Abstract
Noonan syndrome with multiple lentigines (NSML) is primarily caused by mutations in the nonreceptor protein tyrosine phosphatase SHP2 and associated with congenital heart disease in the form of pulmonary valve stenosis and hypertrophic cardiomyopathy (HCM). Our goal was to elucidate the cellular mechanisms underlying the development of HCM caused by the Q510E mutation in SHP2. NSML patients carrying this mutation suffer from a particularly severe form of HCM. Drawing parallels to other, more common forms of HCM, we hypothesized that altered Ca2+ homeostasis and/or sarcomeric mechanical properties play key roles in the pathomechanism. We used transgenic mice with cardiomyocyte-specific expression of Q510E-SHP2 starting before birth. Mice develop neonatal onset HCM with increased ejection fraction and fractional shortening at 4–6 wk of age. To assess Ca2+ handling, isolated cardiomyocytes were loaded with fluo-4. Q510E-SHP2 expression increased Ca2+ transient amplitudes during excitation-contraction coupling and increased sarcoplasmic reticulum Ca2+ content concurrent with increased expression of sarco(endo)plasmic reticulum Ca2+-ATPase. In skinned cardiomyocyte preparations from Q510E-SHP2 mice, force-velocity relationships and power-load curves were shifted upward. The peak power-generating capacity was increased approximately twofold. Transmission electron microscopy revealed that the relative intracellular area occupied by sarcomeres was increased in Q510E-SHP2 cardiomyocytes. Triton X-100-based myofiber purification showed that Q510E-SHP2 increased the amount of sarcomeric proteins assembled into myofibers. In summary, Q510E-SHP2 expression leads to enhanced contractile performance early in disease progression by augmenting intracellular Ca2+ cycling and increasing the number of power-generating sarcomeres. This gives important new insights into the cellular pathomechanisms of Q510E-SHP2-associated HCM.
Keywords: contractility, sarcomeric function, sarco(endo)plasmic reticulum Ca2+-ATPase, cardiac hypertrophy, protein tyrosine phosphatase
hypertrophic cardiomyopathy (HCM) affects ∼1:500 adults (31) and represents one of the most common causes of sudden cardiac death in young individuals (49). Often the disease is detected in early adolescence, when patients develop arrhythmias, exercise intolerance, chest pain, or even sudden cardiac death. Classically, HCM is characterized by concentric myocardial hypertrophy with cardiomyocyte disarray. Outflow tract obstruction resulting from segmental septal hypertrophy or systolic anterior motion of the mitral valve often augments the disease. The majority of the genetic mutations reported in patients with familial HCM affect components of the sarcomere, with multiple sarcomeric proteins implicated in the disease process (10, 47).
Interestingly, nonsarcomeric mutations can also induce HCM. Nonsarcomeric HCM can be clinically indistinguishable from “classic” HCM caused by sarcomeric mutations, and these rare forms of HCM have therefore also been termed “phenocopy” diseases (18). Examples are Fabry's disease, Danon disease, mitochondrial cardiomyopathies, or Noonan syndrome. Why these phenocopy diseases so closely resemble classic HCM is not known, but it suggests that different chains of molecular events may ultimately converge and trigger a common cardiomyopathic mechanism.
To address this question, we focused on elucidating the pathomechanism(s) in Noonan syndrome with multiple lentigines (NSML). This syndrome also is termed LEOPARD syndrome as an acronym for the clinical disease characteristics of multiple lentigines, electrocardiographic abnormalities, ocular telomerism, pulmonic stenosis, abnormalities of the genitalia, retardation of growth, and sensorineural deafness. Although not directly included in the acronym, HCM is a serious concern in these patients and is seen in ∼80% of patients with NSML (26, 27, 41). In ∼90% of cases, NSML is due to mutations in the nonreceptor protein tyrosine phosphatase protein SHP2, which is encoded by protein tyrosine phosphatase, nonreceptor type 11 (PTPN11) (5, 24). In the remaining cases, mutations in BRAF and RAF1 have been identified (20, 32, 37, 40).
SHP2 is an essential positive or negative regulator of multiple signaling pathways (35). Using various in vitro and in vivo models, we and others (8, 17, 30, 43) have previously found that NSML mutations in SHP2 result in increased stimulation of Akt and mammalian target of rapamycin. This leads to enhanced growth signaling and thereby cardiomyocyte hypertrophy. Hyperactivation of Akt has previously been shown to increase intracellular Ca2+ availability and enhance cardiac contractility (3, 4, 19). In classic HCM, changes in calcium handling and Ca2+ sensitivity and/or alterations in the biophysical properties of the contractile apparatus are thought to be critical to the pathomechanism (10). Therefore, we hypothesized that altered Ca2+ handling either alone or in combination with changes in biomechanical characteristics of the sarcomere play a role in NSML. This could be a shared pathomechanism of classic and phenocopy forms of HCM and would explain why these disease variants are clinically similar despite very different genetic causes.
To test our hypothesis, we used a previously generated transgenic (TG) mouse model of NSML-associated HCM. In this model, cardiomyocyte-specific expression of the mutant protein Q510E-SHP2 starting before birth results in neonatal onset HCM (43). This mouse model recapitulates the aggressive form of HCM found in patients carrying the same SHP2 mutation. Importantly, all our prior findings in the Q510E-SHP2 model are consistent with the HCM phenotype described in other NSML models based on Y279C and T468M mutations in SHP2 (30, 45). Because of the severity and early onset of the cardiac phenotype, the Q510E-SHP2 mouse model is ideally suited for proof-of-principle studies. In this investigation, cardiac contractile function early in the course of disease progression was determined in vivo. Subsequently, isolated cardiomyocytes and skinned myofiber preparations from these mouse hearts were used to examine Ca2+-handling and sarcomeric biomechanical properties.
MATERIALS AND METHODS
Animals.
Generation of these TG mice and detailed phenotype analyses were carried out as previously reported (43). For the present study, only 4-wk-old mice of either sex were used. All protocols were in accordance with the Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training of the American Physiological Society and submitted to and approved by the Animal Care and Use Committee of the University of Missouri.
Echocardiography.
Echocardiograms were performed under inhalation anesthesia (1.2–1.8% isoflurane, 0.6-l flow of O2) using a Vevo 2100 ultrasound system (Visualsonics, Toronto, ON, Canada). The echocardiographer was blinded to the mouse genotype. M-mode echocardiography was performed using the parasternal short-axis view of the left ventricle (LV). Guidelines of the American Society of Echocardiography were used for measurements of LV end-diastolic and end-systolic diameters as well as septal and posterior wall thickness. Images were captured digitally, and six consecutive cardiac cycles were measured and averaged for each animal.
Protein analyses.
For total protein extracts, flash-frozen mouse ventricles were homogenized in lysis buffer [150 mM NaCl, 10 mM Tris (pH 7.4), 1% Triton X-100, and 1× HALT Protease & Phosphatase Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO)]. The following antibodies were used for Western blot analysis: Akt, phosphorylated (p)Akt (Ser473), GAPDH, phospholamban (PLB), and pPLB (Ser16/Thr17) from Cell Signaling Technologies (Beverly, MA); sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA), Na+/Ca2+ exchanger (NCX), calsequestrin 2 (CSQ), ryanodine receptor (RyR), and pRyR (Ser2808) from Abcam (Cambridge, MA); cardiac myosin-binding protein-C (cMYBP-C, MYBPC3) and α-myosin heavy chain (α-MHC, MYH1/2/4/6) from Santa Cruz Biotechnology (Santa Cruz, CA); α1C-subunit (Cav1.2) of the L-type Ca2+ channel channel (LTCC) from Alomone Labs (Jerusalem, Israel); and cardiac troponin I (cTnI) from Millipore (Billerica, MA). Phosphorylated and total protein bands were quantified using Bio-Rad ChemDoc or GelDoc imaging systems (Bio-Rad, Berkeley, CA).
Ca2+ measurements.
Hearts were excised from mice anesthetized with 60 mg/kg pentobarbital sodium and perfused via the aorta with Ca2+-free physiological saline solution [containing (in mM) 143 NaCl, 5 KCl, 1 MgCl2, 10 d-glucose, and 10 HEPES (pH 7.4) and supplemented with 2 U/ml heparin] for 10 min. Hearts were then perfused for 10 min with a minimal essential medium-based enzymatic isolation solution containing 10 mM NaHCO3, 2 mM Na-pyruvate, 10 mM HEPES, 8 mM taurine, 20 μM CaCl2, 50 U/ml penicillin-streptomycin (Life Technologies, Grand Island, NY), and 22.5 mg/l Liberase Blendzyme TH (Roche Applied Science, Indianapolis, IN) at pH 7.35 and 37°C. Dissociated LV cardiomyocytes were gradually adapted to Ca2+ (from 50 to 500 μM Ca2+ over 40 min), plated on laminin-coated coverslips, and loaded with 5 μM fluo-4 AM (Life Technologies) for 10 min followed by a 20- to 40-min wash. Coverslips were secured in an imaging chamber, perfused at ∼2 ml/min with physiological saline solution [containing (in mM) 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES, pH 7.4 with NaOH], and imaged using a Leica SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL). Two-dimensional imaging of a central region of the cell was performed at 100 frames/s using the resonant scanhead of the Leica SP5 (HCS PL APO ×40 objective, numerical aperture: 1.25, 512 × 64 pixels at zoom 3.5, bidirectional scanning/2× line averaging, pixel size: 0.22 μm), with excitation at 488 nm and emission recorded from 500 to 580 nm. Cardiomyocytes were electrically stimulated at 0.25, 0.5, or 1 Hz using electrical field stimulation (S48, Grass Instruments, Warwick, RI). Sarcoplasmic reticulum (SR) Ca2+ content was assessed immediately after a 0.5-Hz pacing train by rapidly (∼1 s) applying 10 mM caffeine. All experiments were performed at room temperature (22–24°C). Example traces and summary data are presented as F/F0, where F is the peak fluorescence in response to electrical stimulation or caffeine application and F0 is the baseline fluorescence. The Ca2+ transient recovery time constant (τ) was determined from the decay of the normalized fluorescence signal using an exponential fit between 80% of peak and the baseline. All fluorescence values were background subtracted before analysis.
Cardiomyocyte contractile function.
Cardiac myocyte preparations were obtained by mechanical disruption of mouse hearts as previously described (33). Cardiomyocytes were subsequently skinned in 0.3% Triton X-100 (Pierce Biotechnology, Rockford, IL) in relaxing solution [containing (in mM) 2 EGTA, 5 MgCl2, 4 ATP, 10 imidazole, and 100 KCl, pH 7.0 with the addition of protease inhibitors (Calbiochem, San Diego, CA)]. The experimental apparatus for physiological measurements on myocyte preparations was similar to one previously described (33). Myocyte preparations were attached between a force transducer and torque motor by placing the ends of the myocyte preparation into stainless steel troughs (25-gauge). The ends of the myocyte preparations were secured by overlaying a 0.5-mm length of 3-0 monofilament nylon suture (Ethicon, Somerville, NJ) onto each end of the myocyte and then tying the suture into the troughs with two loops of 10-0 monofilament (Ethicon). The experimental apparatus was mounted on the stage of an inverted microscope (model IX-70, Olympus Instrument) on a pneumatic vibration isolation table. Mechanical measurements were performed using a capacitance-gauge transducer (model 403, sensitivity of 20 mV/mg, plus a 10× amplifier) and resonant frequency of 600 Hz (Aurora Scientific, Aurora, ON, Canada). Resting sarcomere length was set to ∼2.30 μm in pCa 9.0 solution using an IonOptix SarcLen system (IonOptix, Milton, MA), which used a fast Fourier transform algorithm of the video image of the myocyte.
Compositions of relaxing and activating solutions used in mechanical measurements were as follows: 7 mM EGTA, 1 mM free Mg2+, 20 mM imidazole, 4 mM MgATP, 14.5 mM creatine phosphate (pH 7.0), various Ca2+ concentrations between 10−9 M (relaxing solution) and 10−4.5 M (maximal Ca2+ activating solution), and sufficient KCl to adjust ionic strength to 180 mM. The final concentrations of each metal, ligand, and metal-ligand complex at 13°C were determined according to Fabiato (9). Before Ca2+ activations, myocyte preparations were immersed for 30 s in a solution of reduced Ca2+-EGTA buffering capacity, which was identical to normal relaxing solution except that EGTA was reduced to 0.5 mM. This protocol resulted in more rapid development of steady-state force during subsequent activation and helped preserve the sarcomeric integrity during activation.
All mechanical measurements were made at 13 ± 1°C. The protocol for force-velocity and power-load measurements has been previously described (33). Briefly, force-velocity and power-load measurements were made on each cardiomyocyte during maximal Ca2+ activation. The cardiomyocyte was transferred into maximal Ca2+ activating solution, and, after steady-state maximal force was attained, a series of force clamps was performed to determine isotonic shortening velocities. Using a servo system, force was maintained constant for a designated period of time (150–250 ms) while the length change was continuously monitored. After the force clamp, the cardiomyocyte preparation was slackened to reduce force to near zero to allow estimation of the relative load sustained during isotonic shortening; the cardiomyocyte was subsequently reextended to its initial length.
Measurement of force development kinetics was accomplished as previously described (16). In short, a cardiomyocyte in activating solution was allowed to develop steady-state force, after which it was rapidly slacked by 15–20% of the original cardiomyocyte length (L0), held for 20 ms, and then rapidly restretched to a value slightly greater than L0 for 2 ms before it was returned to L0. This slack-restretch maneuver is thought to cause dissociation of cross-bridges and redistribution to preforce-generating states, and thus force redevelopment arises from reattachment of cross-bridges to the thin filament and/or subsequent transition to force-generating states.
PKA enzymatic activity.
PKA was obtained from whole heart cell lysates, and enzymatic activity assessed by incubation with a fluorescent peptide substrate (PepTag assay, Promega, Madison, WI) according to the manufacturer's directions. Phosphorylation by PKA alters the net charge of the substrate (L-R-R-A-S-L-G) from +1 to −1. This allows phosphorylated and nonphosphorylated peptides to be separated on an agarose gel. The phosphorylated species migrates toward the positive electrode, whereas the nonphosphorylated substrate migrates toward the negative electrode. Gels were photographed using the Bio-Rad GelDoc imaging system, and fluorescent bands were quantified with QuantityOne software (Bio-Rad).
PKA backphosphorylation assay.
PKA-induced phosphate incorporation into myofibrillar substrates was determined as previously described (14, 34). Briefly, skinned cardiac myocytes (10 μg) were incubated with the catalytic subunit of PKA (5 μg/ml) and 50 μCi [γ-32P] ATP at room temperature (21–23°C) for 45 min. The reaction was stopped by the addition of electrophoresis sample buffer and heating at 95°C for 3 min. Samples were then separated by SDS-PAGE for 2.5 h at 12 mA, silver stained to control for loading, and subsequently exposed to X-ray film for visualization.
Transmission electron microscopy.
Hearts were perfused with PBS containing 25 mM KCl and 5% dextrose, fixed in 2% paraformaldehyde and 2% glutaraldehyde in 0.1 sodium cacodylate, and processed for thin sectioning. Photomicrographs were obtained using a JEOL 1400 transmission electron microscope (JEOL, Peabody, MA). Photos were taken from each section at ×3,000 magnification in random fashion. To determine sarcomere length, 4–5 sarcomeres/heart were measured using ImageJ (National Institutes of Health, Bethesda, MD) [n = 3 nontransgenic (NTG) and n = 3 TG]. For assessment of the relative area per visual field occupied by sarcomeres, 30–35 randomly chosen, nonoverlapping photos were evaluated using ImageJ.
Quantitative real-time PCR.
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) for first-strand DNA synthesis (Superscript III First-Strand Synthesis System, Invitrogen). SYBR green-based quantitative real-time PCR was carried out on a Bio-Rad MyiQ iCycler. The following primer sequences were obtained from Roche's Universal ProbeLibrary: α-MHC, left 5′-CGCATCAAGGAGCTCACC-3′ and right 5′-CCTGCAGCCGCATTAAGT-3′; cTnI, left 5′-GCAGGTGAAGAAGGAGGACA-3′ and right 5′-CGATATTCTTGCGCCAGTC-3′; cMyBP-C, left 5′-GCATGAAGCAGGATGAAAAGA-3′ and right 5′-TCTTGTGGCCCTTGTTTACC-3′; and GAPDH, left 5′-AGCTTGTCATCAACGGGAAG-3′ and right 5′-TTTGATGTTAGTGGGGTCTCG-3′. Relative expression levels were determined using the 2−ΔΔCT method (where CT is threshold cycle) with GAPDH as the housekeeping gene (28).
Cardiac myofiber purification.
Equal amounts of ventricular tissue (by weight) from NTG and TG mice were used for extraction of cardiac myofibers as previously described (22, 44). Tissue was homogenized in low-salt F-60 buffer (60 mM KCl, 30 mM imidazole, and 2 mM MgCl2, pH 7.4), and soluble proteins were separated by low-speed centrifugation. F-60 washes were repeated without and with 1 mM EGTA and then with 1% Triton X-100 followed by more F-60 washes. The resulting sarcomeric protein pellets were dissolved in equal amounts of high-salt buffer (0.5 M NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.76 mM KH2PO4, pH 7.4), and equal volumes were loaded onto SDS-PAGE gels. Protein bands were stained with GelCode Blue Safe Protein Stain (Thermo Fisher Scientific, Rockford, IL), and the total stain intensity of the entire lane across all molecular weights was quantified (GelDoc imaging systems, Bio-Rad).
Statistical analysis.
Skinned myocyte preparation length traces, force-velocity curves, power-load curves, and rate constants of force redevelopment were analyzed as previously described (16, 33). Frequency-dependent measurements of Ca2+ transients and recovery rates were analyzed using repeated-measures ANOVA with Bonferroni post hoc analysis. All other statistical comparisons were made using unpaired Student t-tests (NTG vs. Q510E-SHP2), with summary data presented as means ± SE. P values of <0.05 were considered significant.
RESULTS
Q510E-SHP2 expression induces hyperdynamic ventricular function in young mice.
We previously generated a TG mouse model that recapitulates NSML-associated HCM with neonatal onset (43). In brief, newborn mice exhibit increased cardiomyocyte cross-sectional areas, heart-to-body weight ratios, interventricular septum thickness, and cardiomyocyte disarray. In adult mice, interstitial fibrosis can be detected and contractile function is depressed.
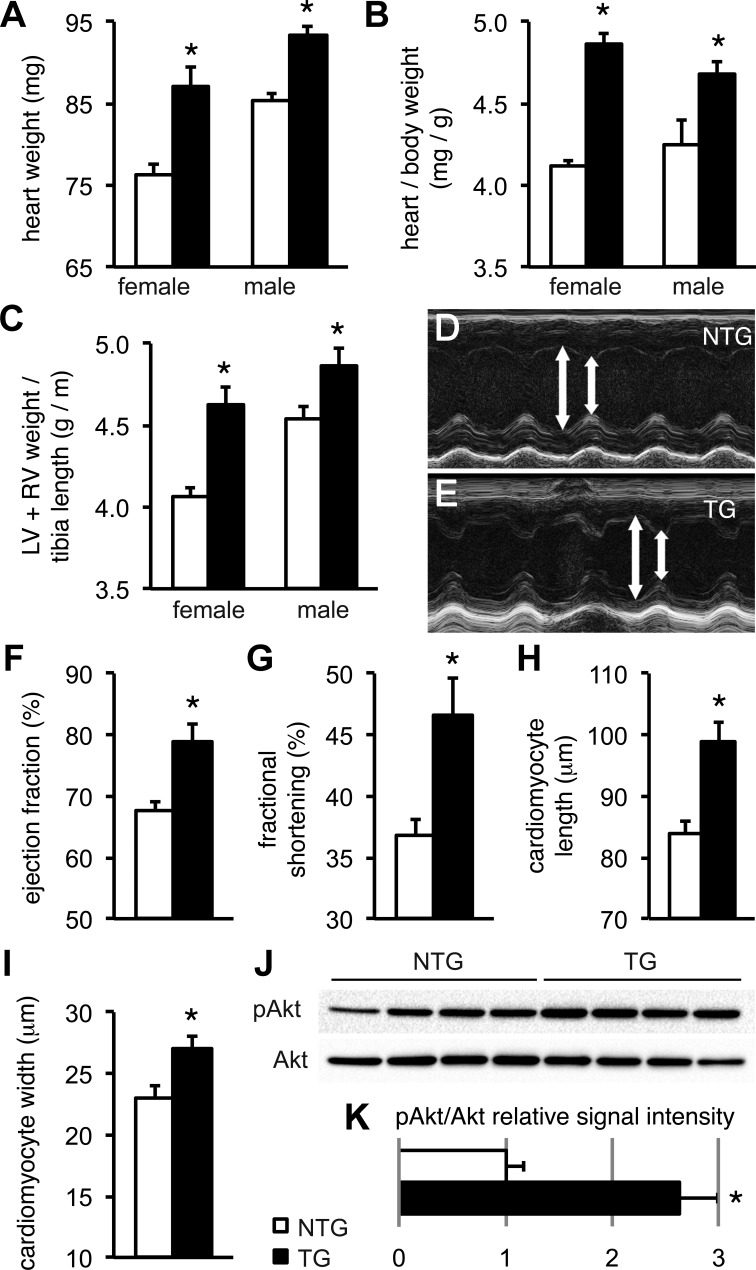
To quantify the degree of cardiac hypertrophy in TG hearts early in the course of disease, we obtained detailed gravimetric data at 4 wk of age for this study. As shown in Fig. 1, A and B, absolute heart weights as well as the heart-to-body weight ratio were significantly increased both in male and female TG mice compared with NTG mice. Since we had noted a small variation in body weight between groups (NTG female mice: 18.55 ± 0.26 g, TG female mice: 17.93 ± 0.36 g, NTG male mice: 20.35 ± 0.56 g, and TG male mice: 19.41 ± 0.8 g) and since atrial enlargement could have substantially contributed to the difference in the heart-to-body weight ratio, we also calculated ventricular weight-to-tibia length ratios (Fig. 1C). Again, this index was significantly increased both in male and female TG mice. Furthermore, we measured isolated cardiomyocyte sizes after enzymatic digestion. Cardiomyocytes obtained from 4-wk-old TG mice exhibited cellular hypertrophy with a significant 18% increase in cell length and a 49% increase in cell width compared with cardiomyocytes from NTG mice (Fig. 1, H and I).
Fig. 1.
Transgenic (TG) Q510E-SHP2 expression induces hypertrophy and increases cardiac contractile function in young mice. A–C: heart weights (A), heart-to-body weight ratios (B), and left ventricular (LV) + right ventricular (RV) weight-to-tibia length ratios (C) were significantly increased in both female and male TG mice at 4 wk of age. n = 22–26 mice/group. Open bars, nontransgenic (NTG) mice; solid bars, TG mice. *P < 0.05 vs. NTG. D and E: echocardiographic end-systolic (D) and end-diastolic (E) LV diameter measurements are indicated by arrows in representative M-mode scans. F and G: LV ejection fraction (F) and fractional shortening (G) were significantly increased in TG mice. n = 4 mice/group. H and I: cardiomyocyte length and width were significantly increased in isolates from 4-wk-old TG mice. n = 59 cells from 3 NTG mice and 53 cells from 3 TG mice. J and K: ventricular tissue homogenates from 4-wk-old mice underwent Western blot analysis to quantify the Akt phosphorylation status. Expression of Q510E-SHP2 increased the relative level of Akt phosphorylation ∼2.6-fold compared with NTG samples. n = 4 per group. pAkt, phosphorylated (p)Akt.
In many cases of HCM, early hypercontractile cardiac function precedes the transition to overt contractile dysfunction (51). To evaluate in vivo cardiac function in early stage disease, we therefore performed echocardiography in 4- to 6-wk-old mice under isoflurane anesthesia. This time point was chosen on the basis of pilot data indicating that contractile function is increased in 4- to 6-wk-old mice and normal in 8-wk-old mice, whereas contractile function is impaired in mice at 11 wk and thereafter (43). Representative M-mode scans obtained in the parasternal short axis at the midpapillary plane are shown in Fig. 1, D and E. In contrast to our previous data obtained in 3-mo-old mice (43), 4- to 6-wk-old TG hearts had enhanced contractile function with significantly increased ejection fraction and fractional shortening compared with NTG littermates (Fig. 1, F and G), indicating hypercontractile function. Next, we confirmed that signaling through Akt is increased in TG mice at this age. Western blot analysis using ventricular tissue showed a 2.6-fold increase of relative Akt phosphorylation over baseline (Fig. 1, J and K).
Q510E-SHP2 expression enhances intracellular Ca2+ cycling.
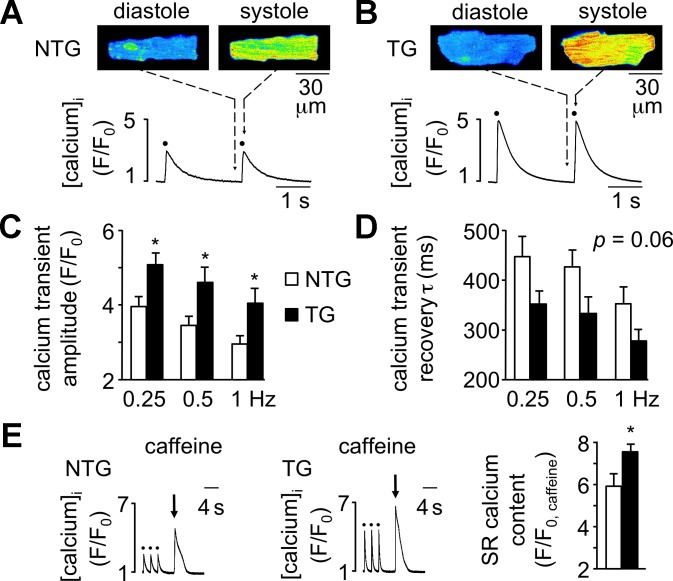
Positive inotropic effects on the heart are primarily mediated via modulation of the amplitude of the systolic intracellular Ca2+ transient during excitation-contraction (E-C) coupling. We therefore examined Ca2+ transients during E-C coupling in enzymatically isolated cardiomyocytes of 4-wk-old TG and NTG mice. Cardiomyocytes loaded with the Ca2+-sensitive indicator dye fluo-4 AM were electrically stimulated at stimulation frequencies of 0.25, 0.5, and 1 Hz. Shown in Fig. 2 are example images and F/F0 profiles of cardiomyocytes from a NTG heart (A) and a TG heart (B) before (diastole) and immediately after (systole) action potential stimulation. The Ca2+ transient amplitude was significantly higher in TG cardiomyocytes at all stimulation frequencies examined (Fig. 2C). Recovery of the Ca2+ transient (i.e., transient τ) trended toward shorter times in cardiomyocytes of TG mice, although this finding did not reach statistical significance (P = 0.06; Fig. 2D). SR Ca2+ content was assessed by rapid application of 10 mM caffeine after cessation of a 0.5-Hz stimulation train and was significantly elevated in cardiomyocytes from TG mice (Fig. 2E).
Fig. 2.
Cardiomyocytes of Q510E-SHP2 TG mice exhibit enhanced Ca2+ signaling during excitation-contraction coupling. A and B: example two-dimensional laser-scanning confocal fluorescence images (fluo-4 AM, top) and F/F0 profiles (bottom) of Ca2+ responses before (cellular diastole) and immediately after 0.5-Hz action potential stimulation (cellular systole, stimuli marked with solid circles in profiles) in cardiomyocytes from 4-wk-old NTG and TG mice. F is the peak fluorescence in response to electrical stimulation or caffeine application, and F0 is the baseline fluorescence C: summary data showing significantly enhanced Ca2+ transients during excitation-contraction coupling in TG cardiomyocytes (solid bars, n = 9 cells from 4 mice) compared with NTG cardiomyocytes (open bars, n = 11 cells from 4 mice). D: recovery of the Ca2+ transient [transient time constant (τ)] trended toward shorter times in cardiomyocytes of TG mice (solid bars), although this finding did not reach statistical significance (P = 0.06). E: sarcoplasmic reticulum (SR) Ca2+ content as assessed by rapid application of 10 mM caffeine (arrow) after cessation of a 0.5-Hz pacing train (solid circles) in NTG and TG cardiomyocytes. Bar graphs show summary data demonstrating elevated SR Ca2+ content in TG cardiomyocytes (solid bars; n = 7–8 cells from 4 mice per group). *P < 0.05 vs. NTG cardiomyocytes.
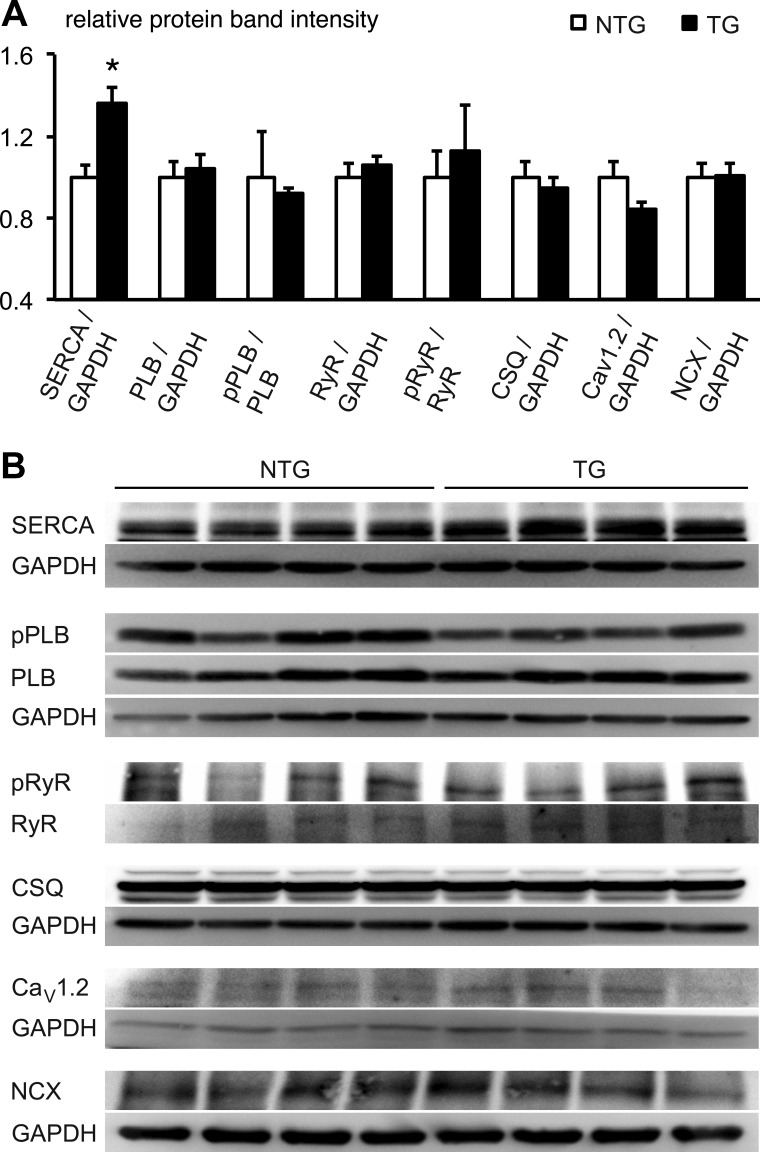
To further investigate the ability of the SR to sequester Ca2+, we monitored protein expression of SERCA and its regulatory accessory protein PLB. Whereas SERCA protein expression was significantly increased in TG hearts, expression of PLB was unchanged between TG and NTG hearts (Fig. 3). Furthermore, the ratio of pPLB to PLB was unchanged when TG and NTG hearts were compared (Fig. 3). Protein expression of RyR and the pRyR-to-RyR ratio were unchanged between TG and NTG hearts. Similarly, protein expression of CSQ, Cav1.2, and NCX were also not altered by Q51E-SHP2 expression (Fig. 3).
Fig. 3.
Cardiac Q510E-SHP2 expression increases sarco(endo)plasmic Ca2+-ATPase (SERCA) protein levels in 4-wk-old mice. A and B: ventricular tissue samples underwent Western blot analysis to assess total protein levels and respective phosphorylation status. GAPDH was used as a loading control. n = 4 per group. The relative expression level of SERCA was significantly increased in TG samples (*P < 0.05). In contrast, expression and phosphorylation levels of phospholamban (PLB), ryanodine receptor (RyR), calsequestrin (CSQ), α1C-subunit of the L-type Ca2+ channel (Cav1.2), and the Na+/Ca2+ exchanger (NCX) were not significantly different between NTG and TG samples.
Q510E-SHP2 expression increases sarcomeric contractile function.
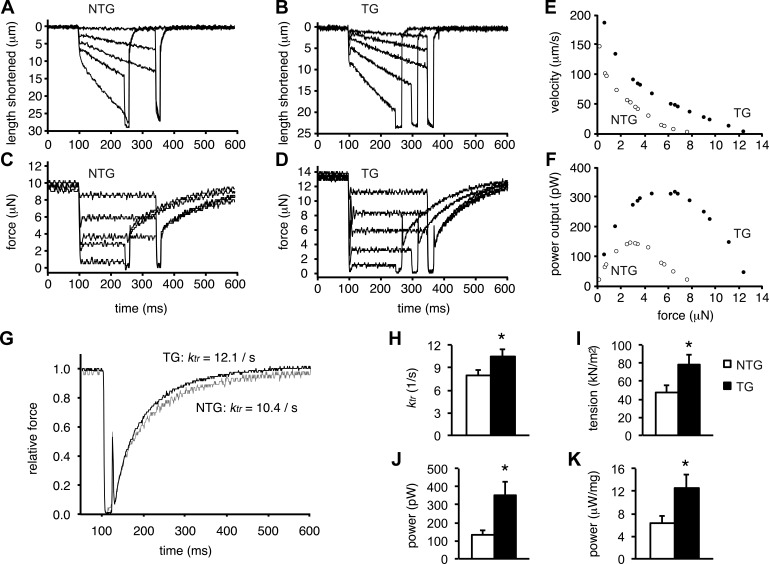
Next, we considered the possibility of other, Ca2+-independent effects of Q510E-SHP2 expression that may enhance contractile function. In particular, we focused on the biomechanical characteristics of the contractile apparatus. Using skinned cardiomyocyte preparations from NTG and TG hearts, the force-generating capability was measured at a fixed Ca2+ concentration. Figure 4, A–D, shows representative recordings of shortening and force over time during a series of force clamps followed by slackening. Figure 4, E and F, shows the respective force-velocity relationships and power-load curves. In skinned cardiomyocytes from TG hearts, both curves were shifted upward compared with preparations from NTG hearts. Furthermore, peak power-generating capacity was increased approximately twofold in cardiomyocytes from Q510E-SHP2 mice compared with cardiomyocytes from NTG mice; this effect was mostly due to the increase in the tension-generating capacity of cardiomyocyte preparations. The rate of force development was measured using a slack-restretch maneuver during maximal Ca2+ activation. Cardiomyocyte preparations from Q510E-SHP2 mice exhibited significantly greater rate constants of force development than cardiomyocytes from NTG mice (Q510E-SHP2: 10.5 ± 0.9 s−1 vs. NTG: 8.0 ± 0.7 s−1, P < 0.05; Fig. 4, G and H). Figure 4, I–K, shows the summary data of the tension and power measurements, indicating that sarcomeric function is substantially increased in TG cardiomyocytes, which is consistent with the hypercontractility seen in echocardiography at the same age.
Fig. 4.
Q510E-SHP2 expression increases force and power generation in skinned cardiac myofibers. A–D: force clamps (for 150–250 ms) and length traces during maximal Ca2+ activation of a cardiomyocyte from NTG and TG mice, respectively. E and F: force-velocity and power-load curves of a myocyte (at maximal Ca2+ activation) from a NTG mouse heart (open circles) and a TG heart (closed circles). G: force redevelopment traces after a slack-restretch maneuver of a NTG cardiomyocyte (shaded trace) and a TG cardiomyocyte (solid trace) during maximal Ca2+ activation. TG cardiomyocyte preparations exhibited faster force redevelopment kinetics than compared with NTG cardiomyocyte preparations. ktr, rate constant of force development H–K: bar plots summarizing rate constants, tension, and power (both absolute and normalized for cardiomyocyte size) generated by cardiomyocyte preparations from NTG and TG hearts. In all bar graphs, n = 9. *P < 0.05 vs. NTG cardiomyocyte preparations.
Q510E-SHP2 expression does not affect PKA activity.
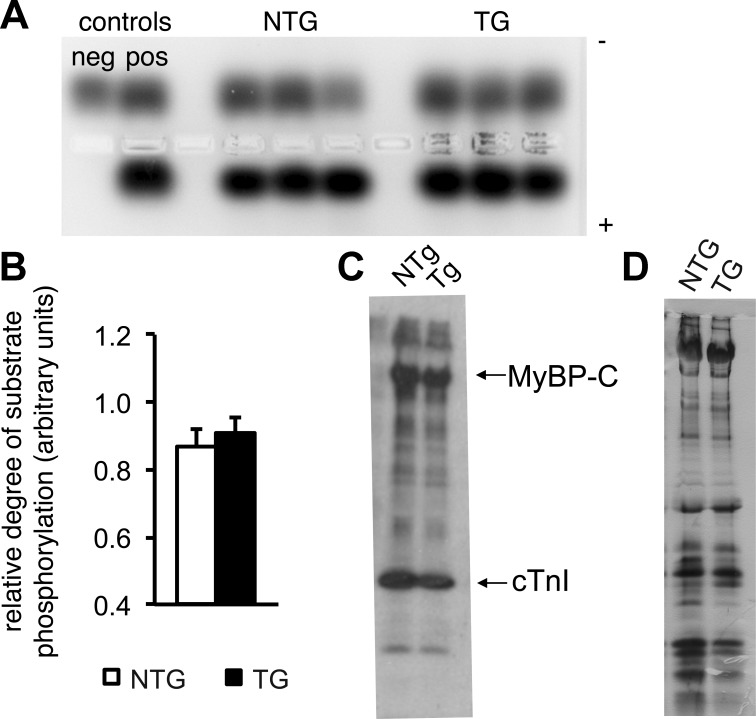
Posttranslational modification of sarcomeric proteins is a major mechanism for fine tuning myofibrillar function. Importantly, PKA-mediated phosphorylation of myofibrillar proteins increases power generation (12). Furthermore, interaction of SHP2 and PKA in a signalosome complex induced by shear stress has been reported (7), suggesting that similar interactions could also play a role in cardiomyocytes expressing Q510E-SHP2. Therefore, we tested whether PKA-dependent posttranslational modification of the sarcomeric proteins is responsible for the increase in contractile function. PKA activity assays were conducted using myocardial tissue samples from NTG and TG hearts. No significant difference was noted in PKA activity as assessed by the relative degrees of phosphorylation of the fluorescently tagged PKA specific peptide (P = 0.45; Fig. 5, A and B). For further confirmation, a backphosphorylation assay was used. Sarcomeric proteins from skinned cardiomyocytes were incubated with active PKA in the presence of radiolabeled ATP and subsequently separated by gel electrophoresis. The degree of radiolabel incorporation in the various sarcomeric proteins did not differ between cardiomyocytes from NTG versus TG hearts (Fig. 5, C and D).
Fig. 5.
PKA activity is normal in Q510E-SHP2-expressing cardiomyocytes. A: ventricular tissue lysates were incubated with a PKA-specific peptide substrate and, subsequently, the degree of substrate phosphorylation was assessed by gel electrophoresis. The phosphorylated species migrated toward the positive electrode, whereas the nonphosphorylated substrate migrated toward the negative electrode. B: quantification of the relative degree of PKA substrate phosphorylation. n = 11 per group. C and D: representative autoradiograms (C) and silver-stained protein gel (D) after PKA backphosphorylation. After normalization for total protein load, the degree of radiolabel incorporation in the various sarcomeric proteins did not differ between cardiomyocytes from NTG versus TG hearts. MyBP-C, myosin-binding protein-C; cTnI, cardiac troponin I. n = 3 per group.
Q510E-SHP2 expression increases the contractile apparatus but not sarcomeric protein mRNA.
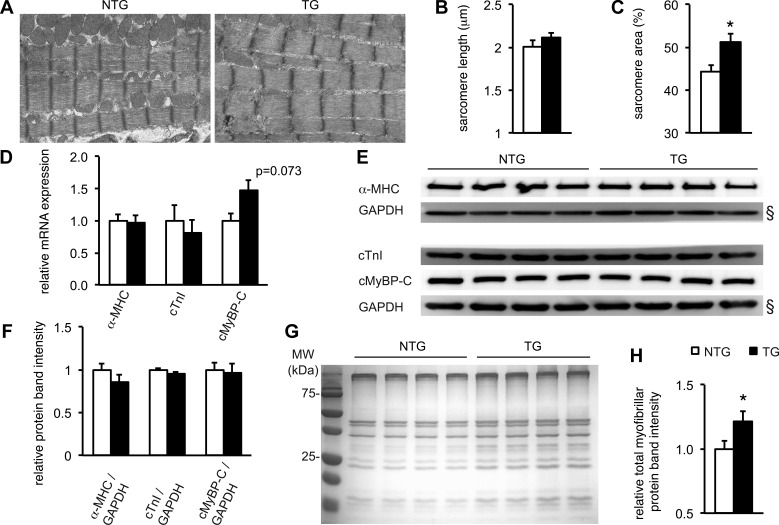
Electron microscopy was used to examine ultrastructural morphological differences in NTG and TG cardiomyocytes that could explain the functional differences. Representative electron microscopy images prepared from 4-wk-old hearts are shown in Fig. 6A. There were no significant differences in sarcomere organization or sarcomere length between NTG and TG hearts (P = 0.22; Fig. 6B). However, the relative area per visual field occupied by sarcomeres was significantly increased in the TG myocardium compared with NTG myocardium (Fig. 6C). This indicates that more thin and thick filaments per cardiomyocyte width may be available for force generation, which is consistent with the increased power developed by skinned TG cardiomyocyte preparations.
Fig. 6.
The contractile apparatus is expanded Q510E-SHP2-expressing hearts. A: representative electron micrographs showing sarcomeres in NTG and TG hearts. B and C: quantification of sarcomere length and relative area per random visual field occupied by contractile fibers. Q510E-SHP2 expression significantly increased the relative area per visual field occupied by sarcomeres. n = 14 images from 3 hearts per group. For both NTG and TG hearts, each image contained five to seven sarcomeres as counted in the longitudinal direction of the myofibers. D: mRNA levels of α-myosin heavy chain (α-MHC), cTnI, and cardiac (c)MyBP-C were quantified using quantitative real-time PCR with GAPDH as a housekeeping gene. There were no significant differences in mRNA expression, although a trend toward increased cMyBP-C mRNA expression in TG compared with NTG hearts was noted. n = 8–10 in all groups. E and F: homogenized ventricular tissue from NTG and TG hearts underwent Western blot analysis. GAPDH was used as loading control. Samples were probed for total α-MHC, cTnI, and cMyBP-C. Signal quantification did not show any difference in relative protein expression levels between NTG and TG hearts. n = 4 per group. (§, of note, the two GAPDH blots shown are the same as in Fig. 3 because the Western blots were run at the same time from the same tissue lysates.) G and H: cardiac myofibers were extracted from equal amounts of NTG and TG ventricular tissue. The total protein yield of the extraction procedure was quantified via gel electrophoresis followed by Coomassie blue staining. The amount of sarcomeric proteins obtained from the TG samples was significantly increased compared with NTG samples. n = 8 per group. *P < 0.05 vs. NTG hearts.
This led to the hypothesis that transcription of sarcomeric proteins may be increased by expression of Q510E-SHP2. To quantify mRNA levels of α-MHC, cTnI, and cMyBP-C, quantitative real-time PCR was used. There were no significant differences in mRNA expression of α-MHC, cTnI, or cMyBP-C when ventricular tissue samples from NTG (n = 5) and TG (n = 4) mice were compared (Fig. 6D), although there was a trend toward significance with increased cMyBP-C mRNA expression in TG mice (P = 0.073). GAPDH expression was used as a reference and did not differ between groups. To determine sarcomeric protein levels, total proteins from ventricular tissue samples underwent Western blot analysis. Again, GAPDH was used as a housekeeping gene to correct for protein loading. As shown in Fig. 6, E and F, there were no significant differences in total α-MHC, cTNI, or cMyBP-C expression.
Since the transmission electron microscopy data had revealed an increase in assembled contractile myofibers, we hypothesized that Q510E-SHP2 does not change total sarcomeric protein content but alters the relative amount of incorporated contractile proteins versus those remaining in a free pool. To test this, we purified cardiac myofibers from equal amounts of TG and NTG ventricular tissue and quantified the total protein yield of the extraction using gel electrophoresis. In TG samples, the total amount of extracted proteins was increased (Fig. 6, G and H).
DISCUSSION
The goal of the present study was to investigate the cellular mechanism(s) underlying the development of HCM in Q510E-SHP2-induced NSML. Cardiac-specific Q510E-SHP2 expression induces a hyperdynamic contractile state in hypertrophied young mouse hearts. Mechanistically, we found two independent mechanisms that synergistically promote hypercontractility. First, intracellular Ca2+ transients were increased in TG cardiomyocytes concurrent with increased SERCA expression and SR Ca2+ content. Second, TG cardiomyocytes exhibited an enlarged contractile apparatus, resulting in increased cardiac myofibrillar force and power generation.
In this NSML model, the early disease stage is characterized by cardiomyocyte hypertrophy with enhanced ventricular function. In the literature, a hyperdynamic contractile state has not yet been described in patients carrying the Q510E mutation in SHP, a finding likely attributed to clinical diagnosis after the onset of heart failure (6, 27, 46). Importantly, normal or hypercontractile function is a common characteristic in early stages of classic HCM due to sarcomeric mutations (51). For example, hypercontractility, identified as an increased ejection fraction and enhanced LV twist, was noted in patients with familial forms of HCM (38, 48) as well as in mice (11). This suggests that hypercontractility early in disease progression could be a common denominator of various forms of HCM, regardless of etiology.
We previously determined and now again confirmed that overexpression of Q510E-SHP2 leads to increased Akt activation in cardiomyocytes (42, 43). Since Akt signaling is known to control Ca2+ handling and thereby contractile function, we assessed Ca2+ transients after action potential stimulation. At all stimulation frequencies examined, Ca2+ transients were elevated by Q510E-SHP2 expression. In mice, the amplitude of the intracellular Ca2+ transient is primarily due to SR Ca2+ release [∼90% release vs. 10% entry (25)], which, in turn, is governed by the content of Ca2+ within the SR (1). Consistent with the increase in Ca2+ transient amplitude, SR Ca2+ content was significantly elevated in Q510E-SHP2 mice. Mechanistically, the increased ability of the SR to sequester Ca2+ was due to an increase in protein expression of SERCA and not due to changes in the expression or phosphorylation status of the SERCA inhibitory protein PLB (21). These data are in agreement with studies using adenoviral or TG overexpression of active Akt in rodents, which increased SERCA expression and enhanced contractile function (3, 19). Notably, cardiac overexpression of SERCA alone results in enhanced Ca2+ transients and hypercontractility (13). SERCA overexpression also accelerates relaxation (13), which is consistent with the trend toward decreased τ that we observed. Therefore, the increase in SERCA expression in the Q510E-SHP2 model is most likely mediated by Akt. In addition to regulating SERCA expression, Akt has been shown to regulate LTCC activity (2, 19). Similar to the findings of Kim et al. (19), LTCC expression was unchanged between TG and NTG groups in our study. We cannot completely rule out that LTCC-mediated Ca2+ influx may be increased, but in the absence of changes in LTCC expression, functional activity is primarily regulated by PKA-dependent phosphorylation. Importantly, we were not able to detect any changes in PKA activity or the phosphorylation status of other target proteins such as sarcomeric proteins. Furthermore, intracellular Ca2+ transport in mice is dominated by SR Ca2+ cycling (25). Therefore, the increase in SERCA expression likely represents the mechanism by which Ca2+ transients are increased in Q510E-SHP2 mice.
Independent of all changes in Ca2+ handling, we found that Q510E-SHP2 increases contractile function of skinned cardiac myofibers. Our initial hypothesis had been that the increase in power generation could be due to altered posttranslational modification of sarcomeric proteins. The biomechanical properties of the contractile proteins are fine tuned by posttranslational modifications such as phosphorylation by PKA. However, our data demonstrate that PKA-induced posttranslational modifications are unlikely to have contributed to the hypercontractile phenotype observed in this NSML model. However, at this point, other modifications, for example, induced by PKC or Ca2+-calmodulin-dependent protein kinase II, cannot be excluded.
Having excluded increased PKA activity as the responsible mechanism, we used electron microscopy to quantify the amount of contractile fibers in the TG myocardium and found that Q510E-SHP2 expression increased the contractile machinery. Consistent with this, we have previously reported that Q510E-SHP2 increases sarcomeric organization as well as overall protein synthesis in neonatal rat cardiomyocytes (42). Our present data are also consistent with electron microscopic findings in a different NSML mouse model based on ubiquitous expression of Y279C-SHP2 (30). As sarcomeres are assembled, strict stoichiometry between the different components appears to be preserved (36). Importantly, sarcomeres are dynamic structures with ongoing incorporation and turnover of the contractile proteins via exchange from a free pool (29, 39, 50). As we could not detect any changes in total sarcomeric protein levels but found increased amounts of myofibers that could be extracted from ventricular tissue, we suspect that Q510E-SHP2 expression leads to alterations in the kinetics of sarcomere assembly. This would be consistent with a recent Xenopus study (23) demonstrating that SHP2 regulates the formation and polarity of cardiac actin fibers during development. On the other hand, it is possible that Q510E-SHP2 expression reduces contractile fiber degradation and turnover. To date, there is no evidence that SHP2 participates in proteasomal pathways, but this remains to be explored.
It is possible that isoform switches of various contractile proteins contribute to the increase in power output in TG hearts. We have previously found that α-skeletal actin mRNA was increased in TG hearts. Interestingly, expression of this isoform has been shown to be associated with increased contractility compared with hearts primarily expressing α-cardiac actin (15). Therefore, this could be another contributing factor enhancing contractility in our NSML model.
Understanding the molecular and cellular mechanisms that induce the NSML phenotype is critical for improving current therapeutic approaches. The mechanistic overlap with classic HCM identified in this study is intriguing and raises the question of whether or not NSML-associated and classic HCM should be treated with the same pharmacological compounds. We recently showed that rapamycin and various other pathway-specific inhibitors are effective against cardiomyocyte hypertrophy induced by Q510E-SHP2 expression (42, 43). This argues for a custom-tailored therapeutic approach for NSML-associated HCM. However, rapamycin treatment started late in the disease process after contractile function had deteriorated did not improve cardiac function in our model (43). Our new data suggest that initiating treatment during the early, hypercontractile stage might be more effective.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-HL-116525 (to M. Krenz), R01-HL-57852 (to K. S. McDonald), and K01-AG-041208 (to T. L. Domeier) and by Mission Enhancement Funding from the School of Medicine, University of Missouri (to M. Krenz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.C., T.L.D., L.M.H., K.S.M., and M.K. conception and design of research; S.A.C., T.L.D., L.M.H., K.S.M., and M.K. performed experiments; S.A.C., T.L.D., L.M.H., K.S.M., and M.K. analyzed data; S.A.C., T.L.D., L.M.H., K.S.M., and M.K. interpreted results of experiments; S.A.C., T.L.D., and M.K. drafted manuscript; S.A.C., T.L.D., L.M.H., K.S.M., and M.K. edited and revised manuscript; S.A.C., T.L.D., L.M.H., K.S.M., and M.K. approved final version of manuscript; T.L.D., L.M.H., K.S.M., and M.K. prepared figures.
ACKNOWLEDGMENTS
The authors thank Dr. Darla Tharp for sharing expertise.
REFERENCES
- 1.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol 268: C1313–C1319, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavα1 protein stability. J Cell Biol 184: 923–933, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cittadini A, Monti MG, Iaccarino G, Di Rella F, Tsichlis PN, Di Gianni A, Stromer H, Sorriento D, Peschle C, Trimarco B, Sacca L, Condorelli G. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther 13: 8–19, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J Jr. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 99: 12333–12338, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, Pizzuti A, Dallapiccola B. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet 71: 389–394, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Digilio MC, Sarkozy A, de Zorzi A, Pacileo G, Limongelli G, Mingarelli R, Calabro R, Marino B, Dallapiccola B. LEOPARD syndrome: clinical diagnosis in the first year of life. Am J Med Genet 140: 740–746, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, Hassid A, Busse R, Fleming I. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res 97: 1236–1244, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Edouard T, Combier JP, Nedelec A, Bel-Vialar S, Metrich M, Conte-Auriol F, Lyonnet S, Parfait B, Tauber M, Salles JP, Lezoualc'h F, Yart A, Raynal P. Functional effects of PTPN11 (SHP2) mutations causing LEOPARD syndrome on epidermal growth factor-induced phosphoinositide 3-kinase/AKT/glycogen synthase kinase 3beta signaling. Mol Cell Biol 30: 2498–2507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378–417, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol 9: 91–100, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Georgakopoulos D, Christe ME, Giewat M, Seidman CM, Seidman JG, Kass DA. The pathogenesis of familial hypertrophic cardiomyopathy: early and evolving effects from an α-cardiac myosin heavy chain missense mutation. Nat Med 5: 327–330, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hanft LM, McDonald KS. Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 296: H1524–H1531, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, Bluhm WF, Meyer M, Sayen MR, Swanson E, Dillmann WH. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest 100: 380–389, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herron TJ, Korte FS, McDonald KS. Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes. Circ Res 89: 1184–1190, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hewett TE, Grupp IL, Grupp G, Robbins J. α-Skeletal actin is associated with increased contractility in the mouse heart. Circ Res 74: 740–746, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Hinken AC, McDonald KS. Inorganic phosphate speeds loaded shortening in rat skinned cardiac myocytes. Am J Physiol Cell Physiol 287: C500–C507, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Ishida H, Kogaki S, Narita J, Ichimori H, Nawa N, Okada Y, Takahashi K, Ozono K. LEOPARD-type SHP2 mutant Gln510Glu attenuates cardiomyocyte differentiation and promotes cardiac hypertrophy via dysregulation of Akt/GSK-3β/β-catenin signaling. Am J Physiol Heart Circ Physiol 301: H1531–H1539, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Jacoby DL, DePasquale EC, McKenna WJ. Hypertrophic cardiomyopathy: diagnosis, risk stratification and treatment. CMAJ 185: 127–134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YK, Kim SJ, Yatani A, Huang Y, Castelli G, Vatner DE, Liu J, Zhang Q, Diaz G, Zieba R, Thaisz J, Drusco A, Croce C, Sadoshima J, Condorelli G, Vatner SF. Mechanism of enhanced cardiac function in mice with hypertrophy induced by overexpressed Akt. J Biol Chem 278: 47622–47628, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Koudova M, Seemanova E, Zenker M. Novel BRAF mutation in a patient with LEOPARD syndrome and normal intelligence. Eur J Med Genet 52: 337–340, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature 298: 182–184, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, Osinska HE, Lorenz JN, Brosseau C, Federico A, Alpert NR, Warshaw DM, Perryman MB, Helmke SM, Robbins J. Analysis of myosin heavy chain functionality in the heart. J Biol Chem 278: 17466–17474, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Langdon Y, Tandon P, Paden E, Duddy J, Taylor JM, Conlon FL. SHP-2 acts via ROCK to regulate the cardiac actin cytoskeleton. Development 139: 948–957, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet 39: 571–574, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol Heart Circ Physiol 274: H1335–H1347, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Limongelli G, Pacileo G, Marino B, Digilio MC, Sarkozy A, Elliott P, Versacci P, Calabro P, De Zorzi A, Di Salvo G, Syrris P, Patton M, McKenna WJ, Dallapiccola B, Calabro R. Prevalence and clinical significance of cardiovascular abnormalities in patients with the LEOPARD syndrome. Am J Cardiol 100: 736–741, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Limongelli G, Sarkozy A, Pacileo G, Calabro P, Digilio MC, Maddaloni V, Gagliardi G, Di Salvo G, Iacomino M, Marino B, Dallapiccola B, Calabro R. Genotype-phenotype analysis and natural history of left ventricular hypertrophy in LEOPARD syndrome. Am J Med Genet A 146A: 620–628, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 29.LoRusso SM, Imanaka-Yoshida K, Shuman H, Sanger JM, Sanger JW. Incorporation of fluorescently labeled contractile proteins into freshly isolated living adult cardiac myocytes. Cell Motil Cytoskeleton 21: 111–122, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, Wu X, Lauriol J, Wang B, Bauer M, Bronson R, Franchini KG, Neel BG, Kontaridis MI. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest 121: 1026–1043, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 92: 785–789, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Quintana E, Rodriguez-Gonzalez F. LEOPARD syndrome: clinical features and gene mutations. Mol Syndromol 3: 145–157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald KS. Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibres. J Physiol 525: 169–181, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald KS, Hanft LM, Domeier TL, Emter CA. Length and PKA dependence of force generation and loaded shortening in porcine cardiac myocytes. Biochem Res Int 2012: 371415, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neel BG, Gu H, Pao L. The “Shp”ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28: 284–293, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Transgenic remodeling of the contractile apparatus in the mammalian heart. Circ Res 78: 504–509, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, Lopez Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet 39: 1007–1012, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Russel IK, Brouwer WP, Germans T, Knaapen P, Marcus JT, van der Velden J, Gotte MJ, van Rossum AC. Increased left ventricular torsion in hypertrophic cardiomyopathy mutation carriers with normal wall thickness. J Cardiovasc Magn Reson 13: 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanger JM, Mittal B, Pochapin MB, Sanger JW. Myofibrillogenesis in living cells microinjected with fluorescently labeled α-actinin. J Cell Biol 102: 2053–2066, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkozy A, Carta C, Moretti S, Zampino G, Digilio MC, Pantaleoni F, Scioletti AP, Esposito G, Cordeddu V, Lepri F, Petrangeli V, Dentici ML, Mancini GM, Selicorni A, Rossi C, Mazzanti L, Marino B, Ferrero GB, Silengo MC, Memo L, Stanzial F, Faravelli F, Stuppia L, Puxeddu E, Gelb BD, Dallapiccola B, Tartaglia M. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Hum Mutat 30: 695–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkozy A, Conti E, Digilio MC, Marino B, Morini E, Pacileo G, Wilson M, Calabro R, Pizzuti A, Dallapiccola B. Clinical and molecular analysis of 30 patients with multiple lentigines LEOPARD syndrome. J Med Genet 41: e68, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schramm C, Edwards MA, Krenz M. New approaches to prevent LEOPARD syndrome-associated cardiac hypertrophy by specifically targeting Shp2-dependent signaling. J Biol Chem 288: 18335–18344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schramm C, Fine DM, Edwards MA, Reeb AN, Krenz M. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am J Physiol Heart Circ Physiol 302: H231–H243, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Solaro RJ, Pang DC, Briggs FN. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta 245: 259–262, 1971. [DOI] [PubMed] [Google Scholar]
- 45.Tajan M, Batut A, Cadoudal T, Deleruyelle S, Le Gonidec S, Saint Laurent C, Vomscheid M, Wanecq E, Treguer K, De Rocca Serra-Nedelec A, Vinel C, Marques MA, Pozzo J, Kunduzova O, Salles JP, Tauber M, Raynal P, Cave H, Edouard T, Valet P, Yart A. LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity. Proc Natl Acad Sci USA 111: E4494–E4503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi K, Kogaki S, Kurotobi S, Nasuno S, Ohta M, Okabe H, Wada K, Sakai N, Taniike M, Ozono K. A novel mutation in the PTPN11 gene in a patient with Noonan syndrome and rapidly progressive hypertrophic cardiomyopathy. Eur J Pediatr 164: 497–500, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev 10: 237–248, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Urbano Moral JA, Arias Godinez JA, Maron MS, Malik R, Eagan JE, Patel AR, Pandian NG. Left ventricular twist mechanics in hypertrophic cardiomyopathy assessed by three-dimensional speckle tracking echocardiography. Am J Cardiol 108: 1788–1795, 2011. [DOI] [PubMed] [Google Scholar]
- 49.van der Werf C, van Langen IM, Wilde AA. Sudden death in the young: what do we know about it and how to prevent? Circ Arrhythm Electrophysiol 3: 96–104, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton 61: 34–48, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wigle ED, Rakowski H, Kimball BP, Williams Hypertrophic cardiomyopathy WG. Clinical spectrum and treatment. Circulation 92: 1680–1692, 1995. [DOI] [PubMed] [Google Scholar]