Abstract
Background and Purpose
Iron chelation therapy is emerging as a novel neuroprotective strategy. The mechanisms of neuroprotection are diverse and include both neuronal and vascular pathways. We sought to examine the effect of iron chelation on cerebrovascular function in healthy aging and to explore whether HIF-1 activation may be temporally correlated with vascular changes
Methods
We assessed cerebrovascular function (autoregulation, vasoreactivity, neurovascular coupling) and serum concentrations of vascular endothelial growth factor (VEGF) and erythropoietin (EPO), as representative measures of HIF-1 activation, during 6 hours of deferoxamine (DFO) infusion in healthy 24 young and 24 older volunteers in a randomized, blinded, placebo-controlled cross-over study design. Cerebrovascular function was assessed using the transcranial Doppler ultrasound. VEGF and EPO serum protein assays were conducted using the Meso Scale Discovery platform.
Results
DFO elicited a strong age- and time-dependent increase in the plasma concentrations of EPO and VEGF, which persisted up to 3 hours post infusion (age effect p=0.04, treatment x time p<0.01). DFO infusion also resulted in a significant time- and age-dependent improvement in cerebral vasoreactivity (treatment x time, p<0.01, age p<0.01) and cerebral autoregulation (gain: age x time x treatment p=0.04).
Conclusions
DFO infusion improved cerebrovascular function, particularly in older individuals. The temporal association between improved cerebrovascular function and increased serum VEGF and EPO concentrations is supportive of shared HIF-1 regulated pathways. Therefore, pharmacological activation of HIF-1 to enhance cerebrovascular function may be a promising neuroprotective strategy in acute and chronic ischemic syndromes, especially in elderly patients.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT013655104.
Keywords: Cerebral hemodynamics, Aging, Transcranial Doppler ultrasound, HIF-1
Introduction
The concept of iron chelation therapy may be a promising therapeutic approach in a number of neurological disorders ranging from chronic neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis, to vascular disorders, such as intracerebral hemorrhage, subarachnoid hemorrhage and ischemic stroke.1–6 Protective mechanisms may be quite varied. In addition to the antioxidant effect, free radical scavenging effect, and inhibition of iron-induced membrane lipid peroxidation, neuroprotection by iron chelators also involve pathways related to hypoxia-inducible transcription factor-1 (HIF-1) activation.7 In animal models, iron chelation and HIF-1 activation have been shown to be neuroprotective in acute ischemic stroke and subarachnoid hemorrhage.8–10
Chronic cerebral hypoxic-ischemic injury, an important factor contributing to many age-related neurodegenerative disorders,11–13 also appears to involve HIF-1 related pathways.14 Interestingly, HIF-1 activation is also reduced in aging.15–18 Although the exact mechanisms underlying this age-dependent reduction of hypoxic/ischemic HIF-1 activation remain unknown, pharmacological activation of HIF-1 with cobalt chloride, a well-established HIF-1 activator, can enhance HIF-1 activation in aged mice.19 Thus, HIF-1 activation may be a promising mechanism for imparting ischemic neuroprotection in age related neurodegenerative disorders.
In addition to neuroprotective effects from direct facilitation of cell survival and inhibition of pro-death apoptotic pathways,20 HIF-1 regulated pathways may also be important for vascular regulation. HIF-1 activation has been shown in cerebrovascular processes such as collateral vascularization and microvascular angiogenesis.16;21;22 However, the effects of iron chelation and HIF-1 activation on cerebrovascular function are not fully understood. This information is particularly relevant to aging, which is associated with impaired cerebrovascular function, even in the absence of vascular risk factors such as hypertension and diabetes.23 Therefore, we sought to better understand the effects of iron chelation therapy on age related cerebrovascular function and to determine if HIF-1 activation may be involved. We used the iron chelator deferoxamine (DFO) to activate HIF-1 regulated proteins erythropoietin (EPO) and vascular endothelial growth factor (VEGF), which have known neuroprotective and vasogenic effects on cerebrovascular hemodynamics and we measured cerebrovascular hemodynamics in young and old healthy volunteers. Iron chelation therapy is a well-established treatment in a number of systemic disorder associated with iron overload such as cardiac siderosis in thalassemia24 as well as myelodysplastic disorders.25 We hypothesized that DFO infusion will improve cerebrovascular function (measured as cerebral autoregulation, cerebral vasoreactivity and neurovascular coupling); that this improvement will be larger in older volunteers; and that this improvement will be temporally correlated with serum levels of HIF-1 regulated proteins, VEGF and EPO.
Methods
Subjects and Experimental Design
Forty eight healthy subjects (45 ± 19 years, range 19 to 80 years old) volunteered to participate in the study. All volunteers were carefully screened with a medical history, physical examination, and electrocardiogram to exclude any acute or chronic medical conditions. Participants were not any vasoactive medications. We also excluded pregnant women. Subjects were nonsmokers and refrained from alcohol for at least 12 hours. The study was approved by the institutional review board, and all subjects gave informed consent.
The study was designed as a blinded, randomized cross-over study. Each subject was admitted twice to the Clinical Research Center (CRC) at the Brigham and Women’s Hospital. Each admission was for 24 hours and scheduled at least 30 days apart. Subjects were randomized to receive either DFO (dissolved in 5% Dextrose-normal saline) or placebo (PLB: 5% Dextrose-normal saline). Randomization was done by the CRC pharmacy, and DFO or placebo solutions were delivered in unmarked containers. Thirty days separation between the two admissions ensured sufficient wash-out between the two admissions.
Participants were admitted between 5–7 pm the night before the study day. On admission, an intravenous catheter was placed in each arm; one for infusion and the other for blood draw to assay for VEGF and EPO levels. Normal saline was infused at a rate of 20cc/hr through each line and continued until the infusion (DFO/PLB) started at 9 am the next day. At this time DFO/PLB infusion was initiated through the “infusion line” at 70cc/hr. The “blood draw line” remained continuously infused with normal saline at 20cc/hr.
The next morning (study day), first meal was served at 6 am. Subject instrumentation (below) was started at 7 am. After baseline data collection (t0) was completed the infusion (DFO/PLB) was started at 9 am. Second meal was served at 10 am and first time-point (3rdhr, t3) data collection was completed at 12 noon. Third meal was served at 1pm and second time-point (6th hour; t6) data collection was completed at 3 pm and infusion (DFO/PLB) ended. Fourth meal was served at 4 pm. Normal saline infusion at 70cc/hr was continued in the “infusion line” until 6 pm when the study ended and post-infusion data collection (9th hour; t9) was completed at 6 pm. The dose of DFO infused was 60mg/kg over 6 hours. Subjects were discharged home after a medical evaluation. All meals and fluid intake (including the first night meal and fluids) where exactly matched throughout each study day and during the two admissions. No caffeine intake was allowed. Between studies subjects remained instrumented, but were allowed to walk around the unit as needed, watch TV or read a book. All measurements were performed while subjects were in seated position. Please see Supplemental Methods and Figure for study schema.
Measurements and Assessment of Cerebrovascular Function
Photoplethysmographic mean arterial blood pressure (MAP) was recorded continuously on the finger (Portapres, Finapres Medical Systems, Netherlands), and cerebral blood flow velocity was measured using the transcranial Doppler ultrasound (MultiDop X, DWL-Transcranial Doppler System Inc., Sterling, VA) bilaterally in the M1 segment of the middle cerebral arteries (MCA) at a depth of 50 – 65 mm. The diameter of the MCA remains relatively constant with changes in blood pressure and arterial gases within physiologic range.26 Thus, mean flow velocity (MFV) was used as a surrogate for flow. Expired CO2 was continuously monitored by an infrared CO2 analyzer (VacuMed #17515 CO2 Analyzer Gold Edition, VacuMed Medical, Ventura, CA) connected to a nasal cannula. All signals were digitized and stored at 500 Hz (DI-720 Series Data Aquisition Systems and Windaq Software, DATAQ Instruments, Inc., Akron, OH).
To assess cerebrovascular function, we quantified its three main components: vasoreactivity, autoregulation, and neurovascular coupling. Cerebrovascular reactivity (VR) was assessed based on the slope of the linear relation between end-tidal CO2 and MCA flow velocity. Cerebral autoregulation was assessed based on transfer function analysis of the relationship between pressure and flow in the MCA as described before.27;28 Confidence intervals and precision of estimates for the transfer function were derived based on the level of coherence.29 We examined coherence, gain, and phase relation between arterial pressure and cerebral flow velocity across 0.03 – 0.07 Hz range, within which autoregulation has been shown to be most active.27;30 Coherence is analogous to linear regression coefficient, and indicates how linearly the fluctuations in pressure are transmitted to cerebral circulation (higher coherence indicates less effective autoregulation). Transfer function gain provides a measure of how much the cerebral blood flow changes per change in pressure (higher gain indicates less effective autoregulation), and transfer function phase provides a measure of the temporal course between arterial pressure and cerebral blood flow responses (lower phase indicates shorter responses, and thus, less efficient autoregulation). Finally, neurovascular coupling was assessed at baseline (t0) and at t6, as the percent change in MFV during a 2-Back task as described before.27;31 The mean percent change in MFV for each MCA was calculated as the percent difference between the MFV during the 2-back and its corresponding 0-Back control period. Please see Supplemental Methods for detailed description of these procedures.
Biomarkers of HIF-1 activation
Ten milliliters of blood was collected from each subject in a sterile glass tube containing 100 IU of preservative-free heparin (Sigma, St Louis, MO, USA) at each time point. Plasma EPO and VEGF were quantified by Meso Scale Discovery Custom Multiplex and Sector Imager 2400 (MSD, Gaithersburg, MD).
Statistics
Log transformations were applied to spectral powers and the inverse hyperbolic tangent to coherence to provide estimates with asymptotically standard distributions.32 The Box-Cox transformation was applied to all other data to ensure normality when necessary. However, for ease of interpretation, all average values and standard errors are presented as standard units.
For statistical analyses, transfer function gain and phase were weighted by their precisions to obtain the most accurate means for statistical analysis. In this way, unreliable estimates received appropriately small weights when group averages and statistics were computed. There were no significant differences in the right and left MCA MFV or measures of cerebrovascular function (vasoreactivity, coherence, and transfer function gain and phase, p > 0.20 for all comparisons). Therefore, to maximize reliability of our statistical analysis and to minimize the risk of committing Type-I error, we treated measures derived from right and left MCA as repeated measures for each individual. Placebo infusions were done in order to control for the potential effect of increased plasma volume on blood pressure, flow, and vascular function measures. All variables of interest were tested for significance via a linear mixed-effect model with age (young vs old), treatment (PLB vs DFO) and time (baseline, 3- and 6-hours of infusion, and 3-hours post-infusion) (including their interaction) as fixed effects and subject and the side of measurement (nested in subject) as random effects. All effects were considered statistically significant at p<0.05 level. All descriptive statistics were reported as mean ± standard error.
Results
Subject Characteristics
There were significant differences in baseline vascular measures between young (18–45 years old) and older (55–80 years old) participants. (Table 1). At baseline, MAP was slightly higher (p=0.07) and MFV was significantly lower (p<0.05) in older participants. Cerebral vasoreactivity was also significantly lower in the older participants (p<0.05). Transfer function coherence was 10% higher in the older volunteers (p<0.05), indicating that fluctuations in arterial pressure were more linearly transmitted to cerebral circulation compared to the young. While gain was not significantly different between age groups (p=0.49), phase was significantly lower (p<0.01) in older individuals, indicating that blood pressure and cerebral blood flow fluctuations were more synchronous compared to young. Therefore, at baseline, cerebral autoregulation was less efficient in older individuals. Although increase in cerebral blood flow in response to cognitive demand (i.e., neurovascular coupling) was somewhat lower in older individuals, this difference was not statistically significant (p=0.63).
Table 1.
Demographic and hemodynamic characteristics of the study participants at baseline
| Young | Old | |
|---|---|---|
| Number | 24 | 24 |
| Age | 27 ± 6 | 63 ± 7† |
| M: F | 12: 12 | 11: 13 |
| Blood pressure (mmHg) | 82 ± 2 | 87 ± 3 |
| MCA cerebral flow velocity (cm/s) | 71 ± 2 | 59 ± 2† |
| Cerebral vasoreactivity (cm/s/%) | 1.63 ± 0.11 | 1.12 ± 0.12† |
| Cerebral autoregulation | ||
| Coherence | 0.46 ± 0.02 | 0.50 ± 0.02† |
| Gain (cm/s/mmHg) | 0.71 ± 0.04 | 0.67 ± 0.05 |
| Phase (radians) | 0.76 ± 0.08 | 0.64 ± 0.07† |
| Neurovascular coupling (cm/s) | 9.1 ± 1.2 | 6.8 ± 2.1 |
p<0.05
Hemodynamic Variables
The effects of DFO on MAP and MFV were minimal (Table 2). At the peak of infusion (t6) MAP was significantly lower and MFV was significantly higher for the young in the DFO compared to the placebo arm, although the magnitude of this difference was very small (~5 mmHg and ~5 cm/s; Table 2). In the older participants, only MAP was slightly lower in the DFO arm as compared to the placebo at t6 (by about ~4 mmHg, p<0.05). Importantly, the magnitude of fluctuations in MAP and MFV did not differ between placebo and DFO infusions at any point (p>0.2 for age, time or treatment effect, or their interaction), suggesting that any significant effect of DFO infusions on measures of cerebrovascular function are unlikely to be due to a change in systemic hemodynamics.
Table 2.
Hemodynamic variables at baseline, (t0) during DFO or placebo infusions (t3 and t6), and 3 hours post-infusion (t9).
| Infusion | Post-infusion | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 3 hours | 6 hours | 9 hours | |||
| Heart rate (bpm) | Young | Placebo | 53 ± 1 | 53 ± 1 | 53 ± 1 | 52 ± 1 |
| DFO | 52 ± 1 | 53 ± 1 | 52 ± 1 | 51 ± 1 | ||
| Old | Placebo | 54 ± 3 | 55 ± 3 | 56 ± 3 | 55 ± 3 | |
| DFO | 56 ± 4 | 56 ± 3 | 55 ± 4 | 52 ± 4 | ||
|
| ||||||
| MAP (mmHg) | Young | Placebo | 82 ± 2 | 81 ± 2 | 85 ± 2 | 86 ± 2* |
| DFO | 85 ± 2 | 81 ± 2 | 81 ± 2† | 84 ± 2 | ||
| Old | Placebo | 87 ± 2 | 87 ± 2 | 89 ± 2 | 91 ± 3* | |
| DFO | 83 ± 2 | 85 ± 2 | 88 ± 2 | 86 ± 2† | ||
|
| ||||||
| MCA flow velocity (cm/s) | Young | Placebo | 71 ± 2 | 68 ± 2 | 65 ± 2 | 69 ± 2 |
| DFO | 71 ± 2 | 68 ± 2 | 72 ± 2† | 73 ± 2† | ||
| Old | Placebo | 59 ± 2 | 58 ± 2 | 58 ± 2 | 62 ± 2 | |
| DFO | 58 ± 2 | 59 ± 2 | 59 ± 2 | 62 ± 2* | ||
|
| ||||||
| End-tidal CO2 (mmHg) | Young | Placebo | 39 ± 1 | 39 ± 1 | 38 ± 1 | 38 ± 1 |
| DFO | 39 ± 1 | 38 ± 1 | 38 ± 1 | 38 ± 1 | ||
| Old | Placebo | 37 ± 2 | 38 ± 1 | 37 ± 2 | 38 ± 1 | |
| DFO | 38 ± 1 | 38 ± 1 | 38 ± 1 | 38 ± 1 | ||
p<0.05 vs baseline;
p<0.05 vs placebo.
The effect of age on MAP and MCA flow velocity (see Table 1) remained throughout the infusions and post-infusion
Cerebrovascular Function (Vasoreactivity, Autoregulation, Neurovascular coupling)
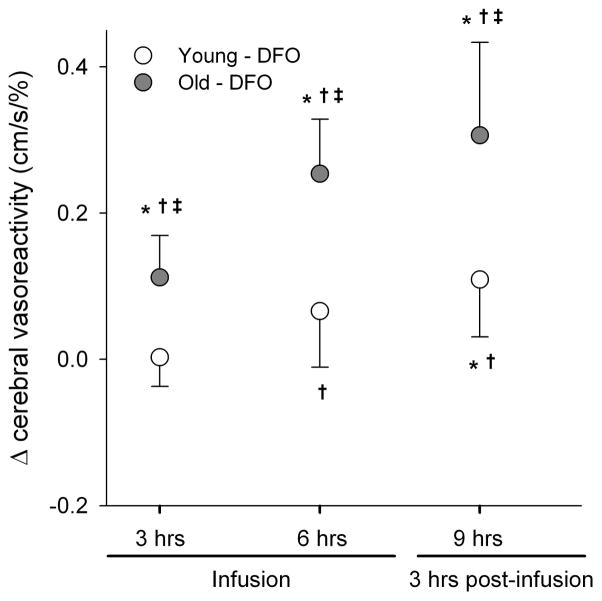
There was no significant effect of placebo infusions on any measure of cerebrovascular function. DFO infusion had a significant time-dependent impact on cerebral vasoreactivity (treatment x time, p<0.01). Compared to baseline (t0) and placebo, DFO infusion resulted in a higher cerebral vasoreactivity (Figure 1). Moreover, there was a significant age effect (p<0.01) such that improvement in cerebral vasoreactivity was greater in older individuals compared to young. The age-related difference in improved cerebral vasoreactivity was present across all time points. Interestingly, the increase in cerebral vasoreactivity was sustained up to 3 hours post-infusion (i.e., at t9).
Figure 1.
Change in cerebral vasoreactivity during (t3 and t6) and after (t9) DFO infusion compared to baseline (t0). *p<0.05 vs baseline, †p<0.05 vs. placebo, and ‡p<0.05 vs. young.
Similar to cerebral vasoreactivity, DFO infusion was also associated with a significant improvement in cerebral autoregulation, more so in the older participants (Figure 2). Both coherence and transfer function gain were reduced throughout the infusions in older individuals. At 3 hours post-infusion (t9), the reduction in coherence was significant in both young and older individuals (age effect p<0.01, treatment effect p=0.09, and time effect p=0.05), and the gain was significantly lower in old, but not in younger individuals (treatment effect p<0.01, age x time p=0.09, age x time x treatment p=0.04) (Figure 2). This indicates a faster and more pronounced effect of DFO in older individuals. Transfer function phase was also higher with DFO infusion across all time points, and this difference reached significance in older individuals at the peak of infusion (t6) and post-infusion (t9) (time effect, age x time interaction, and treatment x time interaction p<0.05) (Figure 2).
Figure 2.
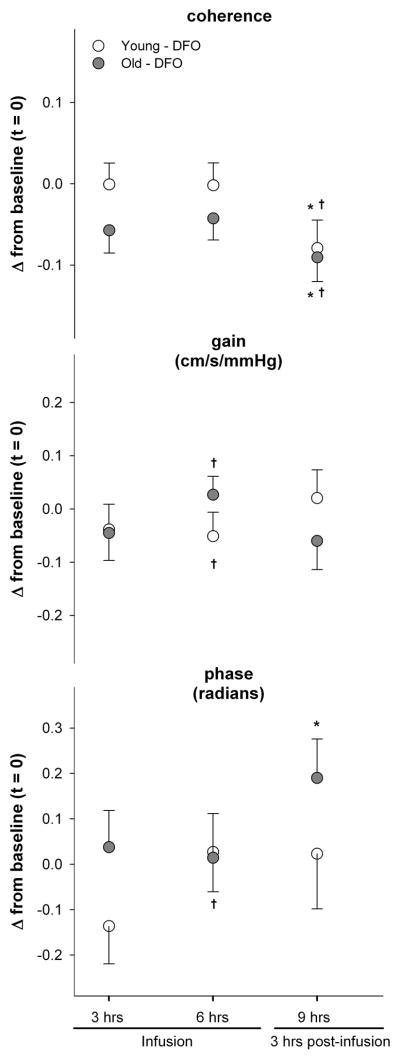
Change in measures of cerebral autoregulation (coherence, transfer function gain, and transfer function phase) during (t3 and t6) and after (t9) DFO infusion compared to baseline (t0). *p<0.05 vs baseline, and †p<0.05 vs. placebo.
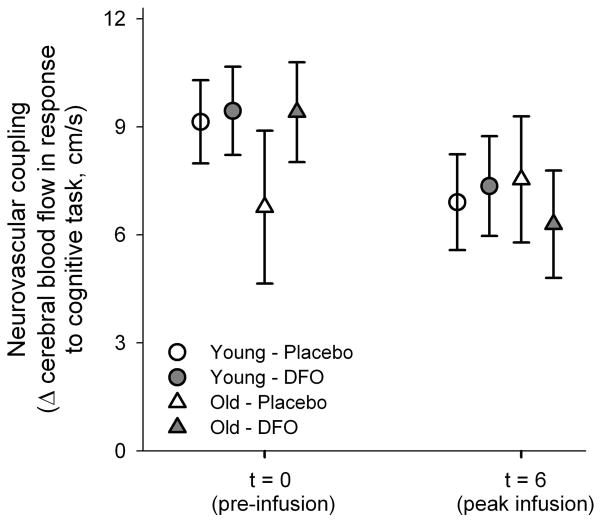
Finally, there was no effect of DFO on neurovascular coupling. Interestingly, the blood flow responses to the cognitive task were lower at t6 across all groups (young and old, placebo or DFO, p=0.06). However, neither the task performance nor the change in blood flow was different between age groups or infusions (p>0.25 for all main effects other than time and interaction) (Figure 3).
Figure 3.
Neurovascular coupling (change in cerebral blood flow in response to 2-Back task at baseline (t0) and at peak infusion (t6).
Biomarkers of HIF-1 Activation
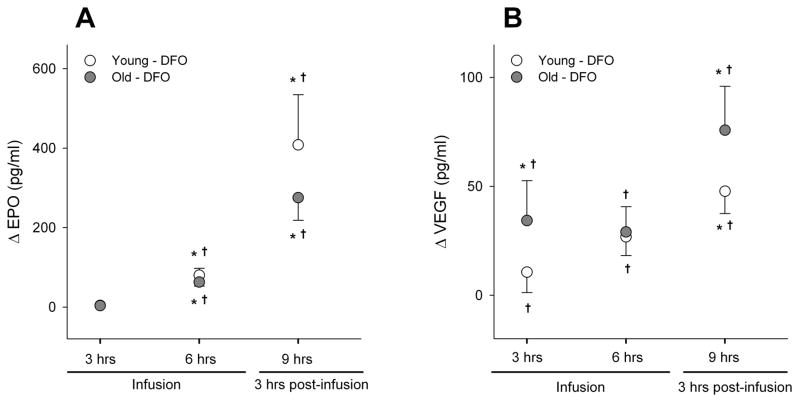
DFO elicited a strong and significant time-dependent increase in the plasma concentrations of EPO and VEGF. In young and old participants, EPO concentrations were significantly increased after 6 hours of DFO infusion, and continued to increase post-infusion (i.e., at the 9th hour; time effect, treatment effect, and time x treatment p<0.01). The magnitude of DFO-induced EPO transcription was somewhat higher in the younger participants, though this difference did not reach significance (age x treatment p=0.26) (Figure 4A). Similar to EPO, there was a significant time-dependent increase in VEGF concentrations in both young and old participants during and after DFO infusion (time effect, treatment effect, and treatment x time p<0.01). However, the magnitude of VEGF transcription was smaller than that of EPO (Figure 4B). VEGF concentration was also somewhat greater in older individuals, but there was no age effect or age interaction with the main effects (p>0.4). There were no changes in VEGF or EPO concentrations during the placebo infusion.
Figure 4.
The change in transcriptional activation during (t3 and t6) and after (t9) DFO infusion compared to baseline (t0). *p<0.05 vs baseline, and †p<0.05 vs. placebo.
Discussion
Our results show that administration of the iron chelator DFO significantly improves two major components of cerebrovascular function (vasoreactivity and autoregulation) and elicits a robust transcriptional activation of HIF-1 regulated proteins (EPO and VEGF). Moreover, the effect of DFO on vascular regulation tended to be more pronounced in the older individuals, who had less effective cerebrovascular function at baseline compared to the young. Thus, our results provide support for one mechanism (cerebrovascular function) underlying DFO mediated-neuroprotection. This finding is particularly relevant for ongoing trials of DFO in neurovascular and neurodegenerative disorders.
Vascular dysfunction is a nearly universal complement to advancing age.33 Consistent with prior studies, cerebrovascular function was less effective in our healthy older participants compared to young. Cerebral vasoreactivity, a potential measure of endothelial function in the brain,34 was lower, and cerebral autoregulation, the ability of cerebral vasculature to buffer against pressure fluctuations, was less efficient in older individuals. It is important to note that cerebrovascular function was diminished in the older participants despite the absence of any of the common age-related vascular risk factors, such as hypertension or diabetes, which have traditionally been tied to cerebrovascular dysfunction. Therefore, age-related decline in vascular function may be a promising therapeutic target in cerebrovascular aging, which is particularly relevant to functional disability in old age.
In fact, the most exciting element of our results is the significant DFO-mediated improvement in cerebrovascular function in older participants. Alterations in cerebrovascular function with healthy aging have been extensively investigated and aging has been shown to be associated with a decline in cerebral vasoreactivity35 and cerebral autoregulation36;37 over time. Yet, to date, very few pharmacological agents have shown any promise in improving some of these functional changes, and available data is equivocal. In a recent study, high-dose atorvastatin therapy significantly improved cerebral vasoreactivity in a small number of patients with impaired baseline vasoreactivity.38 However, a larger study of statins and cerebral vasoreactivity did not show any benefit.39 In animals, both ACE inhibitors and ARBs have been shown to improve cerebral autoregulation40 but the data in humans is limited.41;42. In our study, DFO infusion significantly improved cerebral vasoreactivity and cerebral autoregulation in the older participants. Compared to placebo, the effect of DFO on cerebral vasoreactivity was smaller, and that on cerebral autoregulation was not significant in younger individuals. This is likely to be due to a “ceiling effect”. In other words, DFO may not have appreciable effects on cerebrovascular control in healthy young individuals, whose vascular function is already at or near its peak capacity. We also did not observe an effect of DFO on neurovascular coupling. However, the lack of effect may not be surprising given that measures of neurovascular coupling were similar in the young and older participants at baseline.
Investigations to decipher molecular mechanisms that underlie vascular aging have implicated a regulatory role for the hypoxia-inducible transcription factor 1 (HIF-1). HIF-1, a heterodimeric transcription factor, is responsible for a coordinated genetic program mediating diverse but related functions, such as increased respiratory rate, erythropoiesis, glycolysis, and angiogenesis, in response to hypoxic stress.43 These responses are directed at minimizing the mismatch between substrate supply and metabolic demand. Consistent with studies in senescent animals,18;19 we found that EPO and VEGF, two HIF-1 regulated proteins specifically involved in angiogenesis, neurogenesis, and neuroprotection, were significantly elevated during and following DFO infusion in both young and older individuals. This finding supports the notion that age-related HIF-1 down-regulation, and consequently, vascular aging, may be alleviated via pharmacological intervention.
Our study was not designed to explicitly delineate the mechanistic link between HIF-1 regulated protein expression and cerebrovascular function. However, while our results are based on associative and not causative relations it is important to note that both DFO-mediated EPO and VEGF activation and the improvements in cerebral vasoreactivity and autoregulation followed a similar time course and were sustained up to 3 hours post-infusion. This parallel time course may suggest a mechanistic link involving transcriptional activation and protein synthesis, rather than a direct, transient effect of DFO on the cerebral vessels. If the latter were true, observed improvements in cerebrovascular function would have ended, or at least attenuated with termination of DFO infusion. Instead, the effect did not only last, but was also amplified 3 hours post-infusion (Figures 1 – 2). Thus, our results are strongly suggestive of a causative and mechanistic link between DFO-mediated transcriptional activation of proteins regulating cerebrovascular function and enhanced vascular regulation. Nonetheless, further studies are warranted to identify the exact mechanisms linking DFO to improved cerebrovascular function.
One such link may involve transcriptional activation of another HIF-1 regulated gene, nitric oxide synthase (NOS), which plays an important role in cerebrovascular function. For example, cerebral vasoreactivity is decreased with pharmacologic inhibition of nitric oxide (NO)44 and in individuals with endothelial dysfunction.34 In addition, in healthy individuals, cerebral vasoreactivity is blunted by NOS inhibition via L-NMMA, which can be reversed by L-arginine.44 Basal release of NO is also an important determinant of the cerebral flow,45–47 and NO may partly contribute to cerebral autoregulation.47;48 Nonetheless, further studies are warranted to identify the exact mechanisms linking DFO to improved cerebrovascular function.
Direct measurement of HIF-1 protein levels would have been the most definitive way to prove that DFO can activate HIF-1 protein and pharmacologically ameliorate age-related HIF-1 downregulation. However, given that HIF-1 protein is only expressed in nucleated cells and has a very short half-life (about 4–6 min at room air),49 this assay is difficult in human studies where rapid access to tissue is limited. Therefore, we relied on proxy measures of HIF-1 activation, EPO and VEGF plasma concentrations, which are known to be HIF-1 regulated proteins.43 The relatively small sample size of our study is also a potential limitation. However, we have chosen a robust study design and statistical approach to maximize the reliability of our conclusions and to minimize the risk of committing type-I error. Moreover, it should be noted that most of the statistically significant effects we have seen were large and robust, and differences that did not reach to statistical significance had p values at least 0.20 (mostly>0.50). Therefore, it is highly unlikely that including more subjects would change our main conclusions. Lastly, neurovascular coupling was assessed in response to a cognitive challenge (N-Back test) which has been extensively used in the fMRI literature.50;51 However, while N-back task can assess cognitive function that involves working memory, attention, and cognitive challenge rapidly and repeatedly, one test is clearly not sufficient to assess “neurocognition” as associated alterations in circulation in its entirety. Lastly, our reliance on linear methods to assess cerebral autoregulation may be a potential limitation. While linear approaches can indicate an improvement (or deterioration) in cerebral autoregulation, nonlinearities may play an important role, and linear approaches may not be adequate to fully describe characteristics and effectiveness of cerebral autoregulation.52;53 Thus, while we observed an improvement in cerebral autoregulation in older individuals after DFO infusion, future studies are needed to delineate exactly how DFO enhances vascular function in the brain.
Perspectives
Improving cerebral vascular regulation through DFO infusion and HIF-1 activation holds great promise as a therapeutic strategy for alleviating the deleterious effects of cerebrovascular disease in acute and/or chronic vascular syndromes and disorders such as Alzheimer’s disease and Parkinson’s disease, which also have a strong age-related vascular component. Further insight into the specific HIF-1 regulated pathways responsible for cerebrovascular function may lead to designer HIF-1 activators that can selectively target only the pathways responsible for enhancing cerebrovascular function in response to metabolic and perfusion challenges that the aging brain faces.
Supplementary Material
Acknowledgments
Sources of Funding:
This work was supported by NIH grants K23-AG030967 (FAS), RO1-NS085002 (FAS), P01-AG04390 (LAL) and R01-AG041785 (LAL).
Footnotes
Conflict of interest: none
References
- 1.Chaudhary N, Gemmete JJ, Thompson BG, Xi G, Pandey AS. Iron--potential therapeutic target in hemorrhagic stroke. World Neurosurg. 2013;79:7–9. doi: 10.1016/j.wneu.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Dunaief JL. Ironing out neurodegeneration: iron chelation for neuroprotection. Free Radic Biol Med. 2011;51:1480–1481. doi: 10.1016/j.freeradbiomed.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong JK, Seo JS, Moon MH, Lee YJ, Seol JW, Park SY. Hypoxia-inducible factor-1 alpha regulates prion protein expression to protect against neuron cell damage. Neurobiol Aging. 2012;33:1006–1010. doi: 10.1016/j.neurobiolaging.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Selim M. Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke; a journal of cerebral circulation. 2009;40:S90–S91. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Guo Y, Sun M, Li B, Zhang Y, Li C. Iron is a potential key mediator of glutamate excitotoxicity in spinal cord motor neurons. Brain Res. 2009;1257:102–107. doi: 10.1016/j.brainres.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Zhang K, Du X, Li Y. Neuroprotection of desferrioxamine in lipopolysaccharide-induced nigrostriatal dopamine neuron degeneration. Mol Med Rep. 2012;5:562–566. doi: 10.3892/mmr.2011.671. [DOI] [PubMed] [Google Scholar]
- 7.Sorond FA, Ratan RR. Ironing-out mechanisms of neuronal injury under hypoxic-ischemic conditions and potential role of iron chelators as neuroprotective agents. Antioxid Redox Signal. 2000;2:421–436. doi: 10.1089/15230860050192206. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- 9.Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22:520–525. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hishikawa T, Ono S, Ogawa T, Tokunaga K, Sugiu K, Date I. Effects of deferoxamine-activated hypoxia-inducible factor-1 on the brainstem after subarachnoid hemorrhage in rats. Neurosurgery. 2008;62:232–240. doi: 10.1227/01.NEU.0000311082.88766.33. [DOI] [PubMed] [Google Scholar]
- 11.Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol Aging. 2015;36:27–32. doi: 10.1016/j.neurobiolaging.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez OL, Klunk WE, Mathis C, Coleman RL, Price J, Becker JT, et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83:1804–1811. doi: 10.1212/WNL.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang AJ, Yu XJ, Wang M. The clinical manifestations and pathophysiology of cerebral small vessel disease. Neurosci Bull. 2010;26:257–264. doi: 10.1007/s12264-010-1210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke; a journal of cerebral circulation. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 15.Bazan NG, Palacios-Pelaez R, Lukiw WJ. Hypoxia signaling to genes: significance in Alzheimer’s disease. Mol Neurobiol. 2002;26:283–298. doi: 10.1385/MN:26:2-3:283. [DOI] [PubMed] [Google Scholar]
- 16.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008;8:754–767. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- 17.Poulose N, Raju R. Aging and injury: alterations in cellular energetics and organ function. Aging Dis. 2014;5:101–108. doi: 10.14336/AD.2014.0500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabie T, Kunze R, Marti HH. Impaired hypoxic response in senescent mouse brain. Int J Dev Neurosci. 2011;29:655–661. doi: 10.1016/j.ijdevneu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 19.LaManna JC. Hypoxia in the central nervous system. Essays Biochem. 2007;43:139–151. doi: 10.1042/BSE0430139. [DOI] [PubMed] [Google Scholar]
- 20.Harten SK, Ashcroft M, Maxwell PH. Prolyl hydroxylase domain inhibitors: a route to HIF activation and neuroprotection. Antioxid Redox Signal. 2010;12:459–480. doi: 10.1089/ars.2009.2870. [DOI] [PubMed] [Google Scholar]
- 21.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–1045. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 24.Baksi AJ, Pennell DJ. Randomized controlled trials of iron chelators for the treatment of cardiac siderosis in thalassaemia major. Front Pharmacol. 2014;5:217. doi: 10.3389/fphar.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temraz S, Santini V, Musallam K, Taher A. Iron overload and chelation therapy in myelodysplastic syndromes. Crit Rev Oncol Hematol. 2014;91:64–73. doi: 10.1016/j.critrevonc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke; a journal of cerebral circulation. 2000;31:1672–8. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 27.Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol. 2004;559:965–973. doi: 10.1113/jphysiol.2004.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke; a journal of cerebral circulation. 2010;41:102–109. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koopmans LH. The spectral analysis of time series. New York, NY: Academic Press; 1995. [Google Scholar]
- 30.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 31.Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, et al. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olejnik S, Keselman H. Analysis of covariance (ANCOVA) In: Kattan M, editor. Encyclopedia of medical decision making. Thousand Oaks, CA: SAGE Publications Inc; 2009. pp. 16–22. [Google Scholar]
- 33.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 34.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 35.Sorond FA, Galica A, Serrador JM, Kiely DK, Iloputaife I, Cupples LA, et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology. 2010;74:1627–1633. doi: 10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodie FG, Panerai RB, Foster S, Evans DH, Robinson TG. Long-term changes in dynamic cerebral autoregulation: a 10 years follow up study. Clin Physiol Funct Imaging. 2009;29:366–371. doi: 10.1111/j.1475-097X.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- 37.Oudegeest-Sander MH, van Beek AH, Abbink K, Olde Rikkert MG, Hopman MT, Claassen JA. Assessment of dynamic cerebral autoregulation and cerebrovascular CO2 reactivity in ageing by measurements of cerebral blood flow and cortical oxygenation. Exp Physiol. 2014;99:586–598. doi: 10.1113/expphysiol.2013.076455. [DOI] [PubMed] [Google Scholar]
- 38.Forteza A, Romano JG, Campo-Bustillo I, Campo N, Haussen DC, Gutierrez J, et al. High-dose atorvastatin enhances impaired cerebral vasomotor reactivity. J Stroke Cerebrovasc Dis. 2012;21:487–492. doi: 10.1016/j.jstrokecerebrovasdis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Lavallee PC, Labreuche J, Gongora-Rivera F, Jaramillo A, Brenner D, Klein IF, et al. Placebo-controlled trial of high-dose atorvastatin in patients with severe cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2009;40:1721–1728. doi: 10.1161/STROKEAHA.108.540088. [DOI] [PubMed] [Google Scholar]
- 40.Sigurdsson ST, Paulson OB, Hoj NA, Strandgaard S. Bradykinin antagonist counteracts the acute effect of both angiotensin-converting enzyme inhibition and of angiotensin receptor blockade on the lower limit of autoregulation of cerebral blood flow. J Cereb Blood Flow Metab. 2014;34:467–471. doi: 10.1038/jcbfm.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saura H, Ogasawara K, Suzuki T, Kuroda H, Yamashita T, Kobayashi M, et al. Effect of combination therapy with the angiotensin receptor blocker losartan plus hydrochlorothiazide on brain perfusion in patients with both hypertension and cerebral hemodynamic impairment due to symptomatic chronic major cerebral artery steno-occlusive disease: a SPECT study. Cerebrovasc Dis. 2012;33:354–361. doi: 10.1159/000335836. [DOI] [PubMed] [Google Scholar]
- 42.Walters M, Muir S, Shah I, Lees K. Effect of perindopril on cerebral vasomotor reactivity in patients with lacunar infarction. Stroke; a journal of cerebral circulation. 2004;35:1899–1902. doi: 10.1161/01.STR.0000131748.12553.ed. [DOI] [PubMed] [Google Scholar]
- 43.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmetterer L, Findl O, Strenn K, Graselli U, Kastner J, Eichler HG, et al. Role of NO in the O2 and CO2 responsiveness of cerebral and ocular circulation in humans. Am J Physiol. 1997;273:R2005–R2012. doi: 10.1152/ajpregu.1997.273.6.R2005. [DOI] [PubMed] [Google Scholar]
- 45.Dahl A, Russell D, Nyberg-Hansen R, Rootwelt K. Effect of nitroglycerin on cerebral circulation measured by transcranial Doppler and SPECT. Stroke; a journal of cerebral circulation. 1989;20:1733–1736. doi: 10.1161/01.str.20.12.1733. [DOI] [PubMed] [Google Scholar]
- 46.Hancock SM, Eastwood JR, Mahajan RP. Effects of inhaled nitrous oxide 50% on estimated cerebral perfusion pressure and zero flow pressure in healthy volunteers. Anaesthesia. 2005;60:129–132. doi: 10.1111/j.1365-2044.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 47.White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clin Sci (Lond) 2000;99:555–560. [PubMed] [Google Scholar]
- 48.Hamner JW, Tan CO. Relative contributions of sympathetic, cholinergic, and myogenic mechanisms to cerebral autoregulation. Stroke; a journal of cerebral circulation. 2014;45:1771–1777. doi: 10.1161/STROKEAHA.114.005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moroz E, Carlin S, Dyomina K, Burke S, Thaler HT, Blasberg R, et al. Real-time imaging of HIF-1alpha stabilization and degradation. PLoS One. 2009;4:e5077. doi: 10.1371/journal.pone.0005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 51.Walter H, Bretschneider V, Gron G, Zurowski B, Wunderlich AP, Tomczak R, et al. Evidence for quantitative domain dominance for verbal and spatial working memory in frontal and parietal cortex. Cortex. 2003;39:897–911. doi: 10.1016/s0010-9452(08)70869-4. [DOI] [PubMed] [Google Scholar]
- 52.Tan CO. Defining the Characteristic Relationship between Arterial Pressure and Cerebral Flow. J Appl Physiol. 2012;113:1194–1200. doi: 10.1152/japplphysiol.00783.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan CO, Taylor JA. Integrative physiologic and computational approaches to understand autonomic control of cerebral autoregulation. Exp Physiol. 2014;99:3–15. doi: 10.1113/expphysiol.2013.072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.