Abstract
Renal denervation for the treatment of hypertension has proven to be successful; however, the underlying mechanism/s are not entirely clear. To determine if preautonomic neurons in the paraventricular nucleus (PVN) respond to afferent renal nerve (ARN) stimulation, extracellular single-unit recording was used to investigate the contribution of the rostral ventrolateral medulla (RVLM)-projecting PVN (PVN-RVLM) neurons to the response elicited during stimulation of ARN. In 109 spontaneously active neurons recorded in the PVN of anesthetized rats, 25 units were antidromically activated from the RVLM. Among these PVN-RVLM neurons, 84% (21/25) were activated by ARN stimulation. The baseline discharge rate was significantly higher in these neurons than those PVN-RVLM neurons not activated by ARN stimulation (16%, 4/25). The responsiveness of these neurons to baroreflex activation induced by phenylephrine and activation of cardiac sympathetic afferent reflex (CSAR) was also examined. Almost all of the PVN neurons that responded to ARN stimulation were sensitive to baroreflex (95%) and CSAR (100%). The discharge characteristics for nonevoked neurons (not activated by RVLM antidromic stimulation) showed that 23% of these PVN neurons responded to ARN stimulation. All the PVN neurons that responded to ARN stimulation were activated by N-methyl-d-aspartate, and these responses were attenuated by the glutamate receptor blocker AP5. These experiments demonstrated that sensory information originating in the kidney is integrated at the level of preautonomic neurons within the PVN, providing a novel mechanistic insight for use of renal denervation in the modulation of sympathetic outflow in disease states such as hypertension and heart failure.
Keywords: sympathetic activity, cardiovascular, paraventricular nucleus, afferent renal nerves
the sympathetic nervous system has been identified as a major contributor to the complex pathophysiology of hypertension and heart failure both in experimental models and in patients with these diseases (24, 37). Ablation of the renal sympathetic nerves remains an attractive therapeutic approach in hypertension and heart failure (29–31, 49). It has been suggested that renal afferent nerve input may be important in the establishment and maintenance of certain forms of experimental hypertension (25). However, the underlying mechanism for this effect is not entirely clear.
The kidneys have a dense afferent sensory and efferent sympathetic innervation and are thereby strategically positioned to be the origin as well as target of sympathetic nervous system activation (48). Renal afferent nerve signals are centrally integrated, and their activation results in an increase in sympathetic tone, which is not only directed toward the kidneys, thereby inducing increased sodium retention and renin secretion, but also toward other organs that have a dense sympathetic innervation resulting in an increase in sympathetic outflow to cause a rise in blood pressure (25, 36, 2, 40, 47). It is well known that the mammalian kidney contains several distinct classes of sensory receptors: mechanoreceptors sensitive to changes in renal arterial, venous, or ureteral pressure and chemoreceptors (46). These receptors transmit information to the central nervous system via the afferent renal nerves (ARN).
The paraventricular nucleus (PVN) of the hypothalamus is an important site that integrates and responds to a variety of neural and humoral signals regulating sympathetic drive and extracellular fluid volume (42). Previous studies demonstrated that the discharge frequency of putative vasopressinergic magnocellular neurosecretory neurons in the PVN is increased during stimulation of ARN (11) and during the activation of specific renal receptors. Taken together these observations suggest that afferent information from the kidney is important in the coordination of neural and hormonal activity concerned with body fluid balance and the regulation of arterial blood pressure (28, 37). In addition, neurons containing Fos-like immunoreactivity after ARN stimulation were found in the PVN, indicating that the PVN neurons were activated by ARN stimulation (50). The PVN includes neuroendocrine-related functional neurons that project to the median eminence, posterior pituitary and preautonomic neurons that send long descending projections to the brainstem and spinal cord regions that are important in dictating autonomic outflow (3, 52). There are a number of PVN neurons that project to the rostral ventrolateral medulla (RVLM), which have been shown to temporally correlate with renal sympathetic nerve activity (RSNA), suggesting that they contribute to sympathetic activation (10). Thus the present study focused on RVLM-projecting PVN (PVN-RVLM) neurons.
In this respect it is of interest to understand if preautonomic neurons in the PVN respond to ARN stimulation and how the PVN integrates signals from the ARN, baroreceptors, and cardiac afferent nerves to influence sympathetic tone.
In the present study, extracellular single-unit recording was used to investigate the effect of ARN stimulation on firing of PVN-RVLM neurons. In addition, the responses of these PVN-RVLM neurons to baroreflex and cardiac sympathetic afferent reflex were also examined. Finally, the responsiveness of these neurons to endogenous glutamate was tested.
METHODS
Male Sprague-Dawley rats weighing between 250 and 300 g were obtained from SASCO Breeding Laboratories (Omaha, NE). This study was approved by the Institutional Animal Care and Use Committee of the University of Nebraska and was carried out under the guidelines of the American Physiological Society and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
General procedures.
Each rat was anesthetized by injection of urethane (0.75–1.5 g/kg ip) and α-chloralose (70–140 mg/kg ip). Adequate depth of anesthesia was assessed by the absence of corneal reflexes and paw withdrawal response to a noxious pinch. Supplemental doses of anesthesia were administered to maintain an adequate depth of anesthesia during the experiment. The right femoral artery and femoral vein were cannulated for the recording of arterial blood pressure and administration of chemicals, respectively. The mean arterial pressure and heart rate were simultaneously recorded on a PowerLab data acquisition system (8SP; ADInstruments, Colorado Springs, CO). Body temperature was maintained at ∼37°C with an animal temperature controller [ATC 1000; World Precision Instruments (WPI), Sarasota, FL].
Extracellular single-unit recording in vivo.
Rats were placed in a stereotaxic apparatus (model 620; Stoelting, Chicago, IL). The stereotaxic coordinates for the PVN were determined according to Paxinos and Watson's atlas (41). Typically, three tracks were explored for extracellular recording in each rat, from −1.4 to −2.1 mm caudal to bregma, at 0.4 mm lateral (right side) to the midline, and with a depth of 7.4–8.6 mm ventral to the dorsal surface. Extracellular single-unit recording was carried out using a single micropipette (resistance: 5–15 MΩ) filled with 0.5 M sodium acetate containing 2% pontamine sky blue. The glass micropipettes were advanced using a microdrive controller (type 860; Hugo Sachs Elektronik) into the PVN. The spontaneous activity of neurons was amplified (gain: 1,000) with an AC/DC differential amplifier (model IX1; Dagan, Minneapolis, MN) with a low-frequency cut-off at 30 Hz and a high-frequency cut-off at 3 kHz. The neuronal discharge was recorded on a PowerLab data acquisition system (8/30; ADInstruments). The frequency of the neuronal discharge was analyzed with special software (SpikeHistogram; ADInstruments).
Identification of RVLM-PVN neurons.
Antidromic stimulation was used to identify RVLM-PVN neurons in vivo. The RVLM (−12.5 mm caudal to the bregma, 2.1 mm left of the midline, and 9.2 mm ventral to the surface of cerebellum) was functionally identified by a 15- to 20-mmHg blood pressure response to microinjection of NMDA (100 pmol in 50 nl) during a 10-s period (33). Subsequently, a concentric bipolar electrode (500-μm outer diameter, tip tapered at 60°; WPI) was placed at the same location in the RVLM to allow for antidromic stimulation (model A310; WPI). RVLM stimulation was ipsilateral to PVN recording.
When a spontaneously active neuron was recorded, standard tests were performed to ensure its antidromic nature (34). First, we examined if the neurons responded to RVLM stimulation (pulse width: 3–10 ms; current intensity: 0.1–1 mA; stimulation frequency: 0.5 Hz) with consistent onset latency spikes at a discrete stimulus threshold. Second, we examined if the neurons responded to each pulse in a high-frequency 300-Hz stimulus train. The collision test was performed to examine whether the stimulus-evoked spikes could be canceled by spontaneous action potentials. A window discriminator (model 74-60-1C; WPI) and oscilloscope (model OS-5100RA; LG) were used to discriminate the PVN spontaneous action potentials, and the signal was translated to TTL pulse as a trigger with an adjustable latency to evoke the RVLM stimulation. In a few cases RVLM stimulus intensity was increased gradually above threshold to determine if a discontinuous decrease (>2 ms) in the antidromic onset latency could be detected. Such antidromic latency “jumps” were interpreted to indicate the presence of a terminal arborization in the vicinity of the RVLM stimulating electrode as described in our previous study (53). At the end of the experiment, the stimulation sites in the RVLM were marked by passing 1 mA of anodal direct current for 20 s.
Stimulation of ARN.
To stimulate ARN, the left kidney was exposed through a left retroperitoneal flank incision. A branch of the renal nerve was isolated through a retroperitoneal incision while it was under an operating microscope. The distal end of the nerve was ligated, and the nerve was placed on a bipolar platinum electrode. The nerve-electrode junction was insulated electrically from the surrounding tissues with gel (Wacker, St. Louis, MO). The stimulus to ARN consisted of brief trains of stimuli (3–5 pulses at 200 Hz, 2- to 3-ms pulse durations) applied once every 2 s. In a subset of experiments, a low-frequency stimuli (20 Hz) was used to stimulate ARN. The current intensity was one to five times the threshold current (50–200 μA) required to elicit an increase in mean arterial pressure of ∼10 mmHg using a 15-s train of pulses at 40 Hz and 0.5-ms pulse duration (7, 19). To exclude the possibility that any effect observed during the stimulation of ARN was due to current spread, in some experiments a second stimulating electrode was firmly attached to an adjacent structure. Onset latency of response to the stimulation of ARN was determined as the time interval from the stimulus artifact to a 30% change with respect to control in discharge frequency on peristimulus time histograms. This criterion was based on previous studies (6, 7, 8, 11).
Cardiac sympathetic afferent reflex induced by epicardial application of chemicals.
A limited left lateral thoracotomy was performed to expose the heart, and the pericardium was removed. The cardiac afferents were activated by application of a piece of filter paper (3 × 3 mm) containing capsaicin (Cap; 1.0 nmol in 2.0 μl) or bradykinin (BK; 0.3 nmol in 2.0 μl) to the epicardial surface of anterior wall of the left ventricle. Each piece of paper was removed 1 min later, and the epicardium was rinsed with 10 ml of warm normal saline (38°C) three times. Successive applications of chemicals were separated by at least 15 min.
Baroreflex activation.
Baroreceptor challenges were induced by intravenous injection of phenylephrine (PE; 5–25 μg/kg) or sodium nitroprusside (SNP; 4–20 μg/kg) to increase or decrease arterial blood pressure, respectively.
Iontophoretic injection with extracellular unit recording in vivo.
To combine PVN single-unit recording with iontophoretic injection, multibarrel glass micropipettes (3 barrels) were used. Single micropipettes were pulled for recording. Two ejection pipettes were pulled (Multi-pipette Puller PMP-107; MicroData Instruments, South Plainfield, NJ) from thick-walled glass capillary tubing with a calibrated narrow inner diameter (outer diameter = 1.0 mm, thick wall = 0.5 mm; Micropipette Glass 50610; Stoelting), which was redefined to a final outer diameter of ∼30 μm (Microprocessor-controlled Microforge DMF1000; WPI). The recording and ejection pipettes were then assembled in a specially designed electrode holder (HMD-2; Narishige) to permit the tips and as long a part of the tapering ends as possible to touch one another (1). The tip of the recording pipette was advanced 30 μm beyond the ejection tip. The iontophoretic system was a Neurophore Model BH-2 control unit (WPI). The current used was −50 to −100 nA for the NMDA pipette and −100 to −200 nA for the AP5 pipette. These parameters for iontophoretic injection were based on the studies from others (56).
Histology of recording sites within the PVN.
At the end of the experiment, pontamine sky blue was iontophoresed (−15 μA, 10 min) to mark the site of the last recorded neuron, and other recording sites were extrapolated from the marked point according to Paxinos and Watson's atlas (41). Then, the rat was euthanized with an overdose of anesthesia. The rat brains were then removed, frozen, and sectioned. The dye spots for recording sites in the PVN and the sites of electrolytic lesion in the RVLM were identified with a light microscope. Rats whose recording sites were within the boundaries of the PVN were used for data analysis. The location of the center of the dye spot was transferred to a histological map based on the rat atlas Paxinos and Watson's atlas (41).
Statistical analysis.
Data are presented as means ± SE. Differences between groups were determined by a two-way ANOVA followed by the Newman-Keuls test for post hoc analysis of significance (StatView II, Berkeley, CA). P < 0.05 was considered statistically significant.
RESULTS
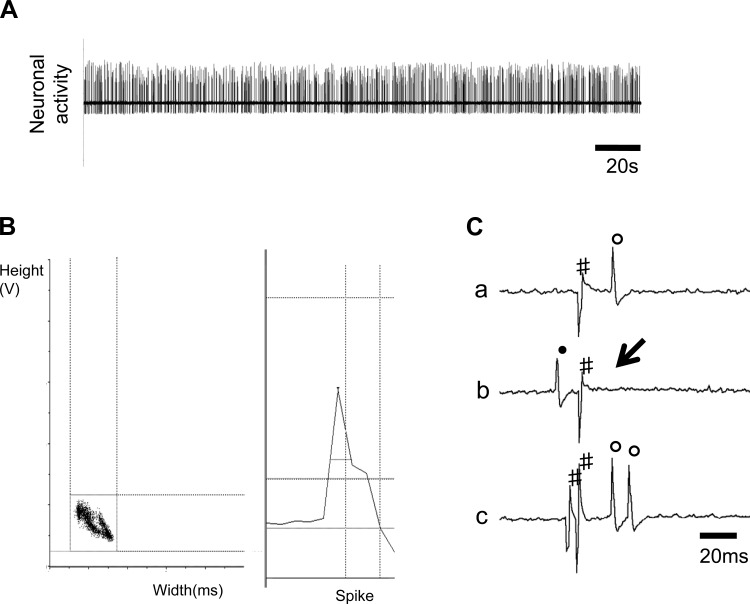
In 109 spontaneously active neurons recorded in the PVN, 25 units were antidromically activated from the RVLM in 32 rats. Figure 1, A–C, shows one neuron that had antidromic spikes evoked by stimulation of the RVLM with constant latency (31 ms), and the antidromic spike was canceled when the interval between the spontaneous action potential and the stimulation was reduced to <31 ms. We then performed a high-frequency (333 Hz, 3 ms) following test. These results verify that we were recording from RVLM-PVN neurons.
Fig. 1.
A and B: segments of original recordings and spike discriminator output demonstrating a single-unit discharge from rostral ventrolateral medulla (RVLM)-projecting PVN (PVN-RVLM) neuron. C: this neuron (a–c) was identified as a PVN-RVLM neuron with consistent onset latency using the collision test; a: neuronal activity with constant latency (31 ms) antidromic spikes evoked by stimulation of RVLM; b: RVLM stimulation evoked an antidromic spike that was cancelled when the interval between spontaneous action potentials and stimulation was reduced to <30 ms; and c: high-frequency (333 Hz, 3 ms) following test. All segments, a–c, represent 2 superimposed sweeps: ↓, electric stimulation; ●, spontaneous action potentials; #, stimulus artifacts; ○, antidromic spikes.
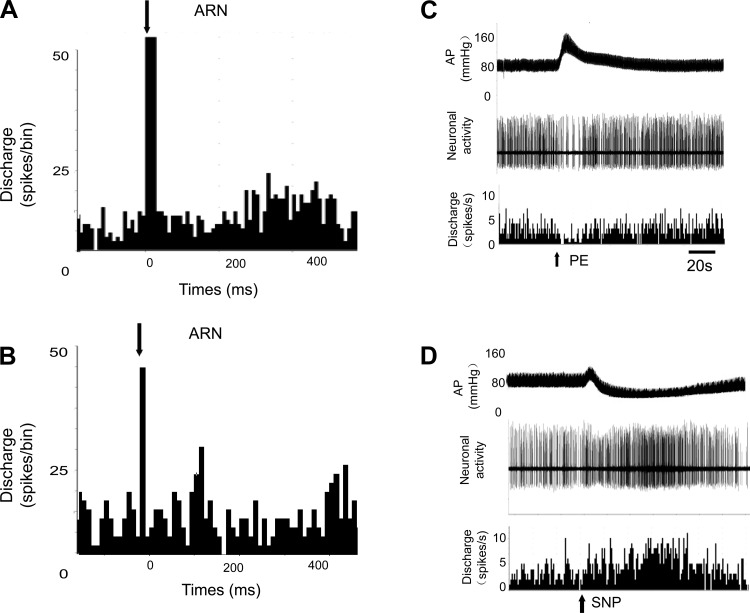
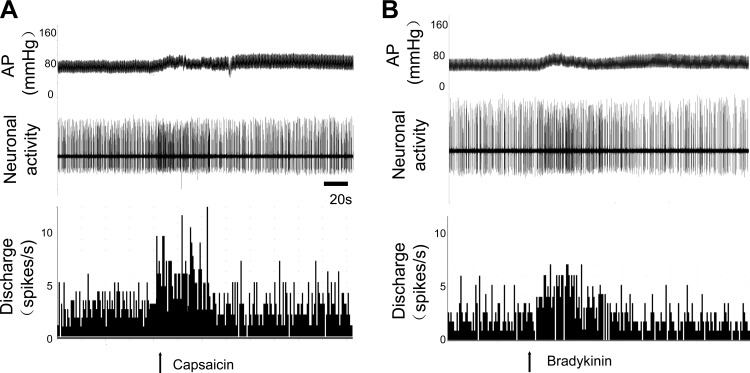
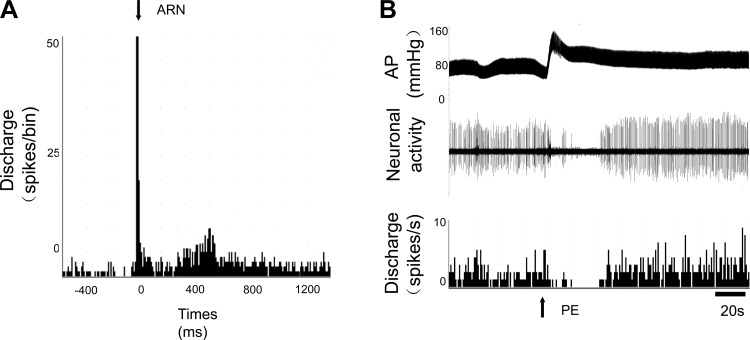
As shown in Table 1, among units classified as PVN-RVLM neurons, 84% (21/25) of these neurons were activated by ARN stimulation (ARN input to PVN neurons). In addition, almost all the PVN neurons that responded to ARN stimulation were also sensitive to baroreflex activation (95%) induced by intravenous injection PE or SNP and to the CSAR induced by epicardial application of Cap (100%). An example of an antidromically identified single unit in PVN responding to stimulation of the ARN and baroreflex activation is shown in Fig. 2, A and B. The same neuron also responded to the CSAR. The CSAR was induced by epicardial application of BK or Cap (Fig. 3). The mean onset latency of response of PVN-RVLM neurons to ARN stimulation (3–5 pulses at 200 Hz, 2- to 3-ms pulse durations) was 129 ± 8 ms, n = 11 (from 66 to 207 ms). In the experiments given a low-frequency stimuli (20 Hz) to stimulate ARN, the mean onset latency of response of PVN-RVLM neurons to ARN stimulation was 171 ± 14 ms, n = 8 (from 115 to 228 ms), which was significantly longer compared with high-frequency ARN stimulation (P < 0.05).
Table 1.
Summary of discharge characteristics for PVN-RVLM neurons
| Neuronal Type | n | Baseline Discharge, spikes/s | Inhibited by PE | Excited by Cap | Axonal Conduction Velocity of PVN-RVLM Neurons, m/s |
|---|---|---|---|---|---|
| ARN | 21/25 (84%) | 2.6 ± 0.4* | 17/19 (95%) | 5/5 (100%) | 0.9 ± 0.1 |
| No renal afferent input | 4/25 (16%) | 0.7 ± 0.3 | 0/4 (0%) | 0/3 (0%) | 0.4 ± 0.1 |
Values are means ± SE; n = 25/109. PE, phenylephrine; Cap, capsaicin; PVN, paraventricular nucleus; RVLM, rostral ventrolateral medulla; ARN, afferent renal nerve.
Fig. 2.
Peristimulus histogram of spike occurrence triggered by electrical stimulation of the afferent renal nerve (ARN) with 50 sweeps at 20 Hz (A) and 200 Hz (B). Segments of original recordings in a single PVN-RVLM neuron of changes in discharge after intravenous injection of phenylephrine (PE) to increase mean arterial pressure (AP; C) or sodium nitroprusside (SNP) to lower mean arterial pressure (D).
Fig. 3.
Original recordings of blood pressure, neuronal activity, and discharge rate (spikes/s) from an identified PVN-RVLM neuron in response to capcaicin (Cap; A) or bradykinin (BK; B), respectively.
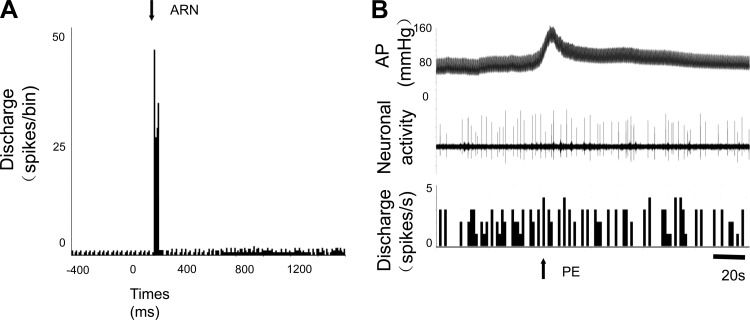
We also found that 16% (4/25) of PVN-RVLM neurons were not activated by ARN stimulation (non-ARN input neurons). Figure 4 shows one example of a PVN-RVLM neuron that was not sensitive to ARN stimulation and baroreflex activation. Interestingly, the baseline discharge rate in PVN-RVLM neurons activated by ARN stimulation was significantly higher than PVN-RVLM neurons that could not be activated by ARN stimulation. The axonal conduction velocity of PVN-RVLM neurons activated by ARN stimulation was significantly faster compared with PVN neurons that were not activated by ARN stimulation (Table 1).
Fig. 4.
A: peristimulus histogram of spike occurrence triggered by electrical stimulation of the ARN with 50 sweeps in a nonresponsive PVN-RVLM neuron. B: segment of original recordings of changes in discharge after intravenous injection of PE to increase mean arterial pressure in the same neuron.
A summary of discharge characteristics for nonevoked neurons that represented 77% (84/109; not activated by RVLM antidromic stimulation) of the total number of recorded neurons is shown in Table 2. Among the units classified as nonevoked neurons by RVLM stimulation, only 23% (8/35) of the PVN neurons were activated by ARN stimulation. Eighty-three percent of these neurons that responded to ARN were sensitive to baroreflex activation but not to the CSAR. The majority of the neurons that did not respond to ARN stimulation (27/35) were also not very responsive to baroreflex activation (27%; 4/15) and were not sensitive to the CSAR (0/5). No differences were observed in the baseline discharge rate between the two groups. Figure 5 shows one example of a neuron that was not evoked by RVLM antidromic stimulation yet was activated by ARN stimulation and was also sensitive to baroreflex activation.
Table 2.
Summary of discharge characteristics for nonevoked neurons
| Neuronal Type | n | Baseline Discharge, spikes/s | Inhibited by PE | Excited by Cap |
|---|---|---|---|---|
| ARN | 8/35 (23%) | 2.4 ± 0.4 | 5/6 (83%) | 0/3 |
| No renal afferent input | 27/35 (77%) | 1.8 ± 0.2 | 4/15 (27%) | 0/5 |
Values are means ± SE; n = 84/109.
Fig. 5.
A: peristimulus histogram of spike occurrence triggered by electric stimulation of the ARN with 50 sweeps in nonevoked PVN neuron by antidromic stimulation of the RVLM. B: segment of original recordings of changes in discharge after intravenous injection of PE to increase mean arterial pressure in the same nonevoked PVN neuron.
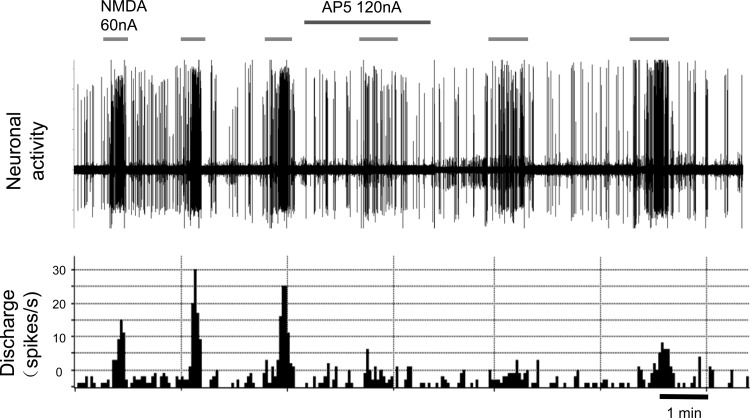
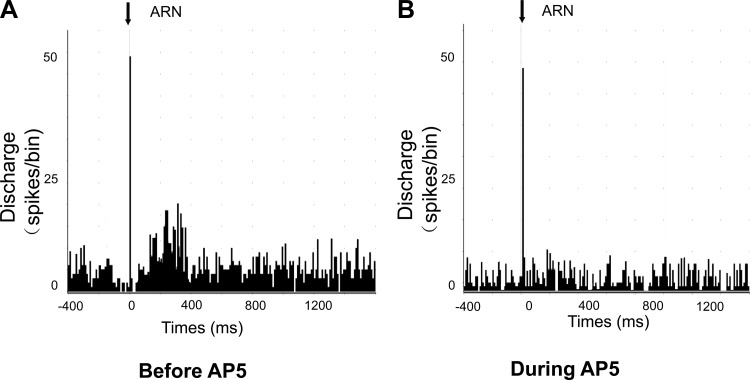
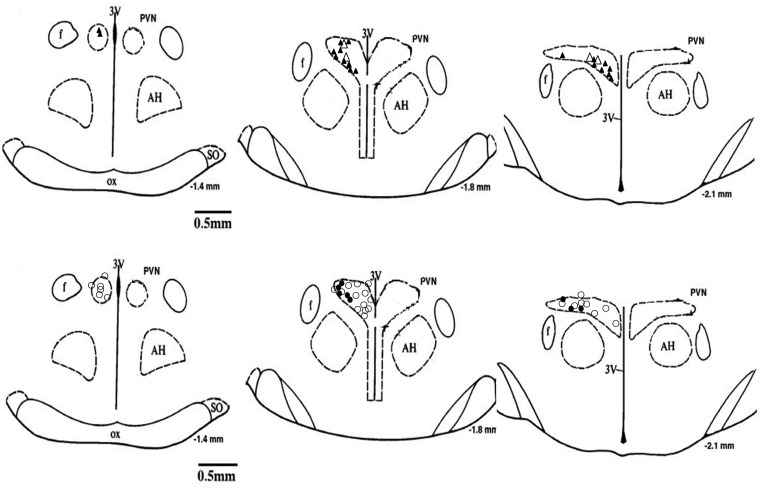
A subset of PVN neurons that responded to ARN stimulation (n = 5) could all be activated by NMDA, and these responses were attenuated by the glutamate receptor blocker AP5 (Fig. 6). In addition, the responses of PVN neurons (n = 3) to ARN stimulation were blocked during iontophoretic application of AP5 (Fig. 7). Figure 8 shows a schematic distribution of recorded neurons within the PVN.
Fig. 6.
Segment of original recordings of changes in discharge of a ARN-responsive PVN-RVLM neuron after repeated iontophoretic injections of N-methyl-d-aspartate (NMDA; 60 nA) before, during, and after AP5 (120 nA) injection.
Fig. 7.
Peristimulus histogram of spike occurrence triggered by electrical stimulation of the ARN with 50 sweeps. A: before iontophoretic application of the NMDA receptor antagonist. B: during iontophoretic application of AP5.
Fig. 8.
Approximate locations of the PVN neurons. Top: △, units antidromically activated from the RVLM only; ▲, units activated by ARN stimulation and by RVLM stimulation. Bottom: ○, units not antidromically activated from the RVLM; ●, units activated by ARN stimulation but not by RVLM stimulation. The distance (in mm) posterior to bregma is shown for each section. AH, anterior hypothalamic nucleus; f, fornix; 3V, 3rd ventricle; OX, optic tract; SO, supraoptic nucleus.
DISCUSSION
This study provides direct electrophysiological evidence to indicate that afferent information originating from the kidney contributes to activation of preautonomic neurons within the PVN. This conclusion is based on the observation that electrical stimulation of ARN increases the discharge rate of PVN neurons that projected directly to the RVLM. Furthermore, selective activation of baroreceptors or cardiac chemoreceptors also influenced the activity of the majority of these RVLM-PVN neurons. Taken together, these data suggest that there is a prompt and direct interaction of renal afferent input with baroreceptor and cardiac chemoreceptors at the level of the PVN to dictate overall sympathetic tone.
The PVN is an important integrative (32) site in the regulation of sympathetic outflow and cardiovascular function, particularly via its projections to principal centers of sympathetic drive, the RVLM and the intermediolateral cell column (IML) of the spinal cord (4, 15, 17, 23). It is well known that baseline sympathetic outflow mediated by the RVLM is mainly dependent on spontaneous activity of preautonomic neurons (16, 18). Previous studies have demonstrated that PVN axons have terminal sites closely associated with spinally projecting RVLM neurons, many of which are likely to terminate on sympathetic preganglionic neurons (43, 44). Recently, Chen and Toney (10) demonstrated that the activity of both PVN-RVLM and PVN-RVLM/IML neurons is temporally correlated with RSNA and may contribute to basal sympathetic nerve activity. Electrical stimulation of ARN elicits an increase in arterial blood pressure and heart rate (51). The hypertensive response is presumably due to the activation of the sympathetic nervous system leading to peripheral vasoconstriction (51). Therefore, the present data provide electrophysiological evidence indicating that afferent information originating from renal receptors contributes to neural mechanisms selectively controlling sympathetic nerve activity. A previous study demonstrated that ARN stimulation excites magnocellular neurosecretory neurons in PVN antidromically identified to project to the neurohypophsis and suggest that this renal-paraventricular reflex loop may contribute to the elevated arterial pressure and vasopressin release during conditions when ARN are activated (11). We also observed that part of the nonantidromically identified neurons responded to the stimulation of the ARN. It is possible that this population of PVN neurons represent the projection to the neurohypophysis to influence the AVP release observed previously (8).
In the present study we found that the mean onset latency of response in PVN-RVLM neurons to ARN stimulation was comparable to that reported in cats (9) and rats (19) with a fairly wide range of values. Furthermore, the onset latency of PVN-RVLM neurons to low-frequency ARN stimulation was longer compared with high-frequency ARN stimulation. One possible explanation for these observations is the differences in conduction velocities of the different afferent fibers. This evidence is provided by studies in the cat showing that ARN contain a mixture of slow-conducting fibers comprised of both unmylinated and thinly myelinated and fast-conducting small mylinated fibers (27). The renal afferent A fibers were elicited by stimulation with trains of pulses at low voltage and high frequency, and the renal afferent C fibers were stimulated by trains of pulses at high voltage and low frequency (5, 22). It is thought that mechanoreceptor information is carried by the mylinated fibers and the chemoreceptor information is transmitted by the unmylinated fibers (5, 11). Alternatively, the long variable latency of the responses suggests that ARN input to the PVN-RVLM neurons may be due to ARN information being transmitted possibly via a polysynaptic pathway. The precise pathway by which ARN information is relayed to the PVN neurons is not entirely clear. A probable path may involve the nucleus tractus solitarius and the ventrolateral medulla, since single units in these areas have been shown to alter their rate of firing in response to ARN stimulation (6, 22). Furthermore, neurons in these areas have been shown to relay cardiovascular afferent information directly to the PVN (13, 14). Taken together, the longer mean onset latency of response in PVN-RVLM neurons to ARN stimulation is probably due to both the difference in conduction velocities as well as the polysynaptic pathway from the ARN to the preautonomic neurons within the PVN.
The excitatory influence from renal afferents may represent an enhancement of tonic input from the kidneys or perhaps an activation of quiescent renal afferents in response to a variety of stimuli, including alterations in renal arterial pressure, ischemia, renal venous occlusion, ureteral occlusion, compression of the kidney, and changes in the ionic composition of the pelvic urine, since these maneuvers have been shown to produce alterations in renal afferent nerve activity (45, 46). However, this study did not identify the specific modality of the input from the kidney, only to suggest that both mechanoreceptor afferent and chemoreceptor afferent information from the kidney is directly relayed to preautonomic neurons within the PVN.
Several lines of evidence indicate that there is a possible interaction between the baroreceptor input and the renal afferent input at the level of the hypothalamus (12). Evidence in support of an interaction between renal afferents and baroreceptor afferents is provided by the studies showing that renal afferent fibers project to hypothalamic sites known to influence neurohormonal control of the circulation and fluid balance (12). In addition, a majority of single units in the hypothalamus that respond to stimulation of ARN also respond to electrical stimulation of arterial baroreceptor afferent fibers (6). Therefore, it is conceivable that activation of ARN may affect neuronal mechanisms within the hypothalamus, specifically within the PVN that, in turn, may be involved in modulating the neurohormonal control of the circulation. It has also been shown that there is interaction between the excitatory influences from the renal afferents and inhibitory input from the baroreceptors in terms of the noradrenergic activity within the hypothalamus (39). Furthermore, it has been shown that removal of renal nerves prevents the acute neurogenic hypertension (26), characterized by increased sympathetic outflow, induced by aortic depressor transection (38); that is, removal of baroreceptor input. These results demonstrate that removal of renal afferents resulted in a reduction in sympathetic activation initiated by removal of baroreceptor-mediated inhibition (26). The results in the present study are consistent with these observations and further provide direct electrophysiological evidence to indicate that afferent input from the kidney activates the same preautonomic neurons within the PVN that are influenced by baroreceptor input. These data, taken together, suggest that there is direct interaction between the baroreceptor input and renal afferent input at the level of the preautonomic neurons within the PVN to dictate overall sympathetic outflow. In disease conditions such as hypertension and heart failure, known to have altered baroreceptor function, it is conceivable that renal afferent input may play an important and critical role in the initiation of enhanced sympathetic outflow and, therefore, is more amenable to therapeutic approaches, such as catheter-based therapeutic renal denervation, which is shown to be effective (29–31, 49).
It is well known that the CSAR is a sympatho-excitatory reflex, which can be initiated by increases in cardiac pressure and volume as well as various substances such as adenosine, BK, and hydrogen peroxide released in the myocardium in the state of myocardial ischemia or chronic heart failure (21, 35). Recent studies indicate that catheter-based therapeutic renal denervation may improve cardiac function in chronic heart failure conditions, possibly by a reduction in renal afferent input that causes attenuation in sympatho-excitation (49). Thus it is crucially important to understand the details of the underlying mechanism/s involved in the renal denervation-mediated decrease in arterial pressure. In the present study almost all the PVN neurons (95%) that were responsive to ARN stimulation were sensitive to baroreflex activation induced by increasing arterial pressure with PE. Interestingly, we observed that in a subset of all of PVN neurons responsive to ARN stimulation (n = 5), 100% of these neurons were also sensitive to CSAR induced by epicardial application of Cap. Furthermore, the majority of the PVN neurons that do not respond to ARN stimulation were also not sensitive to the baroreflex and CSAR. These results imply that neurons within the PVN that are sensitive to ARN are also influenced by baroreflex stimulation and the CSAR. It is well known that the kidneys communicate with integral structures in the central nervous system via the renal sensory afferent nerves (25). Intrarenal stimuli or pathology, such as ischemia or hypoxia, results in an increase in renal afferent nerve activity (54, 55). Renal sensory afferent nerve activity directly influences sympathetic outflow to the kidneys and other organs such as the heart and peripheral blood vessels, which are also modulated by the PVN (20). Thus renal denervation is likely to be valuable in the treatment of several clinical conditions such as hypertension and chronic heart failure characterized by increased overall and particularly renal sympathetic nerve activity.
Previous studies have indicated that NMDA NR1 receptor mRNA expression and protein in the PVN are significantly increased in chronic heart failure, which may contribute to the elevated sympatho-excitation during chronic heart failure (32). As shown in the present study, NMDA activated all of ARN inputs to PVN neurons, and these responses were attenuated during iontophoretic application of the glutamate receptor blocker AP5. Thus it is possible that the upregulation of the NMDA receptor and the subsequent increase in glutamate activity within the PVN may be contributing to the altered compensatory responses in disease states such as hypertension and chronic heart failure. However, this remains to be investigated.
In summary, these experiments demonstrate that sensory information originating in the kidney excites preautonomic neurons in PVN and suggest that this renal-PVN afferent pathway may contribute to elevated sympathetic nerve activity. Furthermore, this afferent renal input integrates with both baroreflex and CSAR input at a single neuron level within the PVN to dictate activity of preautonomic neurons in the PVN. It is thus conceivable that an enhanced afferent renal input in disease conditions such as hypertension and heart failure may be critically involved in interacting with altered baroreflex and CSAR to produce elevated sympathetic nerve activity commonly observed in these states.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R56-HL-124104 and P01-HL-62222.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.X. and K.P.P. conception and design of research; B.X. and X.L. performed experiments; B.X. and X.L. analyzed data; B.X., H.Z., X.L., and K.P.P. interpreted results of experiments; B.X., H.Z., X.L., and K.P.P. prepared figures; B.X., H.Z., and K.P.P. drafted manuscript; H.Z. and K.P.P. edited and revised manuscript; H.Z. and K.P.P. approved final version of manuscript.
REFERENCES
- 1.Akaoka H, Saunier CF, Chergui K, Charlety P, Buda M, Chouvet G. Combining in vivo volume-controlled pressure microejection with extracellular unit recording. J Neurosci Methods 42: 119–128, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Ammons WS. Bowditch Lecture. Renal afferent inputs to ascending spinal pathways. Am J Physiol Regul Integr Comp Physiol 262: R165–R176, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience 5: 1931–1958, 1980. [DOI] [PubMed] [Google Scholar]
- 4.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28: 95–99, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Calaresu FR, Ciriello J. Projections to the hypothalamus from buffer nerves and nucleus tractus solitarius in the cat. Am J Physiol Regul Integr Comp Physiol 239: R130–R136, 1980. [DOI] [PubMed] [Google Scholar]
- 6.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst 3: 311–320, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol Regul Integr Comp Physiol 254: R531–R543, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Caverson MM, Ciriello J. Effect of stimulation of afferent renal nerves on plasma levels of vasopressin. Am J Physiol Regul Integr Comp Physiol 252: R801–R807, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Caverson MM, Ciriello J, Calaresu FR. Cardiovascular afferent inputs to neurons in the ventrolateral medulla projecting directly the central autonomic area of the thoracic cord in the cat. Brain Res 274: 354–358, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol 275: R1745–R1754, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Ciriello J, Calaresu FR. Hypothalamic projections of renal afferent nerves in the cat. Can J Physiol Pharmacol 58: 574–576, 1980. [DOI] [PubMed] [Google Scholar]
- 13.Ciriello J, Calaresu FR. Monosynaptic pathway from cardiovascular neurons in the nucleus tractus solitarii to the paraventricular nucleus in the cat. Brain Res 193: 529–533, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Ciriello J, Caverson MM. Ventrolateral medullary neurons relay cardiovascular inputs to the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 246: R968–R978, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Dampney RA. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol 42: 197–227, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Day TA, Ciriello J. Afferent renal nerve stimulation excites supraoptic vasopressin neurons. Am J Physiol Regul Integr Comp Physiol 249: R368–R371, 1985. [DOI] [PubMed] [Google Scholar]
- 20.DiBona GF. Neural control of the kidney: past, present, and future. Hypertension 41: 621–624, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Du YH, Chen AF. A “love triangle” elicited by electrochemistry: complex interactions among cardiac sympathetic afferent, chemo-, and baroreflexes. J Appl Physiol (1985) 102: 9–10, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Felder RB. Excitatory and inhibitory interactions among renal a cardiovascular afferent nerves in dorsomedial medulla. Am J Physiol Regul Integr Comp Physiol 250: R580–R588, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus–a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12: 717–727, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension 54: 690–697, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Grisk O, Rettig R. Interactions between the sympathetic nervous system and the kidneys in arterial hypertension. Cardiovasc Res 61: 238–246, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kline RL, Patel KP, Ciriello J, Mercer PF. Effect of renal denervation on arterial pressure in rats with aortic nerve transection. Hypertension 5: 468–475, 1983. [DOI] [PubMed] [Google Scholar]
- 27.Knuepfer MM, Schramm LP. The conduction velocities and spinal projections of single renal afferent fibers in the rat. Brain Res 435: 167–173, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Kopp UC. Neural Control of Renal Function. San Rafael, CA: Univ. of Iowa Carver College of Medicine, Dept. of Veterans Affairs Medical Center, 2011. [Google Scholar]
- 29.Krum H, Schlaich MP, Whitbourn R, Sobotka P, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler MD. Catheter-based renal sympathetic denervation for resistant hypertension. Lancet 373: 1275–1281, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Lambert GW, Hering D, Esler MD, Marusic P, Lambert EA, Tanamas SK, Shaw J, Krum H, Dixon JB, Barton DA, Schlaich MP. Health-related quality of life after renal denervation in patients with treatment-resistant hypertension. Hypertension 60: 1479–1484, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet 380: 591–600, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Matsumura K, Kagiyama S, Fukuhara M, Fujii K, Iida M. Chronic administration of olmesartan attenuates the exaggerated pressor response to glutamate in the rostral ventrolateral medulla of SHR. Brain Res 1058: 161–166, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981. [DOI] [PubMed] [Google Scholar]
- 35.Malliani A, Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension 39: 63–68, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Malpas SC, Ramchandra R, Guild SJ, McBryde F, Barrett CJ. Renal sympathetic nerve activity in the development of hypertension. Curr Hypertens Rep 8: 242–248, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 33: 1058–1066, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Patel KP, Ciriello J, Kline RL. Noradrenergic mechanisms in brain and peripheral organs after aortic nerve transection. Am J Physiol Heart Circ Physiol 240: H481–H486, 1981. [DOI] [PubMed] [Google Scholar]
- 39.Patel KP, Kline RL. Influence of renal nerves on noradrenergic responses to changes in arterial pressure. Am J Physiol Regul Integr Comp Physiol 247: R615–R620, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Patel KP, Knuepfer M. Effect of afferent renal nerve stimulation on blood pressure and heart rate and noradrenergic activity in conscious rats. J Auton Nerv Syst 17: 121–130, 1986. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando, FL: Academic, 1986. [Google Scholar]
- 42.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38: 197–208, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience 88: 949–957, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res 120: 164–172, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Recordati GM, Moss NG, Genovesi S, Rogenes PR. Renal receptors in the rat sensitive to chemical alterations of their environment. Circ Res 46: 395–405, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Recordati GM, Moss NG, Waselkov L. Renal chemoreceptors in the rat. Circ Res 43: 534–543, 1978. [DOI] [PubMed] [Google Scholar]
- 47.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 54: 1195–1201, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 20: 933–939, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Sobotka PA, Krum H, Bohm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep 14: 285–292, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res 753: 102–119, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Stella A, Weaver L, Golin R, Genovesi S, Zanchetti A. Cardiovascular effects of afferent renal nerve stimulation. Clin Exp Hypertens A 9, Suppl 1: 97–111, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of the projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. [DOI] [PubMed] [Google Scholar]
- 53.Xu B, Zheng H, Patel KP. Enhanced activation of RVLM-projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 302: H1700–H1711, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens 11: 723–728, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Ye S, Zhong H, Yanamadala V, Campese VM. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am J Hypertens 15: 717–724, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol 511.3: 733–745, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]