Abstract
Exercise intolerance in heart failure has been linked to impaired skeletal muscle oxidative capacity. Oxidative metabolism and exercise capacity are regulated by PPARδ signaling. We hypothesized that PPARδ stimulation reverts skeletal muscle oxidative dysfunction. Myocardial infarction (MI) was induced in C57BL/6 mice and the development of ventricular dysfunction was monitored over 8 wk. Mice were randomized to the PPARδ agonist GW501516 (5 mg/kg body wt per day for 4 wk) or placebo 8 wk post-MI. Muscle function was assessed through running tests and grip strength measurements. In muscle, we analyzed muscle fiber cross-sectional area and fiber types, metabolic gene expression, fatty acid (FA) oxidation and ATP content. Signaling pathways were studied in C2C12 myotubes. FA oxidation and ATP levels decreased in muscle from MI mice compared with sham- operated mice. GW501516 administration increased oleic acid oxidation levels in skeletal muscle of the treated MI group compared with placebo treatment. This was accompanied by transcriptional changes including increased CPT1 expression. Further, the PPARδ-agonist improved running endurance compared with placebo. Cell culture experiments revealed protective effects of GW501516 against the cytokine-induced decrease of FA oxidation and changes in metabolic gene expression. Skeletal muscle dysfunction in HF is associated with impaired PPARδ signaling and treatment with the PPARδ agonist GW501516 corrects oxidative capacity and FA metabolism and improves exercise capacity in mice with LV dysfunction. Pharmacological activation of PPARδ signaling could be an attractive therapeutic intervention to counteract the progressive skeletal muscle dysfunction in HF.
Keywords: heart failure, skeletal muscle, metabolism, PPARδ
skeletal muscle weakness and exercise intolerance are hallmarks of advanced heart failure (HF) and have been shown to correlate with a reduction in oxidative metabolism and protein catabolism (5, 37). This metabolic derangement results not only from a decrease in oxygen and nutrient supply to skeletal muscle but also from intrinsic changes in skeletal muscle gene expression, cytokine activation, and mitochondrial dysfunction (24, 33). These changes result in a shift of muscle fibers from oxidative toward more glycolytic fibers and an impaired oxidative capacity of skeletal muscle in HF (5). Effective treatments for skeletal muscle dysfunction in HF are lacking; only aerobic exercise training has been shown to improve skeletal muscle function and exercise intolerance, which was linked to an associated improvement in oxidative metabolism and mitochondrial function following exercise (10, 25).
Peroxisome proliferator-activated receptors (PPARs) are a family of nuclear receptors that sense metabolic status and engage the cell in lipid uptake and utilization (28, 31). Among the three members of this family (PPARα, -γ, and -δ), PPARδ is the predominant isoform in skeletal muscle (1, 3, 16). PPARδ levels are higher in oxidative type I compared with glycolytic type II muscle fibers (34), and PPARδ gene deletion mice have a lower proportion of type I muscle fibers (23). Mice overexpressing constitutively active PPARδ show an increase of type I fibers (34). Wild-type mice receiving a PPARδ agonist elicited a similar type I fiber gene expression profile in their skeletal muscle and showed an increase in expression of genes related to oxidative function of mitochondria and fatty acid oxidation (34). The specific PPARδ agonist GW501516 upregulates genes involved in fatty acid transport, beta-oxidation, and mitochondrial respiration (29).
Therefore, we hypothesized that in mice with left ventricular dysfunction following myocardial infarction (MI) and signs of skeletal muscle dysfunction, treatment with the PPARδ agonist GW501516 improves oxidative metabolism, skeletal muscle catabolism, and endurance function.
METHODS
Animal model.
Left ventricular (LV) dysfunction was induced in C57BL/6 mice by ligature of the left coronary artery and confirmed by echocardiography (n = 5). Sham-operated animals underwent the same procedure without ligation of the coronary artery (n = 5). To test the impact of PPARδ agonism, starting at 8 wk after surgery, an additional group of mice received the PPARδ agonist GW501516 (5 mg/kg body wt dissolved in DMSO and 0.5% carboxymethylcellulose per day for 4 wk, n = 10) or placebo (DMSO only, n = 10) via gavage. Animals were euthanized 12 wk post-MI after 4 h of starvation.
For the endurance running test, mice were accustomed to the treadmill by running 5 min/day for 3 days at a 0° incline and a belt speed of 10 m/min. At the time of the test (on the day before being euthanized), mice ran on a treadmill set at a 20° incline with an initial belt speed of 10 m/min. The speed was increased to 14 m/min at minute 10 and to 18 m/min at minute 15. Mice ran until exhaustion at 18 m/min speed. Exhaustion was judged by refusal of the mice to continue running on the treadmill belt after being stimulated to run by gently being pushed forward three times.
This study and its protocols were approved by the local IACUC institutional review board of Columbia University.
Echocardiography.
Cardiac function was assessed by echocardiography at baseline, 8 wk post-MI, and before being euthanized in order to assess successful MI induction and the possible effect of the treatment on myocardial function and remodeling. Mice were anesthetized with inhalational isoflurane anesthesia. Anesthesia was induced by 1.5–2% isoflurane, and reduced to 0.5–1% once the mouse was asleep. The heart was visualized by using a 30-MHz high-frequency ultrasound transducer (Visualsonics Vevo770, Toronto, Canada).
Muscle fiber histomorphometry.
Quadriceps muscles were excised and fixed in ∼20 times its volume in 10% buffered formalin phosphate (Fisher Scientific) and then stored in 70% ethanol with refrigeration until embedding procedure. Hematoxylin and eosin (H and E) staining was performed in skeletal muscle sections and used in cross-sectional area analysis. ImageJ (NIH) software was used to measure cross sectional areas in 10× magnification. Pictures were taken using a Leica microscope and Spot camera and software (Diagnostic Instruments) of at least 400 quadriceps fibers per animal.
Muscle fiber typing.
Quadriceps were excised and fixed in 20 times its volume in 10% buffered formalin phosphate (Fisher Scientific) and then stored in 70% ethanol with refrigeration until embedding procedure. Paraffin-enclosed sections were fixed in 10% buffered formalin phosphate for 30 min and then deparaffinized in Histo-Clear, histological clearing agent (National Diagnostics) for 5 min, 3 times followed by rehydration from 100% to 75% ethanol. Antigen unmasking was performed by boiling in 1 × Antigen retrieval solution (BioGenex, Fremont, CA) for 30 min and then slides were blocked with 6% normal goat serum (Vector) in phosphate-buffered solution (PBS) for 30 min. Monoclonal antibody directed against skeletal muscle fast fiber type myosin (Sigma M4276) diluted 1:400 in 6% in normal goat serum (Vector)-PBS was used for incubation overnight at 4°C. After washing in 0.1% Tween PBS and PBS, slides were incubated in 3% hydrogen peroxide for 10 min and then washed again before incubation with secondary biotinylated Anti-mouse IgG made in goat (Vector) diluted 1:400 in 6% in normal goat serum (Vector)-PBS for 30 min at room temperature. Slides were then washed and incubated for 30 min with Vecstatin ABC kit (Vector) at room temperature following manufacturer instructions followed by washing and developed using NovaRED (Vector) diaminobenzidine substrate. Reaction was stopped in distilled water and dehydrated in ethanol solutions from 75% to 100% and cleared with Histo-Clear, histological clearing and finally mounted in Permount (Fisher Scientific). Magnification (10×) pictures were analyzed for cross-sectional area.
Citrate synthase activity assay.
Skeletal muscle pieces of about 20 mg were homogenized 20% (wt/vol) homogenization buffer (20 mM HEPES, 10 mM EDTA, pH 7.4) on ice. Homogenates were frozen for 1 h and then diluted 1:10. The reaction was done in 20 mM HEPES, 1 mM EDTA, 220 mM, 40 mM KCl, 0.1 mM 2-nitrobenzoic acid, 0.1 mM acetyl-CoA, pH 7.4, at 25°C. Reaction was started by adding 0.05 mM oxaloacetate and OD was measured at 412 nm for 3 min.
Succinate dehydrogenase activity assay.
Skeletal muscle tissue was homogenized in 250 mM saccharose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.2, centrifuged for 10 min, and 4°C at 600 g. The supernatant was centrifuged at 12,000 g for 10 min, 4°C. The pellet was resuspended in incubation medium (5 mM KPO, pH 7.2, 220 mM saccharose, 20 mM, KCl, 10 mM HEPES, pH 7.2). Reaction was assayed in 100 mM triethanolamine (HCl), pH 8.3, 0.5 mM EDTA, 2 mM KCN, 2 mM iodonitrotetrazolium chloride (INT), 2 g/l cremophor EL, 20 mM succinate, previously adjusted to pH 7.4. The reduction of INT to a formazan was followed at 500 nm at 30°C for 6 min.
Calculations were performed according to the respective assay protocol (Sigma Aldrich, Citrate Synthase Assay Kit, Catalog No. CS0720; Sigma Aldrich, Succinate Dehydrogenase Activity Colorimetric Assay Kit, Catalog No. MAK197) with an abundant substrate concentration. All analyses are based on end point measurements. The kinetic parameter Km was not measured.
Mitochondrial DNA copy number.
The number of mitochondria was assessed by the ratio of cytochrome c oxidase subunit 2 (a mitochondrion-encoded gene) mRNA expression levels corrected for cyclophilin A (nuclear-encoded gene) mRNA expression levels and compared between groups.
ATP content analysis.
Skeletal muscle was excised and immediately frozen in liquid nitrogen. Tissue was cut into 20 mg pieces and immediately homogenized in 10 volumes of 0.25% trichloroacetic acid, 2 mM EDTA using 1.0 mm Zirconia/Silica beads (BioSpec Products). Homogenates were spun at 4,500 rpm for 10 min at 4°C. The supernatant was diluted 1:10 with 250 mM Tricine buffer, pH 7.8, 50 mM MgSO4, 1 mM EDTA, and 1 mM sodium azide. ATP concentration was determined in the diluted supernatant using a firefly luciferase bioluminescence-based ATP determination kit (Invitrogen) following manufacturer instructions. Protein concentration was determined by BCA method.
Fatty acid oxidation measurements.
Thirty millligrams of quadriceps muscle was assayed for fatty acid oxidation activity as previously described (12) with modifications as follows. Tissue pieces were placed in a flask containing 1 ml low-glucose DMEM cell culture medium (Cellgro), 1.5% delipidated bovine serum albumin fraction V (Sigma), 0.2 mM oleic acid, and 1 μCi/ml [14C]oleic acid and were incubated at 37°C for 2 h. The filter papers were soaked with 1 N KOH and the reaction was stopped injecting 200 μl of 70% perchloric acid and incubating for 1 h with shaking. At the end of the incubation, the filter papers were transferred to vials containing scintillation liquid, and counts per minute (cpm) were measured using a scintillation liquid counter. The total oleic acid oxidation reaction rate was evaluated as 14CO2 released and analyzed from cpm in the filter papers.
RT-PCR.
Gene expression was analyzed by real time PCR (iCycler, BioRad) using specific oligonucleotides: PDK4, 5′-AACGCAACACAAAACCAAGC-3′ [forward (f)], 5′-CATTGCCAAAGGAGAAGCAG-3′ [reverse (r)]; CD36, 5′-CAGCCTCCTTTCCACCTTTT-3′ (f), 5′-GCATTGGCTGGAAGAACAAA-3′ (r); CPT1, 5′-CCTGCAAGAATGGCCTGTTA-3′ (f), 5′-GCTCACCCACACAGTGTCCT-3′ (r); PGC1a, 5′-CACGCAGCCCTATTCA-3′ (f), 5′-GTCGTACCTGGGCCTA-3′ (r); LPL, 5′-GCTGGTGGGAAATGATGTG-3′ (f), 5′-TGGACGTTGTCTAGGGGGTA-3′ (r); ANGPTL4, 5-GGAAAAGATGCACCCTTCAA-3′ (f), 5-TGCTGGATCTTGCTGTTTTG-3′ (r); ATG1, 5′-TTCAGCAGCCTGAACTACGA-3′ (f), 5′-GGCAGTCGAGAAGTCCAGTC-3′ (r); UCP2, 5′-ACAAGACCATTGCACGAGAG-3′ (f), 5′-CATGGTCAGGGCACAGTGGC-3′ (r); COXII, 5′-CAGGCCGACTAAATCAAGCAAC-3′ (f), 5′-CTAGGACAATGGGCATAAAGCT-3′ (r); cyclophilin A, 5′-TTCCTCCTTTCACAGAATTATTCCA-3′ (f), 5′- CCGCCAGTGCCATTATGG-3′ (r); FABP3, 5′-CATGACCAAGCCTACCACAAT-3′ (f), 5′-CCCCAACTTAAAGCTGATCTCTG-3′ (r).
Cell culture.
Mouse C2C12 myoblasts were cultured in Dulbecco's modified essential medium high glucose (Gibco) with 10% fetal calf serum, 1% penicillin-streptomycin at 37°C, 5% CO2. After reaching 80–90% confluence, cells were washed with PBS removing the media and incubated with DMEM containing 3% horse serum (Sigma) to induce differentiation into myotubes for 2–5 days.
Statistical analysis.
Statistical analysis was performed using Student's t-test for comparison between two groups and ANOVA with subsequent post hoc analysis for more than two groups. A P value < 0.05 was considered statistically significant. Analysis was performed using GraphPad Prism (version 5). All values are presented as means ± SE.
RESULTS
Animal model.
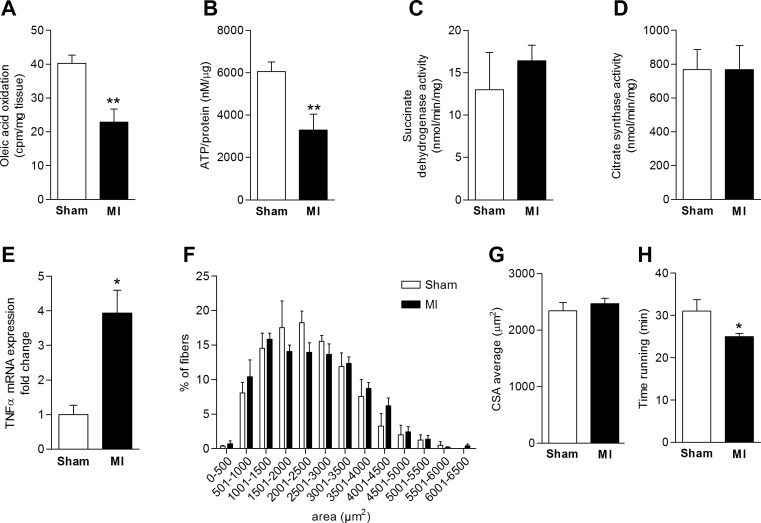
MI by ligation of the left coronary artery was confirmed by echocardiography [8 wk post-surgery: fractional shortening 31.6 ± 2.9 in the sham group vs. 18.5 ± 4.2% in the MI group, P = 0.041, left ventricular end-diastolic diameter (LVEDD) 4 ± 0.2 vs. 5 ± 0.3 mm, P = 0.033]. Skeletal muscle histomorphometry showed no significant changes in oxidative fiber proportion in the MI group; also, cross-sectional area analysis revealed no changes (Fig. 1, F and G). Exercise endurance function was reduced (Fig. 1H; running to exhaustion: 31 ± 2.7 vs. 25 ± 0.7 min, P = 0.046) without changes in grip strength (4.8 ± 0.4 vs. 5.5 ± 0.5 g/g body wt; P = 0.25).
Fig. 1.
Skeletal muscle dysfunction in an animal model with left ventricular dysfunction following myocardial infarction. A: reduced oleic acid oxidation rate in skeletal muscle of animals 8 wk post-myocardial infarction (post-MI). B: decreased ATP content in skeletal muscle tissue in MI mice compared with the sham-operated group. C and D: no differences in succinate dehydrogenase (SDH) or citrate synthase (CS) activity were noted between groups. E: increased TNFα mRNA expression levels in skeletal muscle of MI mice. F and G: muscle fiber cross-sectional area (CSA) analysis. No significant changes in CSA were observed between sham and MI mice. H: decreased running time in the MI group compared with sham-operated mice (*P < 0.05, **P < 0.01, n = 5 per group).
Characterization of abnormal skeletal muscle oxidative capacity post-MI.
Ex vivo oleic acid oxidation measurements in quadriceps muscle showed a decrease in fatty acid oxidation rates in HF animals compared with control (−57%, P = 0.005, Fig. 1A). Expression of proinflammatory TNFα was increased in skeletal muscle of animals following MI (fold change 1 ± 0.27 n = 3 vs. 3.94 ± 0.66 n = 3, P = 0.015) (Fig. 1E). Analysis of skeletal muscle ATP content revealed significant differences in ATP content in skeletal muscle of animals following MI compared with sham surgery (−54%, P = 0.009, Fig. 1B).
To address whether mitochondrial function was abnormal at the level of Krebs cycle enzymatic activity, we assessed the activity of citrate synthase (CS), a rate-limiting enzyme of the Krebs cycle, and succinate dehydrogenase (SDH), which is pivotal for electron chain transfer, in skeletal muscle. No difference between sham-operated mice and the MI group was found (Fig. 1, C and D). Of note, citrate synthase has been established as a marker of skeletal muscle mitochondrial density (9, 17).
Analysis of PPAR signaling in C2C12 cells.
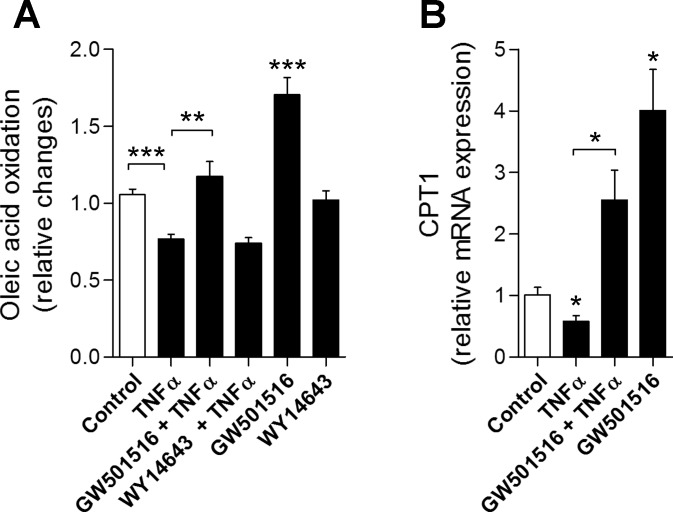
We next assessed the role of PPAR signaling on cytokine-induced changes of oxidative capacity in C2C12 myotubes. TNFα treatment (25 ng/ml, 16 h) reduced oleate oxidation rates in vitro by 28.9% (P < 0.001 vs. unstimulated cells, Fig. 2A). Costimulation with the PPARδ agonist GW501516 [5 μM (2)] at baseline and in the presence of TNFα increased oleate oxidation rates by 65% (P < 0.001 vs. unstimulated cells) and 40.8% (P < 0.01 vs. TNFα-stimulated cells). Costimulation with the PPARα agonist WY14643 [80 μM (17)] had no effects at baseline and did not correct the TNFα-induced decrease in FA oxidation rates (Fig. 2A). Further, expression of the marker gene and rate-limiting enzyme of FA oxidation, carnitine palmitoyltransferase-1 (CPT1), decreased in the presence of TNFα (−43%, P = 0.045) and increased upon costimulation with the PPARδ agonist GW501516 (TNFα vs. TNFα + GW501516: +196%, P = 0.017) (Fig. 2B).
Fig. 2.
In vitro analysis of effects of peroxisome proliferator-activated receptor δ (PPARδ) stimulation. A: reduced fatty acid oxidation rates in response to TNFα stimulation is corrected by PPARδ agonist (GW501516) coincubation but not in response to PPARα agonism (WY14643). B: expression of carnitine palmitoyltransferase-1 (CPT1) decreases under TNFα treatment and increases with coincubation using the PPARδ agonist (C2C12 skeletal muscle myotubes, n = 3 independent experiments per condition. *P < 0.05, **P < 0.01, ***P < 0.001).
Analysis of in vivo effects of PPARδ stimulation post-MI.
To investigate whether GW501516 improves skeletal muscle oxidative capacity in an animal model with left ventricular dysfunction following MI, 8-wk-old C57BL/6 mice were subjected to ligation of the left coronary artery leading to MI. Eight weeks after the surgery, animals with echocardiographic evidence of left ventricular dysfunction were randomized to GW501516 or placebo via daily gavage for a total of 4 wk.
Echocardiography revealed that 4 wk of treatment with the PPARδ agonist GW501516 had no significant effect on fractional shortening (FS) 12 wk following MI (FS: 15.5 ± 3.5 before vs. 19.4 ± 3.7% after treatment, P = 0.46; LVEDD: 5.6 ± 0.3 vs. 5.2 ± 0.5 mm, P = 0.323).
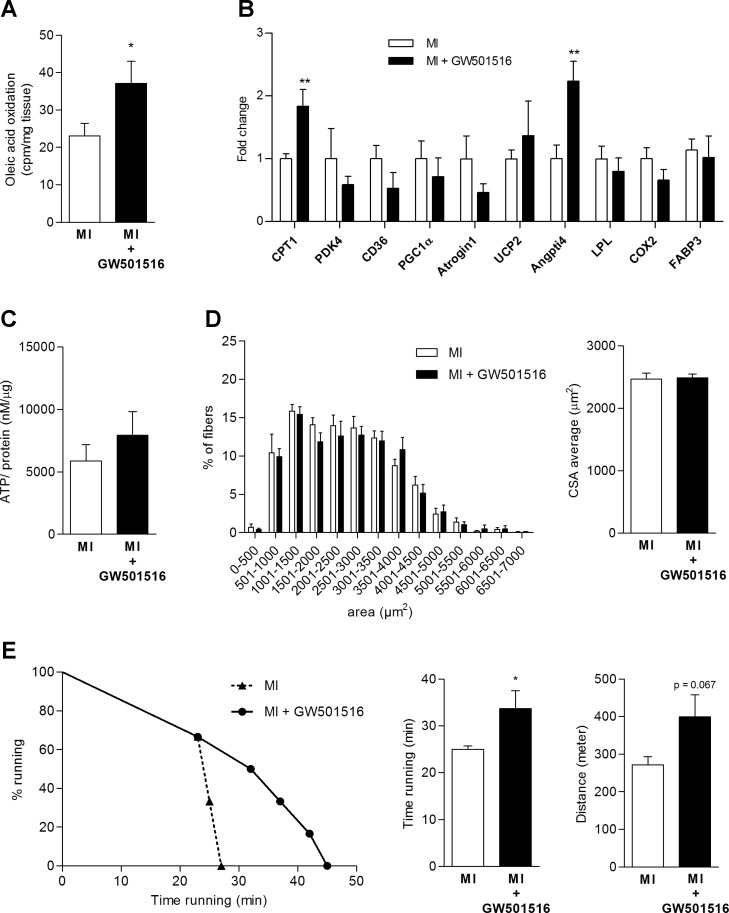
Oleic acid oxidation rates increased in skeletal muscle of animals treated with GW501516 compared with placebo (+47.6%, P = 0.048, Fig. 3A). This was accompanied by changes in skeletal muscle gene expression showing increased CPT1 and Angptl4 levels in the treatment group (CPT1: +101%, P < 0.01; Angptl4: +78.2%, P < 0.01, Fig. 3B) and a nonsignificant increase of ATP normalized for protein content in skeletal muscle (Fig. 3C). Analysis of skeletal muscle histomorphometry demonstrated no differences in muscle CSA (Fig. 3D) or fiber type composition. No statistical differences in the number of mitochondria as assessed by DNA copy number (ratio mitochondrial DNA copy number/genomic DNA copy number: 0.51 ± 0.11 vs. 0.74 ± 0.16, P = 0.27) were found between groups while a numerical trend toward higher numbers in response to GW501516 treatment was noted.
Fig. 3.
Effects of PPARδ treatment in an animal model of chronic left ventricular dysfunction following MI. A: increased fatty acid oxidation rate in response to PPARδ stimulation. B: changes in skeletal muscle gene expression with PPARδ stimulation. C: skeletal muscle ATP level changes, P = 0.38. D: no differences in skeletal muscle CSA. E: prolonged running time in animals following MI treated with a PPARδ agonist (*P < 0.05, **P < 0.01; placebo n = 5–9 and GW501516 n = 6 per analysis).
We finally evaluated exercise endurance function by performing a treadmill running test. As expected, mice with left ventricular dysfunction following MI had shorter running times compared with sham-operated animals (Fig. 1H). Four weeks of treatment with GW501516 resulted in an increased running time of PPARδ-agonist-treated animals compared with the untreated MI-group, indicating improved endurance exercise function (distance and time) (25.0 ± 0.7 vs. 33.7 ± 3.8 min, P = 0.05; distance: 271.8 ± 21.3 vs. 399.5 ± 58.5 m, P = 0.067; Fig. 3E). Grip strength did not differ between the two groups (5.5 ± 0.48 vs. 4.9 ± 0.31 g/g body wt, P = 0.25).
Analysis of plasma TAG concentrations showed a significant decrease of triglyceride concentrations in the GW501516-treated group compared with placebo (5 ± 0.35 vs. 3 ± 0.62 μg/μl, P = 0.017) (Table 1).
Table 1.
Body morphometry, laboratory data, and echocardiographic assessment of animals with left ventricular dysfunction following MI (treated vs. placebo)
| Placebo (n = 10) | GW501516 (n = 10) | |
|---|---|---|
| Body weight (BW), g | 29.3 ± 0.6 | 28 ± 1.1 |
| Heart weight/BW, mg/g | 5.9 ± 0.4 | 6.1 ± 0.5 |
| Quadriceps weight/BW, mg/g | 13.5 ± 0.8 | 12.9 ± 1.3 |
| Glucose, mg/dl | 167.4 ± 20.7 | 142.6 ± 8.7 |
| Plasma FFA, pmol/μl | 0.8 ± 0.1 | 0.58 ± 0.1 |
| Plasma TAG, μg/μl | 5.0 ± 0.4 | 3.02 ± 0.6 |
| Quadriceps TAG, μg/mg | 72.6 ± 5.4 | 100.2 ± 14.9 |
| Echocardiographic analysis | ||
| FS% before intervention | 18.5 ± 4.2 | 15.5 ± 3.5 |
| FS% after intervention | 20.6 ± 3.3 | 19.4 ± 3.7 |
| HR before intervention, beats/min | 431.4 ± 13 | 444.5 ± 12.2 |
| HR after intervention, beats/min | 423.7 ± 10 | 451.8 ± 11.3 |
| AWT before intervention, mm | 0.61 ± 0.04 | 0.67 ± 0.04 |
| AWT after intervention, mm | 0.72 ± 0.02 | 0.75 ± 0.03 |
| PWT before intervention, mm | 0.83 ± 0.06 | 0.78 ± 0.06 |
| PWT after intervention, mm | 0.76 ± 0.05 | 0.76 ± 0.07 |
| LVESD before intervention, mm | 4.17 ± 0.48 | 4.75 ± 0.45 |
| LVESD after intervention, mm | 4.22 ± 0.47 | 4.22 ± 0.48 |
| LVEDD before intervention, mm | 5.02 ± 0.34 | 5.56 ± 0.34 |
| LVEDD after intervention, mm | 5.27 ± 0.43 | 5.16 ± 0.38 |
Values are means ± SE. MI, myocardial infarction; FFA, free fatty acids; TAG, triacylglycerol; FS, fractional shortening; HR, heart rate; AWT, anterior wall thickness; PWT, posterior wall thickness; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter.
No other significant differences were found in plasma Free fatty acid (FFA) concentrations (FFA: 0.8 ± 0.1 vs. 0.58 ± 0.1 pmol/μl) or other metabolic parameters between placebo-treated and PPARδ-agonist-treated groups of the murine MI model except for a trend (P = 0.14) toward an increase of TAG in skeletal muscle in the GW501516-treated group compared with placebo (100.2 ± 14.9 vs. 72.6 ± 5.4 μg/mg). Treatment with the PPARδ agonist GW501516 did not result in differences in baseline fasting glucose levels compared with placebo treatment (Table 1).
DISCUSSION
In the present study, we show positive effects of PPARδ agonism on skeletal muscle oxidative capacity and endurance function. Our data reveal a potential therapeutic role of PPARδ signaling for the control of skeletal muscle homeostasis and functional improvement in the setting of HF, which is known to be associated with a profound skeletal muscle myopathy (5, 37). We show that the oral PPARδ agonist GW501516 is capable of significantly improving impaired oxidative metabolism in skeletal muscle. Skeletal muscle dysfunction is a central impairment in advanced HF (5, 24, 33, 37). This has been linked to intrinsic skeletal muscle abnormalities that include abnormal metabolic gene expression, reduced oxidative capacity and overall mitochondrial dysfunction, increased cytokine levels, and muscle fiber atrophy and type switching. Type I muscle fibers, with preferential utilization of fatty acids as substrate for oxidative metabolism, are known to have better endurance function compared with type II glycolytic fibers (30) and have been shown to be decreased in HF (5). Further, functional assessments demonstrate the development of a specific HF-related myopathy with reduced endurance function contributing to exercise intolerance in patients with HF and animal models of HF (33, 37). Unfortunately, therapeutic interventions are lacking and the majority of pharmacological and nonpharmacological interventions in HF are focused on improving cardiac function or remodeling (36). The only intervention proven to affect skeletal muscle function, metabolism, and structure is aerobic exercise training, which has been shown to improve (but not correct) mitochondrial dysfunction, skeletal muscle metabolism, and structure (7, 25). Only recently, several studies have implicated PPARδ signaling in favorable changes in skeletal muscle structure and metabolism that were considered beneficial in the setting of HF.
PPARδ agonists mediate a shift of glucose oxidation toward fatty acid oxidation in muscle tissue (11). In vitro experiments in L6 cells using GW501516 showed increased expression of genes involved in fatty acid transport, beta-oxidation, and mitochondrial respiration (29). Wang et al. (34) showed an increase in mRNA of CPT1, troponin I, and cytochrome c oxidase IV in gastrocnemius muscle of mice treated with GW501516. CPT1 is a mitochondrial enzyme crucial for fatty acid transportation into the mitochondrion and an essential, rate-limiting step for fatty acid oxidation overall.
Troponin I is a muscle marker of oxidative fiber types while cytochrome c oxidase IV is involved in mitochondrial electron transport, suggesting that PPARδ agonists increase oxidative metabolism in skeletal muscle. In contrast, neither PPARγ nor PPARα agonists are able to modify fatty acid oxidation capacity of skeletal muscles (29).
PPARδ expression is regulated by exercise. Exercise training and brief bouts of exercise increase PPARδ expression in skeletal muscle (6, 15, 22, 35). Further, PPARβ/δ and PGC-1α mRNA increase early after one session of high-intensity exercise training (20). Similarly, a 12-wk exercise training protocol in rats increased PPARδ in plantaris muscle (26). PPARδ expression is positively correlated with the proportion of type I fibers in skeletal muscle and is essential for maintaining the oxidative fiber type composition that defines muscle endurance function (13).
In our present study, we defined PPARδ signaling-specific effects on skeletal muscle homeostasis. Using the PPARα-specific agonist WY14643 in C2C12 cells, we did not observe any beneficial effects on cellular oxidative function compared with the strong effects of the PPARδ-specific agonist GW501516. These findings are well in line with prior studies by other groups. Tanaka et al. (29) reported that in L6 cell myotubes, fenofibrate, another PPARα agonist, does not increase palmitate oxidation rates in contrast to GW501516, which produced an increase in fatty acid oxidation rates. In another study, the PPARδ-specific agonist GWD0742 was able to stimulate fatty acid oxidation in human skeletal muscle tissue with high potency compared with PPARα stimulation (5). Our results demonstrate that the PPARδ agonist GW501516 induces an increase in free fatty acid oxidation rates in skeletal muscle and an improvement in endurance exercise performance of animals with ischemic LV dysfunction in response to PPARδ agonist treatment compared with placebo. The analysis of fiber type composition failed to reveal significant differences. Other authors described similar results in normal and diabetic animals (18, 29). Increased fatty acid oxidation rates in skeletal muscle correlated with increased expression of CPT1 mRNA indicating an increase of fatty acid transport capacity into mitochondria. CPT1 has a pivotal role in the transport of fatty acids in form of acylcarnitines into the mitochondria and functions as the rate-limiting step. Hence, changes in CTP1-expression are a major indicator for alterations of fatty acid utilization on the mitochondrial level.
As it is known from changes in cardiac metabolism in HF, a switch from oxidative toward more glycolytic metabolism occurs in skeletal muscle in HF. It is, however, unclear whether this is driven by changes in oxygen supply since arteriovenous oxygen gradients are normal while under exercise the venous oxygen saturation decreases suggesting either decreased peripheral flow or increased utilization. On the other hand, local inflammation as highlighted by increased expression of TNFα in our present study might contribute to changes in oxidative capacity. It has been demonstrated that metabolism under inflammation is driven by glycolysis for ATP production.(32) It is, however, unclear whether skeletal muscle substrate utilization of fatty acids, glucose, amino acids, and lactate is deranged in vivo.
Analysis of TNFα mRNA expression levels in skeletal muscle of our treatment groups revealed a significant increase in TNFα expression in the MI group compared with sham, but no effects of GW501516 on TNFα expression levels were observed. This suggests that the effects on skeletal muscle oxidative capacity and structure are either independent or downstream of TNFα signaling in the murine model.
In our study we could not observe significant changes in the number of mitochondria as assessed by DNA copy number nor in activities of enzymes of the Krebs cycle. This is in accordance with previous findings describing that PPARδ agonists do not stimulate mitochondrial gene expression but stimulate gene expression controlling fatty acid oxidation (10, 18).
An intriguing finding of our study is the increased expression of angiopoietin-like 4 (Angptl4) in response to PPARδ stimulation. Angptl4 is a multifunctional protein and known to be a target of PPARδ (21). Angptl4 increases levels of hormone-sensitive lipase, a triglyceride lipase that breaks down intracellular triglyceride levels and increases fatty acid release through enhanced triglyceride turnover. Therefore, an increase in Angptl4 might indicate increased lipid utilization as substrate for oxidative phosphorylation in skeletal muscle (27).
Further qPCR analysis of key metabolic genes revealed no significant effect of PPARδ agonism on these gene expression levels (Fig. 3B). Unchanged levels of PDK4 mRNA expression go well in line with the observed unchanged ATP concentrations in skeletal muscle. PGC1α is a crucial regulator of mitochondrial biogenesis and muscle fiber type determination. Fiber type analysis showed no changes in muscle fiber type composition. We hypothesize that treatment for 4 wk with the PPARδ agonist is either not powerful enough or might have been too short to induce significant structural alterations in muscle fiber composition. Hence, considering the duration of treatment over 4 wk, our study is limited. Atrogin-1 has been linked to skeletal muscle atrophy. Consistent with preserved muscle fiber cross-sectional areas, atrogin-1 expression levels remained unchanged. UCP2, COX, FABP3, and LPL expression levels remained unchanged. UCP2 and COX are linked to mitochondrial oxidation and reactive oxygen species (ROS) production, whereas FABP3 is involved in cellular lipid storage. LPL is essential for lipoprotein metabolism. CD36 is pivotal for cellular fatty acid uptake.
The method used to analyze muscle ATP content is limited in the sense that ATP content could be underestimated due to rapid ATP metabolization. However, due to the consistency in our tissue harvesting protocol, we assume this to be a systematic bias still allowing a comparative analysis between our groups.
In conclusion, we here show beneficial effects of PPARδ stimulation on skeletal muscle oxidative capacity and endurance function in an animal model with left ventricular dysfunction following myocardial infarction. Our data suggest that PPARδ agonism is a novel and unique strategy to enhance skeletal muscle oxidative function counteracting exercise intolerance in HF.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (P30-HL-101272, R01-HL-114813, UL1-RR-024156) and the Herbert and Florence Irving Scholar Award to P. C. Schulze.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.Z., P.J.K., H.A., R.J., I.G., S.H., and P.C.S. conception and design of research; C.Z., P.J.K., H.A., R.J., and E.C. performed experiments; C.Z., P.J.K., and I.G. analyzed data; C.Z. and P.J.K. drafted manuscript; P.J.K., H.A., R.J., E.C., I.G., S.H., and P.C.S. interpreted results of experiments; P.J.K. prepared figures; P.J.K., R.J., E.C., I.G., and P.C.S. edited and revised manuscript; I.G., S.H., and P.C.S. approved final version of manuscript.
REFERENCES
- 1.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137: 354–366, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Coll T, Alvarez-Guardia D, Barroso E, Gomez-Foix AM, Palomer X, Laguna JC, Vazquez-Carrera M. Activation of peroxisome proliferator-activated receptor-delta by GW501516 prevents fatty acid-induced nuclear factor-kappaB activation and insulin resistance in skeletal muscle cells. Endocrinology 151: 1560–1569, 2010. [DOI] [PubMed] [Google Scholar]
- 3.de Lange P, Ragni M, Silvestri E, Moreno M, Schiavo L, Lombardi A, Farina P, Feola A, Goglia F, Lanni A. Combined cDNA array/RT-PCR analysis of gene expression profile in rat gastrocnemius muscle: relation to its adaptive function in energy metabolism during fasting. FASEB J 18: 350–352, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Djouadi F, Aubey F, Schlemmer D, Bastin J. Peroxisome proliferator activated receptor delta (PPARdelta) agonist but not PPARalpha corrects carnitine palmitoyl transferase 2 deficiency in human muscle cells. J Clin Endocrinol Metab 90: 1791–1797, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Fritz T, Kramer DK, Karlsson HK, Galuska D, Engfeldt P, Zierath JR, Krook A. Low-intensity exercise increases skeletal muscle protein expression of PPARdelta and UCP3 in type 2 diabetic patients. Diabetes Metab Res Rev 22: 492–498, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 42: 861–868, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 29: 1067–1073, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs RA, Diaz V, Meinild AK, Gassmann M, Lundby C. The C57Bl/6 mouse serves as a suitable model of human skeletal muscle mitochondrial function. Exp Physiol 98: 908–921, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner S, Nguyen-Tran V, Bare O, Huang X, Spiegelman B, Wu Z. PPARδα agonism activates fatty acid oxidation via PGC-1α but does not increase mitochondrial gene expression and function. J Biol Chem 284: 18624–18633, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A. Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282: 19313–19320, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Yu S, Khan RS, Ables GP, Bharadwaj KG, Hu Y, Huggins LA, Eriksson JW, Buckett LK, Turnbull AV, Ginsberg HN, Blaner WS, Huang LS, Goldberg IJ. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J Lipid Res 52: 732–744, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J 17: 2299–2301, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie LS, Lione L. Harnessing the benefits of PPARbeta/delta agonists. Life Sci 93: 963–967, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19: 1498–1500, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, Winegar DA, Corton JC, Dohm GL, Kraus WE. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem 277: 26089–26097, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Murray AJ. Of mice and men (and muscle mitochondria). Exp Physiol 98: 879–880, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Nakai N, Kawano F, Terada M, Oke Y, Ohira T, Ohira Y. Effects of peroxisome proliferator-activated receptor alpha (PPARalpha) agonists on leucine-induced phosphorylation of translational targets in C2C12 cells. Biochim Biophys Acta 1780: 1101–1105, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell 134: 405–415, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robciuc MR, Skrobuk P, Anisimov A, Olkkonen VM, Alitalo K, Eckel RH, Koistinen HA, Jauhiainen M, Ehnholm C. Angiopoietin-like 4 mediates PPAR delta effect on lipoprotein lipase-dependent fatty acid uptake but not on beta-oxidation in myotubes. PLoS One 7: e46212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4: 407–414, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Schulze PC, Gielen S, Adams V, Linke A, Mobius-Winkler S, Erbs S, Kratzsch J, Hambrecht R, Schuler G. Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulinlike growth factor-I in chronic heart failure. Basic Res Cardiol 98: 267–274, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Schulze PC, Gielen S, Schuler G, Hambrecht R. Chronic heart failure and skeletal muscle catabolism: effects of exercise training. Int J Cardiol 85: 141–149, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Spangenburg EE, Brown DA, Johnson MS, Moore RL. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol Cell Biochem 332: 225–231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Haring HU. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes 58: 579–589, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugden MC, Zariwala MG, Holness MJ. PPARs and the orchestration of metabolic fuel selection. Pharmacol Res 60: 141–150, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 100: 15924–15929, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turpeinen JP, Leppavuori J, Heinonen OJ, Kaila K, Salo J, Lilja M, Kesaniemi YA. Muscle fiber type I influences lipid oxidation during low-intensity exercise in moderately active middle-aged men. Scand J Med Sci Sports 16: 134–140, 2006. [DOI] [PubMed] [Google Scholar]
- 31.van Bilsen M, van der Vusse GJ, Gilde AJ, Lindhout M, van der Lee KA. Peroxisome proliferator-activated receptors: lipid binding proteins controlling gene expression. Mol Cell Biochem 239: 131–138, 2002. [PubMed] [Google Scholar]
- 32.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 555: 1–13, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: An overview. Int J Biochem Cell Biol 45: 2257–2265, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2: e294, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watt MJ, Southgate RJ, Holmes AG, Febbraio MA. Suppression of plasma free fatty acids upregulates peroxisome proliferator-activated receptor (PPAR) alpha and delta and PPAR coactivator 1alpha in human skeletal muscle, but not lipid regulatory genes. J Mol Endocrinol 33: 533–544, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: e240–e327, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Zizola C, Schulze PC. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev 18: 623–630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]