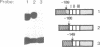Abstract
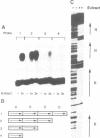
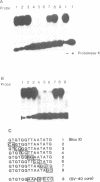
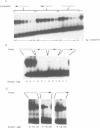
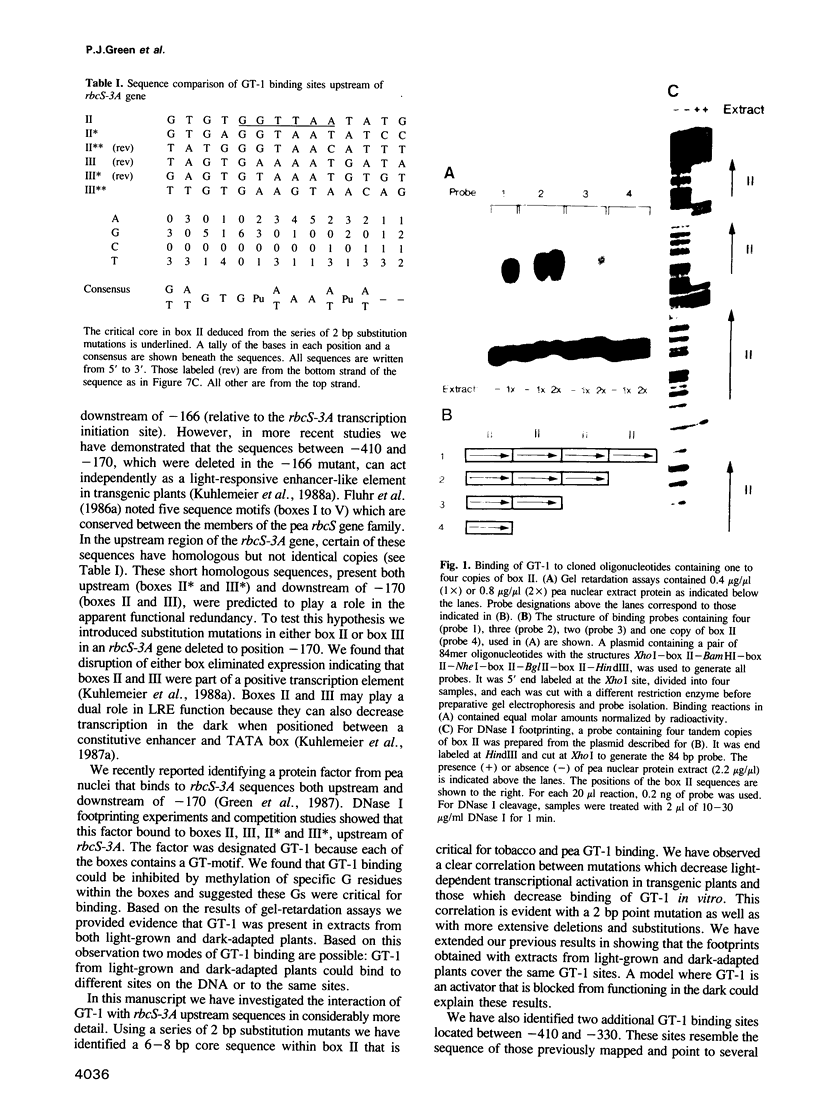
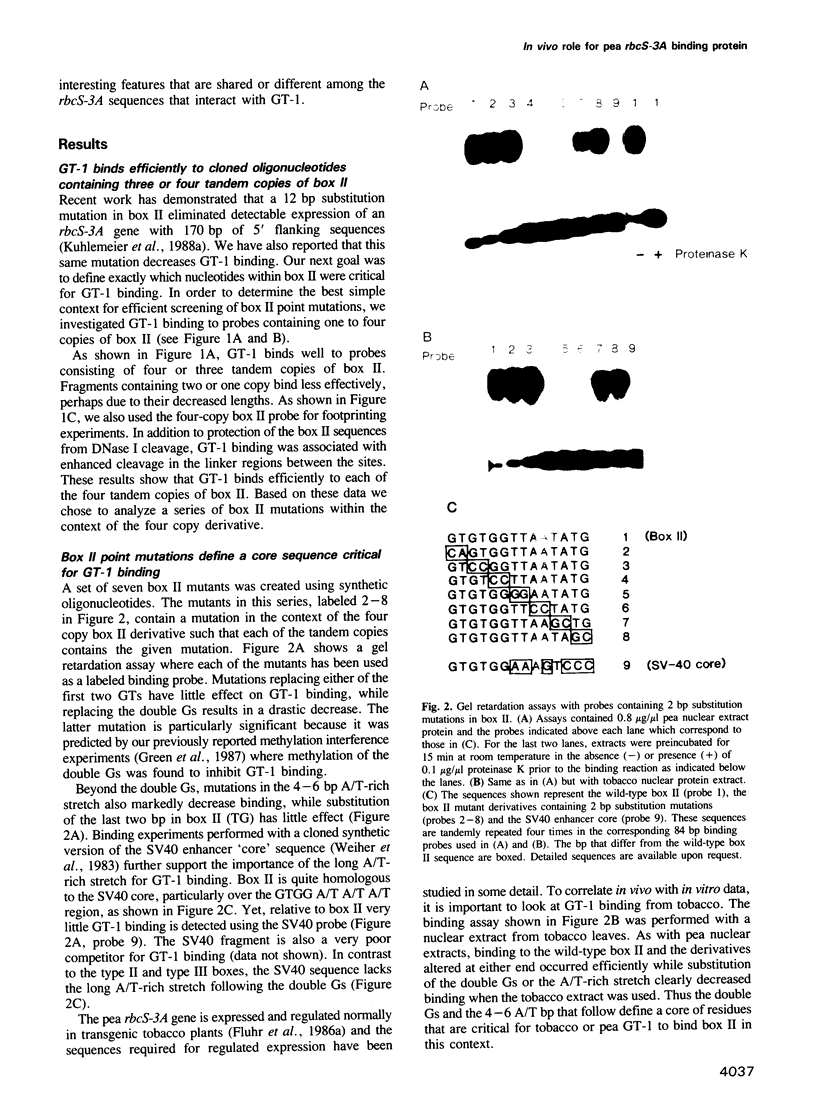
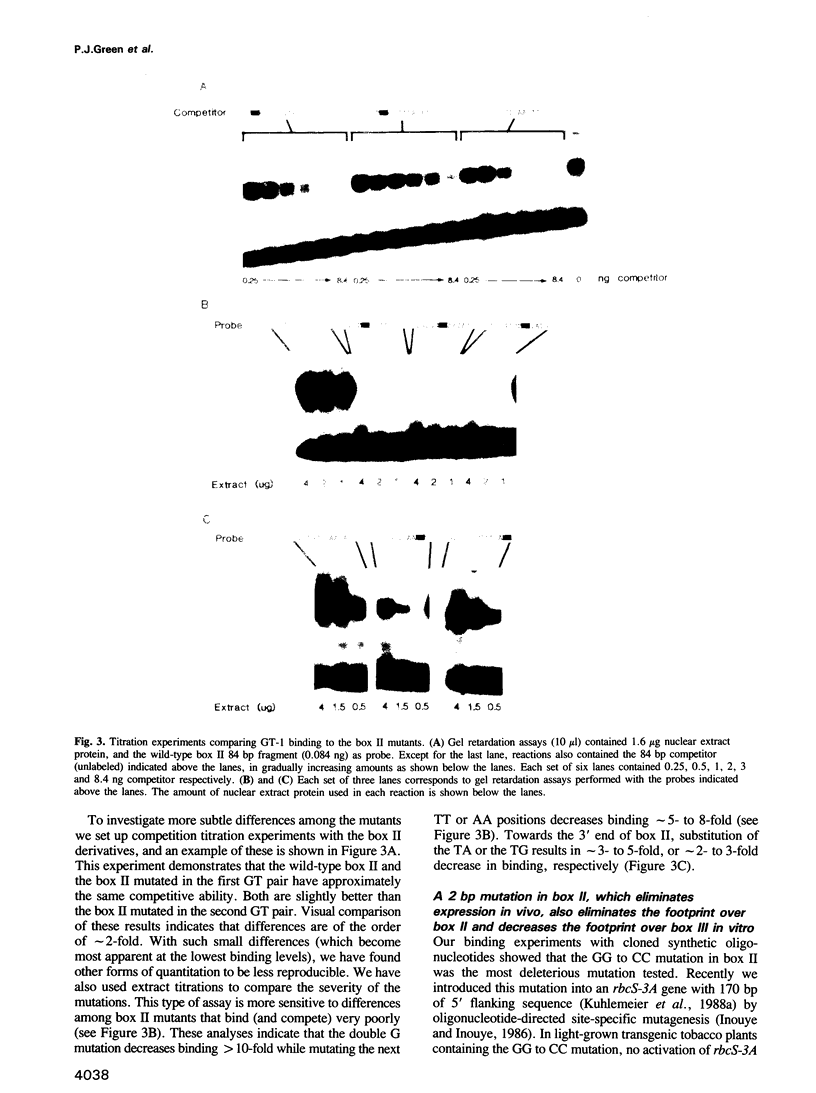
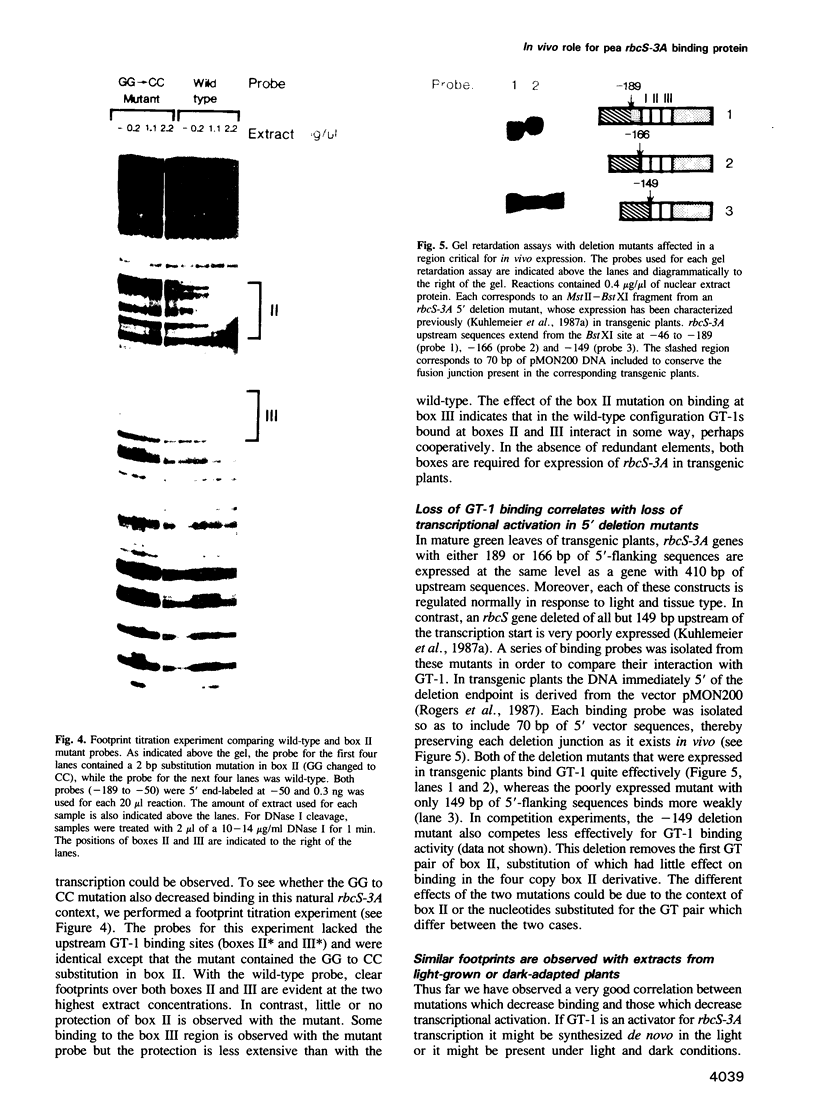
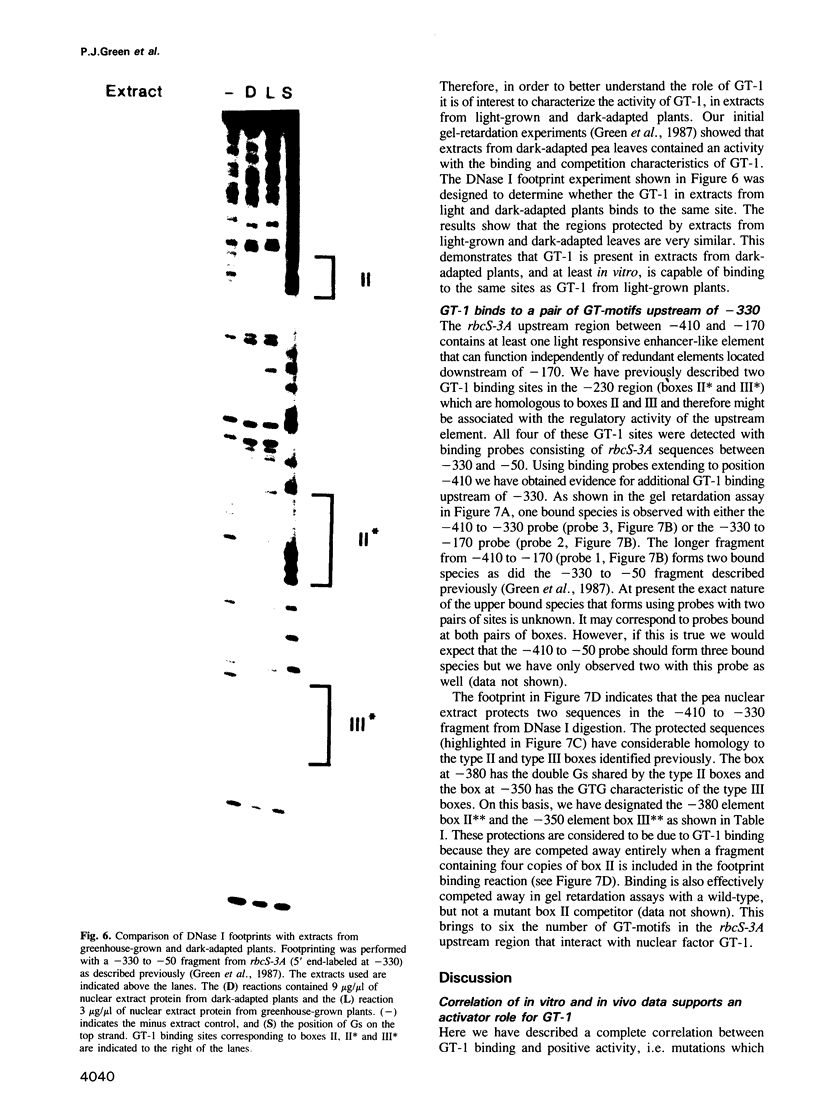
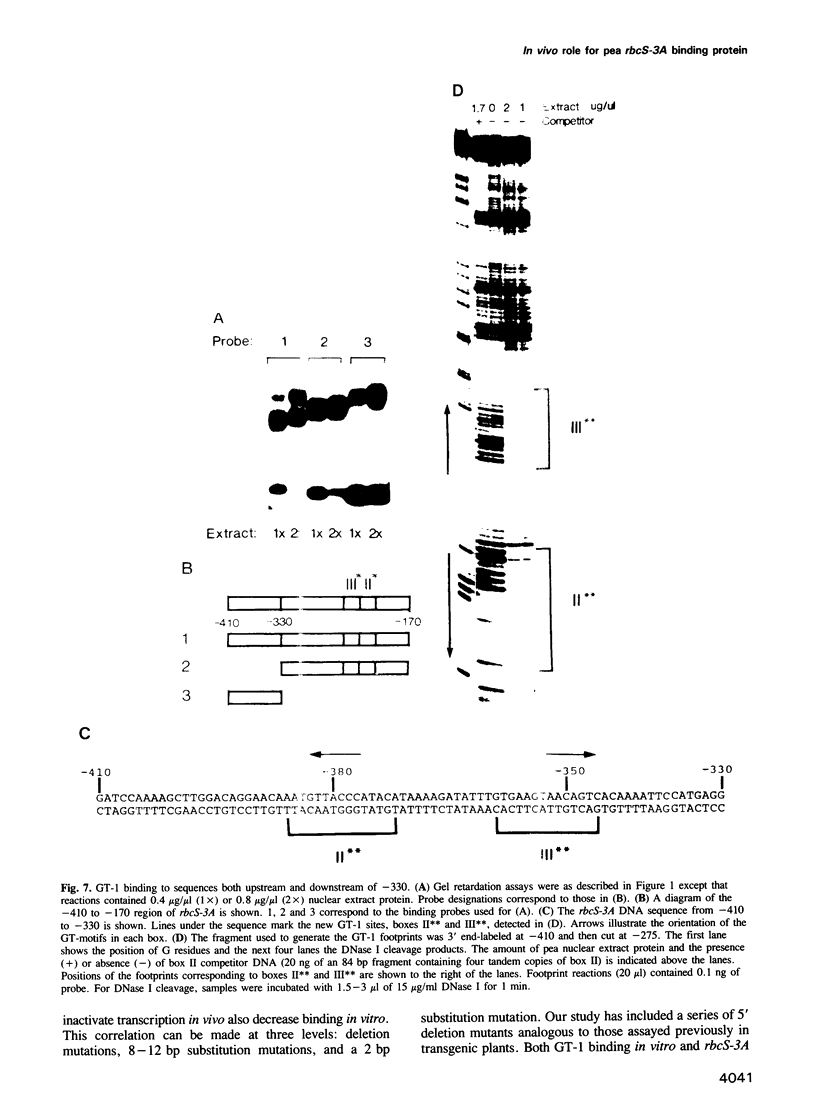
Nuclear protein factor GT-1 binds to sequence boxes II, III, II* and III* upstream of the light-responsive pea rbcS-3A gene. We have shown previously that box II and box III are required for expression of rbcS-3A when redundant elements upstream of -170 (relative to the transcription start site) are removed. Here we present evidence that deletion and substitution mutations downstream of -170 which eliminate expression also decrease binding. Using a series of 2 bp substitution mutations we have defined a core of six residues (GGTTAA) within box II (GTGTGGTTAATATG) that are critical for binding. The most detrimental mutation for binding, which changes the double Gs to Cs, is sufficient to eliminate detectable expression in vivo when only 170 bp of 5' flanking sequences are present. The simplest interpretation of these data is that GT-1 is an activator of rbcS-3A transcription. Footprinting experiments show that GT-1 from both light-grown and dark-adapted plants binds to the same sequences in vitro. Therefore, the lack of expression of rbcS-3A in the dark is not due to the absence of GT-1. In our analysis of the sequence elements upstream of -170, we have mapped two additional GT-1 sites (boxes II** and III**) between -330 and -410. The similarities and differences among the GT-1 sites located upstream and downstream of -170 are discussed in terms of the different sequence requirements for rbcS-3A expression during development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Ruppert S., Schütz G. Genomic footprinting reveals cell type-specific DNA binding of ubiquitous factors. Cell. 1987 Nov 6;51(3):435–443. doi: 10.1016/0092-8674(87)90639-8. [DOI] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot V., Robert L. S., Kavanagh T. A., Bevan M. W., Thompson R. D. Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J. 1987;6(12):3559–3564. doi: 10.1002/j.1460-2075.1987.tb02685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Ferl R. J., Nick H. S. In vivo detection of regulatory factor binding sites in the 5' flanking region of maize Adh1. J Biol Chem. 1987 Jun 15;262(17):7947–7950. [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Fluhr Robert, Moses Phyllis, Morelli Giorgio, Coruzzi Gloria, Chua Nam-Hai. Expression dynamics of the pea rbcS multigene family and organ distribution of the transcripts. EMBO J. 1986 Sep;5(9):2063–2071. doi: 10.1002/j.1460-2075.1986.tb04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Green P. J., Kay S. A., Chua N. H. Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J. 1987 Sep;6(9):2543–2549. doi: 10.1002/j.1460-2075.1987.tb02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. Ø, Marcker K. A., Schell J., Bruijn F. J. Interaction of a nodule specific, trans-acting factor with distinct DNA elements in the soybean leghaemoglobin Ibc(3) 5' upstream region. EMBO J. 1988 May;7(5):1265–1271. doi: 10.1002/j.1460-2075.1988.tb02940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku K. D., Okamuro J. K., Goldberg R. B. Interaction of an embryo DNA binding protein with a soybean lectin gene upstream region. Nature. 1987 Aug 20;328(6132):734–737. doi: 10.1038/328734a0. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C., Cuozzo M., Green P. J., Goyvaerts E., Ward K., Chua N. H. Localization and conditional redundancy of regulatory elements in rbcS-3A, a pea gene encoding the small subunit of ribulose-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4662–4666. doi: 10.1073/pnas.85.13.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C., Fluhr R., Green P. J., Chua N. H. Sequences in the pea rbcS-3A gene have homology to constitutive mammalian enhancers but function as negative regulatory elements. Genes Dev. 1987 May;1(3):247–255. doi: 10.1101/gad.1.3.247. [DOI] [PubMed] [Google Scholar]
- Maier U. G., Brown J. W., Tologcyzki C., Feix G. Binding of a nuclear factor to a consensus sequence in the 5' flanking region of zein genes from maize. EMBO J. 1987 Jan;6(1):17–22. doi: 10.1002/j.1460-2075.1987.tb04712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Mikami K., Tabata T., Kawata T., Nakayama T., Iwabuchi M. Nuclear protein(s) binding to the conserved DNA hexameric sequence postulated to regulate transcription of wheat histone genes. FEBS Lett. 1987 Nov 2;223(2):273–278. doi: 10.1016/0014-5793(87)80303-4. [DOI] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Schäfer E., Apel K. Phytochrome control of in vitro transcription of specific genes in isolated nuclei from barley (Hordeum vulgare). Eur J Biochem. 1985 Feb 15;147(1):137–142. doi: 10.1111/j.1432-1033.1985.tb08729.x. [DOI] [PubMed] [Google Scholar]
- Nagy F., Boutry M., Hsu M. Y., Wong M., Chua N. H. The 5'-proximal region of the wheat Cab-1 gene contains a 268-bp enhancer-like sequence for phytochrome response. EMBO J. 1987 Sep;6(9):2537–2542. doi: 10.1002/j.1460-2075.1987.tb02541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Boutry M., Hsu M. Y., Chua N. H. Phytochrome-controlled expression of a wheat Cab gene in transgenic tobacco seedlings. EMBO J. 1986 Jun;5(6):1119–1124. doi: 10.1002/j.1460-2075.1986.tb04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Chua N. H. Gene regulation by phytochrome. Trends Genet. 1988 Feb;4(2):37–42. doi: 10.1016/0168-9525(88)90064-9. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., VAN Montagu M., Herrera-Estrella L. Photosynthesis-associated gene families: differences in response to tissue-specific and environmental factors. Science. 1986 Jul 4;233(4759):34–38. doi: 10.1126/science.233.4759.34. [DOI] [PubMed] [Google Scholar]
- Stockhaus J., Eckes P., Rocha-Sosa M., Schell J., Willmitzer L. Analysis of cis-active sequences involved in the leaf-specific expression of a potato gene in transgenic plants. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7943–7947. doi: 10.1073/pnas.84.22.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]