Abstract
Background
Patients with severe left ventricular (LV) dysfunction, ischemic heart failure and coronary artery disease (CAD) suitable for coronary artery bypass grafting (CABG) are at higher risk for surgical morbidity and mortality. Paradoxically, those patients with the most severe coronary artery disease and ventricular dysfunction who derive the greatest clinical benefit from CABG are also at the greatest operative risk, which makes decision-making regarding whether to proceed to surgery difficult in such patients. To better inform such decision-making, we analyzed the STICH CABG population for detailed information on perioperative risk and outcomes.
Methods and Results
In both STICH trials (hypotheses), 2136 patients with a left ventricular ejection fraction (LVEF) ≤ 35% and coronary artery disease were allocated to medical therapy, CABG plus medical therapy or CABG with surgical ventricular reconstruction (SVR). Relationships of baseline characteristics and operative conduct with morbidity and mortality at 30 days were evaluated. There were a total of 1460 patients who received surgery, and 346 of them (roughly, one-quarter) of these high-risk patients developed a severe complication within 30 days. Worsening renal insufficiency, cardiac arrest with cardiopulmonary resuscitation, and ventricular arrhythmias were the most frequent complications and those most commonly associated with death. Mortality at 30 days was 5.1% and was generally preceded by a serious complication (65 of 74 deaths). LV size, renal dysfunction, advanced age, and atrial fibrillation/flutter were significant preoperative predictors of mortality within 30 days. Cardiopulmonary bypass time was the only independent surgical variable predictive of 30-day mortality.
Conclusions
CABG can be performed with relatively low 30-day mortality in patients with LV dysfunction. Serious postoperative complications occurred in nearly 1 in 4 patients and were associated with mortality.
Clinical Trial Registration
ClinicalTrials.gov; Unique Identifier: NCT00023595.
Keywords: coronary artery disease, heart failure, revascularization, surgery
Introduction
Patients with severe left ventricular (LV) dysfunction, ischemic heart failure and coronary artery disease (CAD) suitable to coronary artery bypass grafting (CABG) benefit from CABG.1 Because these patients are at higher risk of surgical morbidity and mortality compared to patients with milder forms of LV dysfunction, clinicians remain hesitant to refer these patients for CABG. Paradoxically, there is evidence that higher-risk patients with LV dysfunction and CAD, such as those with a lower LV ejection fraction (LVEF), more LV dilatation and multivessel disease benefit the most from CABG.2 Thus, physicians and patients are left with challenging decisions. Will the potential benefits for a given individual outweigh the short- and long-term morbidity and mortality of the procedure? A number of well-accepted surgical risk scores exist to help guide clinicians and patients in making informed decisions regarding the risks of surgery.3–6 However, although helpful, these scores have not been specifically devised for patients with severe LV dysfunction (LVEF ≤35%).
The Surgical Treatment for Ischemic Heart Failure (STICH) trial evaluated the role of CABG vs. medical treatment and of CABG vs. CABG plus ventricular reconstruction (SVR) in patients with CAD amenable to CABG and a LVEF≤35%.7 In addition to providing information regarding the risk of early (within 30 days) postoperative mortality, STICH provides information on the risk of postoperative complications and their impact on outcomes. Once surgery has been successfully performed and the first critical postoperative period survived, little information exists to inform the health care team regarding the prognosis of an individual patient or the impact of complications on their postoperative course.8 The STICH trial provides an opportunity to evaluate clinical and surgical characteristics that identify patients at risk for both early and late postoperative morbidity and mortality, information that can help influence decisions on how to proceed given a specific patient profile.
The objectives of the present analyses were to: 1) evaluate the association of baseline patient characteristics and operative conduct on 30-day postoperative complications and mortality; and 2) evaluate the incidence of postoperative complications and their association with 30-day mortality.
Methods
The overall objective of the STICH trial was to define the role of revascularization surgery in the management of patients with ischemic cardiomyopathy. STICH tested two hypotheses: 1) Whether CABG is superior to optimal medical treatment alone in improving survival in patients with a LVEF ≤35% and CAD amenable to CABG; and 2) Whether the addition of SVR to CABG in patients with anterior wall akinesis or dyskinesis is superior to CABG alone. The STICH trial Hypothesis 1 results found that while CABG did not significantly reduce all-cause mortality compared to optimal medical therapy alone, it did reduce the combined pre-specified secondary endpoint of all-cause mortality plus cardiovascular hospitalization.9 The results of Hypothesis 2 found that the addition of SVR to CABG alone did not improve mortality or freedom from cardiovascular hospitalization.10
The STICH trial was carefully planned to create a unique cohort of international CABG-eligible patients with CAD and a LVEF ≤35% for whom preoperative, intraoperative and postoperative data were prospectively acquired using structured data forms with standardized descriptions of common operative and postoperative treatment decisions. Past performance of at least 25 CABG operations on patients with a LVEF of 0.40 or less with an operative mortality of 5% or less was required for certification of all STICH surgeons. STICH cardiologists and cardiac anesthesiologists experienced in managing operative and perioperative care of CABG patients helped coordinate preoperative and postoperative patient management decisions. All participating cardiac surgeons comprised the STICH Surgical Committee that met regularly during the active recruitment and treatment phase of the study.7
The Duke University Medical Center Institutional Review Board and the institutional review board or ethics committee for each participating institution approved the study protocol, and all of the patients provided written informed consent.
Patient Population
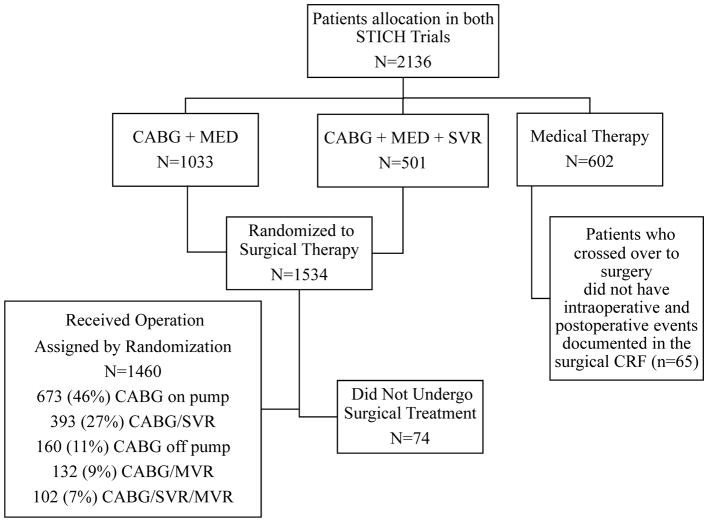
Between July 24, 2002, and May 5, 2007, there were 2136 patients enrolled into the NIH-funded STICH Trial11 and randomized to treatment with medical therapy alone (n=602), medical therapy plus CABG (n=1033), and medical therapy plus CABG and SVR (n=501) (Figure 1). Of 1534 STICH patients randomized to surgery, 74 did not undergo surgery (11 patients died after randomization and before surgery). Among the 1460 patients who received CABG, there were 495 patients who underwent CABG with SVR and 965 patients who underwent CABG alone. Patients randomized to medical therapy that ultimately received CABG during the follow-up period (n=65) were not considered for these analyses as detailed peri- and postoperative data were not collected in these patients.
Figure 1.
Consort diagram of patients enrolled in STICH that had CABG and the type of surgery performed.
All patients randomized in STICH underwent baseline evaluations that included imaging of the LV at randomization. Descriptions of operative conduct and occurrence of perioperative complication events were recorded on the structured surgical treatment clinical report form using explicit definitions (see Supplemental Material), and recorded at the time of hospital discharge post CABG or at 30 days for patients who remained in the hospital for 30 or more days. Follow-up clinical assessment was performed at the time of hospital discharge or at 30 days post operation, and at 4-month intervals for the first year of follow up, and thereafter at 6-month intervals over the remainder of the follow-up period. All postoperative in-hospital morbid events and complications that met a pre-specified definition (Supplemental Material) and occurred within 30 days after operation were tabulated.
The following postoperative complications were documented and considered major: return to the operating room (OR) for bleeding; return to the OR for any reason; mediastinitis; pulmonary edema requiring intubation; new onset ventricular arrhythmia; cardiac arrest requiring cardiopulmonary resuscitation (CPR); worsening renal insufficiency (increase >2 mg/dL and/or 2x baseline creatinine); gastrointestinal complications; respiratory compromise; other major complications; acute myocardial infarction; and stroke. The following postoperative complications were documented and considered non-major: infection other than mediastinitis; new onset atrial fibrillation; and delirium.
Statistical Analyses
Patient and operative conduct characteristics were summarized as number (percentage) for categorical variables and as median (25th, 75th percentile) for continuous variables. The characteristics of patients who did and did not develop major complications and who did and did not die within 30 days were summarized and compared with chi-square, Fisher’s exact, or Wilcoxon rank-sum tests. Mortality rates in patients with different complications within the early postoperative interval are presented as Kaplan-Meier rates at 30 days. The Cox proportional hazards regression model was used in both univariable and multivariable analyses to identify baseline and perioperative factors associated with 30-day mortality. Time to death was censored at 30 days post operation in these analyses. The multivariable analysis employed a backward elimination procedure to determine independently significant prognostic factors. For continuous variables, if needed, appropriate restricted cubic spline functions were used to assess linearity of the relationship of predictors with the log-hazard ratio in the Cox model. Candidate variables included baseline and intraoperative conduct characteristics. P-values less than 0.05 were considered statistically significant. All analyses were performed with SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patient Outcomes
Of the 1460 patients who underwent CABG, 74 patients (5.1%) died within 30 days following surgery (of whom 65 (88%) died following at least 1 major complication). Five patients died in the OR, 45 in an intensive care unit, 15 in the step-down unit and 9 at home or in another facility. A total of 346 patients (23.7%) experienced at least 1 major complication. Of these 346 patients with at least 1 major complication, 65 (18.8%) died within 30 days of surgery. The remaining 1105 (75.7%) patients were alive without major complications at 30 days post operation. Patients without a major complication had <1% mortality, those with one major complication (14.3%) had 7% mortality, those with two major complications (5.8%) had 31% mortality, those with three major complications (1.6%) had 33% mortality, and those having four or more major complications (2.0%) had 59% mortality at 30 days.
Serious Postoperative Complications
The characteristics of patients who developed a serious postoperative complication as compared with patients without serious complications are shown in Table 1. At enrollment, patients with postoperative complications were older, had more renal dysfunction defined as creatinine >1.5 mg/dL, previous CABG, more severe angina (CCS class), more symptomatic heart failure (NYHA class), a higher Duke CAD score, more atrial fibrillation or flutter, a lower LVEF, a lower hemoglobin and a lower 6-minute walk. Surgical characteristics of patients developing postoperative complications included prolonged aortic cross-clamp time, prolonged cardiopulmonary bypass pump time, and less use of cardioplegia. Surgical characteristics that marginally increased the risk of postoperative complications included SVR or mitral valve surgery. Notable characteristics not significantly associated with postoperative complications included diabetes, LV end-systolic volume index (LVESVI), and number of distal anastomoses.
Table 1.
Baseline and surgical characteristics and complications.
| N | No major complications | N | One or more major complications | p* | |
|---|---|---|---|---|---|
| Age at randomization | 1114 | 60.2 (53.3 – 67.5) | 346 | 65.1 (57.5 – 71.3) | <0.0001 |
| Female | 1114 | 148 (13.3) | 346 | 52 (15.0) | 0.4100 |
| Myocardial infarction | 1114 | 918 (82.4) | 346 | 294 (85.0) | 0.2670 |
| Hyperlipidemia | 1111 | 756 (68.0) | 346 | 229 (66.2) | 0.5181 |
| Hypertension | 1114 | 645 (57.9) | 346 | 224 (64.7) | 0.0236 |
| Diabetes | 1114 | 397 (35.6) | 346 | 137 (39.6) | 0.1818 |
| Peripheral vascular disease | 1114 | 156 (14.0) | 346 | 59 (17.1) | 0.1622 |
| Chronic renal insufficiency | 1113 | 57 (5.1) | 346 | 61 (17.6) | <0.0001 |
| Body Mass Index (kg/m2) | 1114 | 27.0 (24.4 – 30.1) | 346 | 27.2 (23.9 – 30.5) | 0.8051 |
| Creatinine (mg/dL) | 1114 | 1.05 (0.90 – 1.20) | 346 | 1.16 (1.00 – 1.40) | <0.0001 |
| Hemoglobin (g/dL) | 1114 | 13.8 (12.7 – 14.9) | 346 | 13.6 (12.4 – 14.7) | 0.0127 |
| Stroke | 1114 | 66 (5.9) | 346 | 31 (9.0) | 0.0477 |
| Previous PCI | 1114 | 174 (15.6) | 346 | 68 (19.7) | 0.0780 |
| Previous CABG | 1114 | 22 (2.0) | 346 | 16 (4.6) | 0.0069 |
| Current Canadian Cardiovascular Society Angina Class |
1114 | 346 | 0.0073 | ||
| None | 313 (28.1) | 108 (31.2) | |||
| I | 113 (10.1) | 31 (9.0) | |||
| II | 317 (28.5) | 70 (20.2) | |||
| III | 313 (28.1) | 107 (30.9) | |||
| IV | 58 (5.2) | 30 (8.7) | |||
| Current NYHA heart failure class | 1114 | 346 | <0.0001 | ||
| I | 111 (10.0) | 25 (7.2) | |||
| II | 546 (49.0) | 131 (37.9) | |||
| III | 420 (37.7) | 160 (46.2) | |||
| IV | 37 (3.3) | 30 (8.7) | |||
| Number of diseased vessels (>75% stenosis) | 1114 | 346 | 0.0650 | ||
| None | 24 (2.2) | 5 (1.4) | |||
| One | 223 (20.0) | 55 (15.9) | |||
| Two | 454 (40.8) | 132 (38.2) | |||
| Three | 413 (37.1) | 154 (44.5) | |||
| Left main stenosis ≥ 50% | 1114 | 147 (13.2) | 346 | 58 (16.8) | 0.0952 |
| Proximal LAD stenosis ≥ 75% | 1113 | 805 (72.3) | 346 | 253 (73.1) | 0.7725 |
| Duke CAD Index (0–100) | 1114 | 65 (39 – 77) | 346 | 65 (43 – 91) | 0.0078 |
| LVEF | 1114 | 28 (23 – 34) | 346 | 26 (22 – 33) | 0.0068 |
| LVESVI | 1114 | 78 (59 – 97) | 346 | 81 (61 – 104) | 0.0705 |
| Distance walked at baseline (meters) | 835 | 357 (280 – 420) | 234 | 331 (240 – 399) | 0.0005 |
| Atrial flutter fibrillation | 1114 | 112 (10.1) | 346 | 58 (16.8) | 0.0007 |
| Statin and at least one of ASA, clopidogrel, or warfarin | 1114 | 768 (68.9) | 346 | 217 (62.7) | 0.0309 |
| Mitral regurgitation | 1111 | 340 | 0.0679 | ||
| None or trace | 417 (37.5) | 110 (32.4) | |||
| Mild (≤2+) | 515 (46.4) | 162 (47.6) | |||
| Moderate (3+) | 150 (13.5) | 51 (15.0) | |||
| Severe (4+) | 29 (2.6) | 17 (5.0) | |||
| Number of distal anastomoses | 1111 | 3 (2 – 4) | 343 | 3 (2 – 4) | 0.4778 |
| Number of distal anastomoses, n (%) | 1111 | 343 | 0.4504 | ||
| 0–1 | 101 (9.1) | 32 (9.3) | |||
| 2–3 | 685 (61.7) | 199 (58.0) | |||
| ≥4 | 325 (29.3) | 112 (32.7) | |||
| More distal anastomoses than conduits | 1114 | 258 (23.2) | 346 | 78 (22.5) | 0.8119 |
| Arterial conduits ≥ 1, n (%) | 1114 | 1019 (91.5) | 346 | 308 (89.0) | 0.1657 |
| On pump surgery | 1114 | 983 (88.2) | 346 | 317 (91.6) | 0.0789 |
| Mitral valve procedure | 1114 | 164 (14.7) | 346 | 70 (20.2) | 0.0147 |
| SVR procedure | 1114 | 362 (32.5) | 346 | 133 (38.4) | 0.0413 |
| Surgery was not elective | 1114 | 130 (11.7) | 346 | 51 (14.7) | 0.1301 |
| Cardioplegia | 1110 | 346 | 0.0039 | ||
| None | 177 (15.9) | 41 (11.8) | |||
| Crystalloid | 254 (22.9) | 62 (17.9) | |||
| Blood | 646 (58.2) | 223 (64.5) | |||
| Both | 33 (3.0) | 20 (5.8) | |||
| Bypass pump time, minutes | 1114 | 95 (65 – 127) | 346 | 112 (75 – 158) | <0.0001 |
| Cardiopulmonary bypass time > 120 minutes | 1114 | 321 (28.8) | 346 | 151 (43.6) | <0.0001 |
| Aorta cross-clamp time, minutes | 1114 | 58 (36 – 83) | 346 | 67 (41 – 95) | 0.0001 |
Note: the entries in this table are median (25th, 75th percentiles) for continuous variables and n (%) for categorical variables.
Wilcoxon rank-sum tests used for continuous variables and chi-square tests used for categorical variables.
The most frequent postoperative complications (Table 2) were worsening renal insufficiency (8.4%), new onset ventricular arrhythmias (7.1%), cardiac arrest requiring CPR (4.7%), return to the OR for bleeding (3.8%), return to the OR for other reasons (3.6%), and pulmonary edema requiring intubation (3.1%). The complications most frequently associated with death were renal insufficiency (37 deaths), cardiac arrest requiring CPR (35 deaths), new onset ventricular arrhythmia (31 deaths), and pulmonary edema requiring intubation (18 deaths). Acute myocardial infarction (MI) was infrequent (0.8%) but resulted in a high death rate (42%). Stroke was also infrequent (1.6%) and was not associated with as great a risk (17%) as most other major complications. Finally, the incidence of mediastinitis was low (1.7%) and associated with a relatively low mortality rate (8%) as compared with other major complications.
Table 2.
Perioperative complications.
| Major complication | n (%) | deaths | 30-day KM mortality rate |
|---|---|---|---|
| Worsening renal insufficiency (incr. >2 mg/dL and 2x baseline creatinine) | 123 (8.4%) | 37 | 30% |
| Cardiac arrest requiring CPR | 69 (4.7%) | 35 | 51% |
| New onset ventricular arrhythmia | 103 (7.1%) | 31 | 30% |
| Pulmonary edema req. intubation | 45 (3.1%) | 18 | 40% |
| Other major complication | 21 (1.4%) | 9 | 43% |
| Return to OR for other reason | 53 (3.6%) | 9 | 17% |
| Return to OR for bleeding | 56 (3.8%) | 7 | 12% |
| Acute MI | 12 (0.8%) | 5 | 42% |
| Stroke | 24 (1.6%) | 4 | 17% |
| Respiratory compromise | 31 (2.1%) | 4 | 13% |
| GI complication | 14 (1.0%) | 2 | 14% |
| Mediastinitis | 25 (1.7%) | 2 | 8% |
| Complications not considered major | |||
| New onset atrial flutter/fibrillation | 329 (22.5%) | 26 | 8% |
| Other infection | 132 (9.0%) | 13 | 10% |
| Delirium | 65 (4.5%) | 7 | 11% |
Early (30 days) Postoperative Mortality
The baseline preoperative characteristics of patients who died within 30 days post operation differed in a number of respects compared to those who survived (Table 3). Patients who died were older, had renal dysfunction, atrial fibrillation or flutter, more symptomatic heart failure, more mitral regurgitation, a lower LVEF, and a lower hemoglobin. In addition, they had a greater LVESVI and more peripheral vascular disease. Differences between the groups in less frequently occurring characteristics such as a history of stroke (12.2% vs 6.3%) did not reach statistical significance. The only operative characteristic associated with 30 day mortality was bypass time. Type of cardioplegia was not associated with mortality.
Table 3.
Baseline and surgical characteristics and 30 day mortality.
| N | Alive at 30 days | N | 30 day death | p* | |
|---|---|---|---|---|---|
| Age at randomization | 1386 | 60.8 (53.9 – 68.1) | 74 | 66.1 (59.9 – 71.7) | 0.0002 |
| Female | 1386 | 190 (13.7) | 74 | 10 (13.5) | 0.9621 |
| Myocardial infarction | 1386 | 1146 (82.7) | 74 | 66 (89.2) | 0.1465 |
| Hyperlipidemia | 1383 | 941 (68.0) | 74 | 44 (59.5) | 0.1244 |
| Hypertension | 1386 | 817 (58.9) | 74 | 52 (70.3) | 0.0532 |
| Diabetes | 1386 | 502 (36.2) | 74 | 32 (43.2) | 0.2216 |
| Peripheral vascular disease | 1386 | 194 (14.0) | 74 | 21 (28.4) | 0.0007 |
| Chronic renal insufficiency | 1385 | 102 (7.4) | 74 | 16 (21.6) | <0.0001 |
| Body Mass Index (kg/m2) | 1386 | 27.0 (24.3 – 30.1) | 74 | 27.3 (23.4 – 30.5) | 0.9595 |
| Creatinine (mg/dL) | 1386 | 1.08 (0.92 – 1.24) | 74 | 1.30 (1.05 – 1.50) | <0.0001 |
| Hemoglobin (g/dL) | 1386 | 13.8 (12.7 – 14.9) | 74 | 13.1 (12.2 – 14.6) | 0.0369 |
| Stroke | 1386 | 88 (6.3) | 74 | 9 (12.2) | 0.0869 |
| Previous PCI | 1386 | 231 (16.7) | 74 | 11 (14.9) | 0.6847 |
| Previous CABG | 1386 | 31 (2.2) | 74 | 7 (9.5) | 0.0023 |
| Current Canadian Cardiovascular Society Angina Class |
1386 | 74 | 0.0302 | ||
| None | 406 (29.3) | 15 (20.3) | |||
| I | 139 (10.0) | 5 (6.8) | |||
| II | 368 (26.6) | 19 (25.7) | |||
| III | 395 (28.5) | 25 (33.8) | |||
| IV | 78 (5.6) | 10 (13.5) | |||
| Current NYHA heart failure class | 1386 | 74 | 0.0001 | ||
| I | 134 (9.7) | 2 (2.7) | |||
| II | 647 (46.7) | 30 (40.5) | |||
| III | 549 (39.6) | 31 (41.9) | |||
| IV | 56 (4.0) | 11 (14.9) | |||
| Number of diseased vessels (>75% stenosis) | 1386 | 74 | 0.1796 | ||
| None | 29 (2.1) | 0 (0) | |||
| One | 269 (19.4) | 9 (12.2) | |||
| Two | 550 (39.7) | 36 (48.6) | |||
| Three | 538 (38.8) | 29 (39.2) | |||
| Left main stenosis ≥ 50% | 1386 | 194 (14.0) | 74 | 11 (14.9) | 0.8342 |
| Proximal LAD stenosis ≥ 75% | 1385 | 996 (71.9) | 74 | 62 (83.8) | 0.0258 |
| Duke CAD Index (0–100) | 1386 | 65 (39 – 77) | 74 | 65 (52 – 91) | 0.0312 |
| LVEF | 1386 | 28 (23 – 34) | 74 | 25 (20 – 30) | 0.0002 |
| LVESVI | 1386 | 78 (59 – 98) | 74 | 94 (71 – 110) | <0.0001 |
| Distance walked at baseline (meters) | 1022 | 350 (274 – 420) | 47 | 310 (224 – 450) | 0.3178 |
| Atrial flutter or fibrillation | 1386 | 151 (10.9) | 74 | 19 (25.7) | 0.0001 |
| Statin and at least one ASA, clopidogrel, or warfarin | 1386 | 945 (68.2) | 74 | 40 (54.1) | 0.0115 |
| Mitral regurgitation | 1378 | 73 | 0.0004 | ||
| None or trace | 513 (37.2) | 14 (19.2) | |||
| Mild (≤2+) | 639 (46.4) | 38 (52.1) | |||
| Moderate (3+) | 187 (13.6) | 14 (19.2) | |||
| Severe (4+) | 39 (2.8) | 7 (9.6) | |||
| Total number of distal anastomoses | 1381 | 3 (2 – 4) | 73 | 3 (2 – 4) | 0.6633 |
| Number of distal anastomoses, n (%) | 1381 | 73 | 0.5555 | ||
| 0–1 | 124 (9.0) | 9 (12.3) | |||
| 2–3 | 843 (61.0) | 41 (56.2) | |||
| ≥4 | 414 (30.0) | 23 (31.5) | |||
| More distal anastomoses than conduits | 1386 | 323 (23.3) | 74 | 13 (17.6) | 0.2533 |
| Arterial conduits ≥ 1, n (%) | 1386 | 1264 (91.2) | 74 | 63 (85.1) | 0.0774 |
| On pump surgery | 1386 | 1231 (88.8) | 74 | 69 (93.2) | 0.2350 |
| Mitral valve procedure | 1386 | 219 (15.8) | 74 | 15 (20.3) | 0.3072 |
| SVR procedure | 1386 | 466 (33.6) | 74 | 29 (39.2) | 0.3243 |
| Surgery was not elective | 1386 | 170 (12.3) | 74 | 11 (14.9) | 0.5086 |
| Cardioplegia | 1382 | 74 | 0.7758 | ||
| None | 204 (14.8) | 14 (18.9) | |||
| Crystalloid | 301 (21.8) | 15 (20.3) | |||
| Blood | 826 (59.8) | 43 (58.1) | |||
| Both | 51 (3.7) | 2 (2.7) | |||
| Bypass pump time, minutes | 1386 | 97 (66 – 131) | 74 | 125 (90 – 176) | <0.0001 |
| Cardiopulmonary bypass time>120 minutes | 1386 | 434 (31.3) | 74 | 38 (51.4) | 0.0003 |
| Aorta cross-clamp time, minutes | 1386 | 60 (37 – 86) | 74 | 72 (32 – 92) | 0.1413 |
Note: the entries in this table are median (25th, 75th percentiles) for continuous variables and n (%) for categorical variables.
Fisher’s exact tests used for stroke and prior CABG. Chi-square tests used for the remaining categorical variables and Wilcoxon rank-sum tests used for continuous variables.
Univariable Relationship of Baseline and Surgical Characteristics (Conduct) with Mortality at 30 Days
The univariable relationships that were strongly predictive of 30-day mortality (Table 4) were creatinine, age, LVESVI, NYHA class, hemoglobin, the presence of baseline atrial fibrillation or flutter, previous CABG, and bypass pump time >120 minutes.
Table 4.
Univariable association between baseline and surgical characteristics and 30 day mortality.
| Characteristic | HR (95% CI) | Chi-Square | p |
|---|---|---|---|
| Age, HR for 10-year increase | 1.60 (1.25, 2.05) | 13.63 | 0.0002 |
| Female | 0.99 (0.51, 1.93) | <0.01 | 0.9732 |
| Myocardial infarction | 1.71 (0.82, 3.56) | 2.06 | 0.1512 |
| Hyperlipidemia | 0.70 (0.44, 1.11) | 2.36 | 0.1244 |
| Hypertension | 1.61 (0.98, 2.66) | 3.55 | 0.0597 |
| Diabetes | 1.33 (0.84, 2.11) | 1.48 | 0.2233 |
| Peripheral vascular disease | 2.35 (1.42, 3.90) | 10.99 | 0.0009 |
| Chronic renal insufficiency | 3.22 (1.85, 5.60) | 17.14 | <0.0001 |
| BMI, HR for 1 kg/m2 increase | 10.39 | 0.0155 | |
| ≤ 25 | 0.83 (0.74, 0.93) | ||
| 25–30 | 1.19 (1.02, 1.37) | ||
| Creatinine, HR for 0.1 mg/dL increase | 35.94 | <0.0001 | |
| ≤1.0 | 0.68 (0.52, 0.89) | ||
| 1.0–1.4 | 1.65 (1.38, 1.97) | ||
| Hemoglobin, HR for 1 g/dL increase | 13.11 | 0.0044 | |
| <12.5 | 1.21 (0.82, 1.78) | ||
| 12.5–15.5 | 0.64 (0.48, 0.84) | ||
| >15.5 | 2.08 (1.32, 3.29) | ||
| Stroke | 2.00 (1.00, 4.02) | 3.80 | 0.0511 |
| Previous PCI | 0.87 (0.46, 1.66) | 0.17 | 0.6774 |
| Previous CABG | 4.16 (1.91, 9.07) | 12.89 | 0.0003 |
| Current angina | 1.62 (0.92, 2.85) | 2.75 | 0.0971 |
| Current NYHA HF class, HR for 1 category increase | 1.80 (1.30, 2.49) | 12.73 | 0.0004 |
| Number of diseased vessels (>75% stenosis) | 1.21 (0.90, 1.63) | 1.54 | 0.2153 |
| Left main stenosis ≥ 50% | 1.07 (0.56, 2.03) | 0.04 | 0.8412 |
| Proximal LAD stenosis ≥ 75% | 1.99 (1.07, 3.69) | 4.76 | 0.0292 |
| Duke CAD Index, HR for 10 unit increase | 7.88 | 0.0194 | |
| ≤ 53 | 1.81 (1.16, 2.82) | ||
| > 53 | 0.95 (0.80, 1.14) | ||
| Ejection fraction, HR for 10% increase | 0.58 (0.44, 0.77) | 14.11 | 0.0002 |
| ESVI, HR for 100 ml increase | 3.19 (1.90, 5.34) | 19.34 | <0.0001 |
| Atrial flutter or fibrillation | 2.70 (1.60, 4.55) | 13.92 | 0.0002 |
| Statin and at least one ASA, clopidogrel, or warfarin | 0.56 (0.36, 0.89) | 6.13 | 0.0133 |
| Moderate or severe mitral regurgitation | 1.99 (1.20, 3.31) | 7.17 | 0.0074 |
| Total number of distal anastomoses | 1.01 (0.82, 1.25) | 0.01 | 0.9036 |
| More distal anastomoses than conduits | 0.71 (0.39, 1.29) | 1.29 | 0.2569 |
| Arterial conduits ≥ 1 | 0.56 (0.30, 1.07) | 3.11 | 0.0777 |
| CABG with CPB | 1.71 (0.69, 4.25) | 1.35 | 0.2450 |
| Mitral valve procedure | 1.34 (0.76, 2.36) | 1.03 | 0.3111 |
| SVR procedure | 1.26 (0.79, 2.02) | 0.97 | 0.3250 |
| Surgery was not elective | 1.24 (0.65, 2.35) | 0.43 | 0.5142 |
| Cardioplegia (reference is none) | 1.12 | 0.7730 | |
| Crystalloid | 0.73 (0.35, 1.52) | ||
| Blood | 0.76 (0.42, 1.39) | ||
| Both | 0.58 (0.13, 2.55) | ||
| Cardiopulmonary bypass time > 120 minutes | 2.27 (1.44, 3.58) | 12.38 | 0.0004 |
Multivariable Model of Baseline and Operative Conduct and 30-day Mortality
The multivariable model (C statistic of 0.83) identified creatinine and LVESVI as the two most powerful baseline and operative conduct characteristics for predicting 30-day mortality (Table 5). Mortality risk increased linearly with creatinine above 1 mg/dL and appeared to level off at 1.6 mg/dL (Figure 2), although the number of patients with more elevated creatinine values was limited. Other strong (p<0.01) characteristics identified included age and moderate or severe mitral regurgitation. Other predictive characteristics were current angina, atrial fibrillation or flutter, hemoglobin, the preoperative use of both statins and ASA, clopidogrel or warfarin (reduced risk), cardiopulmonary bypass time >120 minutes, mitral valve procedure (reduced risk), body mass index (BMI), peripheral vascular disease, and a high Duke CAD index.
Table 5.
Multivariable association between baseline and surgical characteristics and 30 day mortality. (C-statistic=0.826).
| Variable | HR (95% CI) | p |
|---|---|---|
| Creatinine, HR for 0.1 mg/dL increase | <0.0001 | |
| ≤1.0 | 0.67 (0.51, 0.89) | |
| 1.0–1.4 | 1.52 (1.26, 1.82) | |
| ESVI, HR for 100 ml increase | 2.73 (1.50, 4.98) | 0.0010 |
| Age, HR for 10 year increase | 1.47 (1.12, 1.93) | 0.0052 |
| Moderate or severe mitral regurgitation | 2.35 (1.27, 4.34) | 0.0065 |
| Current angina | 2.08 (1.17, 3.70) | 0.0126 |
| Atrial flutter or fibrillation | 1.97 (1.14, 3.39) | 0.0153 |
| Hemoglobin, HR for 1 g/dL increase | 0.0178 | |
| <12.5 | 1.41 (0.91, 2.19) | |
| 12.5–15.5 | 0.68 (0.51, 0.90) | |
| >15.5 | 1.88 (1.19, 2.96) | |
| Cardiopulmonary bypass time > 120 minutes | 1.83 (1.10, 3.04) | 0.0199 |
| Body mass index, HR for 1 kg/m2 increase | 0.0202 | |
| 0–25 | 0.86 (0.76, 0.97) | |
| 25–30 | 1.21 (1.04, 1.41) | |
| Mitral valve procedure | 0.43 (0.20, 0.90) | 0.0251 |
| Peripheral vascular disease | 1.84 (1.07, 3.18) | 0.0278 |
| Statin and at least one ASA, clopidogrel, or warfarin | 0.58 (0.36, 0.95) | 0.0285 |
| Duke CAD Index, HR for 10 point increase to 53 | 1.46 (1.00, 2.13) | 0.0485 |
Figure 2.
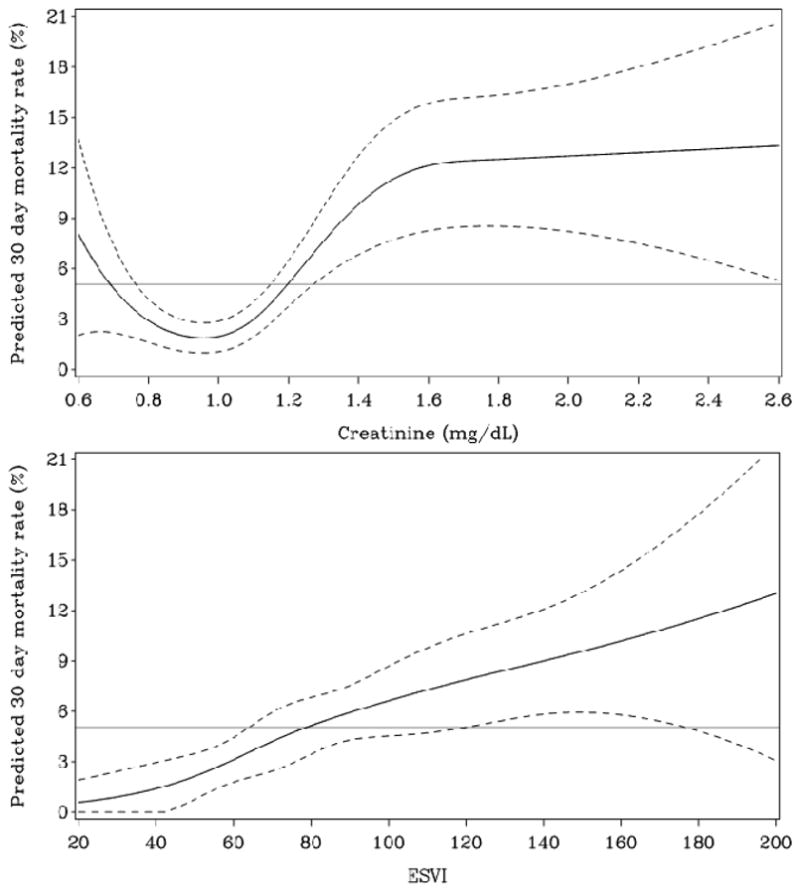
Relationship between creatinine and LVESVI vs. 30-day mortality and the 95% confidence interval (dotted lines).
Discussion
This is the largest international cohort of CABG-eligible patients with CAD and low LVEF (≤35%) for whom preoperative, intraoperative and postoperative data were prospectively acquired permitting the analysis of variables associated with 30-day postoperative mortality and morbidity. Major postoperative complications were relatively frequent (23.7% of all cases) and correlated with death in 88% of fatal cases. Not surprisingly, clinical and operative characteristics predictive of postoperative complication and 30-day mortality were similar, with one notable exception. Increased LVESVI was not significantly associated with risk of postoperative complication, but was one of the two characteristics most predictive of 30-day mortality. The only operative conduct characteristic predictive of 30-day mortality was prolonged cardiopulmonary bypass time, which likely reflects a more difficult or complicated operative procedure.12–14
Postoperative Complications
When a complication occurred, it was frequently followed by at least another complication (137 of 346 patients), and mortality increased markedly as more complications occurred.15 Preoperative renal dysfunction, indices of LV dysfunction including LVEF, and exertional tolerance, such as NYHA class and distance walked, were the patient characteristics most closely associated with the development of major postoperative complications. While it would appear that particular caution should be exercised when considering CABG in these patients, these are precisely the clinical characteristics that identify those patients who may have the most to gain from CABG.2 Strategies to optimize pre- and perioperative renal16 and cardiac function as well as myocardial protection17–19 may be particularly important factors to consider in the management of these complex patients.20
Postoperative 30-day Mortality
Worsening renal function (37 of 74 deaths), cardiac arrest requiring CPR (35 of 74 deaths), the development of ventricular arrhythmias (31 of 74deaths), and pulmonary edema requiring intubation (18 of 74 deaths), were the postoperative complications most frequently associated with death. Worsening renal function probably reflects numerous risk factors and probably also contributed to the development of pulmonary edema requiring intubation. These complications are all generally associated with poor or worsening LV function, which may also be reflective of a complicated, difficult and prolonged operation.
Myocardial infarction, stroke and mediastinitis were reasonably infrequent and associated with a relatively small number of deaths, 5, 4 and 2 respectively. The low major adverse event rate of these three complications may help explain the low 30-day postoperative mortality seen in the STICH trial. 21
Patients undergoing CABG with impaired left ventricular function are among the most challenging patients undergoing coronary surgery.22 Commonly, the EuroSCORE, Society of Thoracic Surgeons (STS) and New York state risk scores are used to assess the risk in patients undergoing CABG.3–6 These risk scores do not specifically and thoroughly address the patient with severe LV dysfunction. However, as with commonly used risk scores, STICH identified advanced age, renal dysfunction, low LVEF, and the presence of more advanced atherosclerosis, whether a higher Duke CAD severity score or the presence of peripheral vascular disease, as important risk factors. Again, although Duke CAD severity score identified patients at higher risk, previous analyses of STICH have also identified these patients to be among those that benefit most by CABG.2 As opposed to commonly used risk scores, gender was not predictive of 30-day mortality in STICH.
The second most powerful predictor of 30-day mortality in STICH was LVESVI. Mortality risk increased linearly with increasing values of LVESVI (Figure 2). This measurement not only reflects reduced LVEF, but reflects the degree of LV remodeling that is itself an independent predictor of poor outcome.23 Well established surgical risk scores have identified LVEF as a very powerful predictor of surgical and 30-day mortality, but due to lack of availability, LVESVI has not been included in these scores. In STICH patients, LVESVI was a stronger predictor of 30-day mortality than LVEF as a significant independent predictor of 30-day mortality. Post-infarct studies have also found LVESVI to be a more important predictor of outcome than LVEF.24 Thus, in considering the surgical risk of death in patients with LV dysfunction, LVESVI should be carefully evaluated as it appears to carry more prognostic significance for risk than does LVEF and also predicts a better outcome with CABG than with medical therapy alone.2
The only operative conduct characteristic predictive of 30-day mortality was prolonged cardiopulmonary bypass time. As mitral valve surgery was associated with reduced mortality, and SVR was not independently associated with increased mortality, it would appear likely that the increased risk associated with cardiopulmonary bypass time is reflective of a more difficult and complex revascularization procedure rather than concomitant surgical procedures. Consistent with this hypothesis is the lack of association between mortality and aortic cross-clamp time found in this and other analyses.12
Mitral valve surgery at the time of CABG was the only surgical characteristic associated with improved 30-day survival. It is unclear why this was associated with improved survival, but it may result from a beneficial effect on patients with significant mitral regurgitation and heart failure.25–26 In STICH, mitral valve repair (221 of 234 mitral valve surgeries) was the overwhelming form of mitral valve surgery performed, such that differential results for mitral valve repair versus replacement cannot be evaluated. The finding of reduced risk with mitral valve surgery in the present analysis is also consistent with the long term benefits demonstrated in STICH when mitral valve repair is performed at the time of CABG in patients with moderate to severe MR.27 These findings also differ from those of a recent trial of 300 patients with moderate ischemic mitral regurgitation randomized to receive CABG plus mitral repair or CABG only.28 In those patients, which had significantly better LV function than in STICH, no significant difference was found in LVESVI, survival or quality of life at 12 months, but there was a 30% lower prevalence of moderate or severe MR, suggesting that mitral valve repair may have greater benefit in patients with more severe LV dysfunction, or that the benefit of MV repair may not be seen until these patients have been followed for a longer period of time.
Clinical Implications
This analysis of the STICH trial data has identified a number of patient characteristics that predict an increased risk of 30-day postoperative complications and mortality risk. Paradoxically some of these risk factors, such as LVESVI, LVEF, and more extensive CAD, also predict which patients will improve most with CABG. The presence of moderate to severe MR also predicts patients at greater risk, but again, MR repair appears to have a marked beneficial effect, both short and long term and should be performed when appropriate. Other factors that increase risk and that are difficult to modify, such as renal dysfunction, advanced age, and the presence of atrial fibrillation or flutter, among others, may encourage surgeons to perhaps choose operative procedures that limit cardiopulmonary bypass time.
Limitations
The results of these analyses need to be viewed within the context of the STICH trial where these procedures were performed by surgical teams with proven excellent results in such high-risk patients. Also, patients in STICH were relatively young (with median age of 61 years) when enrolled and mortality may be higher than that reported in STICH for older more fragile patients and those with other co-morbidities. Finally, this STICH analysis did not consider patients that crossed over from medical therapy to CABG. Although the addition of these 65 patients may have altered the results somewhat because these crossover patients had a particularly good outcome,29 it is probable that their inclusion would have had limited impact on our findings. These analyses identified patient and operative characteristics associated with increased risk of serious postoperative complications and mortality, but did not evaluate interventions that could reduce these risks. Also, other variables known to be important in assessing postoperative risk, such as pulmonary hypertension, that were not prospectively collected and not considered in these analyses may be as important in identifying risk as those considered in the present analyses.
Conclusions
The STICH trial demonstrated that CABG can be performed with a relatively low 30-day mortality in patients with severe LV dysfunction and ischemic heart failure. Despite this low mortality, serious complications are relatively common in these high-risk patients and occur prior to death in the majority of patients dying within 30 days of surgery. Greater renal dysfunction, LVESVI, advanced age, and preoperative atrial fibrillation or flutter are the strongest baseline characteristics predictive of a poor survival. Prolonged cardiopulmonary bypass time is the single operative characteristic independently predictive of poor early outcome.
Supplementary Material
Acknowledgments
The authors thank Seanna Horan and Vanessa Moore for their valuable input in the preparation and editing of this manuscript.
Funding Sources: This work was supported by grants U01HL69015 and U01HL69013 from the National Institutes of Health/National Heart, Lung, and Blood Institute. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures: Dr. Daly has disclosed modest royalties received from NeoChord, Inc. There are no other conflicts to disclose.
References
- 1.Velazquez EJ, Williams JB, Yow E, Shaw LK, Lee KL, Phillips HR, O’Connor CM, Smith PK, Jones RH. Long-term survival of patients with ischemic cardiomyopathy treated by coronary artery bypass grafting versus medical therapy. Ann Thorac Surg. 2012;93:523–530. doi: 10.1016/j.athoracsur.2011.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panza JA, Velazquez EJ, She L, Smith PK, Nicolau JC, Favaloro RR, Gradinac S, Chrzanowski L, Prabhakaran D, Howlett JG, Jasinski M, Hill JA, Szwed H, Larbalestier R, Desvigne-Nickens P, Jones RH, Lee KL, Rouleau JL. Extent of coronary and myocardial disease and benefit from surgical revascularization in LV dysfunction. J Am Coll Cardiol. 2014;64:553–561. doi: 10.1016/j.jacc.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shroyer ALW, Coombs LP, Peterson ED, Eiken MC, DeLong ER, Chen A, Ferguson TB, Grover FL, Edwards FH. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856–65. doi: 10.1016/s0003-4975(03)00179-6. discussion 1864–5. [DOI] [PubMed] [Google Scholar]
- 4.Nashef SA, Roques F, Hammill BG, Peterson ED, Michel P, Grover FL, Wyse RK, Ferguson TB. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg. 2002;22:101–5. doi: 10.1016/s1010-7940(02)00208-7. [DOI] [PubMed] [Google Scholar]
- 5.Hannan EL, Szypulski Farrell L, Wechsler A, Jordan D, Lahey SJ, Culliford AT, Gold JP, Higgins RSD, Smith CR. The New York risk score for in-hospital and 30-day mortality for coronary artery bypass graft surgery. Ann Thorac Surg. 2013;95:46–54. doi: 10.1016/j.athoracsur.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez EJ, Lee KL, O’Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Kini AS, Adams DH. Results and predictors of early and late outcome of coronary artery bypass grafting in patients with severely depressed left ventricular function. Ann Thoracic Surgery. 2007;84:808–816. doi: 10.1016/j.athoracsur.2007.04.117. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL STICH Investigators. Coronary artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RH, Velazquez EJ, Michler RE, Sopko G, OH JK, O’Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL The STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RH, White H, Velazquez EJ, Shaw LK, Pietrobon R, Panza JA, Bonow RO, Sopko G, O’Connor CM, Rouleau JL. STICH (Surgical Treatment for Ischemic Heart Failure) Trial Enrollment. J Am Coll Cardiol. 2010;56:490–498. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisel RD, Nussmeier N, Newman MF, Pearl RG, Wechsler AS, Ambrosio G, Pitt B, Clare RM, Pieper K, Mongero L, Reece TL, Yau TM, Fremes S, Menasché P, Lira A, Harrington RA, Ferguson TB. Predictors of contemporary coronary artery bypass grafting outcomes. J Thorac Cardiovasc Surg. 2014;148:2720–2726. doi: 10.1016/j.jtcvs.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Murphy GS, Hessel EA, Groom RC. Optimal perfusion during cardiopulmonary bypass: An evidence-based approach. Anesth Analg. 2009;108:1394–1417. doi: 10.1213/ane.0b013e3181875e2e. [DOI] [PubMed] [Google Scholar]
- 14.Likosky DS, Goldberg JB, DiScipio AW, Kramer RS, Groom RC, Leavitt BJ, Surgenor SD, Baribeau YR, Charlesworth DC, Helm RE, Frumiento C, Sardella GL, Clough RA, MacKenzie TA, Malenka DJ, Olmstead EM, Ross CS Northern New England Cardiovascular Disease Study Group. Variability in surgeons’ perioperative practices may influence the incidence of low-output failure after coronary artery bypass grafting surgery. Circ Cardiovasc Qual Outcomes. 2012;5:638–644. doi: 10.1161/CIRCOUTCOMES.112.967091. [DOI] [PubMed] [Google Scholar]
- 15.Silber JH, Rosenbaum PR, Trudeau ME, Chen W, Zhang X, Kelz RR, Mosher RE, Even-Shoshan O. Changes in prognosis after the first post-operative complication. Med Care. 2005;43:122–131. doi: 10.1097/00005650-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kohl P. Renal insufficiency after cardiac surgery: a challenging clinical problem. Eur Heart J. 2009;30:1824–1827. doi: 10.1093/eurheartj/ehp282. [DOI] [PubMed] [Google Scholar]
- 17.Guru V, Omura J, Alghamdi AA, Weisel RD, Fremes SE. Is blood superior to crystalloid cardioplegia? a meta-analysis of randomized clinical trials. Circulation. 2006;114(Suppl I):331–338. doi: 10.1161/CIRCULATIONAHA.105.001644. [DOI] [PubMed] [Google Scholar]
- 18.Algarni KD, Weisel RD, Caldarone CA, Maganti M, Tsang K, Yau TM. Microplegia during CABG was associated with less low cardiac output syndrome: a propensity matched comparison. Ann Thorac Surg. 2013;95:1532–1538. doi: 10.1016/j.athoracsur.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 19.Dewey TM, Herbert MA, Prince SL, Magee MJ, Edgerton JR, Trachiotis G, Alexander EP, Mack MJ. Avoidance of cardiopulmonary bypass improves early survival in multivessel coronary artery bypass patients with poor ventricular function. Heart Surg Forum. 2004;7:45–50. [PubMed] [Google Scholar]
- 20.Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med. 2014;370:1379–1381. doi: 10.1056/NEJMp1315538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glance LG, Osler TM, Mukamel DB, Dick AW. Effect of complications on mortality after coronary artery bypass grafting surgery: evidence from New York State. J Thorac Cardiovasc Surg. 2007;134:53–58. doi: 10.1016/j.jtcvs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Topkara VK, Cheema FH, Kesavaramanujam S, Mercando ML, Cheema AF, Namerow PB, Argenziano M, Naka Y, Oz MC, Esrig BC. Coronary artery bypass grafting in patients with low ejection fraction. Circulation. 2005;112:I-344–I-350. doi: 10.1161/CIRCULATIONAHA.104.526277. [DOI] [PubMed] [Google Scholar]
- 23.Michler RE, Rouleau JL, Al-Khalidi HR, Bonow RO, Pellikka PA, Pohost GM, Holly TA, Oh JK, Dagenais F, Milano C, Wrobel K, Pirk J, Ali IS, Jones RH, Velazquez EJ, Lee KL, Di Donato M for the STICH Trial Investigators. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2013;146:1139–1145. doi: 10.1016/j.jtcvs.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Castleberry AW, Williams JB, Daneshmand MA, Honeycutt E, Shaw LK, Samad Z, Lopes RD, Alexander JH, Mathew JP, Velazquez EJ, Milano CA, Smith PK. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation. 2014;129:2547–2556. doi: 10.1161/CIRCULATIONAHA.113.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campwala SZ, Wang N, Bansal RC. Mitral regurgitation progression following isolated coronary artery bypass surgery: frequency, risk factors, and potential prevention strategies. Eur J Cardiothorac Surg. 2006;29:348. doi: 10.1016/j.ejcts.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua Y, Daly R, Senni M, Mokrzycki K, Menicanti L, Oh JK, Michler R, Wróbel K, Lamy A, Velazquez EJ, Lee KL, Jones RH. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–2648. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, Acker MA, Argenziano M, Thourani V, Gammie JS, Miller MA, Pagé P, Overbey JR, Bagiella E, Dagenais F, Blackstone EH, Kron IL, Goldstein DJ, Rose EA, Moquete EG, Jeffries N, Gardner TJ, O’Gara PT, Alexander JH, Michler RE Cardiothoracic Surgical Trials Network Investigators. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. doi: 10.1056/NEJMoa1410490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doenst T, Cleland JG, Rouleau JL, She L, Wos S, Ohman EM, Krzeminska-Pakula M, Airan B, Jones RH, Siepe M, Sopko G, Velazquez EJ, Racine N, Gullestad L, Filgueira JL, Lee KL STICH Investigators. Influence of crossover on mortality in a randomized study of revascularization in patients with systolic heart failure and coronary artery disease. Circ Heart Fail. 2013;6:443–450. doi: 10.1161/CIRCHEARTFAILURE.112.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.