Abstract
Overnutrition/obesity predisposes individuals, particularly women, to diastolic dysfunction (DD), an independent predictor of future cardiovascular disease. We examined whether low-dose spironolactone (Sp) prevents DD associated with consumption of a Western Diet (WD) high in fat, fructose, and sucrose. Female C57BL6J mice were fed a WD with or without Sp (1 mg·kg−1·day−1). After 4 mo on the WD, mice exhibited increased body weight and visceral fat, but similar blood pressures, compared with control diet-fed mice. Sp prevented the development of WD-induced DD, as indicated by decreased isovolumic relaxation time and an improvement in myocardial performance (<Tei index) and septal annular velocity (<E′-to-A′ ratio), as assessed by echocardiography, as well as decreased diastolic relaxation time/increased diastolic initial filling rate, as assessed by MRI. The relationship between passive sarcomere length of cardiac myocytes and ventricular pressure was monitored using di-8-ANEPPS staining of the t-tubule network in hearts ex vivo. Sp administration led to longer sarcomere lengths at each pressure indicative of improved ventricular compliance in WD-fed mice. Sp also prevented left ventricular hypertrophy, interstitial fibrosis, and oxidative stress. Sp prevented the WD-induced increased expression of myocardial proinflammatory M1 macrophage markers monocyte chemoattractant protein-1 and CD11c and increased the expression of the anti-inflammatory M2 macrophage marker CD206. These findings demonstrate that WD-induced DD is associated with increased oxidant stress, fibrosis, and immune dysregulation. Mineralocorticoid receptor antagonism enhanced M2 macrophage polarization and ameliorated oxidant stress and fibrosis. This work supports a novel blood pressure-independent effect of MR antagonism as a strategy to prevent diet-induced DD in women.
Keywords: mineralocorticoid antagonism, low-dose spironolactone, aldosterone, high-fat diet, high-fructose diet, oxidative stress, inflammation, cardiac hypertrophy, myocardial compliance
cardiovascular disease (CVD) is the leading cause of morbidity and mortality in obese, insulin-resistant, type 2 diabetic individuals (29, 40, 61). Importantly, obese diabetic women manifest clinically significant CVD not only more frequently but also more severely than diabetic men (29, 40). This contrasts with the fact that lean nondiabetic premenopausal women exhibit lower incidences of CVD relative to their male counterparts. Diastolic dysfunction (DD), which is often characterized as an asymptomatic abnormality, is one of the early manifestations of diabetic CVD and is increasingly prevalent in women (45, 56, 57), yet is a strong predictor of future CVD events (13, 32, 58). DD is characterized by impaired diastolic relaxation (70) and is associated with insulin resistance, left ventricular (LV) hypertrophy (LVH), inflammation, and cardiac remodeling (54, 57). Moreover, LVH is reported to be more substantial in obese women than in obese men (9). Evidence suggests that both obese women (43–45) and obese diabetic women (9, 51, 53) are at greater risk for the development of DD than their male counterparts (54). Thus, in women, DD, and the eventual progression to heart failure, are major healthcare concerns associated with the ongoing epidemics of obesity, metabolic syndrome, and diabetes, especially in women (12, 13, 17).
Specific prevention or treatment strategies targeting the prevention of DD in the obese diabetic population have yet to emerge; however, given the central role of inappropriate systemic and tissue renin-angiotensin-aldosterone system (RAAS) activation in the pathophysiology of obesity and diabetic cardiomyopathies, antagonists of the RAAS seem promising (58). Emerging evidence indicates that activation of mineralocorticoid receptors (MRs) may play a pivotal role in the development of DD. Cardiac MR activation promotes myocardial inflammation, oxidative stress, fibrosis, and LVH, factors often associated with DD and contributing to functional and structural impairments in the heart (14, 41, 58, 66). We previously demonstrated improved diastolic function with a very low (subpressor) dose of spironolactone (Sp) independent of blood pressure reduction in hypertensive rats (21). In this regard, much interest has focused recently on the preventative utility of adding MR antagonists (MRAs) to target DD given the benefit of MR blockade in treating systolic heart failure (27, 28, 47). Initially, this notion was supported by results from two small randomized clinical trials in obese patients showing an improvement in DD with the MRA Sp (27, 28); however, the larger more recent Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial, conducted in a broader patient population, was negative (47). Thus, given these mixed results, it is likely that some but not all patients with DD would benefit from adjunctive MRA therapy. In this regard, a possible mechanism linking CVD with insulin resistance and obesity in women is the activation of the MR. Indeed, elevations in aldosterone levels have been documented in obese women and are correlated inversely with insulin sensitivity indexes (16). Furthermore, MR activation in the vasculature and heart results in increased inflammation, oxidative stress, and fibrosis (3, 34). Emerging evidence suggests that overnutrition-driven dysregulation of the mammalian target of rapamycin (mTOR)/70-kDa ribosomal S6 kinase (S6K1) signaling pathway contributes to the adverse effects of obesity on cardiac function (68). Moreover, a recent study (6) has implicated increased mTOR/S6K signaling with maladaptive immune responses favoring the polarization of proinflammatory M1 macrophages. Nonetheless, there are no data linking Western diet (WD)-induced cardiac dysfunction to increases in myocardial MR activation, mTOR/S6K signaling, and inflammation. We recently reported that female C57BL6J mice fed a WD high in fat and unrefined sugars, including high-fructose corn syrup and sucrose, become overweight and insulin resistant and underwent a more rapid onset of DD compared with their male counterparts (33). Furthermore, we found higher levels of aldosterone in female mice compared with males (33). Based on these observations, we hypothesized that MR blockade would prevent WD-induced DD, myocardial oxidative stress, macrophage-related inflammation, and LVH in female mice. To test this hypothesis, we administered a low dose of the MRA Sp to female C57BL6J mice fed a WD high in fat and refined carbohydrates. Here, we report that MRs play an important role in the development of WD-induced DD and the contributing cardiac abnormalities, including oxidative stress, dysfunctional immune and inflammatory responses, fibrosis, and LVH. Importantly, these WD-induced abnormalities developed independently of increases in blood pressure and were normalized with a subpressor dose of Sp.
MATERIALS AND METHODS
Animal Models
Three-week-old C57BL6J female mice were purchased from Jackson Laboratories (Bar Harbor, ME) and cared for in accordance with National Institutes of Health guidelines. All procedures were approved in advance by the Institutional Animal Care and Use Committee of the University of Missouri and the Harry S. Truman Memorial Veterans' Hospital. A total of 29 mice were used in the performance of the experiments described here, and, of those, 7 mice were dedicated to ex vivo perfused heart preparations. Beginning at 4 wk of age, mice were randomly distributed into three groups, including 1) mice fed a control diet (CD; Test Diet 58Y2, Purina Diets, Richmond, IN) and implanted with a placebo pellet, 2) mice fed a WD consisting of high fat (46%) and high carbohydrate as sucrose (17.5%) and high fructose corn syrup (17.5%) (Test Diet 58Y1) and implanted with a placebo pellet (WD), and 3) mice fed a WD and implanted with a Sp pellet (WDSp). The treatment period lasted for 4 mo, ending when mice were 20 wk of age. Mice were provided water ad libitum while housed in pairs under a 12-h/day illumination regimen. Sp was administered subcutaneously at a dose of 1 mg·kg−1·day−1 via a slow-release pellet (Innovative Research of America, Sarasota, FL) implanted in the scapular region on the back. The rationale for choosing this dose of Sp was based on previous evidence that this dose does not reduce blood pressure (21) or elicit antiandrogenic and progestogenic effects (8). Indeed, 1 mg·kg−1·day−1 Sp has been shown to inhibit only 35% of in vivo aldosterone binding to MRs (8). Untreated mice received a placebo pellet without Sp. After the 4-mo period, 20-wk-old mice were euthanized while under isoflurane anesthesia (2–4% in 100% O2), and tissue and blood samples were collected and processed for later analysis.
Blood Pressure
At the end of the 4-mo feeding period and immediately before being euthanized, mice were anesthetized with isoflurane (1.75% isoflurane in 100% O2). The right carotid artery was isolated, and a high-fidelity 1.2-Fr mouse pressure catheter (Transonic) was inserted and advanced to a position proximate to the aortic arch. After a brief acclimation period and when blood pressures were stable, average systolic blood pressure, diastolic blood pressure, and mean arterial pressure were determined using a data-acquisition system (Scisense, London, ON, Canada) as previously described (11).
Cardiac Function
Cardiac function was assessed noninvasively by echocardiography and high-resolution cine MRI as previously described (4, 33). Briefly, echocardiography was performed using a GE Vividi system with a 10S-RS 11.5-MHz phased array pediatric probe while MRI scans were performed with a Bruker AVANCE III BioSpec 7 T horizontal bore MRI (Bruker, Billerica, MA) equipped with a four-channel phased array mouse cardiac radiofrequency coil. These procedures were performed on isoflurane-anesthetized mice (1.75% in 100% O2 stream).
Sarcomere Length Measurements in Isolated, Perfused, Pressurized Heart Preparations
Hearts were excised, cannulated via both the aorta and LV, loaded with 10 μM di-8-ANEPPS for ∼5 min, and perfused with aortic perfusion solution containing 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM d-glucose, 10 mM HEPES, 100 μM EGTA, and 6% dextran (pH 7.4 with NaOH). The heart was positioned so that the LV free wall rested gently against the coverslip for two-photon imaging of di-8-ANEPPS fluorescence (inverted Leica SP5/MP, ×40 oil objective, HCX PL APA, numerical aperture: 1.25, excitation at 840 nm and emission at 500–700 nm, 1,024 × 1,024 pixels at 0.19 μm/pixel). The pressure of the LV cannula was adjusted using a calibrated solution column, with the aortic cannula positioned 1 mmHg higher than the LV cannula for each pressure monitored. Measurements were performed at 25°C. Sarcomere length was determined by monitoring the distance of 10–61 consecutive sarcomeres and dividing by the number of sarcomere divisions. Manual analysis (performed by individual blinded to experimental group and pressure) was confirmed by fast Fourier transform analysis of fluorescence profiles using ImageJ software.
Protein Isolation and Quantitation
Briefly, LV free wall proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After 1 h of blockade in 5% BSA, membranes were incubated overnight at 4°C and probed with antibodies to either S6K1 or phospho-Thr389 S6K1 (Cell Signaling Technology, Danvers, MA) diluted 1:1,000 in 5% BSA. After being rinsed, membranes were probed with anti-mouse secondary antibody diluted 1:3,000 (Jackson ImmunoResearch Laboratories) in 5% BSA for 1 h at room temperature. After being washed, SuperSignal West Femto was used to resolve a single band at 70 kDa.
Histological Staining and Immunohistochemistry
A segment of the LV free wall was fixed in 3% paraformaldehyde, dehydrated in ethanol, paraffin embedded, and transversely sectioned in 5-μm slices. Four sections each for 4–5 mice/group were examined. To evaluate cardiac interstitial fibrosis, sections were stained with picrosirius red and Verhoeff-von Gieson stain for the determination of collagen as previously described (4). Staining was quantified as the average percent staining in 5 images/mouse with the aid of the thresholding function in MetaVue software. To detect the presence of reactive nitrogen species in the myocardium, immunostaining for 3-nitrotyrosine (3-NT; AB5411, 1:150 dilution, Millipore, Billerica, MA) was performed as previously described (4). To evaluate cardiomyocyte hypertrophy staining with wheat germ agglutinin was performed on sections obtained from the LV free wall. Two ×40 images from each section were randomly captured using a biphoton confocal microscope. On each image, the average cross-sectional area of 10 cardiomyocytes was measured using MetaVue.
Ultrastructure Analysis With Transmission Electron Microscopy
Details of myocardial tissue preparation, sectioning, staining, and viewing have been previously described (4). A JOEL 1400-EX transmission electron microscope (Joel, Tokyo, Japan) was used to review three fields randomly chosen per mouse to obtain three ×2,000 images/heart.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated using the TRIzol reagent (Sigma) method. The yield of RNA was quantified using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). First-strand cDNA synthesis was performed using 1 μg total RNA with oligo dT (1 μg), 5× reaction buffer, MgCl2, dNTP mix, RNAse inhibitor, and Improm II reverse transcriptase as per the Improm II reverse transcription kit (Promega, Madison, WI). After the first-strand synthesis, real-time PCR was performed using 8 μl cDNA, 10 μl SYBR green PCR master mix (Bio-Rad Laboratories), and forward and reverse primers (10 pM/μl, Integrated DNA Technologies, San Diego, CA) using a real-time PCR system (CFX96, Bio-Rad Laboratories). The primer sequences for M1 macrophage markers included monocyte chemoattractant protein (MCP)-1, forward 5′-GTCTCAGCCAGATGCAGTTAAT-3′ and reverse 5′-CTGCTGGTGATTCTCTTGTAGTT-3′; CD11C, forward: 5′-ATGAAGAACCTCCGGGAAAT3′ and reverse 5′-GCTTAGATCATGGCGTGGTT-3′; and CD86, forward 5′-GACCGTTGTGTGTGTTCTGG-3′ and reverse 5′-GATGAGCAGCATCACAAGGA-3′. Primer sequences for a marker of total macrophage number, CD11b, were forward 5′- CCAAGACGATCTCAGCATCA-3′ and reverse 5′-TTCTGGCTTGCTGAATCCTT-3′. Primer sequences for the M2 macrophage marker CD206 were forward 5′- CAAGGAAGGTTGGCATTTGT-3′ and reverse 5′-CCTTTCAGTCCTTTGCAAGC-3′. Primer sequences for GAPDH were forward 5′-GGAGAAACCTGCCAAGTATGA-3′ and reverse 5′- TCCTCAGTGTAGCCCAAGA-3′. The specificity of each of the primers sets was analyzed by running a melting curve. PCR cycling conditions used were 5 min at 95°C for the initial denaturation and 40 cycles of 30 s at 95°C, 30 s at 58°C, and 30 s at 72°C. Each real-time PCR was carried out using three individual samples, each in triplicate. Results were normalized against the housekeeping gene GAPDH.
Statistical Analysis
Results are reported as means ± SE. Statistical analysis was by one-way ANOVA and post hoc t-tests (Bonferroni) to examine differences in outcomes between treatment groups (Sigma Plot 12.0, Systat Software). All differences were considered significant when P < 0.05.
RESULTS
Body and Fat Pad Weights
Body weights normalized to tibia length of 20-wk-old WD and WDSp mice were similarly heavier compared with those of their respective lean CD counterparts (P < 0.05; Fig. 1A). Percent body weight gain during the 4-mo study period was 72 ± 8%, 122 ± 9%, and 105 ± 10% for CD, WD, and WDSp mice, respectively (P < 0.0001 for CD vs. WD), and Sp administration had no effect on body weight in WD-fed mice (Table 1). Perireproductive fat pad mass was approximately fourfold higher in WD versus CD mice (P < 0.001), and this increase was not altered by Sp (Fig. 1B). Similar trends were observed in retroperitoneal fat pad mass (Table 1).
Fig. 1.
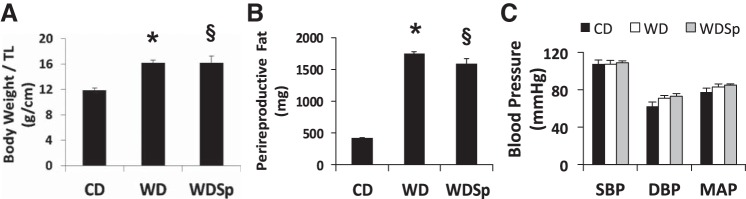
Western diet (WD)-induced increases in body body weight normalized to tibia length (TL; A) and perireproductive fat pad weight (B) were not prevented by spironolactone (Sp). Mice were distributed into the following three groups: 1) mice fed a control diet (CD) and implanted with a placebo pellet, 2) mice fed a WD and implanted with a placebo pellet (WD), and 3) mice fed a WD and implanted with a Sp pellet (WDSp). n = 7 CD mice, 8 WD mice, and 7 WDSp mice. C: blood pressures were not significantly affected by WD or Sp therapy. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure. n = 6 CD mice, 6 WD mice, and 3 WDSp mice. *P < 0.05 for WD compared with CD; §P < 0.05 for WDSp compared with CD.
Table 1.
Body weights of CD, WD, and WDSp mice
| P Value | CD Group | WD Group |
WDSp Group |
|
|---|---|---|---|---|
| Sample size | 7 | 8 | 7 | |
| Pretreatment body weight, g | 0.09 | 12.0 ± 0.8 | 12.7 ± 0.4 | 14.0 ± 0.6 |
| Posttreatment body weight, g | 0.001 | 20.3 ± 0.5 | 28.0 ± 0.8* | 28.7 ± 2.0† |
| Change in body weight, g | 0.001 | 8.3 ± 0.4 | 15.3 ± 0.8* | 14.7 ± 1.7† |
| Tibia length, cm | 0.020 | 1.70 ± 0.00 | 1.73 ± 0.02 | 1.76 ± 0.02† |
| Retroperitoneal fat, mg | 0.001 | 76 ± 13 | 413 ± 38* | 353 ± 78† |
| Heart weight, mg | 0.007 | 81.8 ± 1.4 | 94.2 ± 2.1* | 92.7 ± 2.2† |
Values are means ± SE. Body weights of C57Bl6/J mice fed a Western diet (WD) high in fat and sugars for 4 mo compared with mice fed a control diet (CD) are shown. Although mice on the CD gained weight with age, WD caused an additional increment of body weight gain. Administration of spironolactone (Sp) had no effect on body weight under either dietary regime. WDSp, WD-fed mice that received Sp.
P < 0.05 for CD vs. WD groups;
P < 0.05 for CD vs. WDSp groups (post hoc comparisons).
Blood Pressure
At the end of the treatment period, systolic blood pressure, diastolic blood pressure, and mean arterial pressure were similar between all groups (Fig. 1C).
Sp Improves WD-Induced DD
Consistent with our previous report (33), WD-fed mice exhibited abnormal echocardiography-derived diastolic function parameters versus CD-fed mice (Fig. 2, A–C, and Table 2). Specifically, WD-fed mice exhibited impaired cardiac function, as indicated by an increase in the myocardial performance index, a heart rate- and load-independent measure of global cardiac function. The myocardial performance index increased due mostly to abnormal diastolic function, as indicated by increased isovolumic relaxation time and abnormal diastolic septal annular wall motion (<E′/A′). Cine MRI-generated diastolic parameters demonstrated an increase in relaxation time and reduced initial filling rate in WD-fed mice compared with CD-fed mice (Fig. 2, D–F). The abnormal diastolic parameters were largely prevented in WDSp mice.
Fig. 2.
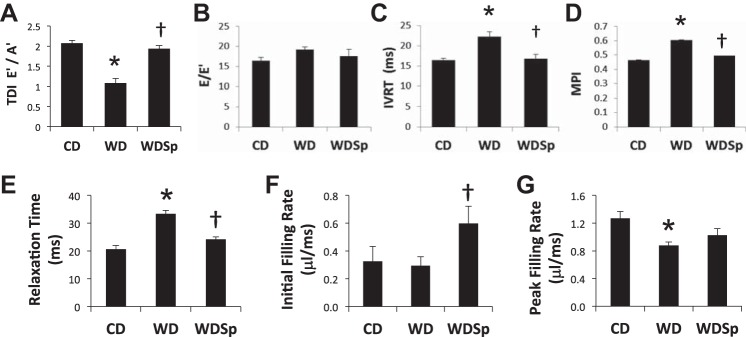
WD caused cardiac diastolic dysfunction that was prevented by Sp therapy (see text for details). Echocardiographic parameters, including tissue Doppler imaging-derived E′-to-A′ ratio (TDI E′/A′; A), E-to-E′ ratio (E/E′; B), isovolumic relaxation time (IVRT; C), and myocardial performance index (MPI; D), were abnormal in WD-fed mice, and these abnormalities were prevented by Sp. n = 7 CD mice, 6 WD mice, and 5 WDSp mice. Cardiac MRI parameters included relaxation time (E), initial filling rate (F), and peak filling rate (G). n = 8 CD mice, 8 WD mice, and 7 WDSp mice. *P < 0.05 for WD compared with CD; †P < 0.05 for WDSp compared with WD.
Table 2.
Summary of cardiac pulse wave and tissue Doppler imaging measurements on 20-wk-old mice fed a CD or WD in the absence or presence of Sp
| Parameter | P Value | CD Group | WD Group | WDSp Group |
|---|---|---|---|---|
| Sample size | 7 | 6 | 5 | |
| Heart rate, beats/min | 0.317 | 419 ± 13 | 385 ± 25 | 432 ± 28 |
| E, m/s | 0.043 | 0.68 ± 0.02 | 0.60 ± 0.04 | 0.74 ± 0.04† |
| A, m/s | 0.129 | 0.47 ± 0.01 | 0.42 ± 0.02 | 0.47 ± 0.02 |
| E/A | 0.518 | 1.41 ± 0.08 | 1.42 ± 0.09 | 1.55 ± 0.06 |
| E′, m/s | <0.001 | 0.041 ± 0.001 | 0.032 ± 0.002* | 0.042 ± 0.002† |
| A′, m/s | 0.002 | 0.020 ± 0.000 | 0.030 ± 0.003* | 0.022 ± 0.002† |
| E/E′ | 0.246 | 16.4 ± 0.8 | 19.3 ± 0.6 | 17.6 ± 1.1 |
| E′/A′ | <0.001 | 2.07 ± 0.07 | 1.08 ± 0.08* | 1.93 ± 0.07† |
| Isovolumic relaxation time, ms | 0.001 | 16.4 ± 0.5 | 22.3 ± 1.1* | 16.8 ± 1.5† |
| Isovolumic contraction time, ms | 0.413 | 9.1 ± 0.9 | 11.2 ± 1.2 | 10.4 ± 1.2 |
| ET, ms | 0.973 | 56.1 ± 1.6 | 55.8 ± 3.1 | 55.2 ± 4.0 |
| Myocardial performance index | 0.011 | 0.46 ± 0.03 | 0.60 ± 0.03* | 0.49 ± 0.04§ |
Values are means ± SE. E, velocity of early mitral flow; A, velocity of late mitral flow; E′, early peak velocity of septal annulus; A′, late peak velocity of septal annulus; E/E′, index of left atrial filling pressure; ET, ejection time.
P < 0.05 for CD vs. WD groups;
P < 0.05 for WD vs. WDSp groups;
P < 0.08 for WD vs. WDSp groups.
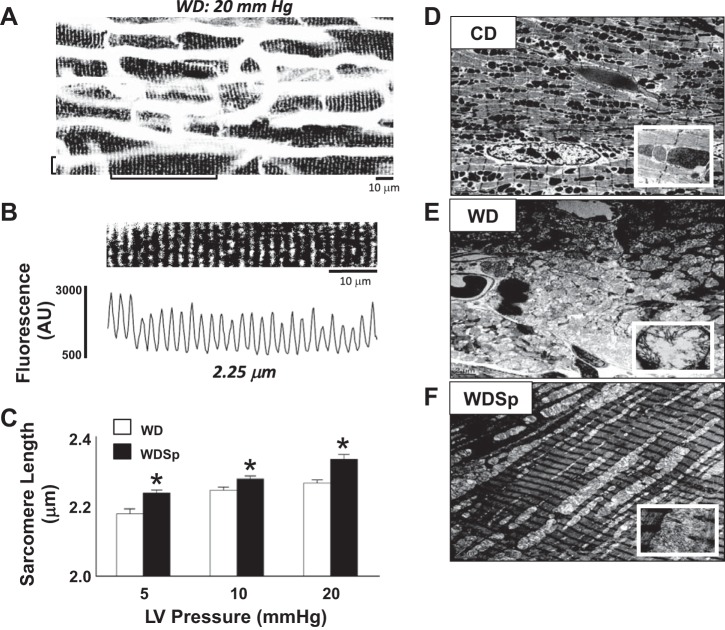
Sp Improves Sarcomere Lengthening of Cardiac Myocytes
Imaging of subepicardial cardiac myocytes of ex vivo perfused hearts subjected to Ca2+-free cardioplegia demonstrated improved cardiac myocyte sarcomere dynamics with Sp administration. In response to physiological pressures of 5, 10, and 20 mmHg, sarcomere lengths of WDSp mice were significantly longer than those of WD mice, consistent with improved ventricular compliance (Fig. 3, A–C). Ultrastructural analysis revealed that WD-induced sarcomere disorganization, interstitial matrix expansion, cellular infiltration, and megamitochondria not seen with CD feeding (Fig. 3, D–F). Representative transmission electron microscopy images of mouse hearts showed that Sp treatment prevented these abnormalities in mitochondrial architecture. WD feeding also increased mitochondrial electron density, and this abnormality was not corrected by Sp.
Fig. 3.
Sp improved cardiac myocyte sarcomere lengthening in response to pressure in perfused hearts. A: representative confocal fluorescence image of left ventricular (LV) subepicardial cardiac myocytes of a WD-fed perfused mouse heart stained with the membrane dye di-8-ANEPPS and pressurized to 20 mmHg. B: expanded image (top) and fluorescence profile (bottom) from the region marked with brackets in A. t-tubule striations were used to determine cellular sarcomere length. AU, arbitrary units. C: comparison of sarcomere lengths in the heart between WD and WDSp mice revealed a significant increase in sarcomere length of hearts held at 5, 10, and 20 mmHg LV pressure, consistent with a reduction in ventricular stiffness with Sp treatment. n = 26–67 individual cardiac cells from 3 (WD) and 4 (WDSp) hearts. *P < 0.05 for WDSp compared with WD. D–F: representative transmission electron microscopy images of mouse hearts showing that WD feeding (E) induced sarcomere disorganization, interstitial matrix expansion, cellular infiltration, and megamitochondria not seen with CD feeding (D). Sp-treated mouse hearts (F) showed a normalization of sarcomere architecture with an absence of intermyofibillar matrix expansion and cellular infiltration and improvement in mitochondrial architecture. WD feeding also increased mitochondrial electron density, which was not corrected by Sp. Insets: magnification of representative mitochondria. Scale bars = 2 μm.
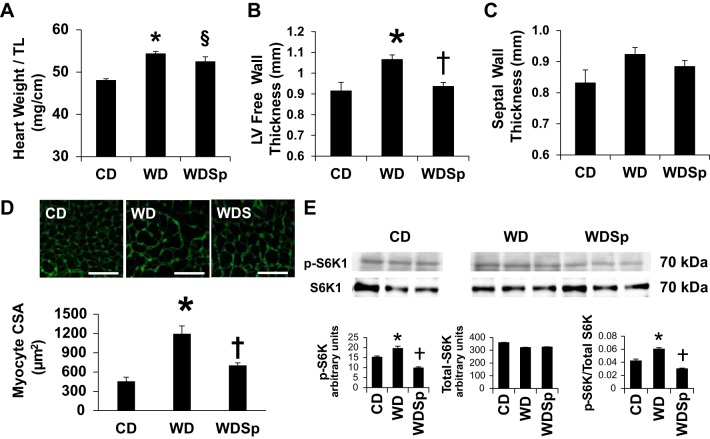
Sp Ameliorates WD-Induced Myocardial Remodeling
LV and cardiomyocyte hypertrophy.
Heart weight (Table 1) normalized to tibial length (Fig. 4C) showed that hearts were similarly heavier in untreated and Sp-treated WD-fed mice compared with normal sized hearts from CD-fed mice. In vivo assessment of LVH by cine MRI indicated thickening of the LV free wall in WD mice compared with CD and WDSp mice (Fig. 4B). The septal wall of WD-fed mice tended to be thicker but did not reach statistical significance (P = 0.07; Fig. 4C). Cardiomyocytes from WD-fed mice were larger in cross-sectional area, suggesting hypertrophy at the level of the cardiomyocyte, and Sp ameliorated this effect (P < 0.001 for CD vs. WD; Fig. 4D). No differences in cardiomyocyte cross-sectional area were detected between CD and WDSp groups (P = 0.245).
Fig. 4.
WD feeding caused LV hypertrophy (LVH) with increased phosphorylation of S6 kinase (S6K), which was ameliorated by Sp. A and B: cardiac MRI measurements of LV free wall thickness (A) and septal wall thickness (B) indicating LVH with WD. C: there was a significant increase in the normalized heart weight in WD-fed mice that was not effected by Sp therapy. D: analysis of cardiomyocyte size showed a significant increase in the cross-sectional area of cardiomyocytes from WD mice, which was prevented by treatment with Sp. n = 5 CD mice, 6 WD mice, and 5 WDSp mice. Scale bars = 50 μm. E: Western blot analysis for phosphorylated S6K (p-S6K) and total S6K (S6K) showed a significant increase in the ratio of p-S6K to S6K with WD feeding, which was prevented by mineralocorticoid receptor (MR) blockade with Sp. All bands are from same blot. Three additional samples of a separate control group of CD mice treated with Sp were run in between the CD and WD groups but were removed for clarity. n = 3 for each group. *P < 0.05 for WD compared with CD; †P < 0.05 for WDSp compared with WD; §P < 0.05 for WDSp compared with CD.
S6K1 signaling.
Activation of myocardial S6K contributes to cardiac hypertrophy (18, 64). To evaluate S6K activation, we determined protein expression of phospho-Thr389 S6K1 and total S6K1. Phospho-Thr389 S6K1 expression was elevated in WD compared with CD mice (P < 0.05), and this increase was prevented by Sp (P < 0.05 for WD vs. WDSp). No differences in total S6K1 expression were observed among groups (P > 0.05). Compared with CD mice, the increase in phospho-Thr389 S6K1 relative to total S6K1 observed in WD mice (P < 0.05) was prevented by Sp (P < 0.05 for WD vs. WDSp; Fig. 4E).
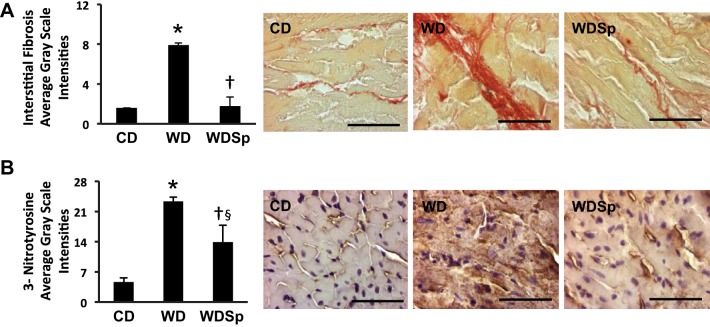
LV fibrosis.
Accumulation of myocardial interstitial collagen, as assessed by picrosirius red staining, was markedly increased with WD feeding (P < 0.001 for WD vs. CD; Fig. 5A), and this was prevented with Sp (P < 0.01 for WDSp vs. WD).
Fig. 5.
WD feeding increased interstitial fibrosis with collagen subtype switching and oxidative stress that was prevented by treatment with Sp. A: interstitial fibrosis was markedly increased with WD feeding but completely abrogated with Sp treatment (left). Micrographs (right) are representative images from CD, WD, and WDSp hearts. n = 4 CD mice, 5 WD mice, and 5 WDSp mice. B: oxidative stress in the heart was significantly increased by WD feeding with significant increases in 3-nitrotyrosine (3-NT) staining in WD heart that was improved by Sp therapy (left). Micrographs (right) show representative 3-NT staining in hearts from each group. n = 5 CD mice, 6 WD mice, and 6 WDSp mice. *P < 0.05 for WD compared with CD; †P < 0.05 for WDSp compared with WD; §P < 0.05 for WDSp compared with CD. Scale bars = 50 μm.
Sp Modulates WD-Induced Oxidative Stress
We previously reported that myocardial oxidative stress precedes or is associated with DD in models of obesity/metabolic cardiomyopathy (2, 4, 10, 69). Analysis of oxidant stress by 3-NT in the myocardium revealed an increase with WD consumption (P < 0.001 for CD vs. WD; Fig. 5C), and Sp significantly lowered 3-NT staining intensity (P < 0.001 for WDSp vs. WD) but not quite to the level in CD mice (P < 0.01 for WDSp vs. CD).
Sp Promotes an Anti-Inflammatory Shift in Immune Cell Markers in the Heart
WD-fed mice showed increased myocardial mRNA expression of M1 markers MCP-1 and CD11c, which was suppressed by Sp (Fig. 6A). We measured another M1 marker, CD86, and although trends among groups were similar to those of MCP-1 and CD11c, no differences were found among groups by two-way ANOVA (data not shown). CD11b, a marker of total macrophage number, was elevated in the myocardium of WD mice, and this was prevented by Sp. Although CD206 expression was not different in WD hearts compared with CD hearts, Sp induced marked expression of CD206. Normalization of the expression of CD206 to MCP-1 or CD11c indicated that Sp induced a relative shift favoring M2 polarization (Fig. 6B). Normalization of myocardial expression of CD206 to CD11b illustrated that the proportion of M2 macrophages to the total number of macrophages increased only in the hearts of WD mice that received Sp.
Fig. 6.
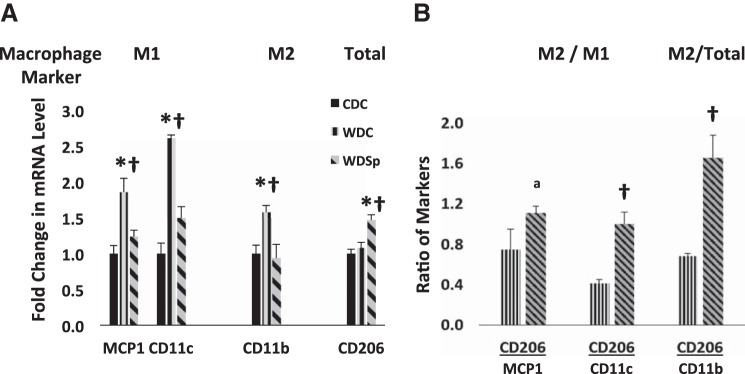
Pro-inflammatory (M1) macrophage marker expression was increased in the myocardium by WD feeding with correction to an M2 anti-inflammatory state by Sp therapy. A: RT-PCR showed significant increases in the levels of M1 macrophage markers MCP-1 and CD11c as well as the (total) macrophage marker CD11b in hearts of WD mice, which were prevented by Sp treatment. The M2 macrophage marker CD206 was significantly increased in hearts of WD mice treated with Sp. B: MR blockade with Sp caused a significant shift to a more anti-inflammatory M2 macrophage population with an increase in expression of CD206 relative to MCP-1, CD11c, and CD11b. n = 3–6 for each group. *P < 0.05 for WD compared with CD; †P < 0.05 for WDSp compared with WD.
DISCUSSION
Lean premenopausal women are generally at lower risk for development of heart and vascular disease compared with men, and this may be due in part to the beneficial cardiovascular effects of sex hormones (37). In contrast, young obese and/or diabetic women are inordinately adversely affected and therefore are at greater risk for the development of heart disease (42–45). The mechanisms for the loss of cardioprotection with these metabolic disorders remain to be elucidated. In this regard, a possible mechanism linking CVD with insulin resistance, obesity, and diabetes in women is MR activation in cardiomyocytes and cardiac fibroblasts. We previously demonstrated that treatment with a subpressor dose of Sp reversed DD in lean renin-overexpressing hypertensive male rats (21) as well as in male Zucker obese rats. Whether Sp could prevent DD in a clinically relevant female model of diet-induced overnutrition/obesity is unknown. To test this hypothesis, we used a recently developed model of WD-induced DD where female mice exhibited accelerated development of DD compared with age-matched male mice (33). Indeed, DD was first detected in WD-fed female mice after 8 wk of WD but not in male mice (33). The main findings of the present study are that MR antagonism with a subpressor dose of Sp prevented the development of DD in WD-fed female mice as well as its associated abnormalities known to contribute to DD, including increased myocardial oxidative stress cardiac fibrosis and LVH. To our knowledge, this is the first study showing that MR antagonism prevents the development of DD in a clinically relevant model of WD-induced DD in the female sex.
DD is often associated with comorbid conditions such as obesity and hypertension. Thus, the efficacy of Sp in preventing DD could have been due to positive effects on these other conditions. Since blood pressure did not increase in female mice in response to 4 mo of WD and Sp did not affect blood pressure, we can conclude that efficacy was unrelated to blood pressure changes. Furthermore, Sp also prevented DD in the absence of changes in body weight or visceral fat weight. In the setting of overweight/obesity, DD is more likely to result from some combination of myocardial inflammation, increased oxidative stress, and enhanced fibrosis and stiffness (10, 60).
The rationale for use of such a low dose of Sp is twofold. First, in addition to MRs, Sp binds to androgen, progesterone, and glucocorticoid receptors; however, binding to non-MRs is likely to require much higher doses of Sp to induce antiandrogenic or progestogenic actions (8). Thus, evidence supports that the use of 1 mg·kg−1·day−1 Sp ensures that the primary site of action of Sp occurs at MRs rather than non-MRs. Second, the addition of low-dose MRA to standard therapy reduces morbidity and mortality among heart failure patients (48, 49). Thus, it could be reasoned that the addition of low-dose MRA could prevent further progression to heart failure in obese females with DD regardless of diabetic status and with a minimal risk of side effects.
We examined the mechanisms underlying the efficacy of MR blockade in preventing WD-induced DD and the associated cardiac structural remodeling. Cardiac remodeling is generally viewed as a deleterious outcome associated with hypertrophy, fibrosis, and DD. There was a blood pressure-independent cardiac hypertrophic response to WD, as indicated by increased heart weight, which was due, in part, to cardiomyocyte hypertrophy. The increases in LV free wall and septal wall thicknesses indicate concentric remodeling, a condition commonly observed in both hypertensive and nonhypertensive obesity (35, 42, 45). Although Sp had no significant effect on whole heart weight, evidence indicated that Sp inhibited the development of cardiomyocyte hypertrophy and LV free wall thickening. LVH in WD-fed mice was accompanied by abnormalities in sarcomere structure and function as well as mitochondrial structure, which were largely prevented by Sp. The mTOR/S6K1 pathway has been implicated in cardiac hypertrophy. mTOR activates S6K1, and S6K1 phosphorylates 40S-ribosomal S6 protein and plays a key role in biogenesis of ribosomes, translation, and hypertrophy (23, 26). Activation of cardiac mTOR/S6K1 occurs in heart failure patients with preserved ejection fraction and in mice with DD and LVH (18). Activation of the mTOR/S6K1 growth pathway also contributes cardiac hypertrophy induced by growth factors, insulin resistance/hyperinsulinemia, and excessive nutrient intake (26, 64). Here, we observed suppression of WD-induced activation of S6K1 with Sp concomitant with decreased cardiac hypertrophy.
Cardiac fibrosis is one of the major determinants of impairment of the passive properties of diastolic relaxation. We observed significant LV accumulation of interstitial collagen, which was suppressed by Sp. Collagen deposited in the extracellular compartment largely consists of collagen types I and III. Collagen type III is the more compliant isoform, whereas the type I isoform is stiffer. Chamber stiffness increases when there is a shift in the ratio of these collagen subtypes from type III to type I (7, 39). Therefore, improved diastolic function with MRAs could be ascribed, in part, to preventing accumulation and stiffening of interstitial collagen. The amelioration of WD-induced interstitial collagen accumulation with Sp could also explain the observed improvement in ex vivo sarcomere lengthening and diastolic function. The association between reductions in S6K activation, interstitial fibrosis, improved sarcomere lengthening, and diastolic function suggests that Sp may inhibit the secretion of collagens into the interstitial space by fibroblasts/cardiomyocytes by suppressing nutrient-driven activation of S6K (50).
Excessive signaling through the MR induces oxidative stress (34, 38), which contributes to cardiac remodeling via activation of signaling cascades driving fibrosis, hypertrophy, and apoptosis (65). We previously reported associations between DD and myocardial oxidative stress in lean and obese rodent models of insulin resistance (4, 10, 11, 21, 69). Thus, evidence supports oxidative stress as an important feature linking metabolic dysregulation to cardiac dysfunction, especially under conditions of overnutrition and diabetes (1, 59, 67, 69). Recent preclinical evidence indicates that low-dose Sp is beneficial in reducing myocardial oxidative stress and DD in lean insulin-resistant male transgenic rats (21). Male mice fed the same WD used in this study exhibit myocardial oxidative stress (4, 24). In this study, the decrease in the magnitude of oxidative stress with Sp suggests that Sp prevents DD, in part, by reducing the level of oxidative stress in the myocardium. Therefore, attenuation of diet-induced myocardial oxidative stress by Sp is likely to be a key factor contributing to the prevention of cardiac hypertrophy and fibrosis. We and others have reported marked increases in the quantity and distribution of the myocardial 3-NT residue in rodent models of cardiomyopathy involving myocardial oxidative stress (21, 24, 36, 63). An ultrastructural localization study (36) of the myocardium indicated increases in 3-NT-labeled immunogold particles in myofibrillar and mitochondrial spaces under conditions of oxidative stress. Indeed, we recently reported disorganization and thinning of sarcomeres as well as mitochondrial structural alterations in association with increased 3-NT staining in WD-fed male mice (24). Nonetheless, a more robust analysis of oxidative stress using multiple markers is necessary to further delineate this issue and warrants further investigation. Relying solely on 3-NT staining as an end point for the measurement of oxidative stress is a limitation of this study. Finally, in humans, increases in myocardial 3-NT has been reported in pathophysiological conditions, such as heart failure, and is considered a myocardial oxidative stress marker (30).
Obesity and type 2 diabetes mellitus are associated with a state of chronic subacute systemic and tissue inflammation (25, 52). Emerging evidence suggests that low-grade inflammation, especially in adipose tissue, may act as a proximate trigger as well as an ultimate modulator of the severity of comorbid conditions associated with obesity, such as insulin resistance, oxidative stress, diabetes, and CVD (52). In this context, MR activation has been implicated in mediating inflammation and immune cell recruitment in multiple tissues, including the heart and vasculature, in rodent models of obesity, diabetes, and hypertension (5, 15, 19), and MR blockade ameliorates inflammation associated with obesity (20, 22, 55). Inflammation in obesity is associated with an increase in M1 proinflammatory macrophages and a decrease in M2 anti-inflammatory macrophages, thereby shifting macrophages to a proinflammatory state (31). We recently reported evidence suggesting such a proinflammatory shift in the hearts of WD-fed male mice (24). Cardiac tissue macrophages are resident in the perivascular area and in direct contact with cardiomyocytes and may even have some level of contact with endothelial cells, suggesting “direct” modulation of cardiomyocyte and endothelial cell morphology and function (46). CD206 (mannose macrophage marker) is a well-accepted marker for identifying M2 macrophages (62). Here, we observed a relative increase in myocardial expression of the anti-inflammatory macrophage marker CD206 in WD mice that received Sp, indicating that Sp promotes a relative shift favoring M2 polarization. Such an increase in the myocardial anti-inflammatory immune cell population is likely to play a role in reducing overnutrition-associated oxidative stress, fibrosis, and DD.
In summary, we demonstrated that a subpressor dose of the MRA Sp prevents WD-associated DD. Moreover, MR blockade involves attenuated cardiac oxidative stress along with reductions in cardiac remodeling and inflammation. Furthermore, our data suggest an anti-inflammatory shift of cardiac immune cell markers after Sp administration, thereby warranting further study of MR-dependent immune modulation as a novel mechanism by which MRs contribute to obesity-associated CVD. Thus, MR inhibition prevents DD induced by inflammation and oxidative stress associated with obesity. However, MRAs may not be beneficial in preventing or treating DD caused by etiologies in which the inciting factors are different. Since MR signaling plays an integral role in the development of DD in obesity, further study of low-dose MRAs to prevent or treat DD specifically in patients with insulin resistance and obesity is warranted.
GRANTS
This work was supported with resources and facilities at the Harry S. Truman Memorial Veterans Hospital (Columbia, MO), including the Biomolecular Imaging Center (BIC for MRI) and Small Animal Ultrasound Imaging Center (SAUIC for Echocardiography).
This work was also supported by American Heart Association Postdoctoral Fellowship 13POST16250010 (to B. Bostick), National Institutes of Health Grants R01-HL073101 and RO1-HL107910 (to J. R. Sowers) and K01-AG-041208 (to T. L. Domeier), Department of Veterans Affairs Biomedical Laboratory Research and Development Grants CDA-2 IK2 BX002030 (to S. B. Bender) and 0018 (to J. R. Sowers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.B., J.H., V.G.D., G.J., T.L.D., M.D.L., A.R.A., R.N., S.B.B., M.G., M.R.H., L.M., C.M.A., and J.R.S. interpreted results of experiments; B.B., V.G.D., G.J., M.D.L., R.N., S.B.B., M.G., L.M., C.M.A., and J.R.S. prepared figures; B.B., V.G.D., G.J., M.D.L., R.N., S.B.B., C.M.A., and J.R.S. drafted manuscript; B.B., V.G.D., G.J., T.L.D., M.D.L., R.N., S.B.B., M.R.H., C.M.A., and J.R.S. edited and revised manuscript; B.B., J.H., V.G.D., G.J., T.L.D., M.D.L., A.R.A., R.N., S.B.B., M.G., M.R.H., L.M., C.M.A., and J.R.S. approved final version of manuscript; J.H., V.G.D., G.J., T.L.D., M.D.L., A.R.A., S.B.B., M.G., L.M., and C.M.A. performed experiments; J.H., V.G.D., G.J., T.L.D., M.D.L., A.R.A., R.N., S.B.B., M.G., M.R.H., L.M., and C.M.A. analyzed data; V.G.D., G.J., M.D.L., A.R.A., S.B.B., C.M.A., and J.R.S. conception and design of research.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Brenda Hunter for editorial assistance and Nathan Rehmer, Alex Meuth, Terry L. Carmack, and Lisa D. Watkinson for technical and administrative support. The authors also thank the Electron Microscope Core Center at the University of Missouri (Columbia, MO) for help with preparation of tissue samples.
REFERENCES
- 1.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin 8: 609–617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M, Whaley-Connell A, Demarco VG. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin resistant male Zucker obese rats. Endocrinology 154: 2501–2513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes 62: 313–319, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism 63: 1000–1011, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown NJ. Aldosterone and vascular inflammation. Hypertension 51: 161–167, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Cobbold SP. The mTOR pathway and integrating immune regulation. Immunology 140: 391–398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels A, van BM, Janssen BJ, Brouns AE, Cleutjens JP, Roemen TH, Schaart G, Van dV, van der Vusse GJ, van Nieuwenhoven FA. Impaired cardiac functional reserve in type 2 diabetic db/db mice is associated with metabolic, but not structural, remodelling. Acta Physiol (Oxf) 200: 11–22, 2010. [DOI] [PubMed] [Google Scholar]
- 8.de Gasparo M, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, Kraehenbuehl C, Biollaz M, Grob J, Schmidlin J, et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther 240: 650–656, 1987. [PubMed] [Google Scholar]
- 9.De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, Lee ET, Howard BV. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens 29: 1431–1438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demarco VG, Ford DA, Henriksen EJ, Aroor AR, Johnson MS, Habibi J, Ma L, Yang M, Albert CJ, Lally JW, Ford CA, Prasannarong M, Hayden MR, Whaley-Connell AT, Sowers JR. Obesity-related alterations in cardiac lipid profile and nondipping blood pressure pattern during transition to diastolic dysfunction in male db/db mice. Endocrinology 154: 159–171, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMarco VG, Johnson MS, Habibi J, Pulakat L, Gul R, Hayden MR, Tilmon R, Dellsperger KC, Winer N, Whaley-Connell AT, Sowers JR. Comparative analysis of telmisartan and olmesartan on cardiac function in the TG(mRen2)27 rat. Am J Physiol Heart Circ Physiol 300: H181–H190, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Stante B, Galandauer I, Aronow WS, McClung JA, Alas L, Salabay C, Belkin RN. Prevalence of left ventricular diastolic dysfunction in obese persons with and without diabetes mellitus. Am J Cardiol 95: 1527–1528, 2005. [DOI] [PubMed] [Google Scholar]
- 13.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 55: 300–305, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funder J. Mineralocorticoids and cardiac fibrosis: the decade in review. Clin Exp Pharmacol Physiol 28: 1002–1006, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol 217: 263–269, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res 7: 355–362, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol 2: 867–874, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Gul R, Demarco VG, Sowers JR, Whaley-Connell A, Pulakat L. Regulation of overnutrition-induced cardiac inflammatory mechanisms. Cardiorenal Med 2: 225–233, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 147: 5363–5373, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 117: 2253–2261, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habibi J, DeMarco VG, Ma L, Pulakat L, Rainey WE, Whaley-Connell AT, Sowers JR. Mineralocorticoid receptor blockade improves diastolic function independent of blood pressure reduction in a transgenic model of RAAS overexpression. Am J Physiol Heart Circ Physiol 300: H1484–H1491, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res 84: 164–172, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol 307: R1198–R1206, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 65: 531–539, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med 33: 26–34, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab 302: E201–E208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart 99: 320–326, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O'Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging 4: 1239–1249, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 23: 962–968, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, dos Remedios C, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation 121: 1474–1483, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci 92: 601–608, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manrique C, Demarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology 154: 3632–3642, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol 350: 256–265, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mensah GA, Treiber FA, Kapuku GK, Davis H, Barnes VA, Strong WB. Patterns of body fat deposition in youth and their relation to left ventricular markers of adverse cardiovascular prognosis. Am J Cardiol 84: 583–588, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Br J Pharmacol 135: 581–588, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol 31: 1871–1880, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oki T, Tabata T, Yamada H, Wakatsuki T, Shinohara H, Nishikado A, Iuchi A, Fukuda N, Ito S. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol 79: 921–928, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Orchard TJ. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med 28: 323–333, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, Demoss AJ, Dence CS, Gropler RJ. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 20: 802–810, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging 1: 424–433, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 43: 1399–1404, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLos One 7: e36814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370: 1383–1392, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Pulakat L, Demarco VG, Whaley-Connell A, Sowers JR. The impact of overnutrition on insulin metabolic signaling in the heart and the kidney. Cardiorenal Med 1: 102–112, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation–mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol 32: 1771–1776, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension 60: 362–368, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 107: 448–454, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Schafer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Luscher TF, Verrey F, Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 34: 3515–3524, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, Vaudo G, Mannarino E. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension 47: 881–886, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin 8: 619–631, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sciarretta S, Paneni F, Palano F, Chin D, Tocci G, Rubattu S, Volpe M. Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond) 116: 467–477, 2009. [DOI] [PubMed] [Google Scholar]
- 59.Selvaraju V, Joshi M, Suresh S, Sanchez JA, Maulik N, Maulik G. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease–an overview. Toxicol Mech Methods 22: 330–335, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 115: 79–96, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol 77: 1017–1020, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 161: 1773–1781, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today 12: 112–124, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: H2181–H2190, 2011. [DOI] [PubMed] [Google Scholar]
- 66.van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 9: 293–302, 2012. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe K, Thandavarayan RA, Harima M, Sari FR, Gurusamy N, Veeraveedu PT, Mito S, Arozal W, Sukumaran V, Laksmanan AP, Soetikno V, Kodama M, Aizawa Y. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy. Curr Cardiol Rev 6: 280–290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, Ming XF. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes Rev 13, Suppl 2: 58–68, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Zhou X, Ma L, Habibi J, Whaley-Connel AT, Hayden MR, Tilmon RD, Brown AN, DeMarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial tissue remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension 55: 880–888, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350: 1953–1959, 2004. [DOI] [PubMed] [Google Scholar]