Details on the growth characteristics of all populations are given in Table 1. The size of the traps did not differ significantly between the habitats (U. vulgaris: 1640 ± 413 µm × 1310 ± 367 µm; U. australis: 1627 ± 366 µm × 1316 ± 300 µm; U. minor: 1341 ± 363 µm × 934 ± 248 µm) but the traps of U. minor were smaller than those of U. vulgaris and U. australis (P < 0·01).
Table 1.
Growth characteristics of the investigated Utricularia populations
| Water body | Dry weight (mg) | Total length (cm) | No. of buds | Leaf density (dm−1) | Trap density (dm−1) | Traps per leaf | N content (%) | C/N ratio (Utricularia) | C/N ratio (control plant) |
|---|---|---|---|---|---|---|---|---|---|
| Seetaler Lake (U. vulgaris) | 216 ± 89 | 50·2 ± 15·0 | 2·9 ± 1·5 | 11·7 ± 1·9 | 439·0 ± 99·3 | 37·9 ± 7·7 | 2·4 ± 0·5 | 17·9 ± 3·8 | 19·9 |
| Seetaler Lake (U. minor) | 21 ± 20 | 40,7 ± 15·9 | 2·8 ± 1·7 | 11·5 ± 1·6 | 138·6 ± 51·3 | 12·8 ± 6·2 | 2·4 ± 0·2 | 16·3 ± 1·2 | |
| Seetaler Lagg (U. minor) | 10 ± 5 | 35·9 ± 12·7 | 6·1 ± 3·0 | 22·0 ± 3·4 | 52·8 ± 21·3 | 2·5 ± 1·0 | 2·1 ± 0·4 | 19·6 ± 5·3 | 18·2 |
| Peat Bog Schrems (U. australis) | 72 ± 43 | 72 ± 42 | 8·3 ± 6·1 | 19·9 ± 3·4 | 163·6 ± 80·2 | 8·9 ± 5·5 | 2·7 ± 0·8 | 16·4 ± 3·8 | 14·3 |
| Winkelauer Pond (U. australis) | 2 ± 2 | 8·2 ± 3·2 | 1·3 ± 0·7 | 28·3 ± 7·7 | 63·3 ± 24·4 | 2·4 ± 1·1 | 5·2 ± 0·7 | 8·2 ± 0·9 | 24·8 |
| Peat Bog Hochstand (U. australis) | 59 ± 60 | 42·5 ± 23·7 | 3·2 ± 1·3 | 15·4 ± 2·7 | 367·6 ± 180·0 | 25·6 ± 15·6 | 2·0 ± 0·3 | 20·5 ± 2·9 | 25·3 |
| Peat Bog Brand (U. australis) | 38 ± 25 | 46·6 ± 28·4 | 4·0 ± 1·7 | 12·6 ± 2·5 | 202·6 ± 54·4 | 17·1 ± 4·4 | 4·2 ± 0·4 | 9·4 ± 0·7 | 21·0 |
| Postteich (U. australis) | 35 ± 25 | 49·4 ± 24·7 | 5·7 ± 3·7 | 23·0 ± 3·7 | 156·4 ± 51·1 | 7·2 ± 3·1 | 1·1 ± 0·1 | 32·6 ± 3·4 | 35·3 |
n = 8–12; data are shown as arithmetic mean ± s.d.
In U. minor (Seetaler Lake) and all populations of U. australis the C/N ratio was lower than in the non-carnivorous control plants (P < 0·01). In U. vulgaris (Seetaler Lake), the C/N ratio did not differ significantly from the non-carnivorous control plants. The same was true for U. minor from the Seetaler Lagg.
Prey composition
In all populations, the prey consisted of animals (mainly microcrustaceans and insect larvae but also rotifers, tardigrads and Acari), pollen grains (mainly Pinus sylvestris and Picea abies, the dominant species in the area surrounding most sites), algae and other objects (protozoa, fungal hyphae or fungal spores). Moss leaflets and other plant particles (≤0·2 % of the total prey) and protozoa (≤0·5 % of the total prey) were of negligible abundance, with the exception of U. australis from Postteich containing 1·5 % plant particles and 1·8 % protozoa. Concerning algae, the same genera were observed as reported in a previous study (Peroutka et al., 2008). Ankistrodesmus, Batrachospermum, Bulbochaete, Microthamnion, Sphaerocystis, Plectonema and Monoraphidium are newly added to the list of algae trapped by Utricularia. In all habitats, algal prey was dominated by filamentous Chlorobionta, mainly Zygnemataceae, followed by coccale Chlorobionta. Other taxonomic groups such as Bacillariophyta, Rhodophyta and Cyanobacteria did occur but were of negligible importance. The Kruskal–Wallis test gave evidence that all categories of prey objects (animals, plants, algae, pollen grains and other objects) were distributed unevenly between the populations (P < 0·01). Table 2 shows the prey composition in the different populations.
Table 2.
Trapping success of the investigated Utricularia populations; all values are objects per trap
| Population | Total objects | Plant particles | Algae | Pollen grains | Animals | Other objects |
|---|---|---|---|---|---|---|
| Seetaler Lake (U. vulgaris) | 31·1 ± 32·9 | 0·0 ± 0·1 (≈ 0·1 %) | 1·9 ± 3·8 (≈ 10·2 %) | 28·0 ± 32·1 (≈ 80·2 %) | 0·6 ± 0·8 (≈ 4·9 %) | 0·6 ± 0·7 (≈ 4·7 %) |
| Seetaler Lake (U. minor) | 13·9 ± 9·7 | 0·0 ± 0·1 (≈ 0·0 %) | 10·8 ± 8·2 (≈ 74·7 %) | 1·8 ± 3·8 (≈ 9·7 %) | 0·4 ± 1·1 (≈ 4·0 %) | 0·9 ± 1·0 (≈ 11·7 %) |
| Seetaler Lagg (U. minor) | 5·6 ± 7·5 | 0·0 ± 0·1 (≈ 0·2 %) | 2·0 ± 5·2 (≈ 35·6 %) | 2·6 ± 5·3 (≈ 34·1 %) | 0·4 ± 0·8 (≈ 10·4 %) | 0·6 ± 0·8 (≈ 19·9 %) |
| Peat Bog Schrems (U. australis) | 20·3 ± 20·8 | 0·0 ± 0·1 (≈ 0·0 %) | 17·7 ± 20·2 (≈ 74·6 %) | 0·6 ± 1·6 (≈ 3·2 %) | 1·1 ± 1·6 (≈ 9·8 %) | 0·9 ± 0·6 (≈ 12·4 %) |
| Winkelauer Pond (U. australis) | 4·2 ± 6·1 | 0·0 ± 0·1 (≈ 0·1 %) | 2·5 ± 5·1 (≈ 37·4 %) | 0·2 ± 1·3 (≈ 4·0 %) | 0·8 ± 1·5 (≈ 19·7 %) | 0·8 ± 1·1 (≈ 38·9 %) |
| Peat Bog Hochstand (U. australis) | 6·0 ± 4·9 | 0·0 ± 0·1 (≈ 0·1 %) | 2·3 ± 2·5 (≈ 34·1 %) | 2·2 ± 2·6 (≈ 26·9 %) | 0·6 ± 1·0 (≈ 12·4 %) | 0·8 ± 0·6 (≈ 26·6 %) |
| Peat Bog Brand (U. australis) | 7·6 ± 5·9 | 0·0 ± 0·1 (≈ 0·1 %) | 2·5 ± 3·9 (≈ 25·6 %) | 0·4 ± 1·0 (≈ 5·5 %) | 4·4 ± 4·0 (≈ 59·1 %) | 0·4 ± 0·6 (≈ 9·8 %) |
| Postteich (U. australis) | 1·7 ± 1·5 | 0·0 ± 0·2 (≈ 1·5 %) | 0·4 ± 0·9 (≈ 12·7 %) | 0·0 ± 0·2 (≈ 1·1 %) | 0·1 ± 0·4 (≈ 4·5 %) | 1·2 ± 1·1 (≈ 81·7 %) |
n ≥ 180 traps per population. ‘Other objects’ include detritus, protozoa, fungal hyphae and spores, Crustacean eggs, and unidentified particles (data are shown as arithmetic mean ± s.d. and as the percentage of the total prey).
In only one water body (Seetaler Lake), two species of Utricularia (U. vulgaris and U. minor) grew simultaneously. In these two species, the composition of prey showed remarkable differences: U. vulgaris caught more total prey (P < 0·01), more pollen grains (P < 0·01) and more animals (P < 0·01) per trap, but fewer algae and other objects (P < 0·01). The most important difference was pollen grains, which counted for 90·2 % of the total prey in U. vulgaris but only 12·6 % in U. minor. However, this dominance of pollen is possibly restricted to the pollen-producing season of Gymnosperm trees.
Most algae trapped by Utricularia were dead and showed signs of decay (Fig. 1). Under controlled conditions, colonies of Scenedesmus within the traps were dead as well whereas the colonies outside the traps of Utricularia were still alive (Fig. 2).
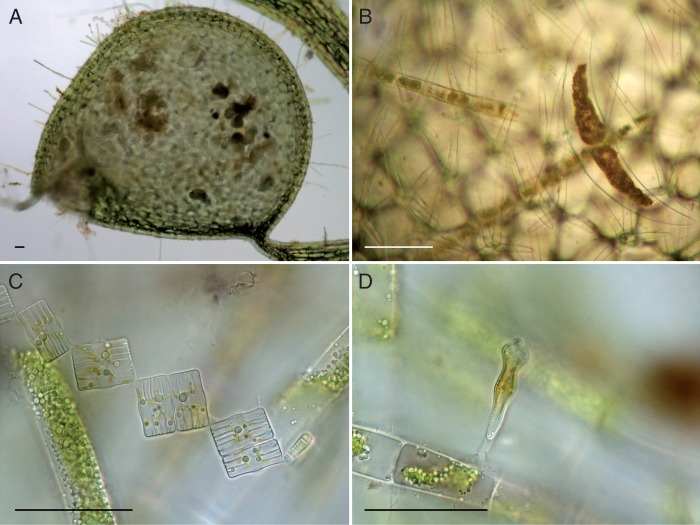
Fig. 1.
Algae trapped by Utricularia. (A) Trap of U. vulgaris from Seetaler See, containing algae and pollen grains. (B) Dead cells of Closterium sp. and Spirogyra sp. within a trap. (C) Dead Tabellaria flocculosa and an undetermined filamentous alga from a trap. (D) Dead Gomphonema constrictum growing on Spirogyra sp. from a trap. Bright field; scale bars ≈ 50 µm.
Fig. 2.
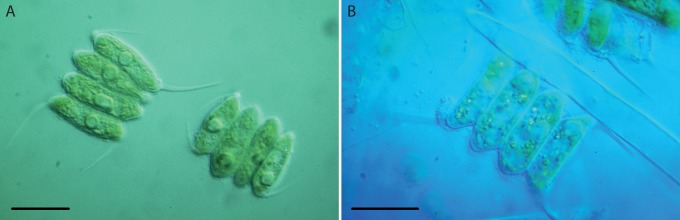
Colonies of Scenedesmus sp., cultivated together with Utricularia australis. (A) Living colony from the medium; (B) decaying colony next to a digestive gland within a trap. Differential interference contrast; scale bars ≈ 10 µm.
Isotope measurements
In the investigated Utricularia populations, δ15N values between +0·1 ‰ (U. australis from the Peat Bog Schrems) to +6·0 ‰ (U. australis from Hochstand) were found. In both U. minor and U. australis, the differences between the various habitats were highly significant. The differences between the three species were not significant, even for U. minor and U. vulgaris which show extremely different growth characteristics.
The proportion of N derived from prey calculated from eqn (1) gave mean values of 71 ± 18 % for U. vulgaris, 68 ± 26 % for U. minor and 120 ± 86 % for U. australis. In U. australis, many unrealistically high values (≥100 %) were calculated, up to 383 %. Some values ≥100 % were also found in U. minor. Detailed results of the isotope measurements are given in Table 3.
Table 3.
Results of the δ15N measurements
| Water body | δ15NUtricularia (‰) | δ15NControl plants (‰) | δ15NZooplankton (‰) | Calculated NPrey (%) |
|---|---|---|---|---|
| Seetaler Lake (U. vulgaris) | 2·6 ± 0·8 | –0·6 | 3·8 | 71 ± 18 |
| Seetaler Lake (U. minor) | 1·5 ± 0·3 | –0·6 | 3·8 | 48 ± 7 |
| Seetaler Lagg (U. minor) | 3·1 ± 0·7 | –0·1 | 3·5 | 89 ± 21 |
| Peat Bog Schrems (U. australis) | 5·1 ± 0·5 | 6·6 | 5·5 | 134 ± 46 |
| Winkelauer Pond (U. australis) | 3·7 ± 0·4 | 1·3 | 2·1 | 303 ± 45 |
| Peat Bog Hochstand (U. australis) | 0·3 ± 0·1 | –2·4 | 2·8 | 51 ± 2 |
| Peat Bog Brand (U. australis) | 1·3 ± 0·2 | –0·2 | 4·3 | 33 ± 4 |
| Postteich (U. australis) | 2·3 ± 0·4 | 4·3 | 2·9 | 1·4 ± 3 |
n = 8–12; data are shown as arithmetic mean ± s.d.
Correlations between growth parameters, prey capture and δ15N
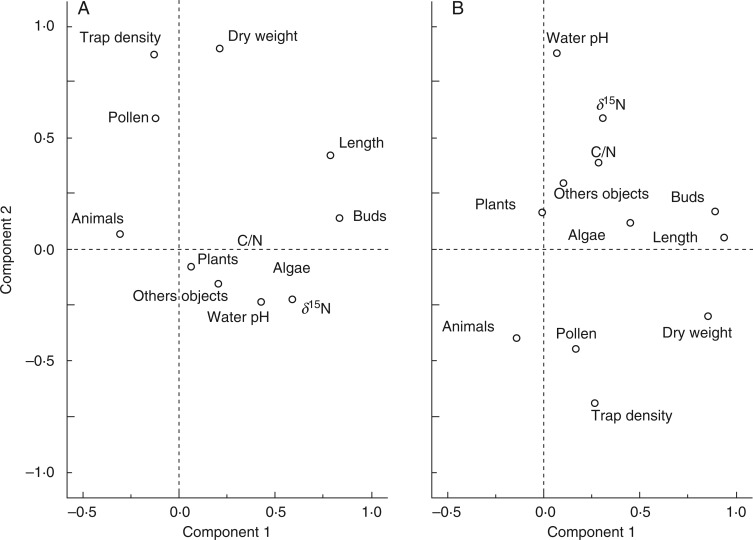
Most growth characteristics as well as δ15N data showed strong correlations both with water chemistry and with trapping success. All data were analysed both for U. australis, the most widespread species used in this study, and for all samples, to investigate the factors determining the growth of Utricularia in general. Figure 3 provides a visualization of these correlations.
Fig. 3.
Main component analyses (Varimax) of various growth parameters and trapping success; (A) all investigated Utricularia samples; (B) U. australis.
Among the total prey, the respective numbers of animals, algae and pollen grains were weakly correlated (R2 ≤ 0·15, P < 0·05). By contrast, the number of plant particles was correlated with the number of other objects (R2 = 0·17, P < 0·01). The same correlations were found in U. australis, with an R2 ≤ 0·21 (P < 0·01) for algae, animals and pollen grains, and R2 = 0·20 (P < 0·01) between plant particles and other objects.
In both test series, the number of total prey objects per trap correlated with the dry weight and explained it to more than 0·30 (P < 0·01). The same was true for shoot length, with a slightly lower R2 of 0·22 (P < 0·01) in both tests. The correlations with the numbers of animal prey were much weaker; in U. australis, in particular, filamentous algae were more strongly correlated with dry weight or shoot length (P < 0·01) than animal prey. The number of trapped pollen grains also correlated with both shoot length and dry weight (P < 0·01). The number of other objects seemed to have little influence.
The number of growing buds, i.e. the branching factor, was not correlated with the capture of animals or pollen grains but with the number of trapped algae in both test series (P < 0·01). In U. australis, the capture of Desmidiaceae explained 0·43 (P < 0·01) of the branching. Leaf density, i.e. the length of internodia, was negatively correlated with the total trapping success (P < 0·01). In this case, the correlation with the number of trapped animals was equal to or stronger than with trapped algae (P < 0·01). Trap density correlated positively with total trapping success (P < 0·01); the strongest correlation was found for the number of pollen grains, followed by the number of animals. Among algae, only the number of filamentous algae correlated with trap density in both test series (P < 0·01). The C/N ratio correlated negatively with the total prey (P < 0·01) and, much more strongly, with the number of trapped animals, especially Crustaceae (P < 0·01). Algae and C/N ratio showed a positive but much weaker correlation (P < 0·01). The number of pollen grains did not correlate with the C/N ratio, whereas other objects showed a positive correlation (P < 0·01). Details on the correlations between growth parameters and trapping success are given in Supplementary Data Table 3.
Water chemistry and plant growth proved to be interlinked as well. In both test series, leaf density was positively correlated with pH, conductivity, carbonate and total hardness (all P < 0·01). Trap density was negatively correlated with pH, conductivity, carbonate and total hardness (all P < 0·01). The C/N ratio was correlated with pH (P < 0·01 for all Utricularia species/P < 0·05 for U. australis only), conductivity, carbonate and total hardness (all P < 0·01) in both test series. Details on correlations between growth parameters and water chemistry are given in Supplementary Data Table 4. Our preliminary data on inorganic nutrients suggest also positive correlations between plant growth and NO3−, PO43– and K+ (data not shown) but require validation by more sensitive analytical techniques. Trapping success also correlated with water chemistry. In both test series, total prey, animals and algae correlated negatively with pH, conductivity, carbonate and total hardness (all P < 0·01). The other prey objects showed the opposite trend. Details on correlations between trapping success and water chemistry are also given in Supplementary Data Table 4.
Particular interest was paid to a possible growth promotion by the capture of algae and pollen grains. However, the number of trapped algae was correlated with the number of trapped animals, and thus any correlation between growth and the capture of algae could be a side effect of successful N uptake from animal prey. Therefore, we tested for partial correlations between plant growth and the capture of algae, using animal prey as the control variable (Table 4). The number of pollen grains was positively correlated with dry weight in both test series (P < 0·01) but the effect was weaker in U. australis. In U. australis, dry weight was also correlated with the number of trapped algae (P < 0·05). Total length and the number of buds correlated with the capture of algae (P < 0·01) but not with the capture of pollen. Trap density was positively correlated with the capture of pollen (P < 0·01) but negatively with the capture of algae (P < 0·05 for all Utricularia species/P < 0·01 for U. australis only).
Table 4.
Partial correlation between the trapping of algae and pollen with plant growth, corrected for animal prey
| All samples |
Utricularia australis |
|||
|---|---|---|---|---|
| Algae | Pollen | Algae | Pollen | |
| Dry weight | – | 0·48** | 0·22** | 0·18** |
| Total length | 0·29** | – | 0·32** | – |
| Number of buds | 0·26** | –0·12** | 0·32** | – |
| Leaf density | – | –0·31** | – | −0·19** |
| Trap density | –0·10** | 0·34** | –0·07* | 0·28** |
| C/N ratio | –0·10** | –0·11** | −0·10** | – |
A dash indicates non-significant correlations. *P < 0·05, **P < 0·01.
A comparison of isotope measurements with trapping success showed that δ15N correlated strongly with the total prey, but even more with the number of algae. The correlation with the number of trapped animals was very weak and sometimes even negative; the same was true for the number of pollen grains. Algae explained 0·37 (P < 0·01) of the δ15N in U. australis. Little or no correlation was found between δ15N and dry weight, shoot length or C/N ratio (Table 5).
Table 5.
Correlations between δ15N and selected growth parameters and the trapping success
| All samples | Utricularia australis | |
|---|---|---|
| Total prey | 0·05* | 0·22** |
| Animal prey | – | –0·10** |
| Algal prey | –0·09** | 0·37** |
| Pollen prey | –0·09** | –0·18** |
| Other objects | 0·05* | 0·14** |
| Dry weight | – | – |
| Total length | – | – |
| Number of buds | 0·29** | 0·28* |
| Leaf density | 0·51** | 0·51** |
| Trap density | –0·38** | –0·52** |
| C/N ratio | – | – |
A dash indicates non-significant correlations. *P < 0·05, **P < 0·01.
DISCUSSION
Algae and pollen grains are the most abundant prey objects
Although Utricularia is commonly regarded as a carnivorous plant, very different kinds of small objects are collected by the traps. In 1844 traps, a total of 20 856 prey objects were found, i.e. 11·3 objects per trap. Among these, only 1912 (9·2 %, i.e. 1·0 per trap) were multicellular animals. Among metazoa, the most important taxa were Crustacea (1640; 7·9 %) and insect larvae (193; 0·9 %). Acari, Nematoda, Tardigrada and Rotifera were rarely found; the same was true for eggs of Crustacea (<50; 0·2 %). Kurbatova and Yershov (2009) confirm the negligible importance of Rotifera but suggest that their fast digestion by the traps may form a bias in quantification.
With 10 483 (50·3 %) individuals, algae formed the most abundant group of prey objects. Coccale algae (7353; 35·3 %) were dominant, followed by filamentous algae (2968; 14·2 %). Flagellates were rare (163; 0·7 %), and capsale and rhizopodiale species were lacking completely. These results are in accordance with a previous study which used partly the same water bodies (Peroutka et al., 2008). Pollen formed another important group of prey objects with 7018 (33·6 %) grains. As all studied habitats except Postteich are surrounded by large coniferous forests, pollen grains are seasonally abundant on the surface of all water bodies. However, not all species of Utricularia are equally efficient in trapping floating pollen grains. In Seetaler See, U. vulgaris contained 28·0 ± 32·1 (maximum 123) pollen grains per trap, whereas in U. minor in the same water body only 1·8 ± 3·8 (maximum 38) grains per trap were found. The position of the traps of U. vulgaris just beneath the water surface may facilitate the capture of pollen floating on the surface. By contrast, U. minor in the same water body trapped highly significantly more algae, especially Desmidiaceae and other coccale algae, as well as more other objects. The position of the leaves and traps within the water body, i.e. in the surface microlayer in U. vulgaris or further down in U. minor, seems to have a signicant influence on the prey spectrum and may be a strategy to reduce competition.
In all investigated traps, only 21 other plant-derived particles were found, exclusively moss leaflets. All other organisms were trapped in negligible amounts as well: 35 protozoa, seven fungal hyphae and 13 fungal spores.
The ratio between the various groups of prey objects differed highly significantly between the different water bodies. The key result of a previous study (Peroutka et al., 2008) was confirmed: the percentage of algae among the total prey correlated negatively with the conductivity of the water [R2 = –0·19 (P < 0·01) for all three species, R2 = –0·13 (P < 0·01) for U. australis]. Thus, algae are trapped especially in nutrient-poor waters. The composition of algal prey from the samples from the Alps was different from those from the Bohemian massif; the same was true for northern Germany, Canada, south-eastern USA or West Africa as described by Alkhalaf et al. (2009, 2011) and Wołkowski et al. (2011). For example, Desmidiacaeae such as Micrasterias, Penium or Staurodesmus were rare in northern Germany, whereas the diversity of Bacillariophyta was higher. Differences in the prey of Utricularia seem to reflect differences in the regional algal diversity.
The low number of protozoa contradicts the results of Płachno et al. (2012) who found a large portion of Paramecium bursaria in U. reflexa. These authors, however, used plants cultivated in plastic tanks, growing in the greenhouse under reduced light conditions and at a higher pH than in the water bodies used in our study. As Utricularia seems to trap small objects from the ambient water unselectively (Ellison and Gotelli, 2001), these conditions may have caused an increase in the abundance of protozoa both in the medium and in the traps.
Algae and pollen grains contribute to the nutrition of Utricularia
As animals play only a limited role within the total prey, the question arises if algae and pollen grains are only useless bycatch which cannot be avoided by the unselective suction traps, or if they contribute to the nutrition of the plant. Concerning algae, Jobson et al. (2000) found that feeding with exclusively Euglena caused a negative effect on the growth of Utricularia. Gordon and Pacheco (2007) suggest that Utricularia would achieve the maximum benefit of a balanced diet consisting of both animals and algae but do not provide experimental evidence for this. Alkhalaf et al. (2009) refer to the uptake of N from algae artificially labelled with 15N, and further suggest (Alkhalaf et al., 2011) that algae may form an important source of N. Concerning pollen grains, the situation is similar: Pinguicula lusitanica was shown to benefit from feeding with pollen grains (Harder and Zemlin, 1968), and the same may be true for some species of Drosera (Lavarack, 1979).
Among the parameters measured in this study, long shoots and a heavy dry weight can be regarded as indicators of good growth. Intensive branching, i.e. the formation of numerous buds, seems to be of specific importance as branching has been shown to correlate with successive sexual reproduction in Utricularia (Adamec, 2011b). Stunted growth, i.e. reduced length of internodia and a high leaf density, indictates low vitality according to Adamec et al. (2010). Similarily, a high C/N ratio indicates poor N supply. A high number of traps was regarded not only as an indicator of good growth but also of high investment into carnivory. Indeed, the measured dry weight correlated positively with total length and the number of buds and traps but negatively with leaf density.
The number of trapped animals correlates strongly negatively and highly significantly with the C/N ratio and strongly and highly significantly with dry weight, but much less strongly with other growth parameters such as shoot length or the number of buds. These factors show a stronger correlation with the capture of algae and pollen grains which contribute little to a low C/N ratio. Pollen of evergreen trees usually exhibits a C/N ratio of 20–45 (Descolas-Gros and Schölzel, 2007). In spite of the increased δ15N in pollen grains (Descolas-Gros and Schölzel, 2007), the number of trapped pollen shows only a very weak correlation with δ15N, thus confirming the limited relevance of pollen for N uptake.
Clearly, animals serve as the most important source of N, possibly besides N-fixing bacteria (Sirová et al., 2014), but this is not the only limiting factor for Utricularia. Algae and pollen grains seem to contribute other essential compounds, possibly phosphorus, trace elements or organic growth factors. The high importance of phosphorus for aquatic Utricularia was stressed by Sirová (2012).
The benefit gained from algae correlates with their size: the numbers of multicellular filamentous algae correlate much better with growth parameters than the small, unicellular Desmidiaceae. Unlike algae and pollen grains, prey particles summarized as ‘other objects’ (protozoa, fungi, moss leaflets and bacterial colonies) appear to be useless bycatch. The number of these objects correlated only weakly or even negatively with the various growth parameters; leaf density and C/N ratio were increased by the capture of this kind of prey.
Trap formation correlated well with other growth parameters; thus, abundant trap formation is only found in healthy, vigorously growing plants. The number of traps was higher in artificial peat bogs that contain more inorganic nutrients than natural fen waters (Loub et al., 1954). A similar correlation between substrate nutrient supply and trap formation was also described by Knight and Frost (1991). Correlation was also found between trap formation and trapping success: plants with many traps caught significantly more animals, filamentous algae and pollen grains, both in total and per trap. Thus, Utricularia seems to benefit simultaneously in three ways from nutrient-rich water: (1) improved nutrient supply from the water enables (2) increased formation of traps which (3) catch more prey objects per trap as nutrient-rich waters are more productive. It remains to be investigated if the dissolved nutrients in the water are the stimulus for trap formation, or if trap formation is promoted by the high trapping success of existing bladders.
It remains of debate whether algae are killed within the traps (Mette et al., 2000; Richards, 2001; Peroutka et al., 2008) or survive for an elongated period and support Utricularia possibly as mutualistic symbionts (Schumacher, 1960; Botta, 1976; Płachno et al., 2012). Our observations, both from the field and from the lab, yielded evidence for death and decay of the majority of algae, regardless of their taxonomic affiliation. More resistant species of algae may be able to survive within the traps but such species seem to be rare in the clean and undisturbed water bodies used in this study. Ongoing studies should resolve this question.
Autonomous firing of traps is confirmed
According to conventional opinion, the suction process in Utricularia is triggered by mechanical stimulation of sensitive hairs next to the trap door (e.g. Lloyd, 1942; Sydenham and Findlay, 1973; Barthlott et al., 2004). Thus, algae and pollen grains could be trapped only as bycatch after stimulation of the trap by animals, and thus a strong correlation between prey animals and other prey objects could be predicted. A first statistical analysis (Peroutka et al., 2008), however, gave little evidence for a correlation between the numbers of trapped algae and animals. In this study, with a much larger sample size, a significant but poor correlation (R2 = 0·15, P < 0·01) between trapped algae and animals was found. The correlation between animals and pollen grains was even weaker (R2 = 0·05, P < 0·05) and the correlation between animals and other objects was negative (R2 = −0·11, P < 0·01). Furthermore, 33·4 % of the traps (616 of 1844) contained algae but no animals. In 954 traps (51·4 %), only immotile or extremely small prey including algae, moss leaflets, pollen grains, protozoa or bacterial colonies were found. Similar results have been described by Mette et al. (2000) and Jobson et al. (2000). Thus, mechanical stimulation of a trap is not a prerequisite for successful prey capture.
Recent studies by Adamec (2011a) and Vincent et al. (2011a, b) provided evidence that the suction traps of Utricularia are capable of autonomous firing under laboratory conditions. After an elongated time without stimulation, the trap door opens spontaneously and sucks in the surrounding water, possibly containing small organisms. The results presented here indicate that this behaviour is of great ecophysiological relevance and significantly contributes to the nutrition of Utricularia.
δ15N data are of limited use in aquatic Utricularia
Some of the δ15N data shown in this study allow for easy interpretation, e.g. the strong correlation between total prey and δ15N. The calculation of prey-derived N, however, leads to serious problems: the established formula which provides excellent results for terrestrial carnivorous plants (Schulze et al., 1991, 2001; Brearley, 2011) is not applicable for aquatic Utricularia because it gave unrealistically high values (≥100 % prey-derived N) for several test populations. Possible reasons include that only one species of floating non-carnivorous plants was available at each site. These control plants were not submerged vascular plants absorbing nutrients via the whole shoot surface like Utricularia but either algae or floating Lemnaceae absorbing nutrient via the root. Thus, 15N uptake from the water is not necessarily comparable between these species.
Besides the evidence discussed previously, the δ15N results further point to the absorption of N from trapped algae. Algae may exhibit very different δ15N compared with vascular plants (Cole et al., 2004). Pollen of evergreen trees also exhibits an increased δ15N (Descolas-Gros and Schölzel, 2007) compared with the control plants used in this study. Thus, the unrealistically high calculated proportion of prey-derived N seems to be caused by the mass capture of algae and pollen grains. Unfortunately, this explanation implies that the established formula for the calculation of animal-derived N is of limited value in systems where other types of captured objects play a major role.
CONCLUSIONS
Prey capture does not compensate completely for an oligotrophic substrate. On the contrary, Utricularia benefits both from high nutrient concentrations in the water and from successful prey capture. Abundant traps are formed only by well-nourished plants growing in waters rather rich in nutrients. In such water bodies, prey objects of all kinds are more abundant as well. Thus, the benefit gained from prey is much higher in mesotrophic than in oligotrophic waters. The contribution of pollen grains and algae to the nutrition of the plant is comparable with the benefit gained from prey animals. Whereas animals are the main source of N, other nutrients or growth factors are derived from algae and pollen. Due to the aberrant δ15N in some algae, δ15N measurements are of limited use for the quantification of prey benefit in Utricularia populations trapping significant amounts of algae.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: Overview of the water bodies sampled. Table S2: Utricularia species and non-carnivorous plants occurring in the studied water bodies and their major chemical characteristics. Table S3: Correlations between selected growth parameters and trapping success. Table S4: Correlations between water chemistry, growth parameters and trapping success.
ACKNOWLEDGEMENTS
Thanks are due to L. Adamec (Czech Academy of Sciences) and I. K. Lichtscheidl (University of Vienna) for proofreading and discussions, and to W. Pois (Evangelischer Friedhof Matzleinsdorf, Vienna), M. Edlinger (Bundesgärten Schönbrunn) and M. Volgger for their help with plant sampling. Isotope measurements were made possible by Dr W. Wanek and G. Theiner (University of Vienna). This project was supported by the Hochschuljubiläumsstiftung der Stadt Wien [Grant H-02319/2007].
LITERATURE CITED
- Adamec L. 1992. Interrelationship between uptake of mineral ions and photosynthesis in submerged aquatic plants. Biologia Plantarum 34: 501. [Google Scholar]
- Adamec L. 2002. Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist 155: 89–100. [DOI] [PubMed] [Google Scholar]
- Adamec L. 2006. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biology 8: 765–769. [DOI] [PubMed] [Google Scholar]
- Adamec L. 2011a. The comparision of mechanically stimulated and spontaneous firings in traps of aquatic carnivorous Utricularia species. Aquatic Botany 94: 44–49. [Google Scholar]
- Adamec L. 2011b. Shoot branching of the aquatic carnivorous plant Utricularia australis as the key process of plant growth. Phyton 51: 133–148. [Google Scholar]
- Adamec L, Sirova D, Vrba J. 2010. Contrasting growth effects of prey capture in two aquatic carnivorous plant species. Fundamental and Applied Limnology 176: 153–160. [Google Scholar]
- Adlassnig W, Peroutka M, Lichtscheidl IK, Lambers H. 2005. Roots of carnivorous plants. Plant and Soil 274: 127–140. [Google Scholar]
- Alkhalaf IA, Hübener T, Porembski S. 2009. Prey spectra of Utricularia species (Lentibulariaceae) in northeastern Germany: the role of planctonic algae. Flora 204: 700–708. [Google Scholar]
- Alkhalaf IA, Hübener T, Porembski S. 2011. Microalgae trapped by carnivorous bladderworts (Utricularia, Lentibulariaceae): analysis, attributes and structure of the microalgae trapped. Plant Diversity and Evolution 129: 125–138. [Google Scholar]
- Barthlott W, Porembski S, Fischer E, Gemmel B. 1998. First protozoa-trapping plant found. Nature 392: 447.9548248 [Google Scholar]
- Barthlott W, Porembski S, Seine R, Theisen I. 2004. Karnivoren. Biologie und Kultur fleischfressender Pflanzen, 1st edn Stuttgart: Eugen Ulmer. [Google Scholar]
- Benzing DH. 1987. The origin and rarity of botanical carnivory. TREE 2: 364–369. [DOI] [PubMed] [Google Scholar]
- Botta SM. 1976. Sobre las trampas y las víctimas o presas de algunas especies argentinas del género Utricularia. Darwiniana (Buenos Aires) 20: 127–154. [Google Scholar]
- Brearley FQ. 2011. Natural abundance of stable isotopes reveals the diversity of carnivorous plant diets. Carnivorous Plant Newsletter 40: 84–87. [Google Scholar]
- Büsgen M. 1883. Die Bedeutung des Insektenfanges für Drosera rotundifolia L. Botanische Zeitung 35: 569–577. [Google Scholar]
- Chandler GE, Anderson JW. 1976. Studies on the nutrition and growth of Drosera species with reference to the carnivorous habitat. New Phytologist 76: 129–141. [Google Scholar]
- Christensen L. 1976. The role of carnivory in Sarracenia flava with regard to specific nutrient deficiences. Journal of the Elisha Mitchell Scientific Society 92: 144–147. [Google Scholar]
- Cole ML, Valiela I, Kroeger KD, et al. 2004. Assessment of a δ15N isotopic method to indicate anthropgenic eutrophication in aquatic ecosystems. Journal of Environmental Quality 33: 124–132. [DOI] [PubMed] [Google Scholar]
- Descolas-Gros C, Schölzel C. 2007. Stable isotope ratios of carbon and nitrogen in pollen grains in order to characterize plant functional groups and photosynthetic pathway types. New Phytologist 176: 390–401. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. 2001. Evolutionary ecology of carnivorous plants. TRENDS in Ecology & Evolution 16: 623–629. [Google Scholar]
- Englund G, Harms S. 2001. The functional response of a predatory plant preying on swarming zooplankton. Oikos 94: 75–181. [Google Scholar]
- Englund G, Harms S. 2003. Effects of light and microcrustacean prey on growth and investment in carnivory in Utricularia vulgaris. Freshwater Biology 48: 786–794. [Google Scholar]
- Garbini A. 1898. La vittime della Utricularia neglecta. [Google Scholar]
- Givnish TJ. 1989. Ecology and evolution of carnivorous plants. In: Abrahamson WG, ed. Plant–animal interactions . New York: McGraw-Hill, 243–290. [Google Scholar]
- Gordon E, Pacheco S. 2007. Prey composition in the carnivorous plants Utricularia inflata and U. gibba (Lentibulariaceae) from Paria Peninsula, Venezuela. Revista de Biologia Tropical 55: 795–803. [DOI] [PubMed] [Google Scholar]
- Harder R, Zemlin I. 1968. Blütenbildung von Pinguicula lusitanica in vitro durch Fütterung mit Pollen. Planta 78: 72–78. [DOI] [PubMed] [Google Scholar]
- Hegner RW. 1926. The interelations of protozoa and the utricles of Utricularia. Biological Bulletin 50: 239–270. [Google Scholar]
- Jobson RW, Morris EC, Burgin S. 2000. Carnivory and nitrogen supply affect the growth of the bladderwort Utricularia uliginosa. Australian Journal of Botany 48: 549–560. [Google Scholar]
- Knight SE, Frost TM. 1991. Bladder control in Utricularia macrorhiza: lake-specific variation in plant investment in carnivory. Ecology 72: 728–734. [Google Scholar]
- Kostytschew S. 1923. Die Photosynthese der Insektivoren. Berichte der Deutschen Botanischen Gesellschaft 41: 277–280. [Google Scholar]
- Krafft CC, Handel SN. 1991. The role of carnivory in the growth and reproduction of Drosera filiformis and D . rotundifolia. Bulletin of the Torrey Botanical Club 118: 12–19. [Google Scholar]
- Kurbatova SA, Yershov I Yu. 2009. Crustaceans and rotifers in the predatory feeding of Utricularia. Inland Water Biology 2: 271–275. [Google Scholar]
- Lavarack PS. 1979. Rainforest Drosera of north Queensland. Carnivorous Plant Newsletter 8: 61–62. [Google Scholar]
- Lloyd FE. 1942. The carnivorous plants, 1st edn New York: Ronald Press. [Google Scholar]
- Loub W, Url W, Kiermayer O, Diskus A, Hilmbauer K. 1954. Die Algenzonierung in Mooren des österreichischen Alpengebietes. Sitzungsberichte der Österreichischen Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse, Abteilung I 153: 447–494. [Google Scholar]
- Maier R. 1973. Produktions- und Pigmentanalyse an Utricularia vulgaris L. Ökosystemforschung: 87–101. [Google Scholar]
- McPherson S. 2010. Carnivorous plants and their habitats, vol. 1, 1st edn Poole: Redfern Natural History Productions. [Google Scholar]
- Mette N, Wilbert N, Barthlott W. 2000. Food composition of aquatic bladderworts (Utricularia, Lentibulariaceae) in various habitats. Beiträge zur Biologie der Pflanzen 72: 1–13. [Google Scholar]
- Oudman J. 1935. Nährstoffaufnahme und Transport durch die Blätter von Drosera capensis L. Proceedings of the Section of Sciences 38: 650–662. [Google Scholar]
- Peroutka M, Adlassnig W, Volgger M, Lendl T, Url WG, Lichtscheidl IK. 2008. Utricularia: a vegetarian carnivorous plant? Algae as prey of bladderwort in oligotrophic bogs. Plant Ecology 199: 153–162. [Google Scholar]
- Płachno BJ, Łukaszek M, Wołowski K, Adamec L, Stolarczyk P. 2012. Aging of Utricularia traps and variability of microorgansisms associated with that microhabitat. Aquatic Botany 97: 44–48. [Google Scholar]
- Richards J. 2001. Bladder function in Utricularia purpurea (Lentibulariaceae): is carnivory important? American Journal of Botany 88: 170–176. [PubMed] [Google Scholar]
- Sasago A, Sibaoka T. 1985a. Water extrusion in the trap bladders of Utricularia vulgaris. I. A possible pathway of water across the bladder wall. Journal of Plant Research 98: 55–66. [Google Scholar]
- Sasago A, Sibaoka T. 1985b. Water extrusion in the trap bladders of Utricularia vulgaris. II. A possible mechanism of water outflow. Journal of Plant Research 98: 113–124. [Google Scholar]
- Schulze E-D, Gebauer G, Schulze W, Pate JS. 1991. The utilization of nitrogen from insect capture by different growth forms of Drosera from Southwest Australia. Oecologia 87: 240–246. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Schulze I, Oren R. 2001. Quantification of insect nitrogen utilization by the venus fly trap Dionaea muscipula catching prey with highly variable isotope signatures. Journal of Experimental Botany 52: 1041–1049. [DOI] [PubMed] [Google Scholar]
- Schumacher GJ. 1960. Further notes on the occurence of desmids in Utricularia bladders. Castanea 25: 62–65. [Google Scholar]
- Seine R, Porembski S, Balduin M, Theisen I, Wilbert N, Barthlott W. 2002. Different prey strategies of terrestrial and aquatic species in the carnivorous genus Utricularia (Lentibulariaceae). Botanische Jahrbücher für Systematik 124: 71–76. [Google Scholar]
- Shearer G, Kohl DH. 1988. Estimation of N2 fixation in ecosystems: the need for and basis of the 15N natural abundance method. In: Rundel PW, Ehleringer JR, Nagy KA, eds. Stable isotopes in ecological research . Berlin: Springer Verlag, 342–375. [Google Scholar]
- Sirová D. 2012. Hunters or gardeners? Plant–microbe interactions in rootless carnivorous Utricularia. Ph.D. Thesis Series of the University of South Bohemia 8: 1–22. [Google Scholar]
- Sirová D, Borovec J, Šantrůčková H, Šantrůček J, Vrba J, Adamec L. 2010. Utricularia carnivory revisited: plants supply photosynthetic carbon to traps. Journal of Experimental Botany 61: 99–103. [DOI] [PubMed] [Google Scholar]
- Sirová D, Šantrůček J, Adamec L, et al. 2014. Dinitrogen fixation associated with shoots of aquatic carnivorous plants: is it ecologically important? Annals of Botany 114: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydenham PH, Findlay GP. 1973. The rapid movement of the bladder of Utricularia sp. Australian Journal of Biological Sciences 26: 1115–1126. [Google Scholar]
- Thum M. 1988. The significance of carnivory for the fitness of Drosera in its natural habitat. 1. The reactions of Drosera intermedia and D. rotundifolia to supplementary feeding. Oecologia 75: 472–480. [DOI] [PubMed] [Google Scholar]
- Ulanowicz RE. 1995. Utricularia’s secret: the advantage of positive feedback in oligotrophic environments. Ecological Modelling 79: 49–57. [Google Scholar]
- Vincent O, Marmottant P. 2011. Carnivorous Utricularia. The buckling scenario. Plant Signaling & Behavior 6: 1752–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent O, Roditchev I, Marmottant P. 2011a. Spontaneous firings of carnivorous aquatic Utricularia traps: temporal patterns and mechanical oscillations. PLoS ONE 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent O, Weiβkopf C, Poppinga S, et al. 2011b. Ultra-fast underwater suction traps. Proceedings of the Royal Society (London) 278: 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wołkowski K, Piβtek J, Płachno B. 2011. Algae and stomatocysts associated with carnivorous plants. First report of chrysophyte stomatocysts from Virginia, USA. Phycologia 50: 511–519. [Google Scholar]
- Zamora R, Gómez JM, Hódar JA. 1998. Fitness responses of a carnivorous plant in contrasting ecological scenarios. Ecology 79: 1630–1644. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.