Abstract
Recent epidemiological evidence suggests that there are two forms of Crohn disease (CD): perforating and nonperforating. We hypothesized that, just as with tuberculoid and lepromatous leprosy, differences in the two forms of CD would be both identified and determined by differences in the host immune response. Resected intestinal tissue from control patients as well as perforating and nonperforating CD patients was evaluated for mRNA levels. We employed 32P PCR amplification with published or custom-designed primers of a housekeeping gene (beta-actin); a human T-cell marker (CD3-delta); and the cytokines tumor necrosis factor alpha, transforming growth factor beta, granulocyte/macrophage colony-stimulating factor, interleukin (IL) 1 beta, IL-1ra, and IL-6. Differences were identified with IL-1 beta (control = 162 +/- 57 vs. perforating = 464 +/- 154 vs. nonperforating = 12,582 +/- 4733; P < or = 0.02) and IL-1ra (control = 1337 +/- 622 vs. perforating = 2194 +/- 775 vs. nonperforating = 9715 +/- 2988; P < or = 0.02). These data corroborate the epidemiological observation that there are two forms of CD. Nonperforating CD, the more benign form, is associated with increased IL-1 beta and IL-1ra mRNA expression. We conclude that it is the host immune response that determines which form of CD becomes manifest in any given individual and discuss the investigative, diagnostic, and therapeutic implications of these observations.
Full text
PDF
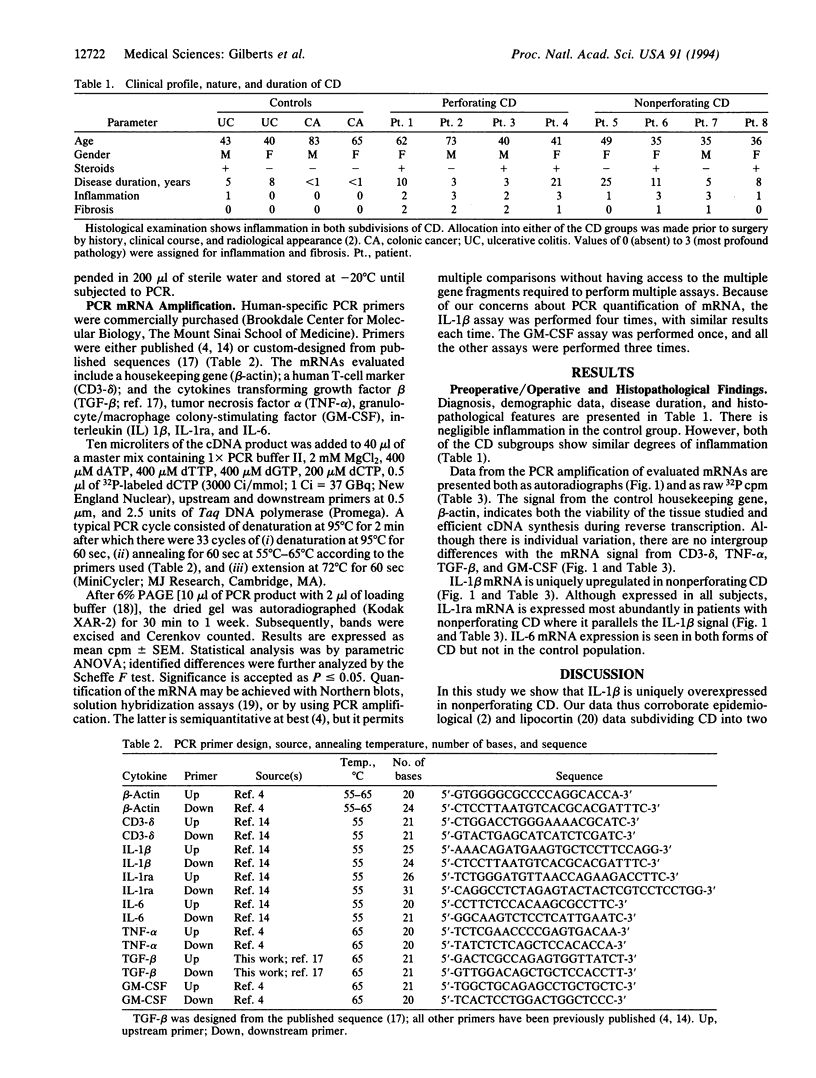
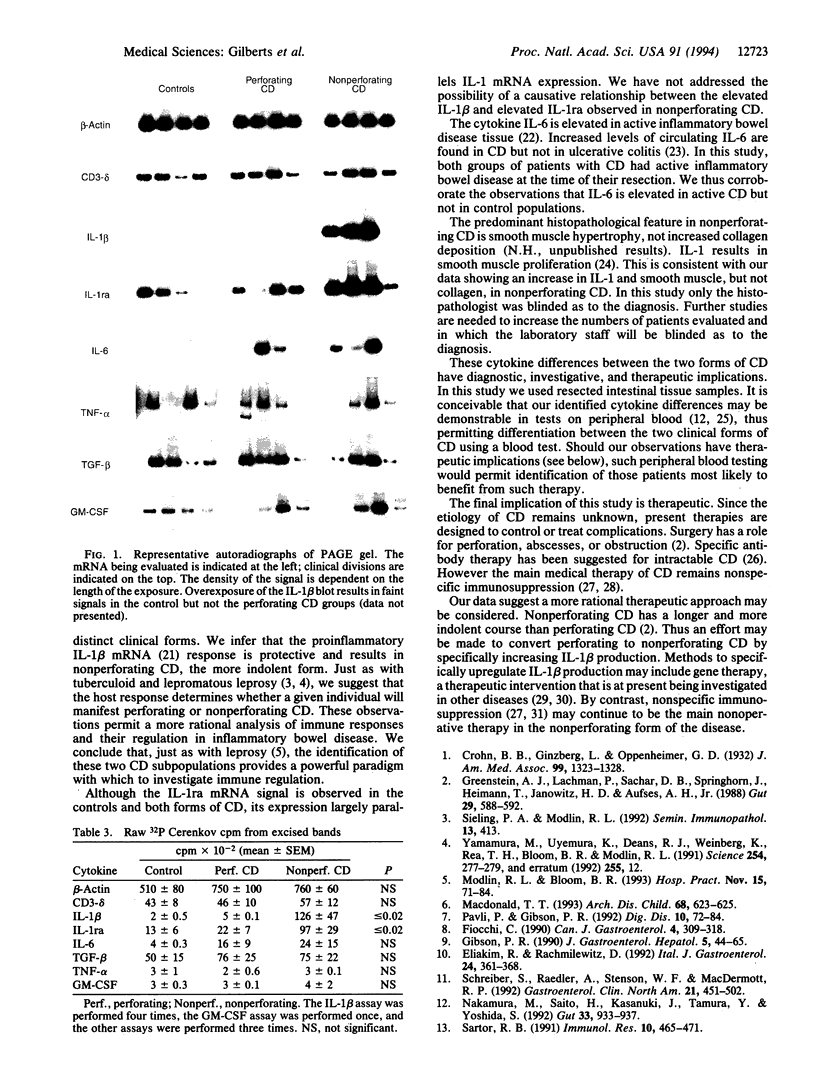

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brynskov J. The role of ciclosporin therapy in Crohn's disease. A review. Dig Dis. 1991;9(4):236–244. doi: 10.1159/000171308. [DOI] [PubMed] [Google Scholar]
- Derkx B., Taminiau J., Radema S., Stronkhorst A., Wortel C., Tytgat G., van Deventer S. Tumour-necrosis-factor antibody treatment in Crohn's disease. Lancet. 1993 Jul 17;342(8864):173–174. doi: 10.1016/0140-6736(93)91375-v. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Rachmilewitz D. Inflammatory mediators and the pathogenesis of inflammatory bowel disease. Ital J Gastroenterol. 1992 Jul-Aug;24(6):361–368. [PubMed] [Google Scholar]
- Gibson P. R. Current concepts in the pathogenesis of Crohn's disease. J Gastroenterol Hepatol. 1990 Jan-Feb;5(1):44–65. doi: 10.1111/j.1440-1746.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Greenstein A. J., Lachman P., Sachar D. B., Springhorn J., Heimann T., Janowitz H. D., Aufses A. H., Jr Perforating and non-perforating indications for repeated operations in Crohn's disease: evidence for two clinical forms. Gut. 1988 May;29(5):588–592. doi: 10.1136/gut.29.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein R. J., Isola L., Gordon J. Differential cholecystokinin gene expression in brain and gut of the fasted rat. Am J Med Sci. 1990 Jan;299(1):32–37. doi: 10.1097/00000441-199001000-00008. [DOI] [PubMed] [Google Scholar]
- Hanauer S. B., Smith M. B. Rapid closure of Crohn's disease fistulas with continuous intravenous cyclosporin A. Am J Gastroenterol. 1993 May;88(5):646–649. [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T. Aetiology of Crohn's disease. Arch Dis Child. 1993 May;68(5):623–625. doi: 10.1136/adc.68.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Kurlac L., Gallagher A., Hawkey C. J. High circulating concentrations of interleukin-6 in active Crohn's disease but not ulcerative colitis. Gut. 1991 Dec;32(12):1531–1534. doi: 10.1136/gut.32.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Maruta K., Burkart V., Gillis S., Kolb H. IL-1 and IFN-gamma increase vascular permeability. Immunology. 1988 Jun;64(2):301–305. [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Bloom B. R. Immune regulation: learning from leprosy. Hosp Pract (Off Ed) 1993 Nov 15;28(11):71-4, 77-80, 83-4. doi: 10.1080/21548331.1993.11442874. [DOI] [PubMed] [Google Scholar]
- Morgan R. A., Anderson W. F. Human gene therapy. Annu Rev Biochem. 1993;62:191–217. doi: 10.1146/annurev.bi.62.070193.001203. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Saito H., Kasanuki J., Tamura Y., Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992 Jul;33(7):933–937. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M. W., Grisham M. B. Cytokines increase proliferation of human intestinal smooth muscle cells: possible role in inflammation-induced stricture formation. Inflammation. 1993 Aug;17(4):481–487. doi: 10.1007/BF00916587. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Jones S. D., Bond B., Yamamoto K. R. The immunoglobulin octanucleotide: independent activity and selective interaction with enhancers. Science. 1987 Mar 20;235(4795):1498–1501. doi: 10.1126/science.3029871. [DOI] [PubMed] [Google Scholar]
- Pavli P., Gibson P. R. Pathogenic factors in inflammatory bowel disease. 2. Crohn's disease. Dig Dis. 1992;10(2):72–84. doi: 10.1159/000171346. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanoue Y., Horai T., Okamoto T., Hatada T., Shoji Y., Fujita S., Kusunoki M., Utsunomiya J. Lipocortin-present perforating and lipocortin-absent nonperforating Crohn's disease. Am J Surg. 1992 Oct;164(4):341–344. doi: 10.1016/s0002-9610(05)80901-8. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Pathogenetic and clinical relevance of cytokines in inflammatory bowel disease. Immunol Res. 1991;10(3-4):465–471. doi: 10.1007/BF02919743. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Raedler A., Stenson W. F., MacDermott R. P. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992 Jun;21(2):451–502. [PubMed] [Google Scholar]
- Schürmann G., Betzler M., Post S., Herfarth C., Meuer S. Soluble interleukin-2 receptor, interleukin-6 and interleukin-1 beta in patients with Crohn's disease and ulcerative colitis: preoperative levels and postoperative changes of serum concentrations. Digestion. 1992;51(1):51–59. doi: 10.1159/000200875. [DOI] [PubMed] [Google Scholar]
- Sieling P. A., Modlin R. L. T cell and cytokine patterns in leprosy skin lesions. Springer Semin Immunopathol. 1992;13(3-4):413–426. doi: 10.1007/BF00200538. [DOI] [PubMed] [Google Scholar]
- Stark M. E., Tremaine W. J. Maintenance of symptomatic remission in patients with Crohn's disease. Mayo Clin Proc. 1993 Dec;68(12):1183–1190. doi: 10.1016/s0025-6196(12)60070-6. [DOI] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M. L., Zanker B., Muggia A., Antonioli D., Peppercorn M. A., Strom T. B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992 Jun;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Tolstoshev P. Gene therapy, concepts, current trials and future directions. Annu Rev Pharmacol Toxicol. 1993;33:573–596. doi: 10.1146/annurev.pa.33.040193.003041. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]