Abstract
Background and Aims Environmental temperature regulates plant regeneration via seed in several superimposed ways, and this complex regulation will be disrupted by climate change. The role of diurnally alternating temperatures (ΔT) in terminating dormancy will be a major factor in this disruption, as its effects on seed germination are immediate.
Methods The effect of ΔT on seed germination was modelled using two populations of the wetland sedge Carex diandra, one from a montane site and one from a subalpine site. A cardinal-temperature model was fitted to germination results obtained from a thermal gradient plate, and the model was used to simulate changes in germination under two possible future climate scenarios (RCP2·6 and RCP8·5, for representative concentration pathways) as defined by the Intergovernmental Panel on Climate Change.
Key Results Scenario RCP2·6 projected moderate increases in average temperatures and ΔT, whereas RCP8·5 projected greater warming and higher ΔT. Increasing ΔT decreased the base temperature for seed germination and the thermal time required for germination. The effect of higher ΔT together with the higher temperatures increased germination under both climate scenarios.
Conclusions Carex diandra germination is highly responsive to potential changes in ΔT, and thus this study highlights the role of ΔT in seed responses to climate change. Comprehensive cardinal-temperature models, encompassing the different effects of temperature on seed germination, are needed to understand how climate change will affect plant regeneration.
Keywords: Cardinal-temperature models, climate change, germination thermal threshold models, physiological dormancy, plant–climate interactions, plant regeneration, representative concentration pathways, seed ecology
INTRODUCTION
Climate change (IPCC, 2013) will disrupt the many interactions between biology and climate, from enzymatic reactions to ecological patterns. Climate thoroughly controls key processes such as plant regeneration, as is exemplified by the thermal regulation of seed germination (Probert, 2000; Fenner and Thompson, 2005). Temperature drives local adaptation (Wagmann et al., 2012; Fernández-Pascual et al., 2013a) and phenotypic plasticity (Fenner, 1991; Hoyle et al., 2008; Figueroa et al., 2010; Chiang et al., 2011; Fernández-Pascual and Jiménez-Alfaro, 2014) in germination traits, as well as the physiological processes of dormancy loss (Pritchard et al., 1996; Batlla and Benech-Arnold, 2003, 2004, 2005; Finch-Savage and Leubner-Metzger, 2006; Steadman and Pritchard, 2004) and germination elicitation (García-Huidobro et al., 1982; Pritchard and Manger, 1990; Hardegree, 2006). In seasonal climates, germination traits interplay with annual temperature cycles to ensure that seed emergence and seedling establishment occur in the most favourable season (Vleeshouwers et al., 1995; Donohue et al., 2010). Given the significance of germination in the life history of a plant, it is not surprising that its timing is a central scenario for natural selection (Donohue et al., 2005a, b, c). However, the complex thermal control of germination timing is highly responsive to climate change (Walck et al., 2011). New environmental temperatures may not match the temperatures that alleviate dormancy (Orrù et al., 2012) and elicit germination (Cochrane et al., 2011). This mismatch could alter recruitment from the soil seed bank (Ooi et al., 2009; Ooi, 2012; Hoyle et al., 2013) and shift germination timing (Mondoni et al., 2012), compromising plant regeneration and community composition.
Nonetheless, many aspects of the potential seed response to climate change remain unstudied. Together with a steady increase in average temperatures, climate change projections point to more frequent hot and fewer cold daily extremes (IPCC, 2013). Depending on the relative change in daily maxima and minima, diurnal thermal oscillations could actually rise, fall or remain unchanged, affecting the physiological processes of species (Vasseur et al., 2014). It has been known for a long time that diurnally alternating temperatures (hereafter ΔT) promote germination in many plant species (Morinaga, 1926; Probert, 2000; Fenner and Thompson, 2005). This germination response to ΔT is especially common in wetland and disturbed habitats, where it detects favourable establishment environments with regard to burial depth, soil perturbation or water level (Thompson and Grime, 1983; Liu et al., 2013). Physiologically, ΔT may act through a decrease in the ratio of abscisic acid to gibberellins, which reduces the embryo’s water potential and promotes radicle growth (Huarte and Benech-Arnold, 2005, 2010; Huarte, 2013). The effect of thermal oscillations is almost immediate, and on the first day after transfer from constant to ΔT regimes imbibed seeds undergo a large transcriptome change involving metabolism, plant hormones and the circadian clock (Foley et al., 2010). ΔT is thus an ‘immediate’ signal for dormancy alleviation which marks that emergence conditions are immediately favourable, contrasting with slower dormancy loss associated with seasonal change, for example overwintering (Finch-Savage and Leubner-Metzger, 2006). This immediacy means that if climate change modifies ΔT, plant regeneration will be strongly affected as small changes in amplitude will translate into quick changes in emergence.
Recent research has begun to explore seed responses to climate change using cardinal-temperature models (Orrù et al., 2012; Porceddu et al., 2013). In this approach, germination experiments are employed to fit germination models based on two thresholds, namely (1) the base temperature (Tb) below which the germination rate is zero and (2) the thermal time (θ) or degrees-day above the Tb required to reach successive percentage germination increases in a seed population (Hardegree, 2006; Porceddu et al., 2013). Subsequently, the models are tested under average temperature projections to simulate potential emergence patterns in the future (Orrù et al., 2012). In terms of cardinal-temperature models, increasing ΔT decreases the base temperature and the thermal time for germination, i.e. germination is faster (Qiu et al., 2006), similar to other dormancy-breaking factors such as chilling (Steadman and Pritchard, 2004; Pritchard et al., 1996, 1999). It is therefore clear that in those species with a germination response to ΔT, an accurate simulation will require the incorporation of the dormancy-breaking effect of ΔT into the models. To our knowledge this step in model design and testing has not yet been taken.
Here we describe for the first time a simulation of seed germination under projected diurnal thermal oscillations according to climate change scenarios. To do so we chose as a study species the wetland sedge Carex diandra (Cyperaceae). C. diandra is a widely distributed northern hemisphere sedge that occupies transitional mire soils (Habitats Directive 7140 Transition mires and quaking bogs; Jiménez-Alfaro et al., 2014) in the margins of lakes and ponds (Tutin et al., 1980; Castroviejo et al., 2007), habitats where the germination response to ΔT seems to be a pervasive adaptation. We used seeds from two contrasting altitudes to establish comparative cardinal-temperature models describing the fresh germination response to ΔT. We then used the models to simulate germination changes under the ΔT projected in representative concentration pathway (RCP) scenarios (Vuuren et al., 2011). RCPs define different pathways of total radiative forcing produced by accumulation of all sources of human greenhouse gas emissions. RCPs do not represent specific scenarios of environmental/economic projections; instead each of them could result from different combinations of economic, technological, demographic, policy and institutional futures (http://sedac.ipcc-data.org/ddc/ar5_scenario_process/RCPs.html). We chose RCP2·6 (lowest radiative forcing, i.e. lowest climatic alteration) and RCP8·5 (highest radiative forcing, i.e. highest climatic alteration). We address two specific hypotheses in freshly dispersed seeds with conditional dormancy: (1) increasing ΔT will lower the base temperature and shorten the thermal time for germination; and (2) simulated germination timing will change under the ΔT projections of the RCPs.
MATERIALS AND METHODS
Study system
Carex diandra Schrank produces abundant seeds that disperse in summer and show conditional physiological dormancy, being able to germinate at warm temperatures immediately after dispersal and acquiring the capacity to germinate at cooler temperatures as they lose dormancy (Fernández-Pascual et al., 2013b). In late July 2012, ripe C. diandra seeds were collected from two ponds of the Cantabrian Mountains, the montane site ‘La Mina’ (43°16′N, 4°59′W, 1110 m asl) and the subalpine site ‘La Recoleta’ (43°1′N, 6°7′W, 1780 m asl) (Molina et al., 2009). The day after collection, seeds were cleaned and kept in controlled conditions (15 °C, 15 % relative humidity) until further use.
To calculate present and future values of environmental average temperatures and ΔT at the population sites, present model-derived monthly maximum and minimum temperatures at the population sites were downloaded from WorldClim (Hijmans et al., 2005), and projections of the same variables for the year 2070 under the RCP2·6 and RCP8·5 scenarios were downloaded from the HadGEM2-ES model (Jones et al., 2011).
Germination experiment
The germination experiment began in November 2012. For each population, 25 seeds were sown onto 169 Petri dishes (1 % agar). Each population was placed on a bidirectional thermal gradient plate (Model GRD1, Grant Instruments, Cambridge, UK). While one gradient remained constant, the other changed direction every 12 h, providing 169 thermoperiod combinations with averages from 7·5 to 37·5 °C and ΔT from 0 to 30 °C (Fig. 1). The thermoperiods were coupled with a 12/12-h light–dark photoperiod, half of the dishes having a warmer day regime and half a warmer night regime. Actual temperatures in the gradient were recorded with five temperature probes (one in the centre and one in each corner of the thermal gradient plate), placed into the agar Petri dishes and set to record every 10 min for the duration of the experiment. From these records we calculated the average day and night temperatures of each Petri dish, and used these averages in further calculations. Germination (radicle emergence) was scored daily, and the dishes were replenished periodically to prevent the agar from drying. The experiment lasted 5 weeks by which time no further germination was observed. A random sample (n = 300 per population) of non-germinated seeds were cut open to determine the occurrence of empty and fungus-infected seeds. The percentage on non-viable seeds was low (4 %) and therefore the germination percentages were calculated over the total number of seeds sown (25).
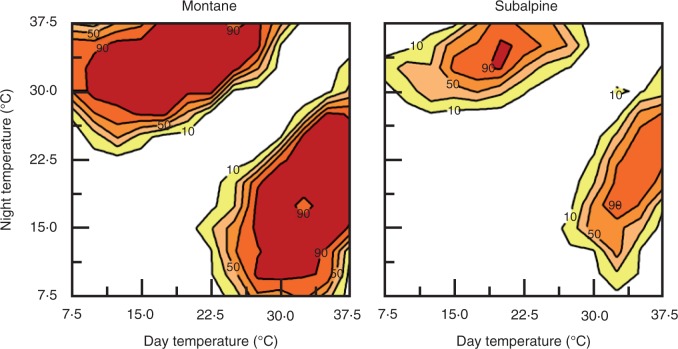
Fig. 1.
Germination of C. diandra on the thermal gradient plate. Contour plots with points of equal percentage germination connected by germination isopleths. The shading represents 20 % germination increases, from lower (lighter filling) to higher (darker filling). Seeds were collected from a montane site (left panel) or subalpine site (right panel). The transverse axes of symmetry from the bottom-left corner to the top-right corner are constant thermal regimes. Points above and below the axes represent diurnally alternating temperature regimes, with greatest amplitude at the top-left (warm night, cold day) and bottom-right (warm day, cold night) corners.
Cardinal-temperature model
Cardinal-temperature models (García-Huidobro et al., 1982; Pritchard and Manger, 1990; Hardegree, 2006; Qiu et al., 2006; Orrù et al., 2012; Porceddu et al., 2013) were used to describe the germination thermal thresholds under different ΔT regimes. For each dish, the time (tg, days) taken for cumulative germination to reach successive 10 % increases (g) was calculated by linear interpolation on the germination curves. Data from dishes were grouped according to their regime of ΔT (± 2 °C), and for each regime the rate of germination (1/tg) was plotted against the average germination temperature. Available treatments did not allow analysis of the supra-optimal temperature range, which would not be relevant ecologically for the environment of the Cantabrian Mountains (see Discussion). The suboptimal germination temperature range was identified by visual inspection of the germination rate plots, and a linear regression was fitted to it according to:
The average x-intercept among g percentiles was the Tb for each ΔT, and Tb was allowed to vary among g to consider variation among individual seeds.
Subsequently the thermal time required to reach successive g (θg, °Cd) was estimated as the inverse of the slope of the sub-optimal linear regression equations. Values of g were transformed into probits, and new linear regressions were fitted between probit (g) and θg expressed as both linear θg and log θg. Linear θg gave higher R2 values, and therefore linear equations were used to describe the germination of seeds as a function of thermal time under each ΔT regime:
The reciprocal of the slope (σ) represents the sensitivity of the population to thermal time; flatter slopes indicate higher variability among individual seeds in their response. K is the intercept when θ is zero.
Statistical analysis
To determine how the thermal thresholds varied depending on the ΔT regime, the seed population and the phase when temperatures were higher (day or night), Generalized Linear Models (gamma regression) were fitted to the values of Tb and θ50. Then, considering only the dishes with a warmer day regime (the ecologically meaningful situation) and separately for each population, linear or logarithmic equations were used to estimate Tb and θ50 as a function of ΔT values at present and in the RCP2·6 and RCP8·5 scenarios. Finally, the thermal thresholds and the accumulation of degrees-day for germination were simulated under (1) present average monthly temperatures and ΔT, (2) RCP average monthly temperatures but present ΔT, and (3) RCP average monthly temperatures and ΔT.
RESULTS
Germination response to temperature
Germination of C. diandra did not occur under constant thermoperiods; a ΔT of at least 2 °C was necessary for some germination to take place (Fig. 1). The highest germination percentages occurred with an average (day + night) temperature of around 25 °C and a ΔT of 10–14 °C. The montane population had higher germination (a total of 1798 germinated seeds) than the subalpine (687 germinated seeds). These differences were due to dormancy and not seed quality, as the subalpine seeds were able to produce >95 % germination in some treatments (e.g. 35/20 °C). Germination was possible both under warmer day and warmer night regimes, and although slightly more seeds germinated when the warm phase coincided with light, the differences were minimal (1258 seeds vs. 1227 seeds, averaging both populations).
Cardinal-temperature model and effect of ΔT
Tb was significantly different among the two populations, being estimated at 16·8 ± 0·5 °C for the montane seeds and 20·3 ± 0·6 °C for the subalpine seeds whereas the phase of higher temperatures did not affect Tb significantly (Table 1). Conversely, θ50 was not significantly different among populations but was significantly higher in warmer day regimes (258·8 ± 27·5°Cd), compared with warmer night regimes (162·1 ± 19·0°Cd) (Table 1).
Table 1.
Sources of variation in the thermal thresholds, base temperature for germination (Tb) and thermal time for 50 % germination (θ50): results of a main effects Generalized Linear Model (gamma regression) that included diurnal thermal amplitude (ΔT), population of origin and warmer phase (i.e. whether the warmer phase of the photoperiod was coupled to the light or the dark phase) as fixed predictors
| Effect |
Tb |
θ50 |
||
|---|---|---|---|---|
| F | P | F | P | |
| ΔT | 36·626 | <0·001 | 19·01 | <0·001 |
| Population | 20·404 | <0·001 | 1·943 | 0·172 |
| Warmer phase | 0·092 | 0·763 | 8·739 | 0·005 |
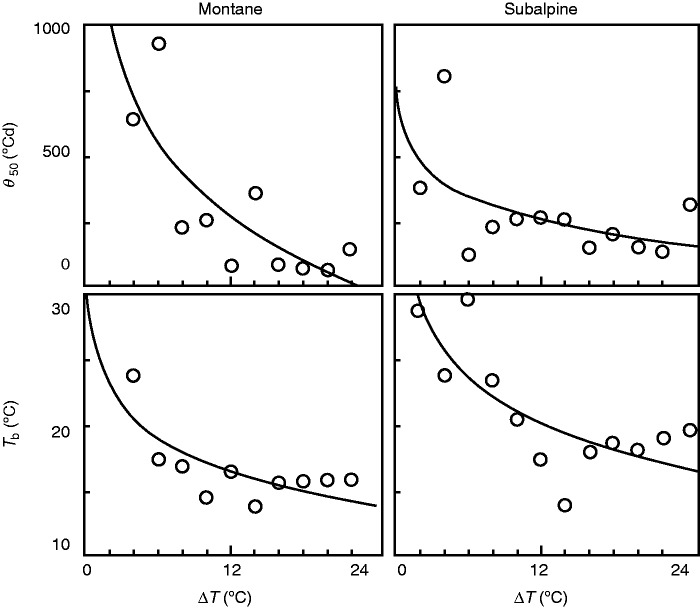
We were able to establish sub-optimal cardinal-temperature models for ΔT regimes from 2 to 24 °C. ΔT significantly influenced both Tb and θ50 (Table 1). In both cases the effect followed a logarithmic function (Fig. 2), with a sharp decrease as ΔT increased from 0 to 10–14 °C and stabilization with higher values. Tb followed a linear decrease between 0 and 14 °C; as this matched the range of ΔT found in the temperatures of the models, we used a linear equation to estimate the monthly Tb under the monthly ΔT projected at present and under the two RCPs. For θ50, we estimated an annual average using a logarithmic equation and the annual average ΔT under each scenario.
Fig. 2.
Effect of diurnal thermal amplitude (ΔT) on the base temperature for germination (Tb) and the thermal time for 50 % germination (θ50) for seeds from montane and subalpine sites. The symbols represent the average values of Tb and θ50 at each ΔT regime; the lines are the logarithmic fits to the data.
Simulated germination under climate change
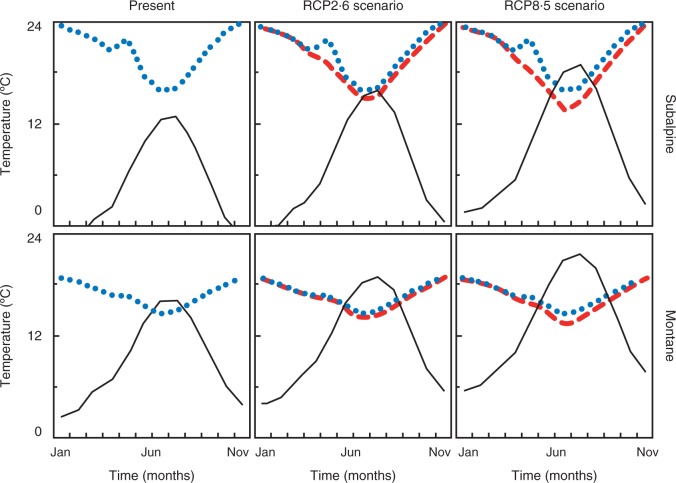
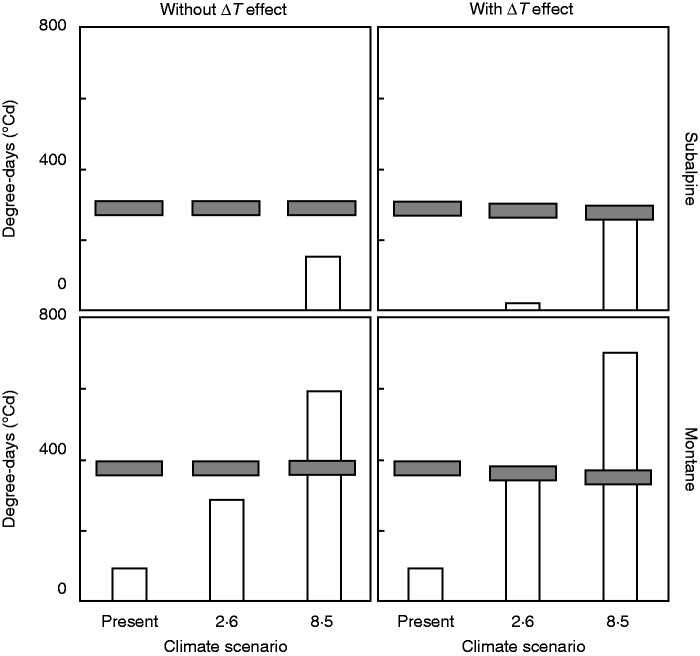
The average annual temperature at present according to models was 6·7 °C with a ΔT of 9·5 °C. The RCP2·6 projected an average of 8·8 °C with a ΔT of 9·9 °C. The RCP8·5 projected an average of 11·0 °C with a ΔT of 10·3 °C. As a consequence of the increasing ΔT of the RCPs, Tb (averaging the two populations) was projected to decrease from 18·6 °C at present to 18·2 °C in RCP2·6 and to 17·8 °C in RCP8·5 (Fig. 3). Present Tb and environmental temperatures would allow some accumulation of degree-days for fresh germination at the montane site, but prevent it at the subalpine site (Fig. 4). Under RCP2·6, the accumulation of degree-days would increase notably in the montane population, but would still not occur in the subalpine population (Fig. 4). Under RCP8·5, accumulation would be high in both populations (Fig. 4). When the lowering of Tb as a consequence of the new ΔT is incorporated into the simulation, the accumulation of degree-days increases in both sites and RCPs, the effect being more marked in the subalpine site where accumulation appears under RCP2·6 and doubles under RCP8·5 (Fig. 4).
Fig. 3.
Simulated environmental temperature and base temperature (Tb) for germination at present and under the RCP2.6 and RCP8.5 scenarios. Each panel shows the results for one population under a given scenario. The solid line is the environmental monthly average temperature, the dotted line represents Tb projected on the basis of the present diurnal thermal amplitude (ΔT) and the broken line is for Tb projected in relation to the ΔT of the scenario.
Fig. 4.
Annual accumulated environmental heat sum for germination (EHR) and thermal time required for 50 % germination (h50) at present and under the RCP2·6 (2·6) and RCP8·5 (8·5) scenarios for montane and subalpine populations. The vertical columns are EHR and the grey horizontal bars are h50. The results of the simulation are shown without (left panels) and with (right panels) taking into account the effect of the diurnal thermal oscillations (ΔT).
The value of θ50 would also vary with the ΔT of each climatic scenario, although the change would be minimal (Fig. 4). At the montane site it was projected to decrease by 4 % from the present value in RCP2·6 and by 8 % in RCP8·5. At the subalpine site the decrease would be comparatively smaller, 3 % in RCP2·6 and in 5 % RCP8·5. Nonetheless, the combined effects of a higher accumulation of degree-days and a lower θ50 would change seed germination patterns in both sites (Fig. 4). In the montane site, 50 % fresh germination would occur in the summer season of dispersal under either scenario. The subalpine population would be unaffected under RCP2·6, but under RCP8·5 significant fresh germination would occur at dispersal, 50 % if the ΔT effect is considered.
DISCUSSION
Germination response to ΔT
Germination in C. diandra has an absolute requirement for ΔT. Such a strong germination response to ΔT is a common trait of sedges (Schütz, 1997, 2000; Schütz and Rave, 1999; Budelsky and Galatowitsch, 1999) and wetland species in general (Thompson and Grime, 1983; Maas, 1989; Baskin and Baskin, 1998; Patzelt et al., 2001; Carta et al., 2013). In the case of C. diandra, it could allow seeds to detect a fall of the water table in the pond margins it colonizes. ΔT marks the beginning of the favourable season for dormancy-breaking and germination, at least in related fen soils of the Cantabrian Mountains where amplitude is minimal in winter, increases in spring and peaks in summer (unpubl. result). Nevertheless, there are differences among sites and ΔT is less successful in alleviating dormancy in the subalpine population, following a pattern of deeper dormancy at higher altitudes that is common to many species (Cavieres and Arroyo, 2000; Fernández-Pascual et al., 2013a; Zhou and Bao, 2014). Conditional dormancy in C. diandra can also be alleviated in the long term by cold-overwintering (Fernández-Pascual et al., 2013b), and this signal of slow seasonal change (Finch-Savage and Leubner-Metzger, 2006) is probably more significant in the subalpine site, where winter is harsher and premature emergence riskier.
These dormancy differences operate through Tb, mirroring the positive latitudinal correlation that has been found in the Tb of Aesculus hippocastanum L. populations (Daws et al., 2004). On the other hand, θ50 does not vary among populations, contrasting with the negative correlation between altitude and θ50 that has been found in Vitis vinifera L. (Orrù et al., 2012). Tb, as well as the total number of germinated seeds, does not vary depending on whether the light phase coincides with the warm or the cool phase of the thermoperiod. This agrees with previous work that shows that the amplitude of the thermal oscillation is the main parameter that explains the germination response to ΔT (Roberts and Totterdell, 1981; Benech-Arnold et al., 1990a, b; Batlla et al., 2003). Conversely, θ50 is significantly lower when the night is warmer than the day, i.e. seeds germinate faster in that case. It is difficult to interpret this abnormal and ecologically unrealistic situation, but it could be related to the role of the phytochrome in the response to ΔT (Probert, 2000).
A main effect of ΔT in C. diandra is that Tb decreases as ΔT increases, thus facilitating the accumulation of degree-days for germination (i.e. under a given average temperature, more degrees above Tb can be accumulated each day). The relationship between Tb and ΔT is linear up to 10–14 °C, whereas it becomes asymptotic with larger ΔT values, as was found in certified seeds of Dactylis glomerata L. (Qiu et al., 2006). ΔT above the optimal amplitude did have a negative effect in wild European populations of D. glomerata (Probert et al., 1986). However, supraoptimal values do not seem realistic in C. diandra field conditions and therefore lack ecological significance as temperatures above 25 °C will not be reached in the Cantabrian Mountains, even under the more extreme climatic scenarios (Álvarez García et al., 2009). The optimal ΔT in C. diandra and D. glomerata contrasts with what was found in grassland species living in disturbed soils at very high altitudes in the Tibetan Plateau, where the optimum ΔT was 20 °C and 10 °C had no effect (Liu et al., 2013). Such high amplitudes can be expected in the extreme Tibetan conditions, and therefore the optimal ΔT might be closely related to the thermal range of the specific habitat.
Implications for climate change
Simulation of C. diandra germination under RCP scenarios points to an increase in emergence parallel to the intensity of the RCP scenario and to the associated increase in average temperatures. However, the full magnitude of the change is only appreciated when the response to ΔT is incorporated into the simulation, as this increases the daily accumulation of degree-days at the same time that it decreases the cumulative degree-days needed for the population to germinate. The effect of incorporating ΔT is particularly relevant at the subalpine site, where the accumulation of degree-days doubles under the RCP8·5 scenario. Total germination increases are higher at the montane site, where a high percentage of the seeds are not expected to overwinter and join the soil seed bank even under the moderate RCP2·6 scenario. However, as germination at dispersal at the montane site seems to be possible at present, it is to be expected that seedlings might survive winters there. Conversely, the harsher winters at the subalpine site make premature germination in autumn or during early winter a more deleterious trait, and accordingly dormancy there is deeper. Therefore, the change could have more negative consequences at the subalpine site in the RCP8·5 scenario, as germination in the same summer of dispersal would change from negligible to affecting half the seed population. Although our findings are based on a simulation of how one species might respond across two sites, they do highlight the probable importance of ΔT on regeneration under climate change of the many plant species that show a response to ΔT. The latest climate change analysis at the C. diandra study sites reports a higher increase of daily maxima in comparison with daily minima (Álvarez García et al., 2009), so ΔT could already be modifying germination patterns.
Note that our simulation is a simplification of nature that concentrates on the effect of ΔT on fresh germination, whereas in the field temperature will influence germination in other ways. A new climate will modify germination traits through phenotypic plasticity (Fenner, 1991; Daws et al., 2004; Hoyle et al., 2008; Chiang et al., 2011; Fernández-Pascual et al., 2013a; Fernández-Pascual and Jiménez-Alfaro, 2014). For example, present differences in Tb between the montane and subalpine sites are larger (16·8 vs. 20·3 °C) than the differences in Tb projected among scenarios (18·6–17·8 °C). Therefore, if all the present variation among sites is attributable to phenotypic plasticity, this plasticity would accommodate the effect produced by climate change (Cochrane et al., 2014). However, our experimental design does not at this stage allow for differentiation between phenotypic plasticity and genetically based local adaptation (Fernández-Pascual et al., 2013a).
Furthermore, our simulation is only valid for the germination of fresh seeds, i.e. seed germination between dispersal in summer and the beginning of the winter season in November. In the field, temperatures during overwintering would alleviate dormancy (Fernández-Pascual et al., 2013b), probably slowly lowering Tb (Pritchard et al., 1996, 1999; Batlla and Benech-Arnold, 2003; Steadman and Pritchard, 2004; Porceddu et al., 2013) and reducing relative sensitivity to ΔT (Benech-Arnold et al., 1990a, 2000; Batlla et al., 2003). Such anticipated changes require consideration. Finally, in situ soil temperatures can be expected to vary from those projected by air models, especially in fen soils where the buffer effect of the spring water is strongly felt (unpubl. res.). Overall, further complexity is needed in the development of cardinal-temperature models before they can encompass all the thermal determinants of seed germination and accurately predict plant regeneration under climate change.
ACKNOWLEDGEMENTS
T. García Gutiérrez, E. Lázaro González and P. Martín Pevida contributed to seed collection, which was authorized by the governments of Asturias and Castile-Leon. Special thanks to P. Lewis and O. Tierney for experimental support. J. Adams, N. Keogh and L. Queste also helped with laboratory work. We thank three anonymous reviewers for their suggested improvements to the manuscript. The research was funded by the Department of Education and Science of the Government of Asturias (Convenio entre la Administración del Principado de Asturias y la Universidad de Oviedo, para el Desarrollo del Plan Estratégico de Viabilidad y Conversión a Campus de Excelencia Internacional) and had the institutional support of the Royal Botanic Gardens, Kew, and Defra. E.F.P. was supported by the Government of Asturias (Grant BP09-107, Programa de Ayudas Predoctorales ‘Severo Ochoa’, Plan de Ciencia, Tecnología e Innovación del Principado de Asturias).
LITERATURE CITED
- Álvarez García MÁ, De Castro M, Cruz Guerrero R, Gómez Borrego V, Pérez Muñuzuri V, Stoll H. 2009. Clima. In: Anadón R, Roqueñí N, eds. CLIMAS. Evidencias y efectos potenciales del cambio climático en Asturias . Oviedo: Consejería de Medio Ambiente, Ordenación del Territorio e Infraestructuras. [Google Scholar]
- Baskin CC, Baskin JM. 1998. Seeds. Ecology, biogeography, and evolution of dormancy and germination . San Diego: Academic Press. [Google Scholar]
- Batlla D, Benech-Arnold RL. 2003. A quantitative analysis of dormancy loss dynamics in Polygonum aviculare L. seeds: development of a thermal time model based on changes in seed population thermal parameters. Seed Science Research 13: 55–68. [Google Scholar]
- Batlla D, Benech-Arnold RL. 2004. A predictive model for dormancy loss in Polygonum aviculare L. seeds based on changes in population hydrotime parameters. Seed Science Research 14: 277–286. [Google Scholar]
- Batlla D, Benech-Arnold RL. 2005. Changes in the light sensitivity of buried Polygonum aviculare seeds in relation to cold-induced dormancy loss: development of a predictive model. New Phytologist 165: 445–452. [DOI] [PubMed] [Google Scholar]
- Batlla D, Verges V, Benech-Arnold RL. 2003. A quantitative analysis of seed responses to cycle-doses of fluctuating temperatures in relation to dormancy: development of a thermal time model for Polygonum aviculare L. seeds. Seed Science Research 13: 197–207. [Google Scholar]
- Benech-Arnold RL, Ghersa CM, Sanchez RA, Insausti P. 1990a. A mathematical model to predict Sorghum halepense (L.) Pers. seedling emergence in relation to soil temperature. Weed Research 30: 91–99. [Google Scholar]
- Benech-Arnold RLG, Ghersa CM, Sanchez RA, Insausti P. 1990b. Temperature effects on dormancy release and germination rate in Sorghum halepense (L.) Pers. seeds: a quantitative analysis. Weed Research 30: 81–89. [Google Scholar]
- Benech-Arnold RL, Sánchez RA, Forcella F, Kruk BC, Ghersa CM. 2000. Environmental control of dormancy in weed seed banks in soil. Field Crops Research 67: 105–122. [Google Scholar]
- Budelsky RA, Galatowitsch SM. 1999. Effects of moisture, temperature, and time on seed germination of five wetland Carices: implications for restoration. Restoration Ecology 7: 86–97. [Google Scholar]
- Carta A, Bedini G, Müller J, Probert R. 2013. Comparative seed dormancy and germination of eight annual species of ephemeral wetland vegetation in a Mediterranean climate. Plant Ecology 214: 339–349. [Google Scholar]
- Castroviejo S, Luceño M, Galán A, Jiménez Mejías P, Cabezas F, Medina L. 2007. Flora iberica vol. XVIII Cyperaceae-Pontederiaceae . Madrid: Real Jardín Botánico, CSIC. [Google Scholar]
- Cavieres LA, Arroyo MTK. 2000. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae). Plant Ecology 149: 1–8. [Google Scholar]
- Cochrane A, Daws MI, Hay F. 2011. Seed-based approach for identifying flora at risk from climate warming. Austral Ecology 36: 923–935. [Google Scholar]
- Cochrane A, Yates CJ, Hoyle GL, Nicotra AB. 2014. Will among-population variation in seed traits improve the chance of species persistence under climate change? Global Ecology and Biogeography. doi:10.1111/geb.12234. [Google Scholar]
- Chiang GC, Bartsch M, Barua D, et al. 2011. DOG1 expression is predicted by the seed-maturation environment and contributes to geographical variation in germination in Arabidopsis thaliana. Molecular Ecology 20: 3336–3349. [DOI] [PubMed] [Google Scholar]
- Daws MI, Lydall E, Chmielarz P, et al. 2004. Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytologist 162: 157–166. [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. 2005a. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution 59: 740–757. [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. 2005b. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evolution 59: 758–770. [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. 2005c. Niche construction through germination cueing: life-history responses to timing of germination in Arabidopsis thaliana. Evolution 59: 771–785. [PubMed] [Google Scholar]
- Donohue K, de Casas RR, Burghardt L, Kovach K, Willis CG. 2010. Germination, postgermination adaptation, and species ecological ranges. In: Futuyma DJ, Shafer HB, Simberloff D, eds. Annual Review of Ecology, Evolution, and Systematics 41. Palo Alto: Annual Reviews Inc. [Google Scholar]
- Fenner M. 1991. The effects of the parent environment on seed germinability. Seed Science Research 1: 75–84. [Google Scholar]
- Fenner M, Thompson K. 2005. The ecology of seeds . Cambridge: Cambridge University Press. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B. 2014. Phenotypic plasticity in seed germination correlates differentially with overwintering and flowering temperatures. Seed Science Research 24: 273–280. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, Caujapé-Castells J, Jaén-Molina R, Díaz TE. 2013a. A local dormancy cline is related to the seed maturation environment, population genetic composition and climate. Annals of Botany 112: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, Díaz TE. 2013b. The temperature dimension of the seed germination niche in fen wetlands. Plant Ecology 214: 489–499. [Google Scholar]
- Figueroa R, Herms DA, Cardina J, Doohan D. 2010. Maternal environment effects on common groundsel (Senecio vulgaris) seed dormancy. Weed Science 58: 160–166. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Foley ME, Anderson JV, Chao WS, Doğramacı M, Horvath DP. 2010. Initial changes in the transcriptome of Euphorbia esula seeds induced to germinate with a combination of constant and diurnal alternating temperatures. Plant Molecular Biology 73: 131–142. [DOI] [PubMed] [Google Scholar]
- García-Huidobro J, Monteith JL, Squire GR. 1982. Time, temperature and germination of pearl millet (Pennisetum typhoides S. & H.): I. Constant temperature. Journal of Experimental Botany 33: 288–296. [Google Scholar]
- Hardegree SP. 2006. Predicting germination response to temperature. I. Cardinal-temperature models and subpopulation-specific regression. Annals of Botany 97: 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hoyle GL, Steadman KJ, Daws MI, Adkins SI. 2008. Pre- and post-harvest influences on seed dormancy status of an Australian Goodeniaceae species, Goodenia fascicularis. Annals of Botany 102: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GL, Venn SE, Steadman KJ, et al. 2013. Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Global Change Biology 19: 1549–1561. [DOI] [PubMed] [Google Scholar]
- Huarte HR. 2013. Mecanismos fisiológicos y moleculares involucrados en la terminación de la dormición de semillas expuestas a las temperaturas alternadas . PhD thesis, University of Buenos Aires. [Google Scholar]
- Huarte HR, Benech-Arnold RL. 2005. Incubation under fluctuating temperatures reduces mean base water potential for seed germination in several non-cultivated species. Seed Science Research 15: 89–97. [Google Scholar]
- Huarte HR, Benech-Arnold RL. 2010. Hormonal nature of seed responses to fluctuating temperatures in Cynara cardunculus (L.). Seed Science Research 20: 39–45. [Google Scholar]
- IPCC. 2013. Climate Change 2013. The physical science basis. Summary for policymakers . Geneva: Intergovernmental Panel on Climate Change. [Google Scholar]
- Jiménez-Alfaro B, Hájek M, Ejrnaes R, et al. 2014. Biogeographic patterns of base-rich fen vegetation across Europe. Applied Vegetation Science 17: 367–380. [Google Scholar]
- Jones CD, Hughes JK, Bellouin N, et al. 2011. The HadGEM2-ES implementation of CMIP5 centennial simulations. Geoscientific Model Development 4: 543–570. [Google Scholar]
- Liu K, Baskin JM, Baskin CC, Bu H, Du G, Ma M. 2013. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the eastern Tibet Plateau. PloS ONE 8: e69364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas D. 1989. Germination characteristics of some plant species from calcareous fens in southern Germany and their implications for the seed bank. Holartic Ecology 12: 337–344. [Google Scholar]
- Molina A, Acedo C, Lence C, et al. 2009. Estado de conservación y recatalogación UICN de Carex diandra Schrank en España. In: Llamas F, Acedo C, eds. Botánica pirenaico-cantábrica en el siglo XXI. León: University of León. [Google Scholar]
- Mondoni A, Rossi G, Orsenigo S, Probert RJ. 2012. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany 110: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga T. 1926. Effect of alternating temperatures upon the germination of seeds. American Journal of Botany 13: 141–158. [Google Scholar]
- Ooi MKJ. 2012. Seed bank persistence and climate change. Seed Science Research 22: 53–60. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. 2009. Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology 15: 2375–2386. [Google Scholar]
- Orrù M, Mattana E, Pritchard HW, Bacchetta G. 2012. Thermal thresholds as predictors of seed dormancy release and germination timing: altitude-related risks from climate warming for the wild grapevine Vitis vinifera subsp. sylvestris. Annals of Botany 110: 1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt A, Wild U, Pfadenhauer J. 2001. Restoration of wet fen meadows by topsoil removal: vegetation development and germination biology of fen species. Restoration Ecology 9: 127–136. [Google Scholar]
- Porceddu M, Mattana E, Pritchard HW, Bacchetta G. 2013. Thermal niche for in situ seed germination by Mediterranean mountain streams: model prediction and validation for Rhamnus persicifolia seeds. Annals of Botany 112: 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard HW, Manger KR. 1990. Quantal response of fruit and seed germination rate in Quercus robur L. and Castanea sativa Mill, to constant temperatures and photon dose. Journal of Experimental Botany 41: 1549–1557. [Google Scholar]
- Pritchard HW, Tompsett PB, Manger KR. 1996. Development of a thermal time model for the quantification of dormancy loss in Aesculus hippocastanum seeds. Seed Science Research 6: 127–135. [Google Scholar]
- Pritchard HW, Steadman KJ, Nash JV, Jones C. 1999. Kinetics of dormancy release and the high temperature germination response in Aesculus hippocastanum seeds. Journal of Experimental Botany 50: 1507–1514. [Google Scholar]
- Probert RJ. 2000. The role of temperature in the regulation of seed dormancy and germination. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities. Wallingford: CABI, 261–292. [Google Scholar]
- Probert R, Smith R, Birch P. 1986. Germination responses to light and alternating temperatures in European populations of Dactylis glomerata L. V. The principal components of the alternating temperature requirement. New Phytologist 102: 133–142. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bai Y, Coulman B, Romo JT. 2006. Using thermal time models to predict seedling emergence of orchardgrass (Dactylis glomerata L.) under alternating temperature regimes. Seed Science Research 16: 261–271. [Google Scholar]
- Roberts EH, Totterdell S. 1981. Seed dormancy in Rumex species in response to environmental factors. Plant, Cell & Environment 4: 97–106. [Google Scholar]
- Schütz W. 1997. Primary dormancy and annual dormancy cycles in seeds of six temperate wetland sedges. Aquatic Botany 59: 75–85. [Google Scholar]
- Schütz W. 2000. Ecology of seed dormancy and germination in sedges (Carex). Perspectives in Plant Ecology, Evolution and Systematics 3: 67–89. [Google Scholar]
- Schütz W, Rave G. 1999. The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies. Plant Ecology 144: 215–230. [Google Scholar]
- Steadman KJ, Pritchard HW. 2004. Germination of Aesculus hippocastanum seeds following cold-induced dormancy loss can be described in relation to a temperature-dependent reduction in base temperature (Tb) and thermal time. New Phytologist 161: 415–425. [DOI] [PubMed] [Google Scholar]
- Thompson K, Grime JP. 1983. A comparative study of germination responses to diurnally-fluctuating temperatures. Journal of Applied Ecology 20: 141–146. [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, et al. 1980. Flora europaea Volume 5 . Cambridge: Cambridge University Press. [Google Scholar]
- Vasseur DA, DeLong JP, Gilbert B, et al. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proceedings of the Royal Society B: Biological Sciences 281: 20132612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037. [Google Scholar]
- Vuuren DP, Edmonds J, Kainuma M, et al. 2011. The representative concentration pathways: an overview. Climatic Change 109: 5–31. [Google Scholar]
- Wagmann K, Hautekèete N-C, Piquot Y, Meunier C, Schmitt SE, Van Dijk H. 2012. Seed dormancy distribution: explanatory ecological factors. Annals of Botany 110: 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Global Change Biology 17: 2145–2161. [Google Scholar]
- Zhou Z, Bao W. 2014. Changes in seed dormancy of Rosa multibracteata Hemsl. & E. H. Wilson with increasing elevation in an arid valley in the eastern Tibetan Plateau. Ecological Research 29: 693–700. [Google Scholar]